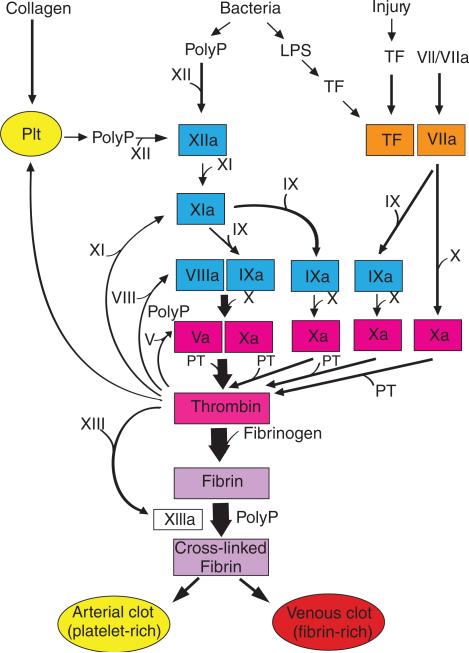
The clotting cascade has been traditionally divided into the extrinsic, intrinsic and common pathways [1,2]. The extrinsic pathway contains two proteins: a receptor called tissue factor and a zymogen/protease called factor VII/VIIa (FVII/FVIIa). A prothrombin time is determined by the addition of high levels of TF (thromboplastin) and phospholipids to plasma. In vivo, formation of a TF-FVIIa complex initiates the clotting cascade and generates small amounts of thrombin (Fig. 1). Thrombin links the initiation phase of the cascade with the propagation phase by activating various proteases, and the cofactors FV and FVIII, as well as platelets (Fig. 1). The extrinsic pathway is rapidly shut down by tissue factor pathway inhibitor, which inhibits the tissue factor-FVIIa complex in a FXa-dependent manner [3]. The intrinsic pathway has several components (FXII, FXI, FIX and FVIII) (Fig. 1). The pathway is activated at different points by FXIIa (also called Hageman factor), thrombin and the TF-FVIIa complex (Fig. 1). FXIIa activates the clotting cascade by sequential activation of FXIa and FIXa. In the presence of anionic surfaces small amounts of FXIIa are generated by a complex of high molecular weight kininogen and plasma kallikrein (referred to as the plasma contact system). Kaolin is used to activate FXIIa in the activated thromboplastin time assay. Prolongation of the clotting time occurs when there are deficiencies in the intrinsic and common pathways, particularly deficiencies in the hemophilic factors FVIII and FIX. Importantly, in contrast to other factors in the intrinsic pathway, FXII deficiency in humans and mice is not associated with clinical bleeding or obvious pathology [4,5]. This led to speculation that FXII was not involved in coagulation in vivo. Formation of the intrinsic tenase complex (FIXa–FVIIIa) and the prothrombinase complex (FVa–FXa) leads to the generation of large amounts of thrombin and cleavage of fibrinogen to fibrin (Fig. 1).
Fig. 1.
Different ways to activate the clotting cascade. The extrinsic pathway consists of TF and FVII/VIIa (orange) and is activated by either vascular injury or by TF expression within the vasculature. The intrinsic pathway consists of FXII, FXI, FIX and FVIII (turquoise) and is activated at various levels by polyP, thrombin and TF-FVIIa. The common pathway consists of FV and FX, and prothrombin (II) (pink). Finally, fibrin is converted into cross-linked fibrin by FXIIIa. Platelets (Plt) contribute to the clotting cascade by releasing polyP, which activates FXII, and by providing a surface for the assembly of the different complexes, such as the intrinsic tenase complex (FVIIIa–FIXa) and the prothrombinase complex (FVa–FXa). The clotting cascade can be activated by the generation of small amounts of thrombin via either the extrinsic pathway (TF-FVIIa to FXa or TF-FVIIa to FIXa to FXa), or via the intrinsic pathway (FXIIa to FXIa to FIXa to FXa). The propagation phase involves the generation of large amounts of thrombin by the prothrombinase complex (FVa–FXa). PolyP also modulates coagulation by accelerating the activation of FV and by enhancing fibrin polymerization.
In 1991, two papers [6,7] reported that thrombin could activate FXI and this activation was greatly enhanced by the presence of negatively charged surfaces. This observation provided an alternative route to activate the intrinsic pathway, and led to a diminished interest in FXII as a clotting factor. However, recent work by Renne and colleagues [8] showed that mice lacking FXII had a reduced thrombosis in various models of arterial thrombosis: collagen-induced thromboembolism; ferric chloride-injured mesenteric arterioles; mechanical injury of the aorta; and ligation of the carotid artery. The thrombi formed in the FXII-deficient mice were unstable. Importantly, all these models lead to the formation of a platelet-rich thrombus. These results were consistent with an earlier report showing that mice lacking FXI had reduced thrombosis in a ferric chloride carotid artery injury model [9]. Another study showed that deficiency or inhibition of FXII protects against ischemic brain injury [10]. In humans, FXI deficiency is a relatively frequent condition that is associated with a reduced incidence of ischemic stroke but not myocardial infarction [11]. No comparable data areavailablefor therelativelyrareFXII deficiency. Nevertheless, these mouse studies sparked renewed interest in the role of FXIIa in the clotting cascade. A number of potential natural activators of FXII have been described over the years, including collagen, RNA and bacterial endotoxin, but their role in activation of the clotting system is still debatable [12].
In 2006, Smith and colleagues [13] made the exciting observation that inorganic polyphosphate (polyP) modulates blood coagulation in several ways, including activating FXII and accelerating the activation of FV. Other studies by this group showed that polyP also enhances fibrin polymerization and binds to the high affinity exosite of thrombin [14,15]. This led to the suggestion that polyP may have utility as a general hemostatic agent [16]. Importantly, polyP is present in the dense granules of human platelets and is released upon activation. PolyP can also be released by bacteria. The paper by Muller and colleagues in the December 2009 issue of Cell [17] provides a detailed description of platelet polyP activation of FXII and how this protease subsequently activates the clotting cascade and proinflammatory pathways. Does this mean that we have found one of the natural activators of FXII? The answer is probably yes.
There are still many questions to answer. For instance, it will be difficult to determine the relative importance of platelet polyP activation of FXII, and possibly collagen activation of FXII, vs. the platelets themselves in arterial thrombosis (Fig. 1). Another important issue in studies with polyP is chain length. Bacteria have longer polymers of polyP (> 500 phosphate units) whereas platelets contain short polymers of polyP (60–100 phosphate units). This would suggest that bacterial infection may activate the clotting cascade via FXII (Fig. 1), which would reduce the dissemination of the bacteria. However, platelets have been shown by several groups to shorten the clotting time in a FXIIa-dependent manner [13,17,18]. Indeed, supplementary data in the Cell paper [17] indicate that a minimal size of 60 phosphate units is required for FXII activation with no enhancement with larger polymers. In contrast, a recent study by the Morrissey group showed that shorter polymers of polyP (mean polymer size of 75 phosphate units) normalized the clotting time in the absence of either FVIII or FIX, suggesting the shorter polymers modulate clotting via an intrinsic pathway-independent mechanism [16]. Clearly, there is more work needed to better understand the role of platelet polyP and other polyPs in the activation of FXII in vivo.
Because FXII participates in thrombosis but not hemostasis in mice, it has been proposed that hemostasis and thrombosis are distinct processes [18]. The extrinsic pathway is often depicted as the driver of hemostatic thrombin generation whereas the intrinsic pathway drives thrombosis. If true, FXII would be an ideal target for antithrombotic therapy because there would be no chance of bleeding. However, this view may be oversimplistic and assumes that thrombosis occurs in the same manner in a variety of conditions. This is unlikely to be correct. An alternative view is that the difference between the formation of a hemostatic clot and a thrombotic clot is the amount of thrombin that is generated. We propose that excessive activation of either the intrinsic pathway via FXIIa or the extrinsic pathway via the TF-FVIIa complex can lead to thrombosis.
What are the requirements for formation of a physiological hemostatic clot? The answer is an intact clotting system and platelets. Figure 1 shows that the clotting cascade has many clotting factors and various pathways that lead to thrombin generation and ultimately to fibrin formation. Platelets are also intimately involved in the clotting cascade by providing a surface for the assembly of the different clotting complexes (Fig. 1). Furthermore, the relative importance of these pathways may be different in different tissues [19]. For instance, the heart contains high levels of TF that maintain hemostasis, whereas skeletal muscle contains low levels of TF and is more dependent on the intrinsic pathway for hemostasis [19].
Results from knock-out mice have allowed us to classify these pathways into either essential or non-essential for embryonic survival and the hemostatic challenge of birth [19]. Components of the extrinsic (TF and FVII) and the common (FV, FX and prothrombin) pathways are essential for embryonic survival. In contrast, some components of the intrinsic pathway (FVIII and FIX) are not essential for survival but adult mice lacking these proteins exhibit excessive bleeding after hemostatic challenge, such as tail transection. A similar phenotype is observed in patients with hemophilia A or B. Mice deficient in FXI have been reported to have no obvious hemostatic defects and to have a normal tail bleeding time. However, D. Gailani has found that FXI-deficient mice have prolonged tail bleeding during treatment with a low dose of the platelet inhibitor clopidogrel compared with wild-type mice treated with the drug (D. Gailani, personal communication). Humans with FXI deficiency (hemophilia C) are either asymptomatic, present with a mild hemostatic defect or may exhibit excessive bleeding after certain hemostatic challenges [12]. Finally, mice and humans deficient in FXII have no hemostatic defects [5,6]. With respect to the intrinsic pathway, the severity of the hemostatic defects is proportional to the position of the factor in the pathway. For instance, FIXa plays a relatively central role in the clotting cascade whereas FXIa is required for an alternative pathway to FIXa generation. A deficiency of FXII would have a very minor effect on thrombin generation that is initiated by the TF-FVIIa complex during hemostasis because there are several alternative routes to FXIa and FIXa. This may explain why FXII deficiency in humans and mice is not associated with bleeding. Interestingly, cetaceans (whales and dolphins) lack FXII and birds lack both FXII and FXI [20], which supports the notion that these proteins play little to no role in hemostasis.
What are the requirements for formation of a pathological thrombotic clot? The answer is excessive thrombin generation and/or uncontrolled platelet activation. Excessive thrombin can come from either the extrinsic pathway or the intrinsic pathway. For instance, rupture of an atherosclerotic plaque will expose blood to large amounts of TF that may trigger thrombosis largely independently of the intrinsic pathway. In addition, TF within the vasculature may trigger thrombosis. Himber and colleagues [21] found that inhibition of TF reduced thrombosis on a collagen-coated thread installed in the jugular vein of rabbits and that thrombi contain leukocyte-associated TF. Other studies suggest that elevated levels of TF on microparticles, which are small membrane vesicles released from activated or apoptotic cells, may induce thrombosis [22]. Microparticle TF may be a major trigger for venous thrombosis in cancer patients [23]. In other pathological conditions, thrombosis may be driven by the intrinsic pathway, particularly in arterial thrombosis because of the proposed role of platelet polyP in the activation of FXII. For instance, administration of an antibody to FXI reduced thrombosis on a TF-coated Teflon graft in baboons [24]. Therefore, it appears that thrombotic clots can be driven by excessive activation of either the extrinsic or intrinsic pathways. It will be very interesting to see if inhibition of FXII or FXI will provide a safe and effective therapy to treat thrombosis.
Acknowledgements
This work was supported by grants from the National Institutes of Health to N. Mackman (HL095096 and HL006350) and to A. Gruber (HL058837 and AHA0850056Z). We would like to thank various colleagues for helpful suggestions (H. Roberts, M. Monroe, A. Wolberg, T. Renne and J. Morrissey) and unpublished data (D. Gailani).
Footnotes
To cite this article: Mackman N, Gruber A. Platelet polyphosphate: an endogenous activator of coagulation factor XII. J Thromb Haemost 2010; 8:865.7.
Addendum
Ruiz et al. were first to show that human dense granules contain inorganic phosphate [25].
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
References
- 1.Macfarlane RG. An enzyme cascade in the blood clotting mechanism, and its function as a biochemical amplifier. Nature. 1964;202:498–9. doi: 10.1038/202498a0. [DOI] [PubMed] [Google Scholar]
- 2.Davie EW, Ratnoff OD. Waterfall sequence for intrinsic blood clotting. Science. 1964;145:1310–2. doi: 10.1126/science.145.3638.1310. [DOI] [PubMed] [Google Scholar]
- 3.Broze GJ. Tissue factor pathway inhibitor. Thromb Haemost. 1995;74:90–3. [PubMed] [Google Scholar]
- 4.Ratnoff OD, Colopy JE. A familial hemorrhagic trait associated with a deficiency of a clot-promoting fraction of plasma. J Clin Invest. 1955;34:602–13. doi: 10.1172/JCI103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pauer HU, Renné T, Hemmerlein B, Legler T, Fritzlar S, Adham I, Müller-Esterl W, Emons G, Sancken U, Engel W, Burfeind P. Targeted deletion of murine coagulation factor XII gene-a model for contact phase activation in vivo. Thromb Haemost. 2004;92:503–8. doi: 10.1160/TH04-04-0250. [DOI] [PubMed] [Google Scholar]
- 6.Gailani D, Broze GJ. Factor XI activation in a revised model of blood coagulation. Science. 1991;253:909–12. doi: 10.1126/science.1652157. [DOI] [PubMed] [Google Scholar]
- 7.Naito K, Fukikawa K. Activation of human blood coagulation factor XI independent of factor XII. Factor XI is activated by thrombin and factor XIa in the presence of negatively charged surfaces. J Biol Chem. 1991;266:7353–8. [PubMed] [Google Scholar]
- 8.Renné T, Pozgajová M, Grüner S, Schuh K, Pauer HU, Burfeind P, Gailani D, Nieswandt B. Detective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202:271–81. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosen ED, Gailani D, Castellino FJ. FXI is essential for thrombus formation following FeCl3-induced injury of the carotid artery in the mouse. Thromb Haemost. 2002;87:774–6. [PubMed] [Google Scholar]
- 10.Kleinschnitz C, Stoll G, Bendszus M, Schuh K, Pauer HU, Burfeind P, Renné C, Gailani D, Nieswandt B, Renné T. Targeting coagulation factor XII provides protection from pathological thrombosis in cerebral ischemia without interfering with hemostasis. J Exp Med. 2006;203:513–8. doi: 10.1084/jem.20052458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seligsohn U. Factor XI deficiency in humans. J Thromb Haemost. 2009;7(Suppl. 1):84–7. doi: 10.1111/j.1538-7836.2009.03395.x. [DOI] [PubMed] [Google Scholar]
- 12.Gailani D, Renné T. The intrinsic pathway of coagulation: a target for treating thromboembolic disease? J Thromb Haemost. 2007;5:1106–12. doi: 10.1111/j.1538-7836.2007.02446.x. [DOI] [PubMed] [Google Scholar]
- 13.Smith SA, Mutch NJ, Baskar D, Rohloff P, Docampo R, Morrissey JH. Polyphosphate modulates blood coagulation and fibrinolysis. PNAS. 2006;103:903–8. doi: 10.1073/pnas.0507195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith SA, Morrissey JH. Polyphosphate enhances fibrin clot structure. Blood. 2008;112:2810–6. doi: 10.1182/blood-2008-03-145755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mutch NJ, Myles T, Leung LL, Morrissey JH. Polyphosphate binds with high affinity to exosite II of thrombin. J Thromb Haemost. 2010;8:548–55. doi: 10.1111/j.1538-7836.2009.03723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith SA, Morrissey JH. Polyphosphate as a general procoagulant agent. J Thromb Haemost. 2008;6:1750–6. doi: 10.1111/j.1538-7836.2008.03104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renné T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1–14. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walsh PN, Griffin JH. Contributions of human platelets to the proteolytic activation of blood coagulation factors XII and XI. Blood. 1981;57:106–18. [PubMed] [Google Scholar]
- 19.Mackman N. Tissue-specific hemostasis in mice. Arterioscler Thromb Vasc Biol. 2005;25:2273–81. doi: 10.1161/01.ATV.0000183884.06371.52. [DOI] [PubMed] [Google Scholar]
- 20.Emsley J, McEwan PA, Gailani D. Structure and function of factor XI. Blood. 2010;115:2569–77. doi: 10.1182/blood-2009-09-199182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Himber J, Wohlgensinger C, Roux S, Damico LA, Fallon JT, Kirchhofer D, Nemerson Y, Riederer MA. Inhibition of tissue factor limits the growth of venous thrombus in the rabbit. J Thromb Haemost. 2003;1:889–95. doi: 10.1046/j.1538-7836.2003.00110.x. [DOI] [PubMed] [Google Scholar]
- 22.Mackman N. The many faces of tissue factor. J ThrombHaemost. 2009;7(Suppl 1):136–9. doi: 10.1111/j.1538-7836.2009.03368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasthuri RS, Taubman MB, Mackman N. Role of tissue factor in cancer. J Clin Oncol. 2009;27:4834–8. doi: 10.1200/JCO.2009.22.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gruber A, Hanson SR. Factor XI-dependence of surface- and tissue factor-initiated thrombus propagation in primates. Blood. 2003;102:953–5. doi: 10.1182/blood-2003-01-0324. [DOI] [PubMed] [Google Scholar]
- 25.Ruiz FA, Lea CR, Oldfeld E, Docampo R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J Biol Chem. 2004;279:44250–7. doi: 10.1074/jbc.M406261200. [DOI] [PubMed] [Google Scholar]