Abstract
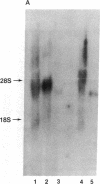
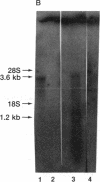
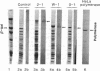
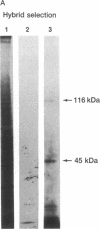
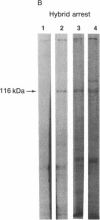
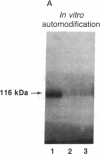
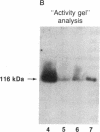
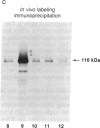
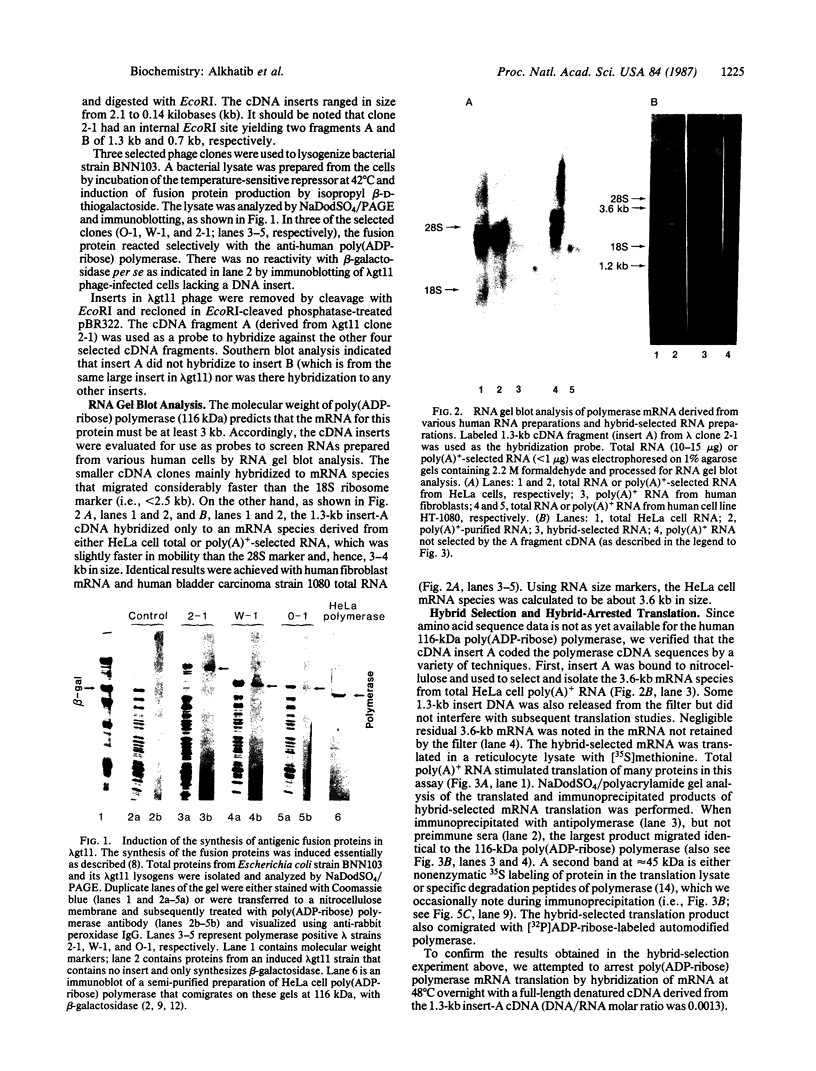
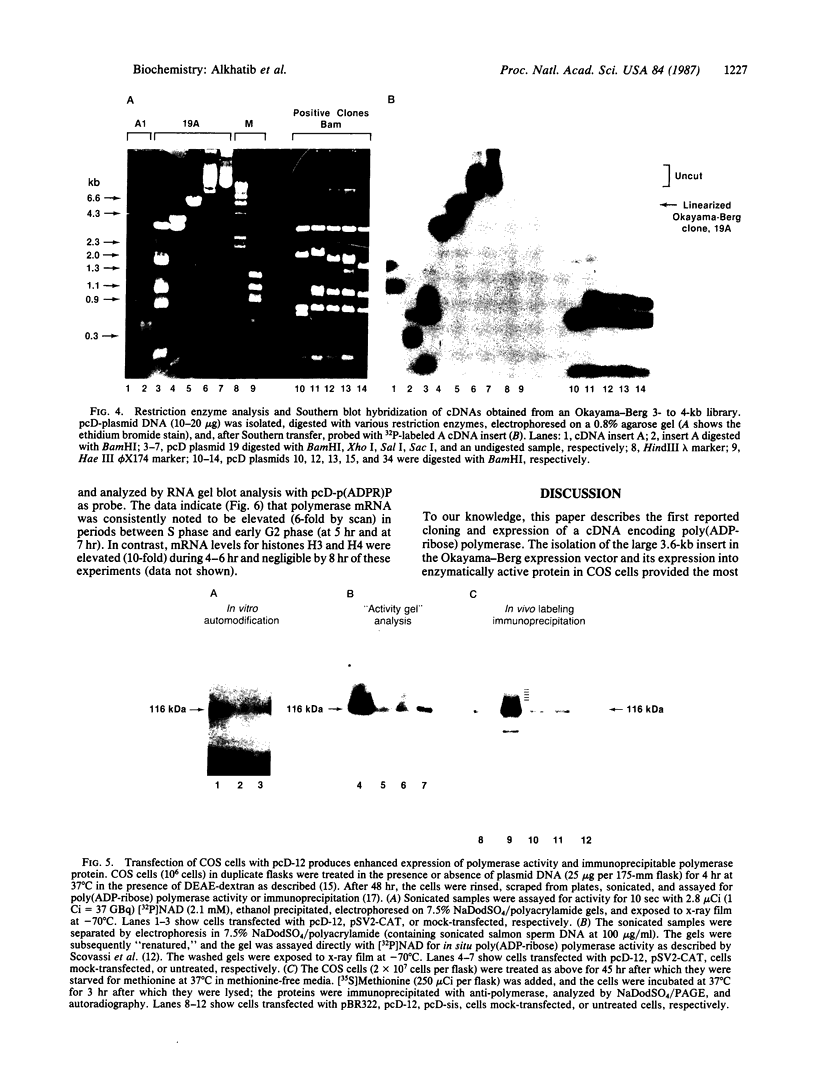
cDNAs encoding poly(ADP-ribose) polymerase from a human hepatoma lambda gt11 cDNA library were isolated by immunological screening. One insert of 1.3 kilobases (kb) consistently hybridized on RNA gel blots to an mRNA species of 3.6-3.7 kb, which is consistent with the size of RNA necessary to code for the polymerase protein (116 kDa). This insert was subsequently used in both in vitro hybrid selection and hybrid-arrested translation studies. An mRNA species from HeLa cells of 3.6-3.7 kb was selected that was translated into a 116-kDa protein, which was selectively immunoprecipitated with anti-poly (ADP-ribose) polymerase. To confirm that the 1.3-kb insert from lambda gt11 encodes for poly(ADP-ribose) polymerase, the insert was used to screen a 3- to 4-kb subset of a transformed human fibroblast cDNA library in the Okayama-Berg vector. One of these vectors [pcD-p(ADPR)P; 3.6 kb] was tested in transient transfection experiments in COS cells. This cDNA insert contained the complete coding sequence for polymerase as indicated by the following criteria: A 3-fold increase in in vitro activity was noted in extracts from transfected cells compared to mock or pSV2-CAT transfected cells. A 6-fold increase in polymerase activity in pcD-p(ADPR)P transfected cell extracts compared to controls was observed by "activity gel" analysis on gels of electrophoretically separated proteins at 116 kDa. A 10- to 15-fold increase in newly synthesized polymerase was detected by immunoprecipitation of labeled transfected cell extracts. Using pcD-p(ADPR)P as probe, it was observed that the level of poly(ADP-ribose) polymerase mRNA was elevated at 5 and 7 hr of S phase of the HeLa cell cycle, but was unaltered when artificial DNA strand breaks are introduced in HeLa cells by alkylating agents.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benjamin R. C., Gill D. M. Poly(ADP-ribose) synthesis in vitro programmed by damaged DNA. A comparison of DNA molecules containing different types of strand breaks. J Biol Chem. 1980 Nov 10;255(21):10502–10508. [PubMed] [Google Scholar]
- Berger N. A., Sikorski G. W., Petzold S. J., Kurohara K. K. Defective poly(adenosine diphosphoribose) synthesis in xeroderma pigmentosum. Biochemistry. 1980 Jan 22;19(2):289–293. doi: 10.1021/bi00543a006. [DOI] [PubMed] [Google Scholar]
- Cherney B. W., Midura R. J., Caplan A. I. Poly(ADP-ribose) synthetase and chick limb mesenchymal cell differentiation. Dev Biol. 1985 Nov;112(1):115–125. doi: 10.1016/0012-1606(85)90125-3. [DOI] [PubMed] [Google Scholar]
- Fagan J. B., Pastewka J. V., Park S. S., Guengerich F. P., Gelboin H. V. Identification and quantitation of a 2.0-kilobase messenger ribonucleic acid coding for 3-methylcholanthrene-induced cytochrome P-450 using cloned cytochrome P-450 complementary deoxyribonucleic acid. Biochemistry. 1982 Dec 7;21(25):6574–6580. doi: 10.1021/bi00268a039. [DOI] [PubMed] [Google Scholar]
- Juarez-Salinas H., Sims J. L., Jacobson M. K. Poly(ADP-ribose) levels in carcinogen-treated cells. Nature. 1979 Dec 13;282(5740):740–741. doi: 10.1038/282740a0. [DOI] [PubMed] [Google Scholar]
- Jump D. B., Butt T. R., Smulson M. Nuclear protein modification and chromatin substructure. 3. Relationship between poly(adenosine diphosphate) ribosylation and different functional forms of chromatin. Biochemistry. 1979 Mar 20;18(6):983–990. doi: 10.1021/bi00573a008. [DOI] [PubMed] [Google Scholar]
- Kidwell W. R., Mage M. G. Changes in poly(adenosine diphosphate-ribose) and poly(adenosine diphosphate-ribose) polymerase in synchronous HeLa cells. Biochemistry. 1976 Mar 23;15(6):1213–1217. doi: 10.1021/bi00651a006. [DOI] [PubMed] [Google Scholar]
- Malik N., Bustin M., Smulson M. Antibody to poly(adenosine diphosphate-ribose) polymerase and its use in chromatin analysis. Nucleic Acids Res. 1982 May 11;10(9):2939–2950. doi: 10.1093/nar/10.9.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik N., Miwa M., Sugimura T., Thraves P., Smulson M. Immunoaffinity fractionation of the poly(ADP-ribosyl)ated domains of chromatin. Proc Natl Acad Sci U S A. 1983 May;80(9):2554–2558. doi: 10.1073/pnas.80.9.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. S., Paterson B. M., Ricciardi R. P., Cohen L., Roberts B. E. Methods utilizing cell-free protein-synthesizing systems for the identification of recombinant DNA molecules. Methods Enzymol. 1983;101:650–674. doi: 10.1016/0076-6879(83)01046-0. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B., Boorstein R. J., Lau C. C. Interference with DNA repair mechanisms of mammalian cells: cell cycle dependence. Princess Takamatsu Symp. 1983;13:287–294. [PubMed] [Google Scholar]
- Scovassi A. I., Stefanini M., Bertazzoni U. Catalytic activities of human poly(ADP-ribose) polymerase from normal and mutagenized cells detected after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1984 Sep 10;259(17):10973–10977. [PubMed] [Google Scholar]
- Slattery E., Dignam J. D., Matsui T., Roeder R. G. Purification and analysis of a factor which suppresses nick-induced transcription by RNA polymerase II and its identity with poly(ADP-ribose) polymerase. J Biol Chem. 1983 May 10;258(9):5955–5959. [PubMed] [Google Scholar]
- Smulson M. E., Schein P., Mullins D. W., Jr, Sudhakar S. A putative role for nicotinamide adenine dinucleotide-promoted nuclear protein modification in the antitumor activity of N-methyl-N-nitrosourea. Cancer Res. 1977 Sep;37(9):3006–3012. [PubMed] [Google Scholar]
- Stein G. S., Borun T. W. The synthesis of acidic chromosomal proteins during the cell cycle of HeLa S-3 cells. I. The accelerated accumulation of acidic residual nuclear protein before the initiation of DNA replication. J Cell Biol. 1972 Feb;52(2):292–307. doi: 10.1083/jcb.52.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thraves P. J., Kasid U., Smulson M. E. Selective isolation of domains of chromatin proximal to both carcinogen-induced DNA damage and poly-adenosine diphosphate-ribosylation. Cancer Res. 1985 Jan;45(1):386–391. [PubMed] [Google Scholar]
- Wong M., Kanai Y., Miwa M., Bustin M., Smulson M. Immunological evidence for the in vivo occurrence of a crosslinked complex of poly(ADP-ribosylated) histone H1. Proc Natl Acad Sci U S A. 1983 Jan;80(1):205–209. doi: 10.1073/pnas.80.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]