Abstract
Improved biodegradable vascular grafts and stents are in demand, particularly for pediatric patients. Poly(l-lactic acid) (PLLA) is an FDA-approved biodegradable polymer of potential use for such applications. However, tissue culture studies have shown that endothelial cell (EC) attachment and growth occurs relatively slowly on PLLA surfaces. This slow growth has been attributed to the fact that PLLA represents a hydrophobic substrate, relatively devoid of active functional groups. As a result, the slow EC recovery on the luminal side of PLLA stents provides an increased risk of induced thrombosis. In the present study, surface modification of PLLA substrates has been examined as a potential route to enhance EC growth. For this purpose, PLLA surfaces were modified via pulsed plasma deposition of thin films of poly(vinylacetic acid). The –COOH surface groups, introduced by the plasma deposition, were employed to conjugate fibronectin (FN), followed by attachment of vascular endothelial growth factor to FN. Pig Aorta ECs (PAE) and kinase-insert domain-containing receptor (KDR)-transfected PAE showed increased cell adhesion and proliferation, as well as substantially improved cell retention under fluidic shear stress on surface-modified PLLA compared with untreated PLLA. Although KDR-transfected PAE exhibited better cell proliferation than PAE, normal EC functions, including EC morphology, nitric oxide production, and KDR expression, were observed when cells were grown on surface-modified PLLA. The results obtained clearly indicate that this combined surface modification technique using poly(vinylacetic acid) deposition, FN conjugation, and vascular endothelial growth factor surface delivery can enhance endothelialization on PLLA, particularly when employed in conjunction with the growth of KDR-transfected ECs.
Introduction
Angioplasty and stenting have been widely used in the United States over the last two decades to treat vascular stenosis. Metals such as stainless steel and nitinol are the major materials for vascular stents; however, they are permanently stable and almost impossible to remove once inserted into an artery. This limits their use for children and young patients whose vasculatures are still growing and having congenital diseases such as branched pulmonary artery stenosis, coarctation of aorta, and superior vene cava stenosis. Although adult vascular stents are often used as an off-label manner in pediatric patients, these stiff metal stents could impair the growth and development of the vasculatures for the young ones.1 Additionally, metal stents could interfere with vasculature remodeling due to their rigidity and permanence.2 Other limitations of metal stents include thrombogenecity and possible erosion.3 To overcome these limitations, biodegradable polymers are receiving increasing attention as new structural materials for vascular stents. In light of the inherent bioabsorbability of such materials, success in this area would help overcome the problems noted above, particularly those involving pediatric care.
Poly(l-lactic acid) (PLLA), a material that has received FDA approval for implants, is the most promising biodegradable synthetic polymers identified to date. For example, its use extends to a wide variety of implantable medical devices, including sutures, dental devices, and orthopedic plates and screws.4–7 Due to its high mechanical strength and comparatively long degradation period, PLLA is an ideal material for use as a fully biodegradable vascular stent. The safety and feasibility of a PLLA stent was first reported by Tamai and his colleagues early in 2000,8 and more recently, the IGAKI-TAMAI® bioabsorbable PLLA stent received approval for use by the European Union in 2007. Additionally, in the United States, Abbott's vascular clinical studies of PLLA-based everolimus-eluting stents exhibited minimal intrastent neointimal hyperplasia after 1 year of implantation.9 Thus current research suggests that PLLA is a promising material for use in biodegradable stents.
Despite the promising results noted above, concerns remain with respect to the biocompatibility of PLLA stents, especially their endothelialization after stent implantation. For example, recovery of vascular endothelial cells (ECs) on the luminal side of stents, also called re-endothelialization, is believed to play an important role in preventing late (>1 year) stent thrombosis.10,11 The EC lining along the blood vessel lumen has natural antithrombotic properties by producing a number of antithrombotic factors such as nitric oxide (NO), prostacylcin, plasminogen, and thrombomodulin.12–14 Therefore, it is generally believed that enhancing the development of functional ECs on vascular prostheses could help prevent late stent thrombosis. Unfortunately, PLLA has a very low affinity for ECs due to its high hydrophobicity, coupled with a lack of active surface functional groups.15 As a result, various surface modification techniques have been employed to enhance PLLA's affinity for these cells. One widely used method is to coat extracellular matrix (ECM) proteins such as collagen or fibronectin (FN) on the PLLA surface. However, physical adsorption of certain proteins is generally fairly weak, and they can be quickly displaced by other proteins. Additionally, the adsorbed molecules are poorly resistant to the fluidic sheer stress presented in arteries. In principle, covalent binding of these biomolecules to the stent surfaces would represent a sufficiently strong interaction to resist desorption and thus retain the ECM molecules. However, a difficulty encountered with respect to covalent attachment of molecules to PLLA is the absence of reactive surface groups. Thus, to increase its covalent binding capacity for ECM proteins, the PLLA substrates must be initially surface modified to provide surfaces with more active groups for this purpose.
In the present study, the feasibility of surface modification to achieve improved endothelialization on PLLA substrates was explored. For this purpose, radio-frequency (RF) plasma polymerization, operated in a pulsed mode, was employed. As shown in several prior studies, the pulsed plasma approach provides a simple and convenient technique to introduce reactive surface functionalities, for example, –NH2 and –COOH groups.16,17 In the current study, –COOH groups were deposited on PLLA in the form of a polymerized vinyl acetic acid (PVAA) thin film, and subsequent molecular tailoring of these surfaces included covalent coupling of FN to plasma-deposited –COOH groups, followed by attachment of growth factor vascular endothelial growth factor (VEGF) to promote EC surface adhesion and proliferation. VEGF is a well-known angiogenic growth factor stimulating EC proliferation and migration.18,19 VEGF exerts its mitogenic function mainly through binding to kinase-insert domain-containing receptor (KDR), also called VEGF receptor-2, on human ECs. We hypothesize that gene-transfected ECs, which overexpress KDR, will promote higher proliferation of ECs and thus enhance endothelialization of PLLA. As detailed below, this surface tailoring approach, particularly when coupled with KDR-transfected ECs, provides a very large increase in cell growth as studied with the use of Pig Aorta ECs (PAE).
Materials and Methods
Materials
PLLA (L210 Resomer, MW = 130,000 g/mol) was purchased from Boehringer Ingelheim, Inc. Human FN was obtained from Chemicom, Inc. Vinylacetic acid (VAA), 2-(N-morpholino)ethanesulfonic acid, and 1-[3-(dimethylamino) propyl]-3-ethylcarbodiimide (EDC) were received from Sigma, Inc. Rabbit anti-human FN polyclonal antibody and bovine anti-rabbit IgG-fluorescein isothiocyanate (FITC) antibody were obtained from Santa Cruz Biotechnology, Inc. VEGF165, human VEGF enzyme-linked immunosorbent assay kit, Measure-iT™ high-sensitive nitrite assay kit, and Picogreen DNA assay were purchased from Invitrogen, Inc. CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay was obtained from Promega, Inc. Mouse anti-human VEGF receptor-2 IgG antibody was purchased from Zymed Invitogen, Inc. Goat anti-mouse IgG-FITC antibody was a product of Jackson ImmunoResearch Laboratories, Inc. All the other chemicals were from Sigma-Aldrich, Inc.
PLLA film preparation
PLLA powder was dissolved in chloroform; a 2.5% (w/v) solution was cast into 12-cm-diameter Teflon Petri dishes and allowed to dry completely. The thickness of the PLLA films was ∼500 μm, and its surface was fairly smooth, as observed with scanning electron microscopy (SEM). PLLA film was cut into round discs (1.5 cm in diameter), glued onto round glass cover slips with medical-grade UV glue, and then placed in 24-well plates. The films were washed with ethanol, de-ionized water, and sterilized under UV for 30–45 min before use. For cell retention studies, PLLA films were cast on glass slides.
Surface modification of PLLA by plasma deposition of PVAA
The PLLA substrates were coated with thin plasma-deposited polymeric films using a bell-shaped RF plasma reactor. Details of the plasma reactor used for this work were described elsewhere.20 An RF power supply (13.56 MHz) was connected capacitively to two external electrodes. The monomer employed, namely, vinyl acetic acid (VAA), was subjected thrice to freeze/thaw cycles to remove dissolved gases before use. The system was evacuated to <5 mtorr and then purged three times with monomer vapor. The working pressure was adjusted to 160 mtorr as controlled by a butterfly valve. The monomer vapor flow rate was 250 cc/min. The system was operated at room temperature. The thickness of all PVAA films was ∼100 nm. In prior studies from this laboratory, it had been demonstrated that the surface density of –COOH groups retained during the polymerization of vinylacetic is readily controlled under pulsed plasma conditions.21 In the present case, depositions were carried using a 2/30 duty cycle (time ON/time OFF in ms) pulsed plasma discharge. Although the peak power was 150 W, the average power was significantly less in view of the pulsed mode operation. The average power is computed from the plasma duty cycle (ratio of ON time to the sum of the ON plus OFF time) multiplied by the peak power. Thus, the average power input was 9.4 W for the 2/30 pulses.
The film compositions were characterized by FT-IR spectroscopy and X-ray photoelectron spectroscopy (XPS). FT-IR spectra of the plasma-deposited PVAA films were obtained using a Brucker Model Vector 22, with spectra recorded using 4 cm−1 resolution. The XPS spectra were acquired using a Kratos Axis Ultra DLD instrument equipped with a monochromator operated at a pass energy of 10.0 eV. A neutralizer was used in these measurements since the samples were nonconductive. The neutralizer was operated at a current of 1.7A and charge balance of 3.4 V. The high-resolution XPS spectra were analyzed (deconvoluted) using Casa XPS software. The binding energy (BE) of the carbon atoms not directly bonded to any heteroatoms was centered at 284.6 eV. XPS data were acquired for the various samples starting with the unmodified PLLA films and continuing through the sequence of surface modifications involving the plasma depositions and attachment of FN.
Static water contact angles measurements were made for the different surfaces using a Rame-Hart goniometer, model number 100-00-115. Distilled water was employed for these measurements. Contact angles were measured at three different spots for each surface examined. Virtually no variation in contact angle (±2%) was observed for each sample. The average value of these three measurements is reported in the Results section.
Conjugation of FN on PLLA film
Fluorescence labeling studies were employed to characterize the extent of covalent coupling of human FN to plasma-modified PLLA substrates. In these studies human FN was conjugated to PLLA substrates using two methods, passive adsorption, and covalent coupling. For passive adsorption, 500 μL FN solution (33 μg/mL) was added on top of each PLLA film and incubated for 4 h at room temperature. For covalent coupling, PVAA-modified PLLA films were treated with EDC (10 mg/mL, in 0.1 M 2-(N-morpholino)ethanesulfonic acid buffer) for 20 min to activate the –COOH groups on the film surface, followed by incubation with 500 μL FN solution (33 μg/mL) for 4 h at room temperature with gentle agitation, a two-step method similar to the protocol published by Bang's Lab.22–24 After conjugation with FN, the PLLA films were rinsed gently with PBS buffer to remove any loosely attached proteins.
To quantify the amount of FN conjugated on these surfaces, the PLLA films were incubated with rabbit anti-human FN polyclonal antibody as the primary antibody, followed by bovine anti-rabbit IgG-FITC antibody as the secondary antibody. After removal of unbound antibodies, by thorough rinsing with PBS, the PLLA films were treated with 2% SDS for 2 h, with constant agitation, to strip off the antibodies. The fluorescence intensity of antibodies, which correlates with the amount of surface-bound FN, was measured using Versafluor™ fluorometer (Bio-Rad).
Conjugation and release of VEGF on PLLA film
The surface conjugation of FN has been shown to permit the subsequent incorporation of VEGF through its specific binding domains for VEGF.25,26 After conjugation with FN as described above, PLLA films were washed once with ice-cold binding buffer (containing 25 mM HEPES and 0.1% BSA, pH 5.5), and then incubated with 250 ng VEGF165 dissolved in the same binding buffer for 2.5 h at 4°C. After incubation, the PLLA films were gently washed with PBS.
To quantify the release of surface-conjugated VEGF, the films were incubated with 1 mL of PBS at 37°C for up to 5 days with constant agitation. At preset times (1, 2, 3, and 5 days), 0.5 mL PBS containing released VEGF were collected and replaced with 0.5 mL fresh PBS. A human VEGF enzyme-linked immunosorbent assay kit was used to measure the VEGF concentration in the releasing medium following the manufacturer's instructions. The VEGF release was plotted as a function of incubation time.
Vascular endothelial and smooth muscle cell proliferation on surface modified PLLA films
PAE and stable KDR-transfected PAE (KDR-PAE) were generous gifts from Dr. Rolf Brekken from the University of Texas Southwestern Medical Center. Using ECs from animals such as porcine to study the cell–substrate interaction is widely accepted. Using ECs from comparatively large animals, such as pigs, in the present study is based on the consideration that we plan to evaluate the effectiveness of transplantation of surface-modified PLLA vascular grafts and/or stents in vivo using pigs in subsequent studies. The KDR-PAE cells were transfected using electroporation. In this process, cell suspensions are transferred to a chamber containing two aluminum electrodes 0.4 cm apart. The cells were subjected to two electrical pulses, as produced by a discharge of 2.5 kV/cm and 0.9 mF, to introduce a full-length KDR cDNA constructed in the cytomegalovirous-based eukaryotic expression vector pcDNAI/Neo as previously described.19 PAE and KDR-PAE were cultured using F-12 cell culture medium supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin. Cells between passages 10 and 13 were used in all experiments. Human aortic smooth muscle cells (HASMCs) were purchased from Cascade Biologics, Inc. They were cultured using Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin. Cells between passages 6 and 8 were used in all experiments.
For the cell adhesion and proliferation studies, three groups of PLLA films were tested: (1) untreated PLLA films; (2) PLLA films treated with PVAA and covalently conjugated with FN via EDC [PLLA-PVAA-(EDC)-FN]; (3) PLLA films treated with PVAA, covalently conjugated with FN via EDC and with VEGF incorporation [PLLA-PVAA-(EDC)-FN-VEGF]. PAE, KDR-PAE, or HASMC cells were seeded on these films at 5000 cells/cm2, and cultured in an incubator at 37°C for up to 5 days. At preset times of 1, 3, and 5 days, cell adhesion and proliferation was assessed using CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay following the manufacturer's instructions.
Measurement of NO production from ECs
PAE and KDR-PAE cells were seeded on untreated PLLA, PLLA-PVAA-(EDC)-FN, or PLLA-PVAA-(EDC)-FN-VEGF films and cultured as described above. The complete DMEM was replaced with phenol red-free complete DMEM (as recommended by the manufacturer of the Nitrite Assay Kit) for this study. At day 1, 3, and 5, the cell culture medium was collected, frozen at −80°C, and freeze-dried. The lyophilized medium was dissolved in 5 μL DI water, and the nitrite concentrations in these samples were measured using Measure-iT high-sensitiviy nitrite assay kit following the manufacturer's instructions.
Immunofluorescence staining of KDR
PAE and KDR-PAE cells were grown on PLLA-PVAA-(EDC)-FN-VEGF films or untreated PLLA films for 3 days. For immunofluorescence staining, the cells were fixed with 2% formaldehyde and treated with mouse anti-human KDR antibody as the primary antibody, followed by goat anti-mouse IgG-FITC antibody as the secondary antibody. Stained cells were observed by a fluorescent microscope (Zeiss).
SEM of cells grown on modified PLLA
PAE grown on untreated PLLA films or KDR-PAE grown on PLLA-PVAA-(EDC)-FN-VEGF films for 5 days were fixed with 1.5% gluteraldehyde for 20 min, postfixed with 1% osmium tetroxide for 1 h, and then dehydrated with alcohol (50%, 75%, 90%, and 100% in sequence) as previously described.27 After complete drying, the samples were sputter-coated with silver and then examined with SEM (Hitachi S-3000N).
EC retention under fluid shear stress
An in vitro parallel flow system was used to assess the cell retention under fluidic shear stress.28 The PAE and KDR-PAE cells were grown on either PLLA-PVAA-(EDC)-FN-VEGF films or untreated PLLA films for 5 days. The cells were then exposed to the cell culture medium flowing over the cells with a shear stress of 15 dyn/cm2 for 30 min. After this flow exposure, the cells remaining on the PLLA films were lysed with 1% Triton X-100. The total amount of cell DNA, which correlates with total cell counts, was measured with PicoGreen DNA assays. Cells not exposed to flow were also analyzed as controls.
Statistical analysis
Results were shown as mean ± SD. Analysis of the results was performed using one sample t-test, and n = 3–6 for each data point. p < 0.05 was regarded as significant differences existing between groups.
Results
Spectroscopic characterization of surfaces employed
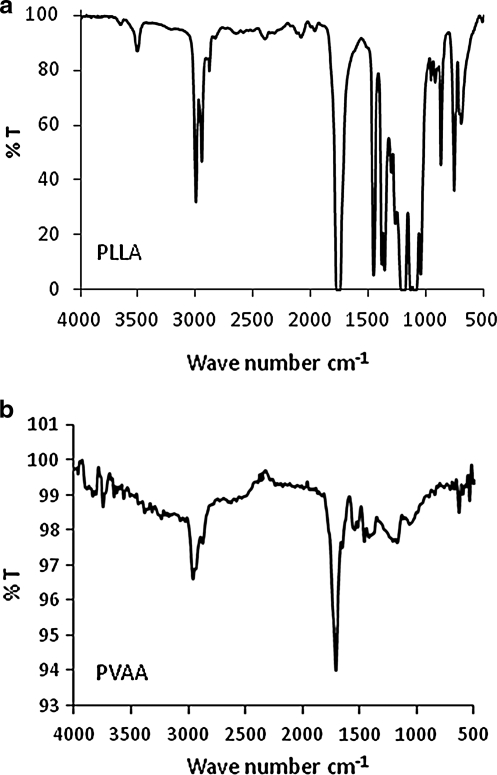
FT-IR spectra of the synthesized PLLA films employed, along with a spectrum of the plasma-generated PVAA film, are shown in Figure 1. The PVAA was deposited on a KBr substrate. These spectra are important with respect to presence and absence of –COOH groups in these films. As shown in the PLLA spectrum (Fig. 1a), there is virtually no absorption in the spectral region extending from around 3400–3000 cm−1. In contrast, the PVAA film exhibits a very broad band, extending from ∼3400 to 2600 cm−1. This broad band is uniquely associated with the presence of –COOH groups.29 The absorbance bands around 3000–2850 cm−1 are those associated with C–H stretching vibrations, as expected from the presence of C–H bonds in each film. The additional bands, including particularly the strong absorptions at ∼1750 cm−1 characteristic of C=O from carboxyl groups, are in accord with the expected film compositions. Thus, the IR data confirm the absence of –COOH in the PLLA and the presence of such groups in the PVAA-modified PLLA.
FIG. 1.
FT-IR absorption spectra of PLLA (a) and PVAA (b) films. PLLA, poly(l-lactic acid); PVAA, poly(vinylacetic acid).
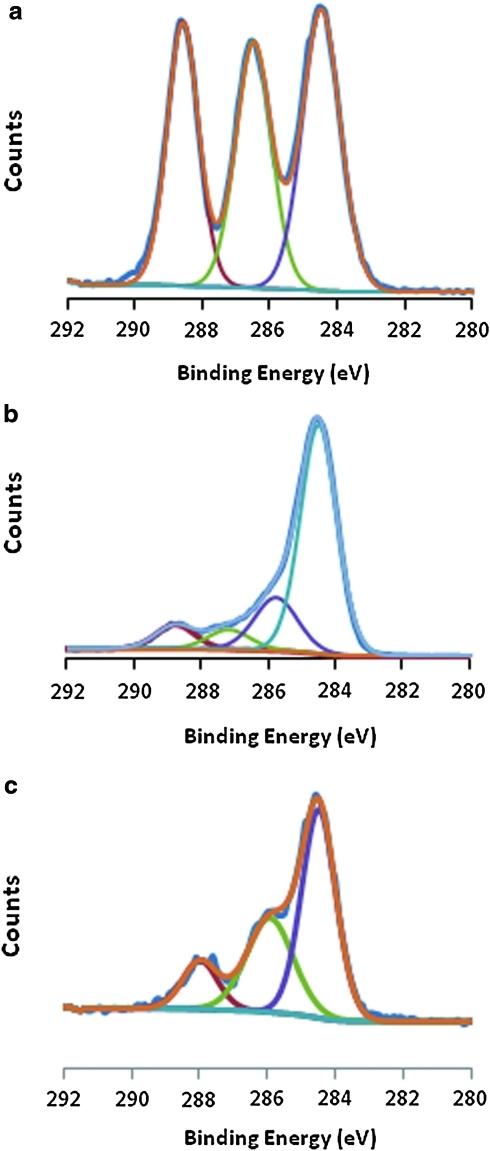
XPS spectra of the various surfaces employed in this study are shown in Figures 2 and 3. The high-resolution C(1s) spectra obtained from pure PLLA (Fig. 2a), PVAA film deposited on the PVAA (Fig. 2b), and PLLA-PVAA after conjugation with FN (Fig. 2c) are presented. The XPS of the pure PLLA film is in excellent accordance with the accepted spectrum of this polymer as previously described.30 The three peaks shown in the C(1s) spectrum corresponded to the presence of saturated C atoms not directly bonded to O atoms (BE, 284.6 eV); C atoms singly bonded to O atoms of a carboxyl group, that is, –C-O-C(=O) (BE 286.5 eV); and C atoms of the carboxyl group –C-O-C(=O) (BE 288.5 eV). Deposition of the PVAA film on the PLLA dramatically changed the C(1s) spectrum, as shown in Figure 2b. The much enhanced peak at 284.6 eV, relative to the other peaks, is consistent with the higher content of saturated C atoms not bonded to O atoms in the VAA monomer to that of lactic acid. The C(1s) peaks at the intermediate binding energies of 285.5 and 287 eV could be assigned to –OH and carbonyl groups produced from partial fragmentation or rearrangement of the VAA during plasma exposure, as generally observed in plasma polymerizations.31 The peak at 288.5 eV, representing 9% of the total carbon content,32 is that of the carboxyl groups of the starting VAA that are retained in the plasma film. A further change in the C(1s) spectrum is easily observed after covalent attachment of the FN, as shown in Figure 2c. Most notable are the increases in the high BE peak at ∼288 eV and the intermediate BE peak around ∼286.5 eV. The relative increased prominence of these peaks, compared with PVAA, is consistent with presence of –COOH and amide groups corresponding to the presence of the FN. Since this protein is presumably present as a monolayer, it is important to recognize that photoelectrons from the underlying PVAA film undoubtedly contribute to this spectrum under the 70° take-off angle employed, thus complicating more quantitative discussion.
FIG. 2.
High-resolution X-ray photoelectron spectroscopy C (1s) spectra of PLLA (a), PVAA (b), and PLLA-PVAA-(EDC)-FN (c). FN, fibronectin; EDC, 1-[3-(dimethylamino) propyl]-3-ethylcarbodiimide. The deconvoluted curves, indicated in each spectrum by colored curves, correspond to photoelectrons from C atoms of differing functionalities, as identified explicitly in the manuscript. Color images available online at www.liebertonline.com/ten.
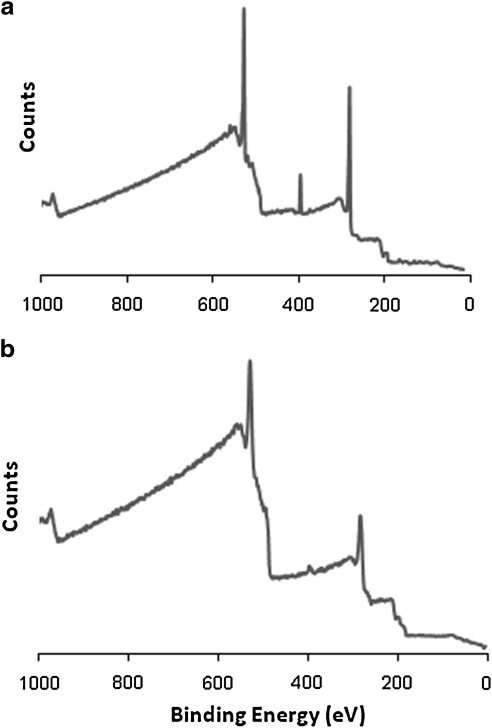
FIG. 3.
X-ray photoelectron spectroscopy survey scan of PLLA-PVAA-(EDC)-FN (a) and PLLA-FN (b).
Additional evidence of successful conjugation of FN to PVAA-modified PLLA surfaces was documented by XPS survey scans, as shown in Figure 3. This Figure shows the comparison of results from a spectrum of FN covalently bonded to the modified surface, PLLA-PVAA-(EDC)-FN (Fig. 3a) compared with that of FN simply adsorbed on the original PLLA surface (PLLA-FN, Fig. 3b). The important distinction between these two spectra is the prominent N(1s) peak at 400 eV in Figure 3a compared with the trace quantity in the spectrum of Figure 3b. The nitrogen atom content of the covalently coupled FN sample was 8.8% as compared with only 2.3% for the physically adsorbed sample. The higher nitrogen atom content of the EDC-coupled FN provides added confirmation of the covalent bonding of the FN to the plasma-modified surface, as opposed to simple physical adsorption. Additionally, the fact that the covalently bonded FN surface does not exhibit a much higher N atom content is consistent with its presence as a monolayer.
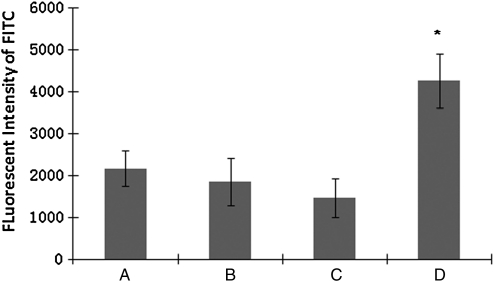
Covalent conjugation of FN on the PVAA-modified PLLA films was also documented by fluorescence measurement of fluorescence (FITC)-labeled antibodies. As shown in Figure 4, the amount of FN passively adsorbed on untreated PLLA (PLLA-FN) or PVAA-modified PLLA (PLLA-PVAA-FN) was essentially the same. In contrast, a marked increase of covalently coupled FN, in excess of 100%, was observed on the PVAA-modified PLLA sample (PLLA-PVVA-(EDC)-FN) compared with other control samples. The control samples included unmodified PLLA (PLLA-FN), PLLA exposed to EDC and FN but not plasma treated (PLLA-(EDC)-FN), and PVAA-modified PLLA exposed to FN in the absence of EDC exposure (PLLA-PVAA-FN).
FIG. 4.
Fluorescent intensity measurement of FN coated on PLLA or PVAA-treated PLLA, with or without EDC coupling, as shown. (A) PLLA-FN, (B) PLLA-PVAA-FN, (C) PLLA-(EDC)-FN, and (D) PLLA-PVAA-(EDC)-FN. n = 4, *p < 0.05 compared with all the other groups.
The successful surface modifications were also evidenced with progressive changes in the surface wettability with each modification procedure. The initial static water contact of the unmodified PLLA film was 78°. After deposition of the PVAA film on the PLLA, the contact angle dropped to 59°. Attachment of the FN to this surface resulted in a further decrease in contact to 52°. Each measurement involved an uncertainty of ± 2%. This sequential decrease in contact angle is in accordance with expectations based on the increasingly polar nature of the molecules involved in the surface modifications. Although, the surface modification reduced the water contact angles, the differences of contact angles among these surfaces are relatively small, and thus unlikely to play a significant role in determining the biocompatibility and cell adhesion of the polymers. Additionally, topographies of the PVAA-treated PLLA films were determined using AFM. These surfaces were found to be very smooth, with roughness (RMS) of 0.3–0.7 nm, similar to that of the original PLLA surfaces. Thus, possible topographical changes introduced by the plasma deposition process can be eliminated in terms of exerting a significant role in the results obtained in this study.
VEGF surface conjugation and release
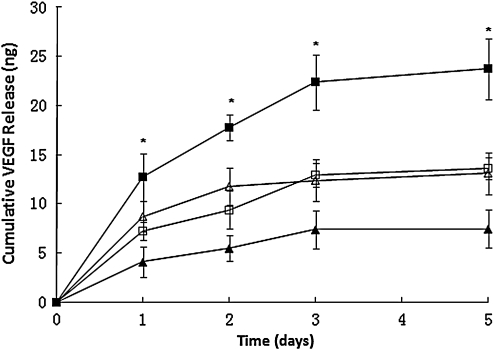
Previous studies have shown that VEGF can bind to FN though specific binding domains,25 and their binding is enhanced in an acidic (pH 5–6) environment.26 In the present study, ice-cold buffer, with pH 5.5, was employed to bindVEGF165 to FN on PLLA and PVAA-modified films. The release of VEGF conjugated on PLLA films to the surrounding medium was measured for up to 5 days, and was plotted as cumulative release curves as shown in Figure 5. The four types of samples employed this study were same as used in the previous FN conjugation study as described above. VEGF conjugated on all four groups showed an initial quick release at the first day, followed by a comparatively slower release thereafter. Plateau was shown on the release curve after day 3, indicating most VEGF was released within the first 3 days. In comparison with other samples, PLLA pretreated with PVAA and covalently conjugated with FN using EDC coupling [PLLA-PVAA-(EDC)-FN] had the highest amount of VEGF conjugation on its surface and subsequent VEGF release. This trend is similar to that of FN conjugation on PLLA films (Fig. 4).
FIG. 5.
Cumulative release of VEGF from PLLA or PVAA-treated PLLA. FN was coated on these substrates by either covalent coupling via EDC or by passive adsorption.  : PLLA-PVAA-(EDC)-FN-VEGF;
: PLLA-PVAA-(EDC)-FN-VEGF;  : PLLA-FN-VEGF;
: PLLA-FN-VEGF;  : PLLA-PVAA-FN-VEGF;
: PLLA-PVAA-FN-VEGF;  : PLLA-(EDC)-FN-VEGF. n = 4. *p < 0.05 compared with all the other groups at the same time point. VEGF, vascular endothelial growth factor.
: PLLA-(EDC)-FN-VEGF. n = 4. *p < 0.05 compared with all the other groups at the same time point. VEGF, vascular endothelial growth factor.
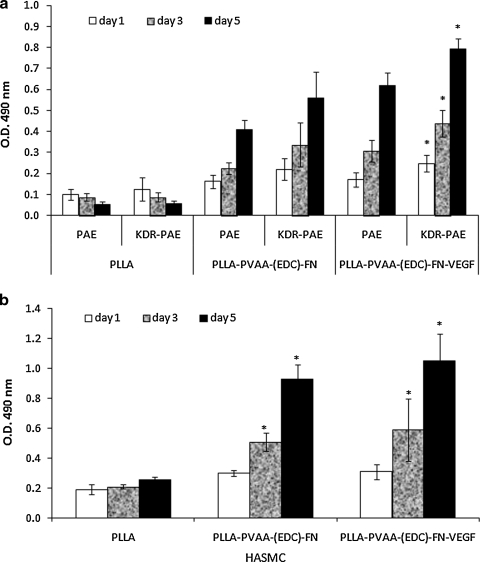
Vascular endothelial and smooth muscle cell adhesion and proliferation on surface-modified PLLA
In these experiments, cell adhesion after 1 day of incubation and cell proliferation after 3 or 5 days of incubation on surface-modified PLLA films were compared with that observed on untreated PLLA films. As shown in Figure 6a, PLLA films modified with PVAA and FN significantly enhanced both PAE and KDR-PAE cell adhesion. In fact, at days 3 and 5, both the PAE and KDR-PAE cells seeded on untreated PLLA exhibited a discernible decline in surface density, suggesting that cells were dying on the untreated PLLA surfaces. In contrast, both PAE and KDR-PAE cells exposed to surface-modified PLLA films, especially to PLLA films releasing VEGF [PLLA-PVAA-(EDC)-FN-VEGF], exhibited fast proliferation. The cell proliferation of KDR-PAE cells appeared to be slightly better than that of the PAE cells, particularly on PLLA-PVAA-(EDC)-FN-VEGF surfaces. Overall, we observed that surfaces modified with the PLLA-PVAA-(EDC)-FN-VEGF sequence were superior to those of PLLA-PVAA-(EDC)-FN with respect to supporting greater EC proliferation, especially with KDR-transfected ECs. Therefore, this best combination was used to compare with the worse combination, that is, untransfected ECs grown on untreated PLLA to illustrate the overall effectiveness of the surface modification technique in the subsequent studies, including immunofluorescence staining and SEM.
FIG. 6.
Endothelial cell (a) or smooth muscle cell (b) adhesion and proliferation on untreated PLLA, PLLA-PVAA-(EDC)-FN, or PLLA-PVAA-(EDC)-FN-VEGF films. n = 4, *p < 0.05 compared with PAE grown on the same film at the same time point. PAE, pig aorta endothelial cells.
HASMCs had a similar growth trend on these films (shown in Fig. 6b). On untreated PLLA films, these cells presented lower adhesion and proliferation rate, whereas on FN-conjugated PLLA films, cells adhered better and had a significantly faster proliferation. However, the presence of VEGF had relatively little effect on the HASMC proliferation.
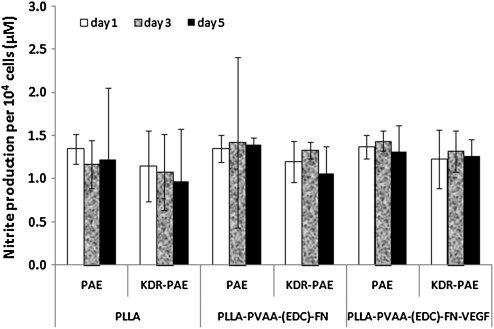
NO production of ECs
NO production is one of the important functions of normal vascular ECs. NO produced by cells is released into the cell culture medium and quickly oxidized to nitrite. In this study, we measured the nitrite concentration in the cell culture medium as a representative of NO produced by the cells. The concentration of nitrite was normalized to the number of cells. As shown in Figure 7, both PAE and KDR-PAE had comparable production of NO when cells were grown on all the substrates exhibiting no statistically significant difference (p > 0.05). Therefore, it can be concluded that cells maintained their normal functions such as NO production despite the significantly higher adhesion and proliferation of ECs grown on the modified PLLA surfaces.
FIG. 7.
Nitric oxide production of endothelial cell grown on untreated PLLA, PLLA-PVAA-(EDC)-FN, or PLLA-PVAA-(EDC)-FN-VEGF films. n = 4.
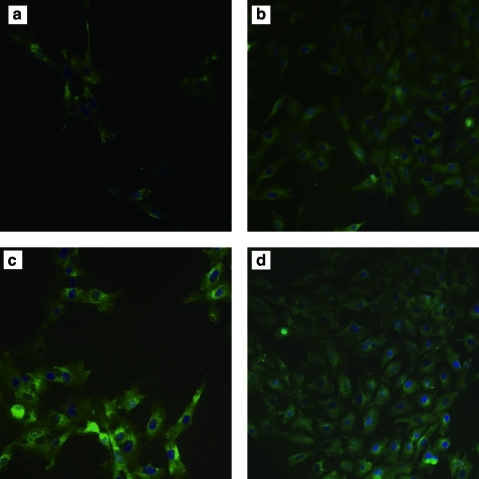
Immunofluorescence staining of KDR
KDR is a large multimeric glycoprotein produced constitutively in vascular ECs, and it is often used as an EC marker and to assess EC function. As shown in Figure 8, both PAE and KDR-PAE cells preserved KDR very well, indicating that they both retained proper EC functions. Slightly stronger KDR expression is observed with the KDR-PAE cells, a reasonable result given the fact these cells are genetically modified to overexpress KDR. Additionally, it is noted that the cells exhibited poor surface coverage and uneven spreading on untreated PLLA films, whereas on our modified PLLA films, the cells were evenly spread and had a far higher extent of coverage.
FIG. 8.
KDR immunofluorescence staining of PAE or KDR-PAE cells grown on untreated PLLA or surface-modified PLLA film. (a) PAE grown on untreated PLLA, (b) PAE grown on PLLA-PVAA-(EDC)-FN-VEGF, (c) KDR-PAE grown on untreated PLLA, and (d) KDR-PAE grown on PLLA-PVAA-(EDC)-FN-VEGF. KDR, kinase-insert domain-containing receptor; KDR-PAE, KDR-transfected PAE. Color images available online at www.liebertonline.com/ten.
SEM of ECs grown on surface-modified PLLA
SEM studies were employed to provide morphological information of cell growth on the different surfaces. Specifically, KDR-PAE cells grown on PLLA-PVAA-(EDC)-FN-VEGF surfaces were compared with that of PAE cells grown on untreated PLLA films, which are relatively smooth (Fig. 9a). As shown in Figure 9c, the KDR-PAE cells grown on surface-modified PLLA films had almost full coverage of the available film surface, with the cells exhibiting a contiguous, flatter morphology. In contrast, PAE cells covered only a small portion of the untreated PLLA film were sporadically located and exhibited irregular morphology (Fig. 9b).
FIG. 9.
Scanning electron microscopic images of untreated PLLA film (a), PAE cells grown on untreated PLLA film (b), and KDR-PAE cells grown on PLLA-PVAA-(EDC)-FN-VEGF film (c). Scale bar = 50 μm.
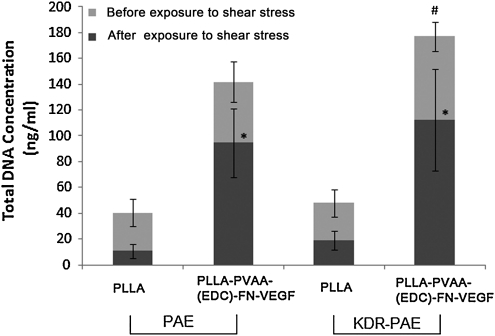
Cell retention under fluidic shear stress
Under physiological conditions, vascular ECs are exposed to shear stress by the flowing blood. For this reason, retention of ECs on PLLA and modified PLLA surfaces when exposed to fluid shear stress was examined. In this study, PAE and KDR-PAE cells grown on untreated PLLA or PLLA-PVAA-(EDC)-FN-VEGF films were exposed to 15 dyn/cm2 flow, which is within the physiological range of shear stress in arteries. As shown in Figure 10, PAE and KDR-PAE grown on untreated PLLA had <40% of cells retained after exposure to 30 min of flow. However, on surface-modified PLLA, both PAE and KDR-PAE cells showed significantly more percentage of cells retained compared with cells grown on untreated PLLA (p < 0.05), with more than 60% of cells were retained after the same flow exposure. In fact, after the flow exposure, PAE and KDR-PAE had approximately nine times cells retained on surface-modified PLLA films than on untreated PLLA films (94.6–112 ng DNA/mL for modified PLLA films versus 10.9–19.2 ng DNA/mL for untreated PLLA films).
FIG. 10.
PAE or KDR-PAE cells retention on PLLA or surface-modified PLLA (PLLA-PVAA-FN-VEGF) after exposed 15 dyn/cm2 shear stress for 30 min. n = 4, *p < 0.05 compared with amount of cells (same type) grown on untreated PLLA.
Discussion
The relatively poor biocompatibility between PLLA surfaces and ECs is well recognized. As a result, a variety of methods have been employed in attempt to improve endothelialization of these surfaces. Such methods include bulk modifications like incorporating copolymers to provide hydrophilic segments in the PLLA as well as surface modifications of the PLLA.15,33 However, modifying the polymer chain creates the risk of changing PLLA mechanical properties, which are critical for certain applications. As a result, surface modifications would appear to be a more desirable route to achieve improved EC-PLLA interactions. In prior reports, ECM proteins such as collagen or FN have been employed to coat PLLA. Although passive coating is relatively easy to do and thus commonly used, weakly adsorbed initial proteins may be replaced quickly by other proteins.34,35 Further, for in vivo endothelialization of vascular prostheses, the physically adsorbed proteins may not be able to withstand the sheer stress created by blood flow. Therefore, stronger adhesion of the ECM proteins to the PLLA surfaces, such as that involved with covalent coupling, is preferred. However, direct covalent coupling of ECM proteins to PLLA surfaces is severely restricted by the paucity of active functional groups on these surfaces.36 Thus, in an effort to provide the requisite covalent protein-surface conjugation, plasma surface modification was employed to provide a relatively high surface density of reactive –COOH groups. This was achieved employing plasma polymerization of vinylacetic acid, under pulsed plasma conditions, in which sufficient –COOH groups, representing some 9% of the surface carbon atoms, were retained for use in attaching FN via standard EDC coupling chemistry. As shown in Figure 4, a more than 100% increase in surface FN was observed on the plasma modified surface compared with the untreated PLLA. The XPS data, shown in Figure 3, also confirm a much higher surface coverage of FN attachment on the plasma modified surface.
The surface-conjugated FN makes possible the subsequent incorporation of VEGF to the PLLA surfaces. VEGF is widely recognized as one of the most potent mitogenic factors for vascular ECs.37,38 ECM proteins including FN,25 fibrin,39 vitronectin,40 and tenascin41 have been reported to posses specific binding domains for VEGF. In this study, a substantially increased surface density of VEGF was found on FN-incorporated PLLA relative to that observed on non-FN containing controls (Fig. 5). Although the required effective concentration of VEGF to enhance EC proliferation is low (1–6 ng/mL),42 high dosages are required for systemic delivery, considering its distribution to the whole blood circulation system. These high dosages are not only costly, but can lead to certain undesirable side effects, such as increasing the permeability of blood vessels.43,44 As a result of these considerations, localized delivery of VEGF to the adjacent cells or tissues is favorable since it requires relatively small amounts to reach the effective concentration at the targeted site. In this study, significantly enhanced proliferation of ECs has been observed with only nanogram quantities of VEGF attached to the PLLA surfaces. Although the adsorbed VEGF was clearly effective in stimulating EC growth, its release from the surface appeared to end after ∼3 days (Fig. 5). Nevertheless, the increased EC proliferation, as a result of the VEGF usage, appears to persist for the 5-day period employed (Fig. 6). These results suggest the possible important role the initial favorable EC-surface interactions exert on maintaining proper cell function and phenotype. Alternately, possibly trace amounts of VEGF remaining in solution can continue to take part in promoting cell growth by reattachment to FN. The period of VEGF release can be extended by various means. For example, fibrin has been used to deliver growth factors in a proteolysis-triggered manner.45,46 Three-dimensional scaffolds, hydrogels, and micro/nanoparticles are also used to increase the growth factor loading capacity depending on the applications.47–49
NO, produced by NO synthase, has multiple functions for vascular systems, including inhibition of platelet aggregation, reduced white blood cell adhesion and penetration, and prevention of abnormal vessel constriction.50 Therefore, the measurement of NO release is generally used as a tool to assess normal EC functions. A number of factors have been found able to alter NO release by the ECs. For example, estrogen, insulin, and exercise can increase NO synthesis and release, whereas oxygen-derived free radicals, aging, and smoking can reduce NO release.13,14,51 In this study, we found that although ECs consisted of significantly higher growth rates on surface-modified PLLA films, their ability to produce NO was not affected by surface modification. The NO production of cells grown on surface-modified PLLA indicates that ECs maintained their phenotypes and subsequent normal functions.
In addition to NO production, KDR has been reported to act as the principal signaling receptor for VEGF to exert its biological activities on ECs.52 Upregulation and activation of KDR are involved in enhanced EC proliferation and angiogenesis.53,54 Additionally, impaired expression of KDR has been related to the ineffectiveness of VEGF for angiogenesis.55,56 In the present study, KDR-PAE cells demonstrated a higher cell proliferating activity than untransfected PAE on surface-modified PLLA-delivering VEGF. This result suggests that overexpression of KDR promotes more efficient use of the VEGF growth factors in achieving EC growth, and thus may be potentially useful to enhance endothelialization.
ECs grown on the luminal side of vascular stents are continuously exposed to fluid shear stress of the blood when implanted. Retention of ECs on the material surface exposed to these sheer forces is obviously an important consideration. Vascular shear stress of arterial vascular network typically ranges between 10 and 70 dyn/cm2.57 In this study, ECs grown on surface modified PLLA had significantly higher percentages of cell retention than on those grown on untreated PLLA after exposure to a physiological level shear stress of 15 dyn/cm2. In fact, the KDR-PAE cell retention on the modified surfaces was an order of magnitude in excess of that observed with untreated PLLA. This result further substantiates the effectiveness of the surface modification method employed in this investigation.
Summation
The surface modification of PLLA involving plasma deposition of PVAA, followed by covalent FN conjugation and VEGF binding, significantly enhanced endothelialization of this biodegradable substrate. The use of KDR-transfected PAE cells, in lieu of unmodified PAE cells, further increased the rate of endothelialization. The results are encouraging since one of the special aspects of plasma polymerization is its versatility to modify materials having various properties and shapes, including porous structures. Vascular grafts and stents are perfect applications for modifying PLLA substrates with plasma deposition of PVAA for subsequent surface modification with FN and VEGF, leading to enhancing endothelialization and, hopefully, inhibition of vascular implant-related thrombosis and restenosis.
Acknowledgments
We acknowledge the support from the NIH (HL082644: K.N.) and UTA Research Area Enhancement grants.
Disclosure Statement
None of the authors has any commercial associations that might create a conflict of interest in connection with this article.
References
- 1.Sutherell J.S. Hirsch R. Beekman R.H. Pediatric interventional cardiology in the United States is dependent on the off-label use of medical devices. Congenit Heart Dis. 2010;5:2. doi: 10.1111/j.1747-0803.2009.00364.x. [DOI] [PubMed] [Google Scholar]
- 2.LaDisa J.F., Jr. Hettrick D.A. Olson L.E. Guler I. Gross E.R. Kress T.T. Kersten J.R. Warltier D.C. Pagel P.S. Stent implantation alters coronary artery hemodynamics and wall shear stress during maximal vasodilation. J Appl Physiol. 2002;93:1939. doi: 10.1152/japplphysiol.00544.2002. [DOI] [PubMed] [Google Scholar]
- 3.Nelken N. Schneider P.A. Advances in stent technology and drug-eluting stents. Surg Clin North Am. 2004;84:1203. doi: 10.1016/j.suc.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Lam K.H. Nijenhuis A.J. Bartels H. Postema A.R. Jonkman M.F. Pennings A.J. Nieuwenhuis P. Reinforced poly(L-lactic acid) fibres as suture material. J Appl Biomater. 1995;6:191. doi: 10.1002/jab.770060308. [DOI] [PubMed] [Google Scholar]
- 5.Harada K. Enomoto S. Stability after surgical correction of mandibular prognathism using the sagittal split ramus osteotomy and fixation with poly-L-lactic acid (PLLA) screws. J Oral Maxillofac Surg. 1997;55:464. doi: 10.1016/s0278-2391(97)90691-1. [DOI] [PubMed] [Google Scholar]
- 6.Matsusue Y. Yamamuro T. Oka M. Shikinami Y. Hyon S.H. Ikada Y. In vitro and in vivo studies on bioabsorbable ultra-high-strength poly(L-lactide) rods. J Biomed Mater Res. 1992;26:1553. doi: 10.1002/jbm.820261203. [DOI] [PubMed] [Google Scholar]
- 7.Partio E.K. Hirvensalo E. Partio E. Pelttari S. Jukkala-Partio K. Bostman O. Hanninen A. Tormala P. Rokkanen P. Talocrural arthrodesis with absorbable screws, 12 cases followed for 1 year. Acta Orthop Scand. 1992;63:170. doi: 10.3109/17453679209154816. [DOI] [PubMed] [Google Scholar]
- 8.Tamai H. Igaki K. Kyo E. Kosuga K. Kawashima A. Matsui S. Komori H. Tsuji T. Motohara S. Uehata H. Initial and 6-month results of biodegradable poly-L-lactic acid coronary stents in humans. Circulation. 2000;102:399. doi: 10.1161/01.cir.102.4.399. [DOI] [PubMed] [Google Scholar]
- 9.Ormiston J.A. Serruys P.W. Regar E. Dudek D. Thuesen L. Webster M.W. Onuma Y. Garcia-Garcia H.M. McGreevy R. Veldhof S. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trial. Lancet. 2008;371:899. doi: 10.1016/S0140-6736(08)60415-8. [DOI] [PubMed] [Google Scholar]
- 10.Daemen J. Wenaweser P. Tsuchida K. Abrecht L. Vaina S. Morger C. Kukreja N. Juni P. Sianos G. Hellige G. van Domburg R.T. Hess O.M. Boersma E. Meier B. Windecker S. Serruys P.W. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007;369:667. doi: 10.1016/S0140-6736(07)60314-6. [DOI] [PubMed] [Google Scholar]
- 11.Stone G.W. Moses J.W. Ellis S.G. Schofer J. Dawkins K.D. Morice M.C. Colombo A. Schampaert E. Grube E. Kirtane A.J. Cutlip D.E. Fahy M. Pocock S.J. Mehran R. Leon M.B. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356:998. doi: 10.1056/NEJMoa067193. [DOI] [PubMed] [Google Scholar]
- 12.Landmesser U. Hornig B. Drexler H. Endothelial function: a critical determinant in atherosclerosis? Circulation. 2004;109:II27. doi: 10.1161/01.CIR.0000129501.88485.1f. [DOI] [PubMed] [Google Scholar]
- 13.van Hinsbergh V.W. The endothelium: vascular control of haemostasis. Eur J Obstet Gynecol Reprod Biol. 2001;95:198. doi: 10.1016/s0301-2115(00)00490-5. [DOI] [PubMed] [Google Scholar]
- 14.Pearson J.D. Normal endothelial cell function. Lupus. 2000;9:183. doi: 10.1191/096120300678828299. [DOI] [PubMed] [Google Scholar]
- 15.Liu X. Ma P.X. The nanofibrous architecture of poly(L-lactic acid)-based functional copolymers. Biomaterials. 2010;31:259. doi: 10.1016/j.biomaterials.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker A.K. Qiu H. Wu Y. Timmons R.B. Kinsel G.R. Studies of peptide binding to allyl amine and vinyl acetic acid-modified polymers using matrix-assisted laser desorption/ionization mass spectrometry. Anal Biochem. 1999;271:123. doi: 10.1006/abio.1999.4141. [DOI] [PubMed] [Google Scholar]
- 17.Yang J. Bei J. Wang S. Enhanced cell affinity of poly (D,L-lactide) by combining plasma treatment with collagen anchorage. Biomaterials. 2002;23:2607. doi: 10.1016/s0142-9612(01)00400-8. [DOI] [PubMed] [Google Scholar]
- 18.Connolly D.T. Heuvelman D.M. Nelson R. Olander J.V. Eppley B.L. Delfino J.J. Siegel N.R. Leimgruber R.M. Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84:1470. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waltenberger J. Claesson-Welsh L. Siegbahn A. Shibuya M. Heldin C.H. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988. [PubMed] [Google Scholar]
- 20.Bhattacharyya D. Pillai K. Chyan O.M. Tang L. Timmons R.B. A new class of thin film hydrogels produced by plasma polymerization. Chem Mater. 2007;19:2222. doi: 10.1021/cm0630688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nair A. Zou L. Bhattacharyya D. Timmons R.B. Tang L. Species and density of implant surface chemistry affect the extent of foreign body reactions. Langmuir. 2008;24:2015. doi: 10.1021/la7025973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.TechNote 205: Covalent Coupling. Bangs Laboratories, Inc. www.bangslabs.com/technotes/205.pdf. www.bangslabs.com/technotes/205.pdf
- 23.Chu B.C. Wahl G.M. Orgel L.E. Derivatization of unprotected polynucleotides. Nucleic Acids Res. 1983;11:6513. doi: 10.1093/nar/11.18.6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lund V. Schmid R. Rickwood D. Hornes E. Assessment of methods for covalent binding of nucleic acids to magnetic beads, dynabeads, and the characteristics of the bound nucleic acids in hybridization reactions. Nucleic Acids Res. 1988;16:10861. doi: 10.1093/nar/16.22.10861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wijelath E.S. Murray J. Rahman S. Patel Y. Ishida A. Strand K. Aziz S. Cardona C. Hammond W.P. Savidge G.F. Rafii S. Sobel M. Novel vascular endothelial growth factor binding domains of fibronectin enhance vascular endothelial growth factor biological activity. Circ Res. 2002;91:25. doi: 10.1161/01.res.0000026420.22406.79. [DOI] [PubMed] [Google Scholar]
- 26.Goerges A.L. Nugent M.A. pH regulates vascular endothelial growth factor binding to fibronectin: a mechanism for control of extracellular matrix storage and release. J Biol Chem. 2004;279:2307. doi: 10.1074/jbc.M308482200. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen K.T. Su S.H. Sheng A. Wawro D. Schwade N.D. Brouse C.F. Greilich P.E. Tang L. Eberhart R.C. In vitro hemocompatibility studies of drug-loaded poly-(L-lactic acid) fibers. Biomaterials. 2003;24:5191. doi: 10.1016/s0142-9612(03)00451-4. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen K.T. Eskin S.G. Patterson C. Runge M.S. McIntire L.V. Shear stress reduces protease activated receptor-1 expression in human endothelial cells. Ann Biomed Eng. 2001;29:145. doi: 10.1114/1.1349700. [DOI] [PubMed] [Google Scholar]
- 29.Socrates G. Infrared Characteristic Group Frequencies. Hoboken, NJ: John Wiley & Sons; 1994. [Google Scholar]
- 30.Beamson G. Briggs D. High-Resolution XPS of Organic Polymers. The Scienta ESCA300 Database. Hoboken, NJ: John Wiley & Sons; 1992. [Google Scholar]
- 31.Timmons R.B., editor; Griggs A.J., editor. Pulsed Plasma Polymerizations. London: Imperial College Press; 2004. [Google Scholar]
- 32.Bhattacharyya D. Xu H. Deshmukh R.R. Timmons R.B. Nguyen K.T. Surface chemistry and polymer film thickness effects on endothelial cell adhesion and proliferation. J Biomed Mater Res. 2010;94:640. doi: 10.1002/jbm.a.32713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quirk R.A. Chan W.C. Davies M.C. Tendler S.J. Shakesheff K.M. Poly(L-lysine)-GRGDS as a biomimetic surface modifier for poly(lactic acid) Biomaterials. 2001;22:865. doi: 10.1016/s0142-9612(00)00250-7. [DOI] [PubMed] [Google Scholar]
- 34.Fang F. Satulovsky J. Szleifer I. Kinetics of protein adsorption and desorption on surfaces with grafted polymers. Biophys J. 2005;89:1516. doi: 10.1529/biophysj.104.055079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szleifer I. Carignano M.A. Fang F. Adsorption of neutral and charged proteins. Abstr Pap Am Chem Soc. 2001;222:U344. [Google Scholar]
- 36.Liu X. Ma P.X. The nanofibrous architecture of poly(L-lactic acid)-based functional copolymers. Biomaterials. 2010;31:259. doi: 10.1016/j.biomaterials.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrara N. The role of VEGF in the regulation of physiological and pathological angiogenesis. EXS. 2005;94:209. doi: 10.1007/3-7643-7311-3_15. [DOI] [PubMed] [Google Scholar]
- 38.Leung D.W. Cachianes G. Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 39.Sahni A. Francis C.W. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood. 2000;96:3772. [PubMed] [Google Scholar]
- 40.Schoppet M. Chavakis T. Al-Fakhri N. Kanse S.M. Preissner K.T. Molecular interactions and functional interference between vitronectin and transforming growth factor-beta. Lab Invest. 2002;82:37. doi: 10.1038/labinvest.3780393. [DOI] [PubMed] [Google Scholar]
- 41.Ikuta T. Ariga H. Matsumoto K. Extracellular matrix tenascin-X in combination with vascular endothelial growth factor B enhances endothelial cell proliferation. Genes Cells. 2000;5:913. doi: 10.1046/j.1365-2443.2000.00376.x. [DOI] [PubMed] [Google Scholar]
- 42.Ferrara N. Houck K. Jakeman L. Leung D.W. Molecular and biological properties of the vascular endothelial growth-factor family of proteins. Endocr Rev. 1992;13:18. doi: 10.1210/edrv-13-1-18. [DOI] [PubMed] [Google Scholar]
- 43.Dvorak H.F. Nagy J.A. Feng D. Brown L.F. Dvorak A.M. Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr Top Microbiol Immunol. 1999;237:97. doi: 10.1007/978-3-642-59953-8_6. [DOI] [PubMed] [Google Scholar]
- 44.Nagy J.A. Benjamin L. Zeng H. Dvorak A.M. Dvorak H.F. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11:109. doi: 10.1007/s10456-008-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ehrbar M. Zeisberger S.M. Raeber G.P. Hubbell J.A. Schnell C. Zisch A.H. The role of actively released fibrin-conjugated VEGF for VEGF receptor 2 gene activation and the enhancement of angiogenesis. Biomaterials. 2008;29:1720. doi: 10.1016/j.biomaterials.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 46.Wilcke I. Lohmeyer J.A. Liu S. Condurache A. Kruger S. Mailander P. Machens H.G. VEGF(165) and bFGF protein-based therapy in a slow release system to improve angiogenesis in a bioartificial dermal substitute in vitro and in vivo. Langenbecks Arch Surg. 2007;392:305. doi: 10.1007/s00423-007-0194-1. [DOI] [PubMed] [Google Scholar]
- 47.Kanczler J.M. Ginty P.J. White L. Clarke N.M. Howdle S.M. Shakesheff K.M. Oreffo R.O. The effect of the delivery of vascular endothelial growth factor and bone morphogenic protein-2 to osteoprogenitor cell populations on bone formation. Biomaterials. 2010;31:1242. doi: 10.1016/j.biomaterials.2009.10.059. [DOI] [PubMed] [Google Scholar]
- 48.Jay S.M. Saltzman W.M. Controlled delivery of VEGF via modulation of alginate microparticle ionic crosslinking. J Control Rel. 2009;134:26. doi: 10.1016/j.jconrel.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Emerich D.F. Mooney D.J. Storrie H. Babu R.S. Kordower J.H. Injectable hydrogels providing sustained delivery of vascular endothelial growth factor are neuroprotective in a rat model of Huntington's disease. Neurotoxicol Res. 17:66. doi: 10.1007/s12640-009-9079-0. [DOI] [PubMed] [Google Scholar]
- 50.Vanhoutte P.M. Shimokawa H. Tang E.H. Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 2009;196:193. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- 51.Moncada S. Palmer R.M. Higgs E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109. [PubMed] [Google Scholar]
- 52.Neufeld G. Tessler S. Gitay-Goren H. Cohen T. Levi B.Z. Vascular endothelial growth factor and its receptors. Prog Growth Factor Res. 1994;5:89. doi: 10.1016/0955-2235(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 53.Fujita Y. Yoshizumi M. Izawa Y. Ali N. Ohnishi H. Kanematsu Y. Ishizawa K. Tsuchiya K. Tamaki T. Transactivation of fetal liver kinase-1/kinase-insert domain-containing receptor by lysophosphatidylcholine induces vascular endothelial cell proliferation. Endocrinology. 2006;147:1377. doi: 10.1210/en.2005-0644. [DOI] [PubMed] [Google Scholar]
- 54.Waltenberger J. Mayr U. Pentz S. Hombach V. Functional upregulation of the vascular endothelial growth factor receptor KDR by hypoxia. Circulation. 1996;94:1647. doi: 10.1161/01.cir.94.7.1647. [DOI] [PubMed] [Google Scholar]
- 55.Fukino K. Sata M. Seko Y. Hirata Y. Nagai R. Genetic background influences therapeutic effectiveness of VEGF. Biochem Biophys Res Commun. 2003;310:143. doi: 10.1016/j.bbrc.2003.08.134. [DOI] [PubMed] [Google Scholar]
- 56.Murota S.I. Onodera M. Morita I. Regulation of angiogenesis by controlling VEGF receptor. Ann N Y Acad Sci. 2000;902:208. doi: 10.1111/j.1749-6632.2000.tb06315.x. [DOI] [PubMed] [Google Scholar]
- 57.Malek A.M. Alper S.L. Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]