Abstract
Treatment of acute ischemic stroke with intravenous tissue-type plasminogen activator is underutilized partly due to the risk of life-threatening hemorrhage. In response to the clinical need for safer stroke therapy, we explored using an aptamer-based therapeutic strategy to promote cerebral reperfusion in a murine model of ischemic stroke. Aptamers are nucleic acid ligands that bind to their targets with high affinity and specificity, and can be rapidly reversed with an antidote. Here we show that a Factor IXa aptamer administered intravenously after 60 minutes of cerebral ischemia and reperfusion improved neurological function and was associated with reduced thrombin generation and decreased inflammation. Moreover, when the aptamer was administered in the setting of intracranial hemorrhage, treatment with its specific antidote reduced hematoma volume and improved survival. The ability to rapidly reverse a pharmacologic agent that improves neurological function after ischemic stroke should intracranial hemorrhage arise indicates that aptamer–antidote pairs may represent a novel, safer approach to treatment of stroke.
Introduction
Stroke is a significant cause of mortality in the world. In the United States a fatal stroke is estimated to occur every 3–4 minutes (Rosamond et al., 2008). Additionally, over half of the patients who survive an acute stroke are left with residual disability at 6 months, making stroke the leading cause of morbidity in the United States (Rosamond et al., 2008). Currently, the only pharmacological agent approved by the Food and Drug Administration for treatment of acute stroke is tissue-type plasminogen activator (TPA), which promotes reperfusion by enhancing the dissolution of intravascular clots. Unfortunately, administration of TPA is not often utilized, with recent reports suggesting that <3% of strokes are treated with TPA (The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group, 1995). Aside from the compressed time frame for administration after symptom onset and TPA-associated neurotoxicity, the most common reason for physician reluctance to use TPA is the risk that it may engender uncontrollable intracranial hemorrhage (Brown et al., 2005; BENARROCH, 2007). For example, although the original NINDS clinical trial demonstrated a rate of symptomatic intracranial hemorrhage after TPA use at 6.4% (The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group, 1995), actual practice rates may reach has high as 15% (Katzan et al., 2000). Unfortunately, no intervention exists that can reverse the effects of TPA in the setting of acute hemorrhage. Although supportive care with fresh frozen plasma and platelets as well as surgical evacuation of the hemorrhage may be undertaken, thrombolysis-related symptomatic intracranial hemorrhage remains associated with an overall mortality rate of 59.7% (Sloan et al., 1998).
In response to this clinical need for improved treatments to promote reperfusion in acute stroke, many therapeutic strategies utilizing combinations of fibrinolytic, antiplatelet, and antithrombotic agents are currently undergoing evaluation in preclinical and clinical trials. Although preliminary data suggest that these treatments may promote recanalization, they do not address the fundamental safety issue of immediate reversibility in the setting of intracranial hemorrhage. Thus, a therapeutic strategy that both facilitates reperfusion and also has the potential for immediate reversal would represent a clear advance for stroke therapy. One method of promoting reperfusion in the setting of acute stroke that has the potential for rapid reversibility is the use of aptamer-based therapeutics. Because aptamers are nucleic acid ligands, complimentary oligonucleotides can be designed to bind to the aptamers, which alter their conformation and thus render them inactive (Rusconi et al., 2002). Therefore, these rationally designed antidotes can be administered as reversal agents.
In the current study, we describe the use of an antithrombotic aptamer-based therapy in a murine model of stroke. The aptamer exerts its antithrombotic actions via specific binding to and inactivation of Factor IXa. According to the cell-based model of hemostasis, activated Factor IX joins with Factor VIIIa on the surface of platelets during the propagation phase of coagulation, forming the tenase complex (Hoffman and Monroe, 2001). This tenase complex then activates Factor X, which combines with Factor Va already present on the activated platelet surface. Together, Factor Xa and Va form the prothrombinase complex that directly converts prothrombin to thrombin. Therefore, we hypothesized that aptamer inhibition of Factor IXa would directly decrease thrombin generation, which is known to increase in the setting of ischemia (Karabiyikoglu et al., 2004). Thrombin is a powerful activator of the inflammatory cascade and as such leads to the upregulation of inflammatory cytokines, chemoattractant molecules and vasoactive substances, which then promote neutrophil adhesion and macrophage activation, in addition to a host of procoagulatory effects (Edmunds et al., 2006). Due to the increased presence of inflammatory cells and activation of the coagulation cascade, the end result of increased thrombin levels is decreased reperfusion. The Factor IXa aptamer should therefore enhance reperfusion by its inhibition of thrombin generation, which is likely responsible for ongoing microvascular thrombosis after reperfustion.
The factor IXa aptamer has demonstrated efficacy as an anticoagulant agent in mice, pigs, and humans, and in each situation its activity was fully reversed upon administration of its antidote oligonucleotide (Rusconi et al., 2004; Dyke et al., 2006; Nimjee et al., 2006). For these reasons, we tested the Factor IXa aptamer in a murine model of ischemic stroke induced by transient middle cerebral artery occlusion (MCAO) to evaluate the aptamer's efficacy as a therapeutic agent for stroke; additionally, we studied drug safety in a subarachnoid hemorrhage (SAH) model to determine the antidote's ability to improve outcome after aptamer-associated hemorrhage. We found that administration of the aptamer after stroke in mice reduced thrombin generation, decreased inflammation, and improved neurological function in the treated animals. Moreover, we describe the use of a matched antidote oligonucleotide to reverse the anticoagulant effects of the aptamer in the setting of aptamer-associated intracranial bleeding. The ability to rapidly reverse an agent administered for treatment of stroke should hemorrhage arise represents a novel and potentially safer approach to enhance reperfusion in the setting of acute stroke.
Materials and Methods
Animal surgical studies
All animals received humane treatment in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health, as approved by the Duke University Animal Care and Use Committee. Before induction of ischemia or hemorrhage, wild-type male C57Bl/6J mice aged 10–12 weeks (Jackson Laboratory) were injected with 2 mg/kg of the cholesterol-modified aptamer generated against Factor IXa, Ch-9.3t, intravenously via tail vein. After waiting 10 minutes for the aptamer to circulate, 200 μL of blood was removed via cardiac puncture. Whole blood activated partial thromboplastin time (aPTT) and activated clotting time (ACT) values were then measured using the Hemochron Jr. Signature Microcoagulation System (ITC). All measurements were performed in duplicate. For experiments involving antidote reversal, 2 mg/kg of Ch-9.3t was administered intravenously, and after 10 minutes of circulation, a 50-fold molar excess of 5-2C antidote (46.8 mg/kg) was given via tail vein injection. After waiting an additional 10 minutes, 200 μL of blood was removed via cardiac puncture and aPTT/ACT values were determined in duplicate as before.
For induction of ischemia, mice were anesthetized with 5% isoflurane in a 30% O2:70% N2 mixture. The trachea was then intubated with a 20-gauge intravenous catheter (Insyte-W; Becton Dickinson). Next, the inspired isoflurane concentration was decreased to 1.5% and the lungs were mechanically ventilated at a rate of 130 breaths per minute at a tidal volume of 0.7 mL. Rectal temperature was monitored and servoregulated at 37°C via surface heating and cooling. A midline neck incision was made and the right common carotid artery was isolated. After isolation and ligation of the right external carotid artery with temporary occlusion of the right common carotid artery, a silicon-coated 6-0 nylon monofilament was inserted into the right external carotid artery and advanced via the internal carotid artery to the bifurcation of the MCA and the anterior cerebral artery. The filament remained in this position for 60 minutes, occluding the origin of the MCA to produce an infarct in its region of perfusion. The filament was then withdrawn and the midline incision was closed via interrupted skin sutures.
For mice undergoing induction of SAH, the surgical procedure was identical to that of induction of ischemia except that of filament placement; in the case of SAH, the filament was advanced further to puncture the MCA/anterior cerebral artery bifurcation to allow for extravasation of blood into the subarachnoid space. In a like manner, the filament was withdrawn and the midline incision was closed via interrupted skin sutures.
Assessment of neurological function
The neurological status of the mice after 60 minutes of ischemia and reperfusion was determined using a Rotarod test of vestibulomotor function (Ugo Basile) (Hamm et al., 1994). In this assay, mice are placed on a rotating rod that accelerates in speed from 4 to 40 rpm. The length of time the mouse is able to remain on the rod without falling (latency period) is recorded, with a maximum time of 300 seconds. All trials were performed in triplicate and the mean latency was calculated. Before assessing neurological status post-stroke, mice were tested and their baseline values were recorded. Mice with a preinjury latency period <180 seconds were excluded from the study, whereas dead mice were assigned the lowest latency score (0).
Determination of thrombin levels
Thrombin levels were indirectly measured via quantification of thrombin–antithrombin (TAT) complex levels. A commercially available Dade Behring Enzygnost® ELISA kit for TAT complexes was purchased from Siemens Healthcare Diagnostics. For sample preparation, ∼900 μL of blood was removed from the mice via cardiac puncture and immediately mixed with 100 μL 0.11 M sodium citrate. The blood was then centrifuged at 1500 g for 10 minutes, and the plasma supernatant was removed, which was used to determine TAT levels; samples were run in duplicate.
Histology and determination of infarct volume
A separate cohort of mice was used for histological determination of infarct volumes. After 60-minute MCAO, mice received intravenous administration of 2 mg/kg Ch-9.3t aptamer or 2 mg/kg mutant aptamer via tail vein. Forty-eight hours after MCAO, the mice were anesthetized with isoflurane and decapitated. The brain was then removed and frozen at −20°C. Using a freezing microtome, 20-μm-thick coronal sections were cut in 440-μm intervals over the rostral-caudal extent of the infarct and mounted on charged slides. After drying overnight, the slides were stained with hematoxylin and eosin. Infarct volume was measured by converting the slides to a digital image using a Photometrics CoolSnap cf digital imager (Roper Scientific) equipped with a micro-nikkor 55-mm lens (Nikon Corporation), utilizing MCID Elite 7.0 (Imaging Research) image analyzer software. An observer blinded to the experimental conditions then outlined the infarct boarders with an operator-controlled cursor. The area (mm2) of the infarct was determined automatically by counting the number of pixels contained in the delineated region, whereas infarct volume (mm3) was calculated by multiplying the infarct area by the interval between sections (440 μm).
Aptamers and antidote
Aptamer Ch-9.3t is a 35-nucleotide-length RNA with sequence 5′-AUG GGG ACU AUA CCG CGU AAU UGC UGC CUC CCC AU-3′ that was modified with 2′-fluoropyrimidines and a 3′ inverted thymidine cap to render nuclease resistance, as well as a 5′ cholesterol moiety to increase its circulating half-life. The mutant aptamer Ch-9.3tM, 5′-AUG GGG ACU GUG CCG CGU AAU UGC UGC CUC CCC AU-3′, is of the same composition as Ch-9.3t with exception of 2 nucleotides that were changed (denoted in bold), which rendered the aptamer unable to bind to Factor IXa and hence inactive as an antithrombotic agent. Antidote oligonucleotide 5-2C is composed of 2′-O-methyl RNA and has a sequence of 5′-CGC GGU AUA GUC CCC AU-3′. Upon binding to Ch-9.3t, 5-2C causes unfolding of the 3-dimensional structure of the aptamer and diminishes binding to Factor IXa, thus reversing aptamer activity. Aptamers were purchased from Agilent Technologies.
Determination of inflammatory markers
Plasma protein levels of interleukin 1β (IL-1β) and matrix metalloproteinase (MMP)-9 after 60-minute ischemia were determined commercially by Thermo Fisher using Pierce SearchLight proteome array technology. Mice underwent MCAO and were allowed to survive for 180 minutes (time measured from filament insertion), followed by cardiac puncture to remove ∼900 μL of blood. The collected blood was immediately mixed with 100 μL 0.11 M sodium citrate and centrifuged at 1500 g for 10 minutes. Next, the plasma supernatant was removed and stored at −80°C until all samples were collected. The samples were then shipped to Thermo Fisher on dry ice for evaluation of inflammatory marker protein levels.
Mortality levels
For mice with induced SAHs, mortality levels were recorded as the number of mice that died within 90 minutes postinduction of hemorrhage; at that time point, all living mice were sacrificed to determine SAH grade.
Assessment of SAH grade
Mice subjected to SAH were sacrificed via decapitation 90 minutes postinduction of hemorrhage. Brains were then dissected and analyzed for determination of SAH grade by an observer blinded to the treatment group. Briefly, the hemorrhage grade was assessed and scored according to the following scale: 0, absence of hematoma; 1, hematoma with a diameter <1 mm; 2, hematoma diameter measured 1–2 mm; 3, hematoma diameter measured 2–3 mm; 4, diameter measured 3–4 mm; 5, diameter measured >4 mm.
Statistical analyses
Data are expressed as mean ± standard error of the mean unless otherwise noted. Repeated-measures analysis of variance (ANOVA) was used for analysis of neurofunctional rotarod results. Mortality was analyzed using Chi-squared analysis, whereas inflammatory protein levels were analyzed using a 2-tailed t-test. TAT complex levels, and aPTT and ACT values were compared using 1-way ANOVA with Fisher's Protected Least Significant Difference (PLSD) or Tukey's post-hoc tests when significant. Nonparametric hemorrhage grade values were analyzed using the Kruskall–Wallis test. P < 0.05 was used as an indication of statistical significance.
Results
Aptamer selected against human FIXa anticoagulates mice
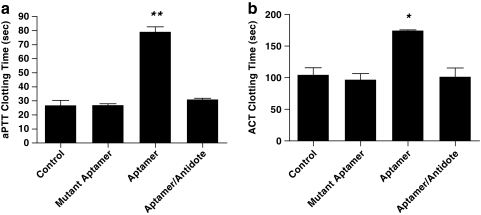
To confirm that the aptamer, selected against human Factor IXa, was able to inhibit the murine Factor IXa activity and effectively anticoagulate mice, we administered a cholesterol modified version of the apamer to mice and performed aPTT and ACT coagulation studies upon blood samples taken from the animals (Rusconi et al., 2002, 2004). The aPTT assay measures deficiencies in the intrinsic as well as common pathways of coagulation, whereas the ACT assay reveals deficiencies in coagulation factors involved in the intrinsic pathway only. Mice were injected with the Factor IXa aptamer Ch-9.3t (2 mg/kg) via tail vein, and after allowing for 10 minutes of circulation, blood was removed via cardiac puncture (Rusconi et al., 2002). aPTT and ACT values were then determined immediately from the whole blood sample. As shown in Fig. 1a, aptamer administration increased the clotting time of the blood as measured by aPTT to 79 ± 3 seconds from 27 ± 3 seconds, the value noted in the control group (P < 0.001). In addition, when the antidote oligonucleotide (50 × molar excess; 46.9 mg/kg) was given 10 minutes after the aptamer, the clotting time decreased to 31 ± 1 seconds, indicating that the antidote was able to fully reverse the activity of the aptamer in a rapid manner. ACT values shown in Fig. 1b confirm the ability of the Factor IXa aptamer to anticoagulate murine blood; aptamer administration increased the clotting time to 175 ± 1 seconds from 105 ± 11 seconds (control group; 1-way ANOVA, P = 0.0132). Antidote administration reversed anticoagulant activity as evidenced by a clotting time of 102 ± 13 seconds.
FIG. 1.
Factor IXa aptamer (Ch-9.3t) effectively anticoagulates mice; antidote oligonucleotide (5-2C) use reverses the activity of the aptamer. Mice were injected via tail vein with the FIXa aptamer (2 mg/kg) or the equivalent amount of a nonactive, mutant aptamer. After 10 minutes, blood was removed via cardiac puncture. Whole blood coagulation measurements were determined using a Hemachron Jr. apparatus. For aptamer–antidote mice, mice were administered the aptamer, 10 minutes passed, and the antidote was then given. After another 10 minutes, blood was removed and tested in a like manner. (a) aPTT measurements; n = 3 for aptamer–antidote group; n = 2 for all other groups. Means ± SEM are shown; 1-way ANOVA; P < 0.0001. **P < 0.01 for aptamer group vs. all other groups. (b) ACT measurements; n = 2 per group. Means ± SEM are shown; 1-way ANOVA; P = 0.0132. *P < 0.05 for aptamer vs. all other groups. aPTT, activated partial thromboplastin time; ACT, activated clotting time; SEM, standard error of the mean; ANOVA, analysis of variance.
Aptamer use reduces neurological deficit after stroke induced by MCAO
To determine the effect of the aptamer on neurological function after stroke, mice were randomized to receive either the Factor IXa aptamer, Ch-9.3t (2 mg/kg, n = 12), or a nonfunctional mutant version of the aptamer, Ch-9.3tM (2 mg/kg; n = 15), immediately after reperfusion of the 60-minute MCAO. Vestibulomotor function was then assessed by measuring Rotarod latency periods for each group. As shown in Fig. 2, aptamer administration was associated with a durable improvement in neurological function as demonstrated by an increase in Rotorod latency (81 ± 30, 152 ± 38, and 164 ± 37 seconds on day 1, 2, and 3 poststroke, respectively). In contrast, mice that received the mutant aptamer had Rotorod latencies of 43 ± 19, 71 ± 27, and 87 ± 31 seconds on day 1, 2, and 3, respectively; P = 0.0153 vs. aptamer.
FIG. 2.
Aptamer administration after MCAO reduces neurological deficit. Mice received either 2 mg/kg of the Ch-9.3t aptamer (n = 12) or 2 mg/kg mutant aptamer (n = 15) after 60-minute filamentous MCAO with reperfusion. Vestibulomotor function was determined by the Rotarod latency period. Data were collected before stroke was induced (day 0) and every 24 hours poststroke, for 3 days (days 1–3). Aptamer use resulted in prolonged Rotarod latencies, indicative of superior neurological function over the mutant aptamer control. Means ± SEM are shown. Repeated measures ANOVA, P = 0.0153, Fisher's PLSD post hoc test, P = 0.0067. *P < 0.05 for aptamer vs. mutant aptamer over 3 days. MCAO, middle cerebral artery occlusion; PLSD, Protected Least Significant Difference.
Aptamer reduces thrombin generation associated with stroke
We next sought to elucidate the underlying mechanism behind the aptamer's efficacy as a therapeutic agent. Therefore, thrombin levels were then quantified via measurement of TAT complex levels to determine if the aptamer was limiting thrombin generation. As demonstrated in Fig. 3a, mice that did not undergo MCAO (sham group) had a TAT complex level of 52.1 ± 24.2 μg/L. Three hours after stroke, the levels increased to 664.6 ± 97.5 μg/L in the mutant aptamer group, but were only slightly increased to 113.3 ± 58.24 μg/L in the aptamer group (P = 0.0001, 1-way ANOVA). As seen in Fig. 3b, TAT complex levels in the mutant aptamer and aptamer groups were not significantly different from sham mice 48-hours after stroke (34.8 ± 14.7 and 28.4 ± 11.9 μg/L vs. 52.1 ± 24.2 μg/L, respectively; P = 0.6, 1-way ANOVA). Therefore, aptamer administration decreased thrombin levels that were acutely elevated due to stroke.
FIG. 3.
Aptamer use mitigates MCAO-associated thrombin generation. TAT complex levels were quantified by enzyme-linked immunosorbent assay using human TAT complexes to generate a standard curve. (a) Mice received either the Ch-9.3t aptamer (2 mg/kg; n = 3) or mutant aptamer (2 mg/kg; n = 3) after 60-minute filamentous MCAO with reperfusion. Sham mice were untreated and did not undergo MCAO (n = 5). Three hours after induction of ischemia, whole blood was removed via cardiac puncture and used to prepare plasma. Administration of the aptamer mitigated the associated increase in thrombin levels associated with MCAO. Means ± SEM are shown. One-way ANOVA; P = 0.0001. **P < 0.01 for mutant aptamer vs. all other groups. (b) Mice received either the Ch-9.3t aptamer (2 mg/kg; n = 7) or mutant aptamer (2 mg/kg; n = 6) after 60-minute filamentous MCAO with reperfusion. Sham mice were untreated and did not undergo MCAO (n = 5). Forty-eight hours after induction of ischemia, whole blood was removed via cardiac puncture and used to prepare plasma. Thrombin levels were not increased 48 hours after MCAO. Means ± SEM are shown. One-way ANOVA; P = 0.6. TAT, thrombin-antithrombin.
Aptamer administration after ischemic stroke reduces inflammatory protein levels
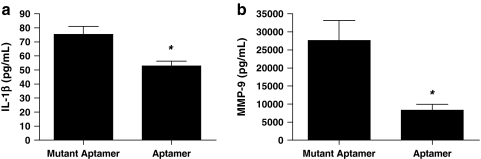
To determine if aptamer administration reduces systemic inflammation in animals that undergo stroke, plasma levels of IL-1β and MMP-9 were quantified. As seen in Fig. 4a, mice that underwent MCAO and received the mutant aptamer had a mean IL-1β level of 75.33 ± 5.59 pg/mL. However, aptamer administration lowered IL-1β to 52.90 ± 3.25 pg/mL (P =0.0256, 2-tailed t-test). A similar pattern was noted during investigation of MMP-9; Fig. 4b shows mutant aptamer and aptamer groups with MMP-9 levels of 27.64 ± 5436 and 8.30 ± 1.57 ng/mL, respectively (P = 0.0268, 2-tailed t-test). Therefore, inhibition of Factor IXa by the aptamer limits the generation of pro-inflammatory agents after cerebral ischemia and reperfusion.
FIG. 4.
Use of Ch-9.3t aptamer after ischemia decreases inflammation. Mice received either the Ch-9.3t aptamer (2 mg/kg; n = 3) or mutant aptamer (2 mg/kg; n = 3) after 60-minute filamentous MCAO with reperfusion. Three hours after induction of MCAO, blood was removed via cardiac puncture and used to prepare plasma. (a) Aptamer administration mitigates ischemia-induced increases in IL-1β levels; means ± SEM are shown. Two-tailed t-test, P = 0.0256; *P < 0.05 for mutant aptamer group vs. aptamer group. (b) Aptamer administration mitigates ischemia-induced increases in MMP-9 levels; means ± SEM are shown. Two-tailed t-test, P = 0.0268; *P < 0.05 for mutant aptamer group vs. aptamer group. IL-1β, interleukin 1β; MMP, matrix metalloproteinase.
Antidote administration reduces hemorrhage grade and mortality after SAH
Next we sought to determine if antidote oligonucleotide (5-2C) administration was able to improve outcome after aptamer use in the setting of active SAH (Rusconi et al., 2004). As depicted in the experimental timeline in Fig. 5a, mice received either aptamer Ch-9.3t (2 mg/kg) or the nonfunctional mutant aptamer (2 mg/kg) via tail vein injection. After induction of SAH, mice that were given the aptamer received either Phosphate Buffered Saline (PBS) (aptamer group) or antidote 5-2C (50 × molar excess; 46.8 mg/kg) to reverse the activity of the aptamer (aptamer/antidote group); mice given the mutant aptamer received PBS post-SAH (mutant aptamer group). Ninety minutes after induction of SAH, the hemorrhage grade of each mouse was assessed. Figure 5b shows the aptamer group with a median hemorrhage grade of 4 as opposed to the mutant aptamer group that had a median hemorrhage grade of 2 (P = 0.001, Kruskal–Wallis test). However, antidote administration after aptamer use and induction of hemorrhage reduced the median hemorrhage grade to 3 and was no longer statistically different from the mutant aptamer group (P > 0.05). Hemorrhages are also seen in Fig. 5c as representative hematomas for each group. In addition, mortality was assessed 90-minutes postinduction of hemorrhage; of the 6 mice in the mutant aptamer group, none died (0%), whereas 5 of the 7 mice died in the aptamer group that did not receive an antidote (71%). However, antidote administration significantly improved survival, as only 2 of the 6 mice in this group died (33%; P = 0.0283, Chi-squared test). These results suggest that, as expected for an anticoagulant, the Factor IXa aptamer may worsen mortality in the setting of active intracranial bleeding; however administration of a specific antidote is associated with rapid reversal of the anticoagulated state, which results in subsequent improvement in survival.
FIG. 5.
Antidote administration after aptamer use and subsequent SAH decreases hemorrhage grade. (a) Schematic of the experimental timeline. The FIXa aptamer (Ch-9.3t) or a nonactive, mutant aptamer was administered to wild-type C57Bl/6 mice via tail vein injection. After induction of hemorrhage, the mice were then given the antidote (5-2C) or PBS. Hemorrhage grades and mortality levels were noted at 90 minutes posthemorrhage induction. (b) Antidote use decreases hemorrhage grade. After sacrifice, SAH grade (range, 1–5) was assessed 90 minutes posthemorrhage induction. Mice that were given the Ch-9.3t aptamer (2 mg/kg) before hemorrhage, but after hemorrhage were administered the antidote (50 × molar excess; 46.8 mg/kg), had significantly smaller hemorrhages. Circles (•), triangles (▴), and squares (▪) represent the hemorrhage grades of mice that received the mutant aptamer, aptamer, or aptamer and antidote, respectively. n = 7, aptamer group; n = 6 for aptamer–antidote and mutant aptamer groups; means ± SEM are shown. Kruskal–Wallis test, P =0.001; **P < 0.01 for aptamer vs. mutant aptamer. (c) Antidote administration mitigates the increase in hemorrhage size after aptamer use and subsequent SAH. Representative pictures of brains with hematomas for the respective groups. SAH, subarachnoid hemorrhage; ACA, anterior cerebral artery; MCA, middle cerebral artery; PBS, Phosphate Buffered Saline. Color images available online at www.liebertonline.com/oli.
Discussion
In this study, we demonstrate the feasibility of using an aptamer-based strategy to inhibit Factor IXa and improve neurofunctional outcome in a murine model of ischemic stroke and reperfusion. Administration of the aptamer also reduced thrombin generation and inflammation. Moreover, the effects of the aptamer could be rapidly and safely reversed with an antidote oligonucleotide. Therefore, in addition to its ability to improve neurological function and reduce inflammation, the aptamer can be rapidly deactivated should hemorrhage arise, which reduces morbidity and mortality in the setting of intracranial hemorrhage. In addition to reversibility, aptamers have been demonstrated to have minimal toxicity, low immunogenicity, and an adjustable bioavailability, all of which confer additional safety (Nimjee et al., 2005). For example, pegaptanib, an aptamer that antagonizes VEGF-mediated vascularization, has been successfully treating macular degeneration since its Food and Drug Administration approval in 2004 (Gragoudas et al., 2004). Moreover, several aptamer-based treatments are currently in clinical trials (Chan et al., 2008a, 2008b). For example, a modified version of aptamer 9.3t has demonstrated the ability to anticoagulate humans without adverse effects, and use of its antidote fully reverses aptamer activity (Dyke et al., 2006). In addition, multiple consecutive Factor IXa aptamer–antidote injections can be administered without major bleeding or other serious adverse events, demonstrating the ability to anticoagulate, reverse, and anticoagulate again if needed (Chan et al., 2008b). Moreover, this aptamer–antidote pair was deemed safe and effective for use in patients with stable coronary artery disease concomitantly using antiplatelet agents (Chan et al., 2008a).
Because aptamer Ch-9.3t was originally developed to target human Factor IXa (Rusconi et al., 2002), we first sought to confirm its ability to function as an effective anticoagulant in mice. We have shown that the aptamer is able to prolong ACT time and increase aPTT values nearly 3-fold, reaching clotting times seen in Factor IXa deficient mice (Kung et al., 1998). In addition, antidote 5-2C is able to fully reverse the activity of the aptamer within the chosen time frame of 10 minutes (Fig. 1). However, a modified version of the aptamer–antidote pair, currently in phase 2 clinical trials, has shown reversal times closer to 1–5 minutes in humans (Dyke et al., 2006).
After demonstrating that the aptamer anticoagulated mice, we determined if aptamer-mediated inhibition of Factor IXa activity could improve neurological function if initiated after stroke. Over 3 days of testing using an objective assay of vestibulomotor function, we saw that aptamer administration significantly reduced the amount of neurological deficit associated with induction of cerebral ischemia (Fig. 2). Upon further investigation we found that the functional improvements were not associated with a decrease in infarct volumes, which were similar between aptamer and control mutant aptamer groups. Given the particular model we used to investigate aptamer activity in stroke, these results were not altogether unexpected. For example, TPA has a much more robust effect on decreasing infarct volumes if a thromboembolic model of stroke is used to incite ischemia as opposed to a filamentous occlusion model (Klein et al., 1999; Orset et al., 2007). Other inciting factors that affect neurological function other than gross infarct volume include enhanced excitotoxic glutamate release leading to secondary hypoxic-ischemic neuronal injury, formation of microthrombi due to disruption of endothelial integrity, and the resulting influx of inflammatory cells. For these reasons, thrombin generation as measured by TAT complex levels was also investigated. We saw that the acute increase in thrombin generation was significantly reduced in animals that had received the aptamer (Fig. 3a), indicating that the acute thrombogenic process was being control by the aptamer.
In addition to inciting thrombus formation, thrombin is a powerful activator of the inflammatory cascade. As such, it leads to the upregulation of inflammatory cytokines, chemoattractant molecules, and vasoactive substances, which then promote neutrophil adhesion and macrophage activation, in addition to a host of procoagulatory effects. All of these processes synergize to produce vascular thrombosis, resulting in what is known as the “no-reflow phenomenon” (Edmunds and Colman, 2006). Nimjee et al. (2006) previously demonstrated the systemic anti-inflammatory properties of this aptamer in a porcine cardiopulmonary bypass model; we therefore chose to investigate pro-inflammatory protein levels after cerebral ischemia and subsequent aptamer treatment. IL-1β was chosen as it has been implicated in stimulation of T-cell proliferation, where increases in MMP-9 are associated with an enhanced risk of hemorrhagic transformation (Feghali and Wright, 1997; Scalia et al., 1999; Zhao et al., 2004). Here we show that both IL-1β and MMP-9 levels are reduced with aptamer administration (Fig. 4). Although we have yet to fully elucidate the mechanism behind improved neurological function with aptamer use, one possible explanation is that decreased inflammation and thrombin levels lead to reductions in microvascular thrombosis and thus allow for enhanced sparing of neurons, leading to improvements in function (Fisher and Tatlisumak, 2005).
The enhanced safety afforded by the ability to reverse the activity of the aptamer should hemorrhage arise is one of the most compelling features of this therapeutic strategy. When placed in an experimental model of SAH, antidote reversal of aptamer activity not only decreased the size of the hematoma as evidenced by decreased hemorrhage grades, but also significantly reduced mortality rates (Fig. 5). One reason why matched antidote administration is superior to addition of procoagulant factors should hemorrhage arise is that the antidote would not carry the risk of rebound thrombosis, as the antidote only binds to the FIXa aptamer, therefore returning the patient to pre-aptamer coagulation values. Thus, hemostasis is restored, as opposed to the possibility of overshooting normal values and thereby inciting thrombosis. These data present the first therapeutic strategy for acute ischemic stroke that can be readily reversed in the event that hemorrhage occurs and suggests that nucleic acid aptamers may be particularly amenable for therapeutic settings where drug safety is a major clinical concern.
Inhibition of Factor IXa has been previously investigated for treatment of stroke in preclinical animal models. For example, Choudhri et al. (1999) utilized an active-site blocked competitive antagonist, FIXai, in a murine model of MCAO. Toomey et al. (2002) expanded on these results, using a humanized murine monoclonal antibody directed against the Gla domain of FIXa in a rat thromboembolic model of stroke. Unfortunately, each of these strategies is limited by the inability to rapidly reverse factor IX inhibition should hemorrhage occur.
In general, anticoagulation strategies have not demonstrated clinical benefit in the setting of acute stroke (Adams et al., 2007) because at least in part, they are associated with an increased risk of intracranial hemorrhage. Therefore, in addition to use in cases of acute ischemic stroke, this aptamer–antidote pair could potentially be used to replace anticoagulation with heparin for treatment or prevention of other cerebrovascular diseases in which anticoagulation is indicated (Albers et al., 2008; REDEKOP, 2008). In general, heparin has been shown to have some beneficial effects in the above-mentioned circumstances by reducing the recurrence of thromboembolic complications, but these effects are often offset by increased rates of hemorrhage (Lyrer and Engelter, 2003; Albers et al., 2008). Although the anticoagulant effect of heparin can be abated with use of protamine, in practice this is rarely performed due to the risk of severe complications. For example, in addition to allergic reactions and hemodynamic compromise, protamine is associated with increased pulmonary artery pressure and decreased systolic and diastolic blood pressure, as well as decreases in myocardial oxygen consumption, cardiac output, heart rate, and systemic vascular resistance (Carr and Silverman, 1999; Nimjee et al., 2006). Thus, in addition to avoiding these adverse events, an aptamer-based strategy may also reduce the risk of other complications associated with the use of heparin, such as thrombocytopenia.
According to the Stroke Therapy Academic Industry Roundtable (1999) committee, experimental treatments of stroke should be tested in more than 1 animal model to increase its likelihood for positive translation to clinical trials. Therefore, following this proof-of-concept study, future directions may include a thromboembolic model of stroke with subsequent safety testing in an SAH model for further investigation of not only the Factor IXa aptamer, but also combinations of antithrombotic aptamers and antiplatelet agents with their respective antidote oligonucleotides. For example, the aptamer–antidote pair selected against von Willebrand factor demonstrated rapid reversibility with potent inhibition of platelet aggregation and could thus aid in enhancing reperfusion by preventing the no-reflow phenomenon after ischemia and reperfusion (Oney et al., 2007).
In conclusion, we report the first application of a reversible, aptamer-based therapeutic strategy for treatment of acute stroke. We show that the aptamer can improve outcome after ischemic stroke in mice and that should hemorrhage arise administration of the aptamer's matched antidote oligonucleotide can reduce mortality.
Footnotes
All work was performed at Duke University Medical Center, Durham, North Carolina.
Acknowledgments
This work was supported by the UNCF-Merck Graduate Science Research Dissertation Fellowship (awarded to C.M.B.), and the National Institutes of Health (1F31NS058273-01 to C.M.B., HL65222 to B.A.S.). B.A.S. declares that he is a scientific founder of Regado Biosciences Inc. The authors would like to thank Keita Faulkner (Duke University Medical Center, Department of Anesthesiology) for her assistance in histological specimen preparation.
References
- ADAMS H.P., Jr. DEL ZOPPO G. ALBERTS M.J. BHATT D.L. BRASS L. FURLAN A. GRUBB R.L. HIGASHIDA R.T. JAUCH E.C. KIDWELL C. LYDEN P.D. MORGENSTERN L.B. QURESHI A.I. ROSENWASSER R.H. SCOTT P.A. WIJDICKS E.F. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- ALBERS G.W. AMARENCO P. EASTON J.D. SACCO R.L. TEAL P. Antithrombotic and thrombolytic therapy for ischemic stroke: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:630S–669S. doi: 10.1378/chest.08-0720. [DOI] [PubMed] [Google Scholar]
- BENARROCH E.E. Tissue plasminogen activator: beyond thrombolysis. Neurology. 2007;69:799–802. doi: 10.1212/01.wnl.0000269668.08747.78. [DOI] [PubMed] [Google Scholar]
- BROWN D.L. BARSAN W.G. LISABETH L.D. GALLERY M.E. MORGENSTERN L.B. Survey of emergency physicians about recombinant tissue plasminogen activator for acute ischemic stroke. Ann Emerg Med. 2005;46:56–60. doi: 10.1016/j.annemergmed.2004.12.025. [DOI] [PubMed] [Google Scholar]
- CARR J.A. SILVERMAN N. The heparin-protamine interaction. A review. J Cardiovasc Surg (Torino) 1999;40:659–666. [PubMed] [Google Scholar]
- CHAN M.Y. COHEN M.G. DYKE C.K. MYLES S.K. ABERLE L.G. LIN M. WALDER J. STEINHUBL S.R. GILCHRIST I.C. KLEIMAN N.S. VORCHHEIMER D.A. CHRONOS N. MELLONI C. ALEXANDER J.H. HARRINGTON R.A. TONKENS R.M. BECKER R.C. RUSCONI C.P. Phase 1b randomized study of antidote-controlled modulation of factor IXa activity in patients with stable coronary artery disease. Circulation. 2008a;117:2865–2874. doi: 10.1161/CIRCULATIONAHA.107.745687. [DOI] [PubMed] [Google Scholar]
- CHAN M.Y. RUSCONI C.P. ALEXANDER J.H. TONKENS R.M. HARRINGTON R.A. BECKER R.C. A randomized, repeat-dose, pharmacodynamic and safety study of an antidote-controlled factor IXa inhibitor. J Thromb Haemost. 2008b;6:789–796. doi: 10.1111/j.1538-7836.2008.02932.x. [DOI] [PubMed] [Google Scholar]
- CHOUDHRI T.F. HOH B.L. PRESTIGIACOMO C.J. HUANG J. KIM L.J. SCHMIDT A.M. KISIEL W. CONNOLLY E.S., Jr. PINSKY D.J. Targeted inhibition of intrinsic coagulation limits cerebral injury in stroke without increasing intracerebral hemorrhage. J Exp Med. 1999;190:91–99. doi: 10.1084/jem.190.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DYKE C.K. STEINHUBL S.R. KLEIMAN N.S. CANNON R.O. ABERLE L.G. LIN M. MYLES S.K. MELLONI C. HARRINGTON R.A. ALEXANDER J.H. BECKER R.C. RUSCONI C.P. First-in-human experience of an antidote-controlled anticoagulant using RNA aptamer technology: a phase 1a pharmacodynamic evaluation of a drug-antidote pair for the controlled regulation of factor IXa activity. Circulation. 2006;114:2490–2497. doi: 10.1161/CIRCULATIONAHA.106.668434. [DOI] [PubMed] [Google Scholar]
- EDMUNDS L.H., Jr. COLMAN R.W. Thrombin during cardiopulmonary bypass. Ann Thorac Surg. 2006;82:2315–2322. doi: 10.1016/j.athoracsur.2006.06.072. [DOI] [PubMed] [Google Scholar]
- FEGHALI C.A. WRIGHT T.M. Cytokines in acute and chronic inflammation. Front Biosci. 1997;2:d12–d26. doi: 10.2741/a171. [DOI] [PubMed] [Google Scholar]
- FISHER M. TATLISUMAK T. Use of animal models has not contributed to development of acute stroke therapies: con. Stroke. 2005;36:2324–2325. doi: 10.1161/01.STR.0000179039.76922.e8. [DOI] [PubMed] [Google Scholar]
- GRAGOUDAS E.S. ADAMIS A.P. CUNNINGHAM E.T., Jr. FEINSOD M. GUYER D.R. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- HAMM R.J. PIKE B.R. O'DELL D.M. LYETH B.G. JENKINS L.W. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J Neurotrauma. 1994;11:187–196. doi: 10.1089/neu.1994.11.187. [DOI] [PubMed] [Google Scholar]
- HOFFMAN M. MONROE D.M., 3rd A cell-based model of hemostasis. Thromb Haemost. 2001;85:958–965. [PubMed] [Google Scholar]
- KARABIYIKOGLU M. HUA Y. KEEP R.F. ENNIS S.R. XI G. Intracerebral hirudin injection attenuates ischemic damage and neurologic deficits without altering local cerebral blood flow. J Cereb Blood Flow Metab. 2004;24:159–166. doi: 10.1097/01.WCB.0000100062.36077.84. [DOI] [PubMed] [Google Scholar]
- KATZAN I.L. FURLAN A.J. LLOYD L.E. FRANK J.I. HARPER D.L. HINCHEY J.A. HAMMEL J.P. QU A. SILA C.A. Use of tissue-type plasminogen activator for acute ischemic stroke: the Cleveland area experience. JAMA. 2000;283:1151–1158. doi: 10.1001/jama.283.9.1151. [DOI] [PubMed] [Google Scholar]
- KLEIN G.M. LI H. SUN P. BUCHAN A.M. Tissue plasminogen activator does not increase neuronal damage in rat models of global and focal ischemia. Neurology. 1999;52:1381–1384. doi: 10.1212/wnl.52.7.1381. [DOI] [PubMed] [Google Scholar]
- KUNG S.H. HAGSTROM J.N. CASS D. TAI S.J. LIN H.F. STAFFORD D.W. HIGH K.A. Human factor IX corrects the bleeding diathesis of mice with hemophilia B. Blood. 1998;91:784–790. [PubMed] [Google Scholar]
- LYRER P. ENGELTER S. Antithrombotic drugs for carotid artery dissection. Cochrane Database Syst Rev. 2003 doi: 10.1002/14651858.CD000255. CD000255. [DOI] [PubMed] [Google Scholar]
- THE NATIONAL INSTITUTE OF NEUROLOGICAL DISORDERS AND STROKE rt-PA STROKE STUDY GROUP. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- NIMJEE S.M. KEYS J.R. PITOC G.A. QUICK G. RUSCONI C.P. SULLENGER B.A. A novel antidote-controlled anticoagulant reduces thrombin generation and inflammation and improves cardiac function in cardiopulmonary bypass surgery. Mol Ther. 2006;14:408–415. doi: 10.1016/j.ymthe.2006.04.006. [DOI] [PubMed] [Google Scholar]
- NIMJEE S.M. RUSCONI C.P. SULLENGER B.A. Aptamers: an emerging class of therapeutics. Annu Rev Med. 2005;56:555–583. doi: 10.1146/annurev.med.56.062904.144915. [DOI] [PubMed] [Google Scholar]
- ONEY S. NIMJEE S.M. LAYZER J. QUE-GEWIRTH N. GINSBURG D. BECKER R.C. AREPALLY G. SULLENGER B.A. Antidote-controlled platelet inhibition targeting von Willebrand factor with aptamers. Oligonucleotides. 2007;17:265–274. doi: 10.1089/oli.2007.0089. [DOI] [PubMed] [Google Scholar]
- ORSET C. MACREZ R. YOUNG A.R. PANTHOU D. ANGLES-CANO E. MAUBERT E. AGIN V. VIVIEN D. Mouse model of in situ thromboembolic stroke and reperfusion. Stroke. 2007;38:2771–2778. doi: 10.1161/STROKEAHA.107.487520. [DOI] [PubMed] [Google Scholar]
- REDEKOP G.J. Extracranial carotid and vertebral artery dissection: a review. Can J Neurol Sci. 2008;35:146–152. doi: 10.1017/s0317167100008556. [DOI] [PubMed] [Google Scholar]
- ROSAMOND W. FLEGAL K. FURIE K. GO A. GREENLUND K. HAASE N. HAILPERN S.M. HO M. HOWARD V. KISSELA B. KITTNER S. LLOYD-JONES D. MCDERMOTT M. MEIGS J. MOY C. NICHOL G. O'DONNELL C. ROGER V. SORLIE P. STEINBERGER J. THOM T. WILSON M. HONG Y. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- STROKE THERAPY ACADEMIC INDUSTRY ROUNDTABLE. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- RUSCONI C.P. ROBERTS J.D. PITOC G.A. NIMJEE S.M. WHITE R.R. QUICK G., Jr. SCARDINO E. FAY W.P. SULLENGER B.A. Antidote-mediated control of an anticoagulant aptamer in vivo. Nat Biotechnol. 2004;22:1423–1428. doi: 10.1038/nbt1023. [DOI] [PubMed] [Google Scholar]
- RUSCONI C.P. SCARDINO E. LAYZER J. PITOC G.A. ORTEL T.L. MONROE D. SULLENGER B.A. RNA aptamers as reversible antagonists of coagulation factor IXa. Nature. 2002;419:90–94. doi: 10.1038/nature00963. [DOI] [PubMed] [Google Scholar]
- SCALIA R. ARMSTEAD V.E. MINCHENKO A.G. LEFER A.M. Essential role of P-selectin in the initiation of the inflammatory response induced by hemorrhage and reinfusion. J Exp Med. 1999;189:931–938. doi: 10.1084/jem.189.6.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLOAN M.A. SILA C.A. MAHAFFEY K.W. GRANGER C.B. LONGSTRETH W.T., Jr. KOUDSTAAL P. WHITE H.D. GORE J.M. SIMOONS M.L. WEAVER W.D. GREEN C.L. TOPOL E.J. CALIFF R.M. Prediction of 30-day mortality among patients with thrombolysis-related intracranial hemorrhage. Circulation. 1998;98:1376–1382. doi: 10.1161/01.cir.98.14.1376. [DOI] [PubMed] [Google Scholar]
- TOOMEY J.R. VALOCIK R.E. KOSTER P.F. GABRIEL M.A. MCVEY M. HART T.K. OHLSTEIN E.H. PARSONS A.A. BARONE F.C. Inhibition of factor IX(a) is protective in a rat model of thromboembolic stroke. Stroke. 2002;33:578–585. doi: 10.1161/hs0202.102950. [DOI] [PubMed] [Google Scholar]
- ZHAO B.Q. IKEDA Y. IHARA H. URANO T. FAN W. MIKAWA S. SUZUKI Y. KONDO K. SATO K. NAGAI N. UMEMURA K. Essential role of endogenous tissue plasminogen activator through matrix metalloproteinase 9 induction and expression on heparin-produced cerebral hemorrhage after cerebral ischemia in mice. Blood. 2004;103:2610–2616. doi: 10.1182/blood-2003-03-0835. [DOI] [PubMed] [Google Scholar]