Abstract
Bardet-Biedl syndrome (BBS) is a rare hereditary autosomal recessive disease associated with several features including obesity, hypertension, and renal abnormalities. The underlying mechanisms of renal defects associated with BBS remain poorly defined. We examined the histological, molecular, and functional renal changes in BBS mouse models that have features of the human disorder. Interestingly, obese hypertensive Bbs4−/− mice exhibited inflammatory infiltration and renal cysts, whereas the obese normotensive Bbs2−/− mice had only minor inflammatory infiltration. Accordingly, the expression level of inducible nitric oxide synthase was elevated in the kidney of both BBS mice with a more marked increase in Bbs4−/− mice. In contrast, endothelial nitric oxide synthase expression was decreased in Bbs4−/−, but not Bbs2−/−, mice. Similarly, the expression levels of transient receptor potential vanilloid 1 and 4 channels as well as β- and γ-subunits of epithelial Na channel were significantly reduced only in the kidney of Bbs4−/− mice. Metabolic studies revealed changes in urine output and urinary concentrations of creatinine, blood urea nitrogen, sodium, and potassium with a more pronounced effect in Bbs4−/− mice. Finally, we found that calorie restriction which prevented obesity in BBS mice reversed the morphological and molecular changes found in Bbs2−/− and Bbs4−/− mice, indicating the kidney abnormalities associated with BBS are obesity related. These findings extend our understanding of the function of BBS proteins and emphasize the importance of these proteins in renal physiology.
Keywords: obesity, hypertension, kidney function
bardet-biedl syndrome (BBS) is a genetically heterogenous, autosomal recessive disorder with 14 Bbs genes identified so far (22, 26). BBS is characterized by several features including obesity, retinopathy, polydactyly, hypogenitalism, and cognitive impairments (3). Hypertension and renal abnormalities are also commonly associated with BBS and renal diseases are a significant cause of death in BBS patients. BBS-related renal abnormalities vary, but they often consist of both structural defects such as cysts formation and functional impairments including reduced glomerular filtration rate, acidification and concentrating defect (10–12). Consequently, end-stage renal disease is frequent in BBS patients requiring hemodialysis maintenance or kidney transplantation.
Defects in renal cilia, highly ordered microtubule organelles, are thought to be the major cause of renal abnormalities in BBS that is consistent with the importance of BBS proteins for ciliary function. Indeed, BBS proteins localize to centrosomes and/or basal bodies (26). In addition, seven BBS proteins (BBS1, BBS2, BBS4, BBS5, BBS7, BBS8, and BBS9) have been shown to form a core complex, known as the BBSome (16). This complex is thought to function as a unit that mediates protein/vesicle trafficking to cilia. Therefore, disruption of ciliary function in BBS may cause morphological and molecular changes in the kidney that perturb many systems relevant to renal physiology and blood pressure control.
We recently generated several mouse models of BBS by individually knocking out the Bbs2, Bbs4, and Bbs6 genes (8, 15, 18). Importantly, these BBS knockout mice phenocopy several features observed in human patients including obesity, retinal degeneration, neurosensory defects, and behavioral changes. In line with the clinical findings (13), we demonstrated that Bbs4−/− and Bbs6−/− mice have elevated blood pressure, whereas Bbs2−/− mice do not (19).
In the present study, we investigated the morphological, molecular, and functional changes in the kidney of BBS mice that may contribute to the hemodynamic and renal defects associated with this syndrome. For this, we studied two BBS mouse models, the obese normotensive Bbs2−/− mice and the obese hypertensive Bbs4−/− mice.
MATERIALS AND METHODS
Materials.
Antibodies against transient receptor potential vanilloid (TRPV)1 and TRPV4 were purchased from Alomone Labs; antibodies against endothelial nitric oxide synthase (eNOS) and secondary antibodies were from Santa Cruz Biotechnology. Antibodies against inducible nitric oxide synthase (iNOS) were from Cell Signaling. Antibody against β-actin was from Abcam.
Animals.
Bbs2−/−, Bbs4−/−, and Bbs6−/− mice used in this study were described previously (8, 15, 18). Homozygous knockout (−/−) and littermate control mice were produced by crossing heterozygous mice with a mixed genetic background of C57BL/6J and 129/SvEv mice. Genotyping was performed by PCR methods as described previously (8, 15, 18). Wild-type littermate mice were used as controls. Obesity was induced in C57BL/6J mice purchased from the Jackson Laboratory (Bar Harbor, ME) using a 45% high-fat diet (D12451, Research Diets, New Brunswick, NJ) as described previously (14, 20). A group of chow-fed C57BL/6J mice was used a lean controls.
Animals were housed in a room maintained at a constant temperature (23°C) and a 12:12-h light-dark cycle (lights turned off at 6 PM) and had free access to standard mouse chow (LM-485; Harlan Teklad Premier Laboratory Diets) and tap water. For caloric restriction, sex- and age-matched controls and BBS mice (6–8 wk old) were housed in individual cages, and Bbs2−/− and Bbs4−/− animals were given 75–80% of the amount of chow consumed by wild-type controls everyday for 3–4 mo. The University of Iowa Animal Research Committee approved all protocols.
Metabolic cage analysis.
Water intake and urine volume were measured by housing the mice in metabolic cages (Nalge Nunc International, Rochester, NY). Mice were acclimated to the cages for 3 days before the 24-h drinking volume and urine output measurements were performed during 2 days.
Radiotelemetry recording of blood pressure.
Arterial pressure was recorded in conscious mice as described previously (19, 21). Briefly, mice were anesthetized with ketamine (91 mg/kg) and xylazine (9.1 mg/kg) and the catheter was inserted in the carotid artery. The transmitter was placed under the skin. The neck incision was closed using silk and further sealed with tissue adhesive. Mice were kept warm on a heating pad and monitored closely until fully recovered from anesthesia. Animals were allowed to recover for several days before arterial pressure was recorded continuously in the conscious unrestrained state for 7 days.
Histological analysis.
Kidneys were collected in Tissue-Tek cassettes, immediately dipped in ice-cold PBS-4% paraformaldehyde, and fixed for 24 h at 4°C. The tissue blocks in paraffin were cut with a microtome, and the sections were stained with periodic acid-Schiff for histological analysis.
Analysis of gene expression by quantitative RT-PCR.
Various tissues were harvested and snap-frozen in liquid nitrogen. Total RNA was isolated using RNeasy Plus Mini Kit from Qiagen. Ten micrograms of total RNA in final volume of 100 μl were used to synthesize first-strand cDNAs with the Super-Script preamplification system. Then, 10 μl of cDNA and 0.4 mmol/l of primers were added in a final volume of 25 μl PCR mixture (iQ SYBR Green supermix, Bio-Rad) and amplified in a iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad). The PCR conditions for all genes were as follows: denaturation for 5 min at 95°C, then 40 cycles for 30 s at 95°C and 30 s at 55°C. The primer set for each gene is also shown in Table S1 (the online version of this article contains supplemental data). β-Actin expression was used as internal control to normalize mRNA expression of these genes.
Western blotting.
Total protein fractions from the kidney, lung, or aorta were prepared and used for Western blotting with specific antibodies. Tissues were homogenized in an ice-cold glass homogenizer in 400–600 μl of lysis buffer [50 mmol/l HEPES, pH 7.5, 150 mmol/l NaCl, 1 mmol/l MgCl2, 1 mmol/l CaCl2, 10 mmol/l NaF, 5 mmol/l EDTA, 1% Triton, 2 mmol/l sodium orthovanadate, and protease inhibitor cocktail (Roche)]. Samples were centrifuged at 9,000 g for 30 min at 4°C, and supernatants were divided in aliquots and stored at −80°C. Protein concentration was determined with Bio-Rad DC Protein Assay Kit.
The expression level of various proteins was determined by Western blotting. Briefly, 20 μg of protein samples were separated by 9% SDS-PAGE and transferred onto PVDF membrane. Each membrane was incubated with primary antibodies (1:1,000) in Tris-buffered saline-tween 20 (TBST) buffer containing 5% skim milk at 4°C overnight followed by secondary horseradish peroxidase-conjugated antibody in TBST buffer with 5% skim milk at room temperature for 1 h. Blots were detected using enhanced chemiluminescence Plus Western Blotting Detection System (GE Healthcare). NIH Image J software was used to quantify band intensity that was expressed as arbitrary values normalized to β-actin.
Biochemical assays.
Urine and plasma sodium, potassium, blood urea nitrogen (BUN), and creatinine concentrations were analyzed using a Vitros 350 Chemistry Analyzer (Ortho Clinical Diagnostics, Raritan, NJ). Vitros Calibrator kit 1 and kit 2 were used for calibration, vitros performance verifier I/II as serum standard control, and Liquichek (Bio-Rad Laboratories, Irvine, CA) urine chemistry control as urine standard control. Plasma albumin concentrations were also measured using Vitros 350 Chemistry Analyzer.
Creatinine clearance was calculated using the formula [(UrineCreatinne × UrineVolume)/PlasmaCreatinine] while fractional excretion of sodium was derived from the formula [(UrineNa+ × PlasmaCreatinine)/(UrineCreatinine × PlasmaNa+)] × 100 (4, 7).
Statistical analysis.
Data are expressed as means ± SE. Data were analyzed using Student's t-test or one-way ANOVA. When ANOVA reached significance, a post hoc comparison was made using Fisher test. A P < 0.05 value was considered to be statistically significant.
RESULTS
Kidney histological analysis.
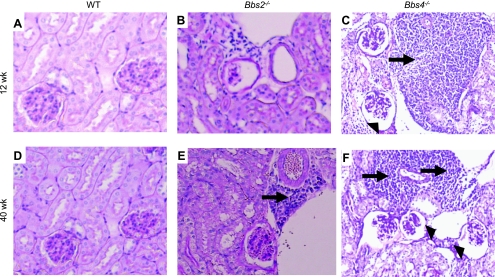
We compared renal morphology between the obese Bbs2−/− and Bbs4−/− mice and age-matched littermate lean controls. Interestingly, 12-wk-old Bbs2−/− and Bbs4−/− mice exhibited inflammatory infiltration (Fig. 1, A–C) with a more marked effect in Bbs4−/− mice. At 40 wk of age, the morphological changes in the kidney of BBS animals, particularly Bbs4−/− mice, were more pronounced. In addition to the enhanced inflammatory infiltration, Bbs4−/−, but not Bbs2−/−, mice displayed significant glomerular changes including glomerular cysts (Fig. 1, D-F). These results suggest an age-dependent penetrance of the morphological alterations in BBS.
Fig. 1.
Periodic acid Schiff-stained sections of the kidney of Bbs2−/−, Bbs4−/− mice, and wild-type (WT) controls. A–C: 12-wk-old mice. D–F: 40-wk-old mice. Arrows indicate the presence of inflammation and arrowheads the cysts. Each photograph is representative of 4–6 animals per group. BBS or Bbs, Bardet-Biedl syndrome.
Gene expression analysis.
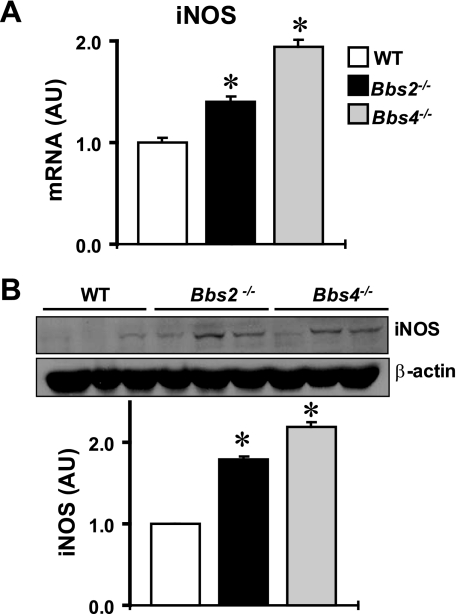
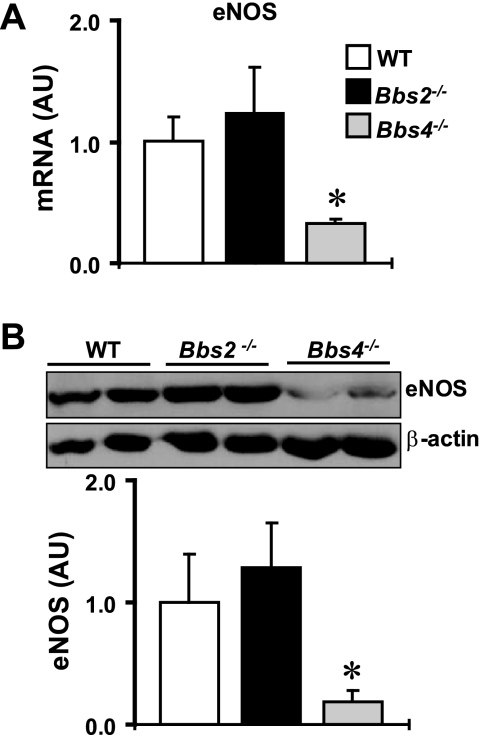
To investigate the potential molecular changes associated with BBS, we compared the expression levels of several renal systems relevant to the kidney function between Bbs2−/−, Bbs4−/− mice and wild-type littermate controls (20–24 wk old). Consistent with the presence of inflammation, the expression of inducible nitric oxide synthase (iNOS) was increased in the kidneys of BBS mice both at the mRNA (Fig. 2A) and protein levels (Fig. 2B). In addition, the increase in renal iNOS was more marked in Bbs4−/− mice compared with Bbs2−/− mice. Conversely, eNOS mRNA and proteins were significantly decreased in the kidneys of Bbs4−/− mice, but not in Bbs2−/− mice (Fig. 3, A and B).
Fig. 2.
Expression level of inducible nitric oxide synthase (iNOS) in the kidney of Bbs2−/−, Bbs4−/− mice, and WT littermate controls at the mRNA (by qRT-PCR; A) and protein (by Western blotting; B) levels. B: example of the Western blot assay is shown at top and quantification data at bottom. *P < 0.05 compared with WT mice; n = 4–5 per group.
Fig. 3.
Expression level of endothelial NOS (eNOS) in the kidney of Bbs2−/−, Bbs4−/− mice, and WT littermate controls at the mRNA (by qRT-PCR; A) and protein (by Western blotting; B) levels. B: example of the Western blot assay is shown at top and quantification data at bottom. *P < 0.05 compared with WT mice; n = 4–6 per group.
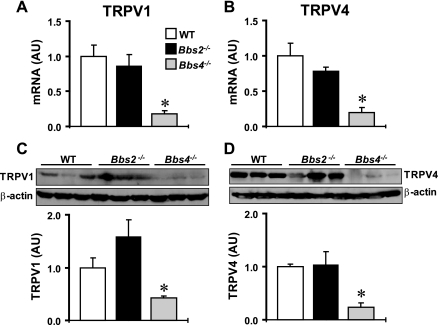
TRPV anion channels, particularly TRPV1 and TRPV4, have recently received attention for their involvement in blood pressure and renal function regulation (23). Of interest, TRPV proteins were found to localize to the cilium of tubular epithelial cells (25). Given the critical role of BBS proteins for ciliary function, we examined the effect of deleting Bbs2 and Bbs4 genes on the expression level of these channels in the kidney. As shown in Fig. 4, A and B, the mRNA level of both TRPV1 and TRPV4 was significantly lower in the kidney of Bbs4−/− mice. In contrast, the TRPV1 and TRPV4 mRNA level in the kidney of Bbs2−/− mice showed no significant change relative to wild-type controls. To corroborate our gene expression findings, we measured the protein levels of TRPV1 and TRPV4 in the kidney of BBS mice. Bbs4−/−, but not Bbs2−/−, mice showed a marked decrease in kidney TRPV1 and TRPV4 proteins (Fig. 4, C and D). To test whether the decrease in TRPV proteins is specific to Bbs4−/− mice, we measured the expression level of TRPV4 in the kidney of Bbs6−/− mice. There was no difference (P = 0.33) in the TRPV4 protein level in the kidney between Bbs6−/− mice (1.12 ± 0.15 AU, n = 3) and littermate controls (1.00 ± 0.16 AU, n = 3). These results indicate that the decrease in renal TRPV expression is specific to Bbs4−/− mice.
Fig. 4.
Expression of transient receptor potential vanilloid (TRPV)1 and TRPV4 in the kidney of Bbs2−/−, Bbs4−/− mice, and WT littermate controls at the mRNA (by qRT-PCR; A and B) and protein (by Western blotting; C and D) levels. C and D: examples of the Western blot assays are shown at top and quantification data at bottom graphs. *P < 0.05 compared with WT mice; n = 4–6 per group.
Next, we tested whether the decrease in TRPV1 and TRPV4 expression in Bbs4−/− mice is restricted to the kidney. For this, we measured the level of TRPV1 and TRPV4 proteins in other tissues. In the aorta, there was no significant change in TRPV1 and TRPV4 expression in Bbs4−/− and Bbs2−/− mice compared with wild-type controls (Fig. S1). Similarly, the expression level of TRPV4 was unaltered in the lung of Bbs4−/− and Bbs2−/− mice relative to littermate controls (data not shown). Together, these data indicate that the decreased level of TRPV1 and TRPV4 in Bbs4−/− mice is specific to the kidney.
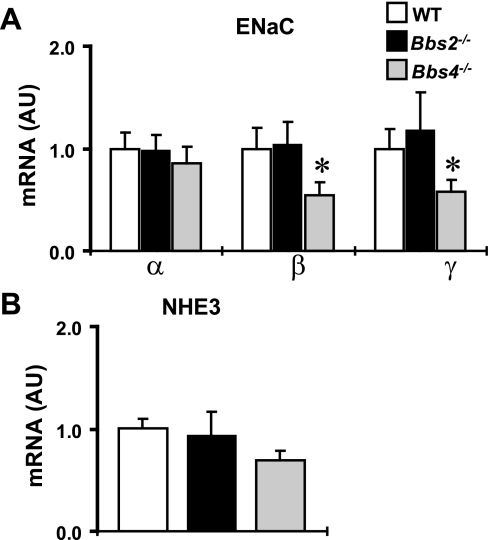
We also examined whether the alteration in gene expression in the kidney of BBS mice is specific to TRPV channels. For this, we assessed whether the expression level of other channels relevant to renal functions was perturbed in BBS mice. Bbs4−/− mice exhibited a significant reduction in the abundance of β and γ, but not α, mRNA subunits of epithelial sodium channel in the kidney (Fig. 5A). In contrast, there was no statistical difference (P = 0.42) in the renal mRNA level of sodium/hydrogen exchanger between Bbs2−/−, Bbs4−/−, and wild-type mice (Fig. 5B), indicating that the changes in gene expression are specific and not general to all ion transporters.
Fig. 5.
Relative mRNA expression (by qRT-PCR) of epithelial sodium channel (ENaC) subunits (A) and sodium hydrogen exchanger type 3 (NHE3; B) in the kidney of Bbs2−/−, Bbs4−/− mice, and WT controls. *P < 0.05 compared with WT mice; n = 5–8 per group.
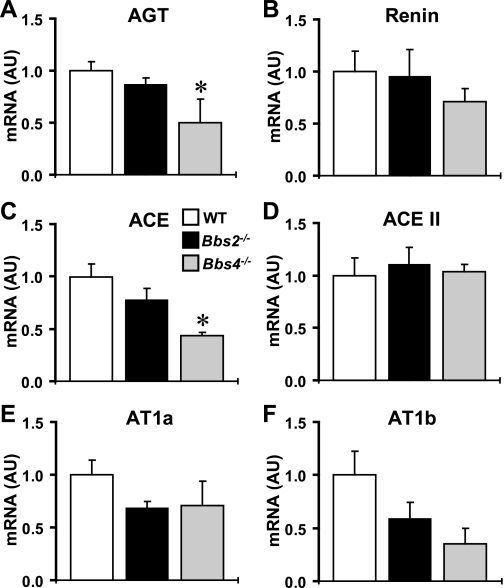
Finally, because the renal renin-angiotensin system (RAS) is critical for the regulation of renal function and blood pressure, we explored a potential pathophysiological role for renal RAS in the abnormalities associated with BBS. For this, we compared mRNA levels of the RAS components in the kidney of Bbs2−/−, Bbs4−/− mice, and littermate controls (Fig. 6). Interestingly, mRNA levels of angiotensinogen and angiotensin converting enzyme were significantly reduced in the kidney of Bbs4−/−, but not Bbs2−/−, mice, whereas no significant difference in the expression levels of renin, angiotensin-converting enzyme II, angiotensin receptor type 1a (AT1a) and AT1b was detected. The suppressed intrarenal RAS in Bbs4−/− mice contrasts with the higher arterial pressure in these animals (19).
Fig. 6.
Relative expression level (by qRT-PCR) of renin-angiotensin system (RAS) components in the kidney of Bbs2−/−, Bbs4−/− mice, and WT controls. Relative mRNA level of angiotensinogen (AGT; A), renin (B), angiotensin-converting enzyme (ACE; C), ACEII (D), angiotensin receptor type 1a (AT1a; E), and AT1b (F) in the kidney of Bbs2−/− and Bbs4−/− mice compared with control littermates. *P < 0.05 compared with WT mice; n = 3–6 per group.
Assessment of renal function.
To determine the functional consequences of the morphological and molecular changes we found in BBS animals, we examined the renal secretory functions of 12- to 24-wk-old Bbs2−/− and Bbs4−/− mice. First, we compared plasma levels of BUN, creatinine, sodium, and potassium and found no significant difference between BBS mice and wild-type controls (Table S2).
There was a tendency for lower water intake in Bbs2−/− and Bbs4−/− mice relative to controls, but this was not statistically significant. On the other hand, urine output was significantly reduced in both BBS mouse models compared with the controls (Table 1). While urine creatinine concentration was increased in both Bbs2−/− and Bbs4−/− mice, creatinine clearance was decreased in these mice relative to controls (Table 1). In contrast, urine concentrations of BUN, sodium, and potassium as well as fractional excretion of sodium were increased only in Bbs4−/− mice. These results demonstrate that the morphological and molecular changes observed in the kidney of BBS mice are associated with alterations in the excretion of water and electrolytes.
Table 1.
Comparison of 24-h water intake, urine volume, urinary concentrations of BUN, creatinine, sodium and potassium, CCreatinine, and FENa+ between Bbs2−/−, Bbs4−/− mice, and WT controls
| Genotype | Water Intake, ml | UVol, ml | UBUN, mg/dl | UCreatinine, mg/dl | CCreatinine, μl/min | UNa+, mmol/l | FENa+, % | UK+, mmol/l |
|---|---|---|---|---|---|---|---|---|
| WT | 2.94 ± 0.54 | 0.90 ± 0.13 | 3,467 ± 160 | 57 ± 2 | 208 ± 8 | 83 ± 8 | 0.16 ± 0.02 | 217 ± 12 |
| Bbs2−/− | 2.25 ± 0.23 | 0.38 ± 0.07* | 4,326 ± 329 | 85 ± 7* | 116 ± 10* | 123 ± 15 | 0.19 ± 0.02 | 244 ± 24 |
| Bbs4−/− | 2.45 ± 0.15 | 0.36 ± 0.08* | 5,301 ± 355* | 72 ± 3* | 104 ± 5* | 199 ± 17* | 0.31 ± 0.03* | 358 ± 20* |
Values are means ± SE. BUN, blood urea nitrogen; CCreatinine, clearance of creatinine; FENa+, fractional excretion of sodium; Bbs, Bardet-Biedl syndrome; WT, wild-type controls.
P < 0.05 compared with WT mice; n = 8-18 per group.
Role of obesity in the renal changes.
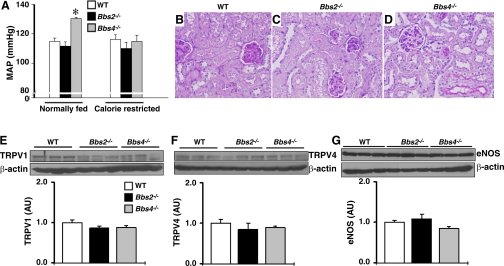
To assess the role of obesity in the renal changes associated with BBS, we performed a histological analysis of the kidneys of calorie-restricted Bbs2−/− and Bbs4−/− mice. Calorie restriction protocol effectively prevented obesity in BBS mice as indicated by the normalized body weight and fat pads (Fig. S2). In addition, calorie restriction eliminated the hypertension associated with Bbs4−/− mice. Indeed, while the obese Bbs4−/− mice displayed higher mean arterial pressure, the calorie-restricted Bbs4−/− mice had normal mean arterial pressure compared with wild-type controls (Fig. 7A).
Fig. 7.
Effect of calorie restriction on hemodynamic and renal changes associated with BBS. A: comparison of mean arterial pressure (MAP; by radiotelemetry) between normally fed (obese) and calorie-restricted (lean) Bbs2−/− and Bbs4−/− mice relative to WT littermate controls. B–D: Periodic acid-Schiff-stained sections of the kidney of calorie-restricted Bbs2−/−, Bbs4−/− mice, and WT controls. E–G: comparison of protein expression level (by Western blot) of TRPV1 (E), TRPV4 (F), and eNOS (G) between calorie-restricted Bbs2−/−, Bbs4−/− mice, and WT controls. E–G: examples of the Western blot assays are shown at top and quantification data at bottom graphs. *P < 0.05 compared with WT mice; n = 4–6 per group.
Histological analysis of kidneys of calorie-restricted Bbs2−/− and Bbs4−/− mice revealed no significant changes (absence of inflammatory infiltration and glomerular cysts) compared with wild-type controls (Fig. 7, B–D), indicating that kidney morphological changes found in obese Bbs2−/− and Bbs4−/− mice can be avoided by preventing obesity.
Finally, we examined the effect of eliminating obesity, by calorie restriction, on the decrease in renal TRPV channels and eNOS in Bbs4−/− mice. There was no difference in the renal expression level of TRPV1, TRPV4, or eNOS between calorie-restricted Bbs4−/−, Bbs2−/− mice, and littermate controls (Fig. 7, E–G), indicating that lower expression of TRPV1, TRPV4, and eNOS in the kidney of Bbs4−/− mice is obesity related. To demonstrate this further, we performed two additional studies. First, we examined the renal expression levels of TRPV1/4 channels in young Bbs4−/− mice (5 wk old) before the development of obesity (body weight: Bbs4−/− mice: 15.62 ± 1.39 g and WT mice: 15.78 ± 1.21 g, P = 0.47, n = 4 each). We found no significant difference in the expression levels of TRPV1 and TRPV4 in the kidneys of young Bbs4−/− mice (1.12 ± 0.26 and 1.05 ± 0.36 AU, respectively, n = 4 each) compared with controls (1.00 ± 0.16 and 1.00 ± 0.31 AU, respectively, n = 4 each). Second, we assessed the renal expression level of TRPV4 in normal C57BL/6J mice rendered obese by high-fat diet. We found a significantly (P = 0.01) reduced TRPV4 protein levels in the kidneys of diet-induced obese mice (0.59 ± 0.07 AU, n = 3) relative to the lean controls (1.00 ± 0.10 AU, n = 3).
DISCUSSION
In the present study, two genetically engineered mouse models of BBS (Bbs2−/− and Bbs4−/−) were used to explore potential dysfunctions in the kidney that may account for the renal and cardiovascular abnormalities associated with BBS. We found that deletion of Bbs2 and Bbs4 genes caused differential changes in the renal systems that convey prohypotensive and protective renal effects such as TRPV1, TRPV4, and eNOS that may contribute to the contrasting arterial pressure levels in the two BBS mouse models. The suppression of these regulatory systems in Bbs4-null mice may contribute to the hypertension associated with this mouse model (19). The reduced renal TRPV1 expression in Dahl salt-sensitive rats that develops hypertension after salt feeding supports a role for the suppression of these TRPV channels in hypertension (24). On the other hand, the normal expression level of these protective renal systems in Bbs2-null mice can explain the protection of these animals from hypertension despite the presence of obesity.
Our data regarding the normal or suppressed renal RAS indicate that this system is not involved in the development of BBS-associated renal abnormalities or hypertension in the case of Bbs4−/− mice, but we cannot rule out a pathophysiological role of systemic RAS or other local RAS including those found in the brain, heart, and vascular system in the pathologies associated with BBS. Also, we cannot exclude the possibility that differences in the expression level of RAS components and other systems between BBS mice and controls in specific renal structures may have been missed due to the use of the whole kidney in our mRNA and protein analysis.
It is possible that the renal changes we found in BBS mice are not caused by loss of BBS proteins in the kidney, but are the consequence of alterations in other tissues/systems that influence the renal physiology such as the sympathetic nervous system. In this regard, we previously demonstrated that Bbs4-null mice have increased renal sympathetic drive, whereas Bbs2-null mice do not (19). Chronic sympathetic nerve activation can induce several defects in the kidney including some of the alterations we describe in the present study (9). However, we found that Bbs6-null mice which like Bbs4-null mice are obese, hypertensive, and have increased renal sympathetic tone do not exhibit similar renal changes as indicated by the normal expression level of TRPV4. Therefore, sympathetic nerve activation may contribute to the renal abnormalities such as those we report in Bbs4-null mice, but do not seem to be the main cause.
Our data in the obese normotensive Bbs2-null mice compared with the obese hypertensive Bbs4-null mice can be interpreted as an indication that the presence of hypertension is necessary for obesity to cause renal abnormalities. In support of this idea is the finding that normotensive melacortin-4 receptor knockout mice do not develop significant renal defects despite obesity (6), mimicking Bbs2-null mice. However, induction of hypertension in these melacortin-4 receptor knockout mice by infusing l-NAME for 8 wk caused only modest renal abnormalities (6). This result combined with the lack of renal changes in the obese hypertensive Bbs6-null mice indicates that hypertension may contribute to the renal abnormalities, but cannot entirely explain the differential renal effects induced by inactivation of Bbs2 and Bbs4 genes.
Growing evidences suggest that BBS proteins are important for ciliary function (3, 26). Primary cilia on renal epithelial cells act as mechanosensors to detect and respond to fluid flow (17). The significance of primary cilia is demonstrated by the fact that ciliary defects alter various physiological functions leading to several disorders including cysts, retinal degeneration, liver fibrosis, anosmia, ataxia, cardiac defects, and hydrocephalus (1, 5). The renal pathologies associated with BBS including those described here are consistent with ciliary defects. In BBS mice, ciliary abnormalities were found in several tissues including the testis, retina, lung, and brain. However, in our previous studies, the renal cilia appeared grossly normal in Bbs4-null mice while only some renal cilia seemed abnormal in shape in Bbs2-null mice (15, 18). Thus, the potential role of ciliary defects in the renal abnormalities associated with BBS mice needs further investigation to explore the possibility that subtle changes in the structure and/or function of renal cilia may account for the effects we describe in BBS mice.
It is intriguing that mice null for Bbs2 and Bbs4 genes exhibited different histological and molecular changes in the kidney given that both BBS2 and BBS4 proteins are part of the BBSome complex (16). These findings suggest that in the kidney the function of the BBSome is differentially affected by the absence of BBS2 vs. BBS4 proteins. Our results may also indicate that in the kidney, BBS4 protein is involved in functions other than the BBSome, suggesting a tissue-specific role for BBS proteins. Such tissue-specific role of BBS proteins would be consistent with the variability in the expression of the phenotypic characteristic of BBS among families.
There has been considerable interest in identifying the genes and determining the pathophysiological mechanisms involved in BBS due to the fact that components of the phenotype including obesity, hypertension, and renal injury are common in the general population. In addition, recent evidence identified Bbs genes as susceptibility genes involved in common obesity and associated diseases such as hypertension (2). Therefore, elucidation of the function of Bbs genes and the mechanisms of BBS pathogenesis may lead to a better understanding of the biochemical and developmental pathways involved in disorders commonly found in the general population.
GRANTS
The author's research was supported by National Institutes of Health (NIH) Grants HL-084207 to K. Rahmouni and V. C. Sheffield and EY-011298 and EY-017168 to V. C. Sheffield. A. M. Beyer was supported by an NIH National Research Service Award research fellowship 5 T32-HL-07121-33. V. C. Sheffield is an investigator of the Howard Hughes Medical Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. H. Li and C. D. Sigmund for assistance with the histological studies; Drs. J. B. Stokes, C. P. Thomas, and U. C. Kopp for the advice and critical reading of the manuscript; and C. Searby, K. Bugge, and P. Shi for expert technical assistance.
REFERENCES
- 1. Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Ann Rev Gen Hum Genet 7: 125–148, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Benzinou M, Walley A, Lobbens S, Charles MA, Jouret B, Fumeron F, Balkau B, Meyre D, Froguel P. Bardet-Biedl syndrome gene variants are associated with both childhood and adult common obesity in French Caucasians. Diabetes 55: 2876–2882, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Blacque OE, Leroux MR. Bardet-Biedl syndrome: an emerging pathomechanism of intracellular transport. Cell Mol Life Sci 63: 2145–2161, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen CC, Pedraza PL, Hao S, Stier CT, Ferriri NR. TNFR1-deficient mice display altered blood pressure and renal responses to ANG II infusion. Am J Physiol Renal Physiol 299: F1141–F1150, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davenport JR, Yoder BK. An incredible decade for the primary cilium: a look at a once-forgotten organelle. Am J Physiol Renal Physiol 289: F1159–F1169, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Do Carmo JM, Tallam LS, Roberts JV, Brandon EL, da Silva AA, Hall JE. Impact of obesity on renal structure and function in the presence and absence of hypertension: evidence from melanocortin-4 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol 297: R803–R812, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eisner C, Faulhaber-Walter R, Wang YH, Leelahavanichkul A, Yuen PST, Mizel D, Star RA, Briggs JP, Levine M, Schnermann J. Major contribution of tubular secretion to creatinine clearance in mice. Kidney Int 77: 519–526, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fath MA, Mullins RF, Searby C, Nishimura DY, Wei J, Rahmouni K, Davis RE, Tayeh MK, Andrews M, Yang B, Sigmund CD, Stone EM, Sheffield VC. Mkks-null mice have a phenotype resembling Bardet-Biedl syndrome. Hum Mol Genet 14: 1109–1118, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Grassi G, Arenare F, Pieruzzi F, Brambilla G, Mancia G. Sympathetic activation in cardiovascular and renal disease. J Nephrol 22: 190–195, 2009 [PubMed] [Google Scholar]
- 10. Green JS, Parfrey PS, Harnett JD, Farid NR, Cramer BC, Johnson G, Heath O, Mcmanamon PJ, Oleary E, Prysephillips W. The cardinal manifestations of Bardet-Biedl syndrome, a form of Laurence-Moon-Biedl syndrome. N Engl J Med 321: 1002–1009, 1989 [DOI] [PubMed] [Google Scholar]
- 11. Harnett JD, Green JS, Cramer BC, Johnson G, Chafe L, Mcmanamon P, Farid NR, Prysephillips W, Parfrey PS. The spectrum of renal disease in Laurence-Moon-Biedl syndrome. N Engl J Med 319: 615–618, 1988 [DOI] [PubMed] [Google Scholar]
- 12. Iannello S, Bosco P, Cavaleri A, Camuto M, Milazzo P, Belfiore F. A review of the literature of Bardet-Biedl disease and report of three cases associated with metabolic syndrome and diagnosed after the age of fifty. Obes Rev 3: 123–135, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Moore SJ, Green JS, Fan YL, Bhogal AK, Dicks E, Fernandez BA, Stefanelli M, Murphy C, Cramer BC, Dean JCS, Beales PL, Katsanis N, Bassett AS, Davidson WS, Parfrey PS. Clinical and genetic epidemiology of Bardet-Biedl syndrome in Newfoundland: a 22-year prospective, population-based, cohort study. Am J Med Genet 132A: 352–360, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morgan DA, Thedens DR, Weiss R, Rahmouni K. Mechanisms mediating renal sympathetic activation to leptin in obesity. Am J Physiol Regul Integr Comp Physiol 295: R1730–R1736, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mykytyn K, Mullins RF, Andrews M, Chiang AP, Swiderski RE, Yang BL, Braun T, Casavant T, Stone EM, Sheffield VC. Bardet-Biedl syndrome type 4 (BBS4)-null mice implicate Bbs4 in flagella formation but not global cilia assembly. Proc Natl Acad Sci USA 101: 8664–8669, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129: 1201–1213, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation 117: 1161–1171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nishimura DY, Fath M, Mullins RF, Searby C, Andrews M, Davis R, Andorf JL, Mykytyn K, Swiderski RE, Yang BL, Carmi R, Stone EM, Sheffield VC. Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proc Natl Acad Sci USA 101: 16588–16593, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rahmouni K, Fath MA, Seo S, Thedens DR, Berry CJ, Weiss R, Nishimura DY, Sheffield VC. Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. J Clin Invest 118: 1458–1467, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rahmouni K, Mark AL, Haynes WG, Sigmund CD. Adipose depot-specific modulation of angiotensinogen gene expression in diet-induced obesity. Am J Physiol Endocrinol Metab 286: E891–E895, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes 54: 2012–2018, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Stoetzel C, Muller J, Laurier V, Davis EE, Zaghloul NA, Vicaire S, Jacquelin C, Plewniak F, Leitch CC, Sarda P, Hamel C, de Ravel TJL, Lewis RA, Friederich E, Thibault C, Danse JM, Verloes A, Bonneau D, Katsanis N, Poch O, Mandel JL, Dollfus H. Identification of a novel BBS gene (BBS12) highlights the major role of a vertebrate-specific branch of chaperonin-related proteins in Bardet-Biedl syndrome. Am J Hum Genet 80: 1–11, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang DH. Transient receptor potential vanilloid channels in hypertension, inflammation, and end organ damage: an imminent target of therapy for cardiovascular disease? Curr Opin Cardiol 23: 356–363, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang YP, Wang DH. A novel mechanism contributing to development of Dahl salt-sensitive hypertension–role of the transient receptor potential vanilloid type 1. Hypertension 47: 609–614, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Woudenberg-Vrenken TE, Bindels RJM, Hoenderop JGJ. The role of transient receptor potential channels in kidney disease. Nat Rev Nephrol 5: 441–449, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Zaghloul NA, Katsanis N. Mechanistic insights into Bardet-Biedl syndrome, a model ciliopathy. J Clin Invest 119: 428–437, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.