Abstract
Urothelium-specific overexpression of nerve growth factor (NGF) in the urinary bladder of transgenic mice stimulates neuronal sprouting in the urinary bladder, produces increased voiding frequency, and results in increased referred somatic hypersensitivity. Additional NGF-mediated pleiotropic changes might contribute to the increased voiding frequency and pelvic hypersensitivity observed in these transgenic mice, such as modulation of other growth factor/receptor systems. Chronic overexpression of NGF in the urothelium was achieved through the use of a highly urothelium-specific uroplakin II promoter. In the present study, we examined NGF, brain-derived neurotrophic factor (BDNF), and associated receptor [p75NTR, tyrosine kinase (Trk)A, TrkB] transcript and protein expression in urothelium and detrusor smooth muscle of NGF-overexpressing (OE) and littermate wild-type mice, using real-time quantitative reverse transcription-polymerase chain reaction, ELISAs, and semiquantitation of immunohistochemistry. We focused on these growth factor/receptors given the established roles of NGF/TrkA, NGF/p75NTR, and BDNF/TrkB systems in bladder function. Increased voiding frequency in NGF-OE mice was confirmed by examining urination patterns. BDNF, TrkA, and TrkB protein expression was significantly (P ≤ 0.01) reduced and p75NTR protein expression was significantly (P ≤ 0.01) increased in urinary bladder of NGF-OE mice. The NGF-OE-induced changes in neurotrophic factor/receptor expression in urinary bladder may represent compensatory changes to reduce voiding frequency in the NGF-OE mouse.
Keywords: detrusor smooth muscle, real-time quantitative reverse transcription-polymerase chain reaction, enzyme-linked immunosorbent assay, immunohistochemistry
many previous studies in rodents have demonstrated the importance of nerve growth factor (NGF) in bladder sensory function and the development of referred hyperalgesia in response to bladder inflammation (10, 11, 14, 22, 26, 63). We recently examined (53) the role of NGF in urinary bladder dysfunction by generating a mouse model of urinary bladder hypersensitivity based on the hypothesis that chronic urothelial NGF overexpression would induce sensory neuronal hypersensitivity and increased urinary bladder reflex function (53). Chronic overexpression of NGF in the urothelium was achieved through the use of a highly urothelium-specific uroplakin II promoter (35, 36).
Our studies (53) revealed that urothelium-specific overexpression of NGF in the urinary bladder of transgenic mice 1) stimulates neuronal sprouting or proliferation in the urinary bladder, 2) produces local inflammatory changes in the urinary bladder, 3) produces increased voiding frequency, and 4) results in increased referred somatic hypersensitivity. Elevated levels of neurotrophins have also been detected in the urine of women with interstitial cystitis (IC)/bladder pain syndrome (BPS) (48) and in the urothelium of individuals with IC/BPS or other painful bladder conditions (43). It has also been recently demonstrated that urinary NGF levels are increased in patients with overactive bladder (OAB) symptoms associated with detrusor overactivity (DO), stress urinary incontinence, or bladder outlet obstruction (BOO) (38–42, 48, 62).
NGF-mediated changes in urinary bladder function and altered referred somatic sensitivity (20–22, 47) may involve changes in urinary bladder expression of nociception-related molecules including neurotrophin/receptor systems. Neurotrophic factors [NGF, brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), neurotrophin (NT)-3, NT-4] (58), tyrosine kinase (Trk) membrane receptors (TrkA, TrkB), and the neurotrophin receptor p75NTR (29, 30) are expressed and modulated in the urinary bladder after urinary bladder inflammation. For this study, we have focused our examination of NGF-induced changes in urinary bladder on BDNF, p75NTR, TrkA, and TrkB because previous studies have demonstrated roles for NGF/TrkA (13, 51, 57, 59, 60), NGF/p75NTR (29, 30), and BDNF/TrkB (50) interactions in urinary bladder function with urinary bladder inflammation.
In the present study, we examined NGF, BDNF, TrkA, TrkB, and p75NTR transcript and protein expression in urothelium and detrusor smooth muscle in NGF-overexpressing (OE) and littermate wild-type (WT) mice, using real-time quantitative reverse transcription-polymerase chain reaction (Q-PCR), enzyme-linked immunosorbent assays (ELISAs), and immunohistochemical approaches.
MATERIALS AND METHODS
Animals
NGF-OE transgenic mice were generated at Roche Palo Alto (material transfer agreement with Roche Palo Alto and Dr. Debra Cockayne) in collaboration with Dr. Henry Sun at New York University Medical School as previously described (9, 17, 53). Animal genotype was confirmed by Southern and/or PCR analyses; all mice have the inbred genetic C57BL/6J background and were derived from F2 to F4 generations maintained through a hemizygous backcross strategy with C57BL/6J WT mice. Mice used in this study were bred locally at the University of Vermont College of Medicine. The litters were of normal size and weight, and behaviors (feeding, drinking, activity patterns) appeared normal. As previously demonstrated (53) and confirmed in this study, urinary bladder weight was significantly (P ≤ 0.01) increased in NGF-OE mice (54.7 ± 4.9 mg) compared with WT mice (21.4 ± 1.8 mg). All experimental protocols involving animal use were approved by the University of Vermont Institutional Animal Care and Use Committee (IACUC no. 08-085). Animal care was under the supervision of the University of Vermont's Office of Animal Care Management in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and National Institutes of Health guidelines. All efforts were made to minimize the potential for animal pain, stress, or distress.
Urination Patterns
We previously characterized voiding function in NGF-OE mice by open voiding cystometry in conscious, unrestrained mice (53). This technique involves the implantation of a bladder catheter into the dome of the bladder, which is secured with a purse string suture (53). In this study, we have determined urination patterns on filter paper in NGF-OE mice, which does not necessitate the need for an intravesical catheter (44). Female WT and NGF-OE littermate mice (n = 12 for each; 5–6 wk of age) were placed individually in standard cages for 1 h (6, 56) in the morning (9–11 AM) with the bedding replaced with Whatman grade 3 filter paper. Food and water were provided ad libitum. Mice were habituated to cages for 1 h in the morning (9–11 AM) on each of two consecutive days before data accumulation. Urine spots were photographed under UV light (56), area (cm2) of spots was determined, and large (0.2–10 cm2) (6) and small (<0.2 cm2) (6) spots were counted. A standard curve of urine volume versus urine spot area was created to estimate void volumes (20 points, r2 = 0.997). Mice were placed into cages and data were analyzed by an individual blinded to mouse strain; groups were decoded after data analysis.
Measurement of Urinary Bladder NGF, BDNF, TrkA, and TrkB by ELISAs
Determination of NGF, BDNF, TrkA, and TrkB content in the urinary bladder of NGF-OE transgenic mice and WT littermate control mice was determined by ELISAs as previously described (9, 53, 58). Whole urinary bladders were homogenized separately in tissue protein extraction agent (T-PER; Roche, Indianapolis, IN), a commercially available, mild zwitterionic dialyzable detergent in 25 mM bicine, 150 mM sodium chloride (pH 7.6) containing a protease inhibitor mix (Sigma-Aldrich, St. Louis, MO; 16 μg/ml benzamidine, 2 μg/ml leupeptin, 50 μg/ml lima bean trypsin inhibitor, and 2 μg/ml pepstatin A), and aliquots were removed for protein assay as previously described (30). The supernatants were used for quantification as previously described (58). Total protein was determined with the Coomassie Plus (Bradford) Protein Assay Kit (Fisher Scientific, Pittsburgh, PA). According to the manufacturer, the NGF E-max or BDNF E-max immunoassay systems (Promega, Madison, WI) demonstrate very low cross-reactivity with structurally related growth factors at concentrations up to 10–100 ng/ml. According to the manufacturer (R&D Systems, Minneapolis, MN), the TrkA or TrkB DuoSets do not show cross-reactivity or interference with other Trk receptors at concentrations up to 100 ng/ml. The standards provided with these systems generated linear standard curves (r2 = 0.996–0.998, P ≤ 0.001). Absorbance values of standards and samples were corrected by subtraction of the background value (absorbance due to nonspecific binding). No samples fell below the detection limits of the assays, and samples were not diluted before assay. Curve fitting of standards and evaluation of NGF, BDNF, TrkA, and TrkB content of samples were performed with a least-squares fit (53, 58).
Euthanasia and Tissue Harvest
Female WT and NGF-OE littermate mice (n = 5–7 for each; 5–6 wk of age) were deeply anesthetized with isoflurane (3–4%) and then euthanized via thoracotomy. The urinary bladder was quickly dissected under RNase-free conditions. The bladder was cut open along the midline and pinned to a silicon-coated plate (Sylgard, Dow Corning, Midland, MI), the urothelium was removed with the aid of fine forceps and a dissecting microscope, and all tissues were snap-frozen on dry ice before processing as previously described (4, 30). Thus dissected, the urothelium also includes suburothelial structures; the term “urothelium” in this article refers to both urothelial and suburothelial structures.
Split Bladder Preparation and Assessment of Potential Contamination of Bladder Layers
NGF, BDNF, TrkA, TrkB, and p75NTR mRNA expression was determined in the urothelium/suburothelium and detrusor smooth muscle layers of the urinary bladder. The urothelium/suburothelium was dissected from the detrusor smooth muscle with fine forceps under a dissecting microscope as previously described (9, 30, 64). To confirm the specificity of our split bladder preparations, urothelium/suburothelium and detrusor samples were examined for the presence of α-smooth muscle actin and uroplakin II by Western blotting or Q-PCR as previously described (9, 12). In urothelium/suburothelium layers, only uroplakin II was present (data not shown). Conversely, in detrusor samples, only α-smooth muscle actin was present (data not shown).
Real-Time Quantitative Reverse Transcription-Polymerase Chain Reaction
Total RNA was extracted with the STAT-60 total RNA/mRNA isolation reagent (Tel-Test “B”, Friendswood, TX) as previously described (4, 18, 29). One to two milligrams of RNA per sample was used to synthesize complementary DNA with SuperScript II reverse transcriptase and a mix of random hexamer and oligo(dT) primers with Moloney murine leukemia virus (MMLV) reverse transcriptase (Promega) in a 25-μl final reaction volume.
The quantitative PCR standards for all transcripts were prepared with the amplified NGF, BDNF, TrkA, TrkB, P75NTR, and 18S cDNA products ligated directly into pCR2.1 TOPO vector with the TOPO TA cloning kit (Invitrogen). The nucleotide sequences of the inserts were verified by automated fluorescent dideoxy dye terminator sequencing (Vermont Cancer Center DNA Analysis Facility). To estimate the relative expression of the receptor transcripts, 10-fold serial dilutions of stock plasmids were prepared as quantitative standards. The range of standard concentrations was determined empirically.
Complementary DNA templates, diluted 10-fold to minimize the inhibitory effects of the reverse transcription reaction components, were assayed with SYBR Green I JumpStart Taq ReadyMix (Sigma, St. Louis, MO) containing 5 mM MgCl2, 200 mM dATP, dGTP, dCTP, and dTTP, 0.64 U of Taq DNA polymerase, and each primer at 300 nM in a final 25-μl reaction volume (4, 18, 29). The Q-PCR was performed (Applied Biosystems 7500 Fast real-time PCR system, Foster City, CA) (4, 18, 29) with the following standard conditions: 1) serial heating at 94°C for 2 min and 2) amplification over 40 cycles at 94°C for 15 s and 60–64°C depending on primer sets for 30 s.
The amplified product from these amplification parameters was subjected to SYBR Green I melting analysis by ramping the temperature of the reaction samples from 60°C to 95°C. A single DNA melting profile was observed under these dissociation assay conditions, demonstrating amplification of a single unique product free of primer dimers or other anomalous products. Oligonucleotide primer sequences for NGF (9, 53), p75NTR (29), and 18S (18, 29) used in these studies have been previously described. We also used the following primer sequences: BDNF upper, GTGACAGTATTAGCGAGTGGG; BDNF lower, GGGTAGTTCGGCATTGC; TrkB upper, TGACGCAGTCGCAGATGCTG; TrkB lower, TTTCCTGTACATGATGCTCTCTGG; TrkA upper, TCCTTCTCGCCAGTGGACGGTAA; TrkA lower, AGTGCCTTGACAGCCACGAGCAT.
For data analyses, a standard curve was constructed by amplification of serially diluted plasmids containing the target sequence. Data were analyzed at the termination of each assay with sequence detection software (Sequence Detection Software, version 1.3.1; Applied Biosystems, Norwalk, CT). In standard assays, default baseline settings were selected. The increase in SYBR Green I fluorescence intensity (ΔRn) was plotted as a function of cycle number, and the threshold cycle was determined by the software as the amplification cycle at which the ΔRn first intersects the established baseline. All data are expressed as the relative quantity of the gene of interest normalized to the relative quantity of the housekeeping gene 18S. WT urothelium samples were set equal to 100%. WT detrusor samples were expressed relative to WT urothelium samples. Data are expressed in this manner to permit comparisons of transcript expression between urothelium and detrusor.
Immunohistochemistry
Bladder cryosections (10 μm) from female WT and NGF-OE mice (n = 5–7; 5–7 wk) were prepared for immunohistochemistry with an on-slide processing technique (4, 29, 30). For immunohistochemical processing, the cryosections were incubated overnight at room temperature with rabbit anti-NGF (1:100; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-TrkA (0.5 μg/ml; Santa Cruz Biotechnology) (51), rabbit anti-TrkB (0.5 μg/ml; Santa Cruz Biotechnology) (51), rabbit anti-BDNF (1:1,000; Santa Cruz Biotechnology), or rabbit anti-p75NTR (1:3,000; Advanced Targeting Systems) (29, 30) diluted in 0.1 M potassium phosphate-buffered saline (KPBS) containing 1% goat serum. After washing, the preparations were incubated with a Cy3-conjugated species-specific secondary antibody (1:500; Jackson ImmunoResearch Laboratories, West Grove, PA) for 2 h at room temperature, rinsed, and mounted with antifade medium (Citifluor, Fisher Scientific) for fluorescent microscopy. In some sections, after washing the slides were stained with YOYO-1 (1:10,000; Molecular Probes, Eugene, OR) to stain RNA and identify cell nuclei and then coverslipped as described above. Control sections incubated in the absence of primary or secondary antibody were also processed and evaluated for specificity or background staining levels. In the absence of primary antibody, no positive immunostaining was observed. Additional controls were also performed, including preabsorption of antisera with appropriate immunogen (1–3 μg/ml) that reduced staining to background levels.
Assessment of Immunohistochemical Staining in Urinary Bladder
Immunohistochemistry and subsequent semiquantification of growth factor and receptor expression in bladder sections were performed on control and experimental tissues simultaneously to reduce the incidence of staining variation that can occur between tissues processed on different days. Staining observed in experimental tissue was compared with that observed from experiment-matched negative controls. Urinary bladder sections or whole mounts exhibiting immunoreactivity that was greater than the background level observed in experiment-matched negative controls were considered positively stained.
Visualization and Semiquantitative Analysis of NGF, BDNF, TrkA, TrkB, and p75NTR Immunoreactivity in Urinary Bladder
Urothelium.
NGF, BDNF, TrkA, TrkB, and p75NTR immunoreactivity (IR) in bladder sections was visualized and images were captured with an Olympus fluorescence photomicroscope. The filter was set with an excitation range of 560–569 nm and an emission range of 610–655 nm for visualization of Cy3. Images were captured, acquired in tagged image file format, and imported into image analysis software (Meta Morph, version 4.5r4, University Imaging, Downingtown, PA) (4, 29). The free hand drawing tool was used to select the urothelium, and the urothelium was measured in total pixels area as previously described (4, 29). A threshold encompassing an intensity range of 100–250 grayscale values was applied to the region of interest in the least brightly stained condition first. The threshold was adjusted for each experimental series, with concomitantly processed negative controls as a guide for setting background fluorescence. The same threshold was subsequently used for all images. Immunoreactivity was considered to be positive only when the staining for the marker of interest (NGF, BDNF, TrkA, TrkB, p75NTR) exceeded the established threshold. Percent marker expression above threshold in the total area selected was calculated. NGF, BDNF, TrkA, TrkB, and p75NTR IR in the urothelium was consistent across all regions (dome, body, neck) of the urinary bladder examined for WT and NGF-OE mice. Semiquantification of growth factor and receptor expression in the urothelium is presented for the bladder neck region.
Detrusor smooth muscle.
Visualization of NGF, BDNF, TrkA, TrkB, and p75NTR IR in detrusor smooth muscle of cryostat sections was identical to that described for the urothelium (above). Semiquantification of IR in detrusor smooth muscle was performed as previously described (9, 28) and modified from Brady et al. (8). Grayscale images acquired in tagged image file format were imported into image analysis software (Image J) (1), and images were thresholded. Images of detrusor smooth muscle were acquired from the dome, body, and neck region of the urinary bladder in WT and NGF-OE mice. A rectangle of fixed dimension (500 × 500 pixels) was placed on the section according to a random selection of x and y coordinates. This process was repeated seven times for each image of detrusor. The average optical density of NGF, BDNF, TrkA, TrkB, or p75NTR in detrusor smooth muscle was then calculated. Growth factor and receptors examined in the detrusor exhibited equivocal expression throughout detrusor of the dome, body, and neck of the urinary bladder; thus data from each region are pooled and presented as NGF, BDNF, TrkA, TrkB, or p75NTR IR above threshold in detrusor smooth muscle.
Digital images were obtained with a charge-coupled device (CCD) camera (MagnaFire SP; Optronics; Optical Analysis, Nashua, NH) and a LG-3 frame grabber (Scion, Frederick, MD). Exposure times, brightness, and contrast were held constant when acquiring images from experimental or control animals processed and analyzed on the same day. Images were imported into a graphics editing program (Adobe Photoshop 7.0, Adobe Systems Incorporated, San Jose, CA), assembled, and labeled.
Statistical Analyses
One-way analysis of variance was used to evaluate differences among groups for Q-PCR. Percentage data from image analysis were arcsin transformed to meet the requirements of this statistical test. Animals processed and analyzed on the same day were tested as a block in the ANOVA. When F ratios exceeded the critical value (P ≤ 0.05), the Newman-Keuls post hoc test was used to compare the experimental means.
RESULTS
Urination Patterns
Urine spots from NGF-OE mice, as quantified on filter paper over a 1-h period, were significantly (P ≤ 0.01) greater in number (42.2 ± 4.5 vs. 14.7 ± 3.5 voids/h) but smaller in area (3.3 ± 1.5 vs. 7.5 ± 1.5 cm2) compared with those made by littermate WT mice. A significant (P ≤ 0.01) increase in the number of both large (0.2–10 cm2)- and small (<0.2 cm2)-diameter urine spots was observed for NGF-OE mice. With a standard curve to estimate urine volumes from urine spot diameter, no difference in total void volume per hour was observed between WT (61.7 ± 3.5 μl) and NGF-OE (63.3 μl ± 4.7 μl) mice. Fluid intake measured over a 24-h period was similar among littermates (7.8 ± 1.0 vs. 7.2 ± 0.8 ml/24 h).
NGF Transcript and Protein Expression and Immunoreactivity in Urothelium and Detrusor of WT and NGF-OE Mice
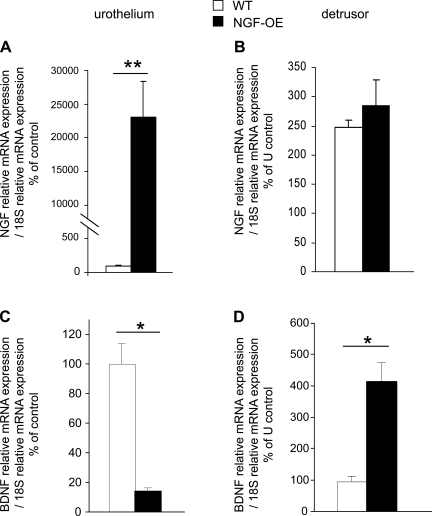
Consistent with our previous studies (9, 17, 53), NGF transcript and protein expression were significantly (P ≤ 0.01) increased in urothelium of NGF-OE mice (Fig. 1A and data not shown); no changes were observed in NGF transcript and protein expression in detrusor smooth muscle between NGF-OE and littermate WT mice (Fig. 1B and data not shown). As previously demonstrated (9, 53), NGF IR was increased in the urothelium of NGF-OE mice but no changes in NGF IR were observed in the detrusor smooth muscle of NGF-OE mice (data not shown).
Fig. 1.
Modulation of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) transcript expression in littermate wild-type (WT) and NGF-overexpressing (NGF-OE) mice in urothelium and detrusor smooth muscle. WT urothelium samples were set equal to 100% and normalized to the relative expression of the housekeeping gene 18S. WT detrusor samples were expressed relative to WT urothelium samples and normalized to the relative expression of the housekeeping gene 18S. A: NGF mRNA expression in urothelium (U). B: NGF mRNA expression in detrusor. C: BDNF mRNA expression in urothelium. D: BDNF mRNA expression in detrusor. Values are means ± SE for sample size (n = 5–7). **P ≤ 0.0001, *P ≤ 0.01 vs. WT.
BDNF Transcript and Protein Expression and Immunoreactivity in Urothelium and Detrusor of WT and NGF-OE Mice
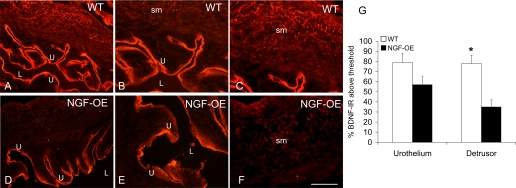
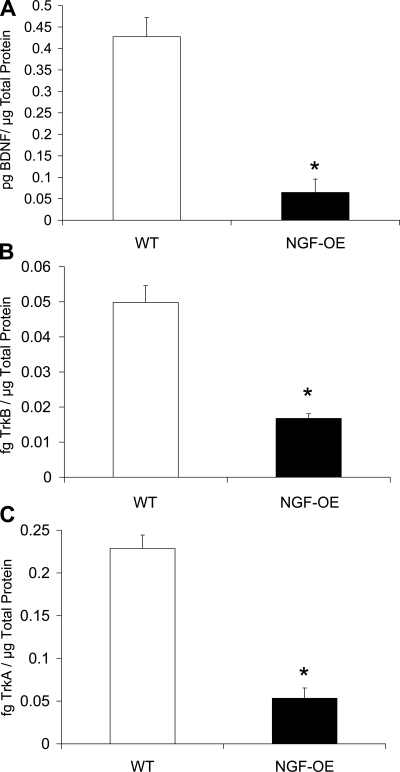
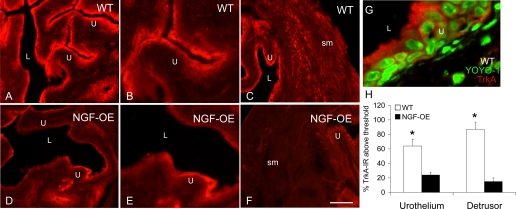
BDNF transcript expression was significantly (P ≤ 0.01) decreased in urothelium of NGF-OE mice (Fig. 1C), whereas BDNF transcript expression in detrusor smooth muscle of NGF-OE mice was increased (P ≤ 0.01) compared with littermate WT mice (Fig. 1D). In contrast to transcript expression, BDNF IR in the detrusor smooth muscle of NGF-OE mice was significantly (P ≤ 0.01) decreased (Fig. 2) and BDNF IR in the urothelium was unchanged (Fig. 2; P ≤ 0.07). In whole urinary bladder, BDNF protein expression was also decreased (P ≤ 0.01) in NGF-OE mice compared with WT mice (Fig. 3A).
Fig. 2.
A–F: BDNF immunoreactivity (IR) in urothelium (U; A, B, D, E) and detrusor smooth muscle (sm) (A, C, D, F) of littermate WT (A–C) and NGF-OE (D–F) mice. Comparable BDNF IR was present in urothelial cells of WT and NGF-OE urothelium (A, B, D, E). BDNF-IR expression was reduced in detrusor sm of NGF-OE mice (D, F) compared with detrusor sm of WT mice (A, C). L, lumen. Calibration bar: 50 μm (B, C, E, F), 125 μm (A, D). G: summary histogram of BDNF expression in U and detrusor sm in WT and NGF-OE mice. Values are means ± SE (n = 5–7). *P ≤ 0.01.
Fig. 3.
BDNF, tyrosine kinase (Trk)B, and TrkA content in the urinary bladders of WT and NGF-OE transgenic mice. BDNF (A), TrkB (B), and TrkA (C) content in whole urinary bladder was determined in WT and NGF-OE. Urinary bladder NGF, TrkB, and TrkA content was significantly (*P ≤ 0.01) decreased in NGF-OE transgenic vs. WT mouse bladders. Values are means ± SE (n = 5–7).
p75NTR Transcript and Protein Expression and Immunoreactivity in Urothelium and Detrusor of WT and NGF-OE Mice
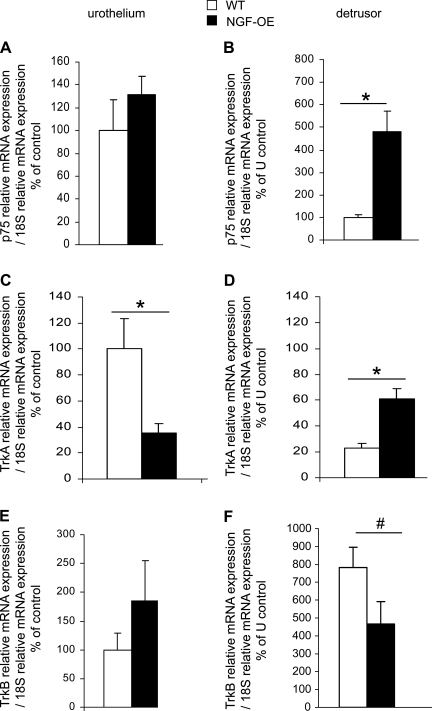
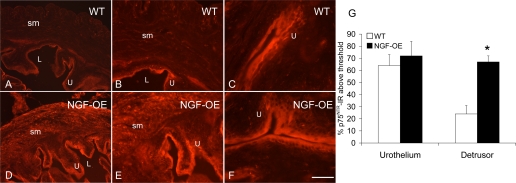
No changes in p75NTR transcript expression or p75NTR IR were observed in urothelium of NGF-OE mice (Fig. 4A; Fig. 5), whereas p75NTR transcript expression (Fig. 4B) and p75NTR IR in detrusor smooth muscle of NGF-OE mice were increased (P ≤ 0.01) compared with littermate WT mice (Fig. 5).
Fig. 4.
Modulation of p75NTR, TrkA, and TrkB receptor transcript expression in littermate WT and NGF-OE mice in urothelium and detrusor smooth muscle. WT urothelium samples were set equal to 100% and normalized to the relative expression of the housekeeping gene 18S. WT detrusor samples were expressed relative to WT urothelium samples and normalized to the relative expression of the housekeeping gene 18S. A: p75NTR mRNA expression in urothelium (U). B: p75NTR mRNA expression in detrusor. C: TrkA mRNA expression in U. D: TrkA mRNA expression in detrusor. E: TrkB mRNA expression in U. F: TrkB mRNA expression in detrusor. Values are means ± SE for sample size (n = 5–7). #P ≤ 0.05, *P ≤ 0.01 vs. WT.
Fig. 5.
p75NTR IR in urothelium (U) (A–F) and detrusor smooth muscle (sm) (A, B, D, E) of littermate WT (A–C) and NGF-OE (D–F) mice. Comparable p75NTR IR was present in urothelial cells of WT and NGF-OE urothelium (A–F). p75NTR IR was expressed in detrusor sm of WT mice (A, B) and increased in detrusor sm of NGF-OE mice (D, E). L, lumen. Calibration bar: 50 μm (B, C, E, F), 125 μm (A, D). G: summary histogram of p75NTR expression in the U and detrusor sm in WT and NGF-OE mice. Values are means ± SE for n = 5–7. *P ≤ 0.01.
TrkA Transcript and Protein Expression and Immunoreactivity in Urothelium and Detrusor of WT and NGF-OE Mice
TrkA transcript expression and TrkA IR were significantly (P ≤ 0.01) decreased in urothelium of NGF-OE mice (Fig. 4C; Fig. 6), whereas TrkA transcript expression in detrusor smooth muscle of NGF-OE mice was increased (P ≤ 0.01) compared with littermate WT mice (Fig. 4D). In contrast to TrkA transcript expression, TrkA-IR in the detrusor smooth muscle of NGF-OE mice was significantly (P ≤ 0.01) decreased (Fig. 6). Consistent with TrkA transcript expression, TrkA IR in the urothelium was significantly (P ≤ 0.01) decreased in NGF-OE mice (Fig. 6). In whole urinary bladder, TrkA protein expression was also decreased (P ≤ 0.01) in NGF-OE mice compared with WT (Fig. 3C).
Fig. 6.
TrkA IR in urothelium (U) (A–F) and detrusor smooth muscle (sm) (C, F) of littermate WT (A–C) and NGF-OE (D–F) mice. TrkA IR was significantly (P ≤ 0.01) reduced in urothelial cells of NGF-OE mice (A–F). TrkA IR was also significantly (P ≤ 0.01) reduced in detrusor sm of NGF-OE mice (C, F). G: higher-power image of TrkA IR in urothelial cells in littermate WT mice. Trk staining was visualized with Cy3-conjugated secondary antibody (red areas). YOYO-1 identified nuclear profiles of urothelial cells and was visualized with Cy2-conjugated secondary antibody (green areas). L, lumen. Calibration bar: 50 μm (B, E), 125 μm (A, C, D, F), 30 μm (G). H: summary histogram of TrkA expression in U and detrusor sm in WT and NGF-OE mice. Values are means ± SE for n = 5–7. *P ≤ 0.01.
TrkB Transcript and Protein Expression and Immunoreactivity in Urothelium and Detrusor of WT and NGF-OE Mice
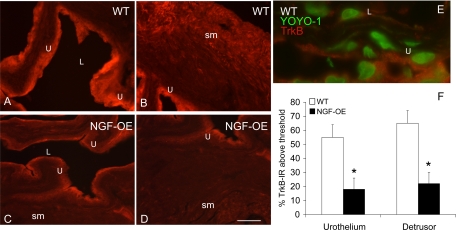
TrkB transcript expression was unchanged (Fig. 4E) in urothelium whereas TrkB IR was significantly (P ≤ 0.01) decreased in urothelium of NGF-OE mice (Fig. 7). TrkB transcript expression (Fig. 4F) and TrkB IR were significantly (P ≤ 0.05) decreased in detrusor smooth muscle of NGF-OE mice (Fig. 7). In whole urinary bladder, TrkB protein expression was also decreased (P ≤ 0.01) in NGF-OE mice compared with WT mice (Fig. 3B).
Fig. 7.
TrkB IR in urothelium (U) (A–D) and detrusor smooth muscle (sm) (B–D) of littermate WT (A, B) and NGF-OE (C, D) mice. TrkB IR was significantly (P ≤ 0.01) reduced in urothelial cells of NGF-OE mice (A, C). TrkB IR was also significantly (P ≤ 0.01) reduced in detrusor sm of NGF-OE mice (B, D). E: higher-power image of TrkB IR in urothelial cells in littermate WT mice. Trk staining was visualized with Cy3-conjugated secondary antibody (red areas). YOYO-1 identified nuclear profiles of urothelial cells and was visualized with Cy2-conjugated secondary antibody (green areas). L, lumen. Calibration bar: 50 μm (A–D), 30 μm (E). F: summary histogram of TrkB expression in the U and detrusor sm in WT and NGF-OE mice. Values are means ± SE for n = 5–7. *P ≤ 0.01.
DISCUSSION
Our recent studies (53) with a transgenic mouse model of urothelium-specific overexpression of NGF represented a novel approach to exploring the role of NGF in urinary bladder inflammation and sensory function. NGF is a potent neurotrophic factor for sensory afferent and sympathetic nerve fibers, and excitatory changes in these neurons underlie NGF-mediated hypersensitivity in a number of target tissues (10, 11, 14, 22, 25, 26, 63). The major findings (53) observed were that NGF overexpression led to marked hyperinnervation of the urinary bladder by sensory afferent and sympathetic nerve fibers as well as an increased number of urinary bladder mast cells, consistent with the known survival and trophic actions of NGF (3, 34). Functionally, NGF-OE mice exhibited frequent urination as well as referred pelvic hypersensitivity (53). Increased voiding frequency in NGF-OE mice was confirmed in the present studies by assessment of urination patterns on filter paper that did not necessitate a bladder catheter implantation as previously published (53). This approach was used in the event that a bladder catheter could artificially augment micturition reflexes in NGF-OE mice that exhibit increased sensory innervation of the urinary bladder. The previous open voiding cystometry studies (53) and present urination pattern results are consistent and support the observation of increased voiding frequency in NGF-OE mice (53).
In this study we have begun to consider additional NGF-mediated pleiotropic changes that might contribute to the NGF-OE phenotype (53). NGF-mediated changes in the excitability and/or sensitization of primary afferents is likely to involve changes in expression of nociception-related molecules—including sensory growth factors (NGF, BDNF) and associated receptors (p75NTR, TrkA, TrkB) (7, 13, 50, 51, 57, 59, 60) previously shown to play roles in urinary bladder function. The present study demonstrates that NGF overexpression in the urothelium modulates transcript and protein expression of BDNF, p75NTR, TrkA, and TrkB in the urinary bladder. BDNF, TrkA, and TrkB protein expression was significantly reduced and p75NTR protein expression was significantly increased in urinary bladder of NGF-OE mice. Given the functional consequences of NGF and BDNF expression and NGF-TrkA (14, 24, 53, 60, 63) and NGF-p75NTR (30) interactions in urinary bladder, the observed changes may represent compensatory, concomitant changes aimed at reducing urinary frequency in the NGF-OE mouse.
The present study demonstrates a number of NGF-mediated pleiotropic changes in BDNF, p75NTR, TrkA, and TrkB protein and transcript expression in the urothelium and detrusor smooth muscle of NGF-OE mice with a combination of approaches including Q-PCR, ELISAs, and semiquantitative immunohistochemistry. Numerous studies demonstrate pleiotropic changes including alterations in growth factors and associated receptors stemming from overexpression of NGF (19, 27), NT-4 (31), artemin (15, 16), or BDNF (33) in specific target tissues. Previous studies (19, 27) using transgenic mice with NGF-OE in the skin demonstrated modulation of TrkA, TrkC, and p75NTR expression in sensory ganglia. As previously published (9, 17, 53) and confirmed in the present study, a notable feature of the NGF-OE mice is the highly urothelium-specific expression of ectopic NGF. Total NGF mRNA in the bladder urothelium/suburothelium of transgenic mice was significantly increased over that observed in WT mice, with no differences detected in the detrusor smooth muscle. We have previously shown (9, 53) that the increased bladder NGF content in NGF-OE mice is evident as early as postnatal days 7–10 and through adulthood, consistent with the onset of expression of the mouse uroplakin II gene.
Altered NGF, NGF-TrkA, and NGF-p75NTR and altered BDNF and BDNF-TrkB interactions are associated with bladder inflammation and urinary bladder dysfunction in both rodents and humans, where it may underlie neurochemical, organizational, and/or electrical property changes of micturition reflex pathways. Experimental studies in animals have shown that increased production of NGF within the bladder is associated with neuronal plasticity changes (10, 11, 14, 22, 24, 26, 63). Clinically, elevated levels of NGF have been detected in the urine and bladder urothelium of patients with IC/BPS (43, 48). A series of recent papers (38–42) have also demonstrated increased urinary NGF levels in patients with OAB symptoms with stress urinary incontinence or BOO, leading to the suggestion that NGF may be a biomarker for OAB. Recent studies (50) have also demonstrated that sequestration of BDNF in a rat model of urinary bladder inflammation induced by cyclophosphamide (CYP) decreased voiding frequency and reduced spinal cord expression of c-Fos triggered by CYP-induced cystitis. Thus altered NGF and BDNF expression in micturition reflex pathways may both contribute to micturition reflex plasticity with urinary bladder inflammation.
Pelvic visceral afferent neurons and the urinary bladder widely express neurotrophic factor receptors, including certain Trk receptors and p75NTR (45, 51, 52, 61). TrkA and TrkB IR as well as Trk phosphorylation status are increased in bladder afferent neurons and urinary bladder after urinary bladder inflammation (46, 51). Intravesical administration of the nonselective tyrosine kinase inhibitor K252a reduced voiding frequency associated with CYP-induced cystitis and referred somatic sensitivity, suggesting a role for NGF-TrkA signaling in CYP-induced changes in voiding frequency and somatic sensitivity (21, 22, 60). Recent studies have shown that CYP-induced cystitis increased p75NTR expression in the lumbosacral spinal cord, dorsal root ganglia (DRG), and urinary bladder (29). The role of p75NTR in bladder function in control and CYP-treated rats was determined with conscious cystometry and immunoneutralization or PD90780, a compound known to specifically block NGF binding to p75NTR (30). Both methods of p75NTR blockade, when delivered intravesically, significantly decreased the intercontraction voiding interval and void volume in control and CYP-treated rats. These studies demonstrate that p75NTR blockade at the level of the urinary bladder produces increased voiding frequency in control and CYP-treated rats. The overall activity of the urinary bladder likely under normal or inflamed conditions likely reflects the balance of NGF-p75NTR and NGF-TrkA signaling (30).
The NGF-induced changes in BDNF, TrkA, TrkB, and p75NTR protein expression in the urinary bladder may represent compensatory changes that would result in an overall improvement in bladder function by reducing NGF-induced increases in voiding frequency. In the present study, NGF-OE in the urothelium reduced BDNF, TrkA, and TrkB protein expression but increased p75NTR protein expression in the urinary bladder. Given the previous evidence that increased NGF bladder content and NGF-TrkA interactions (14, 24, 32, 60, 63) and increased BDNF bladder content and BDNF-TrkB interactions (50) increase voiding frequency, the observed decreases in BDNF, TrkA, and TrkB protein content in the urinary bladder of NGF-OE mice may represent adaptive mechanisms aimed at reducing voiding frequency. In addition, NGF-p75NTR interactions have been shown to reduce bladder activity in CYP-treated and control (no inflammation) rats (30). Therefore, increased p75NTR protein expression in urinary bladder may promote NGF-p75NTR signaling representing an additional compensatory mechanism to reduce voiding frequency in NGF-OE mice. Compensation may also be aimed at reducing pelvic hypersensitivity in the NGF-OE mice that also exhibit increased pelvic but not hindpaw sensitivity (53). Urinary bladder inflammation and hyperactivity induced by CYP (30, 58) or intravesical acrolein (22) or NGF (21) also produce hindpaw sensitivity that is attenuated by blockade of NGF by TrkA-IgG (26) or immunoneutralization (21) or by blockade of Trk receptors with K252a (21, 22). However, the role(s) of NGF-TrkA or NGF-p75NTR signaling or BDNF expression and BDNF-TrkB signaling has not been specifically addressed for pelvic sensitivity. Although NGF-induced changes in BDNF, TrkA, Trk, and p75NTR expression in the urinary bladder may be compensatory in nature, it is clear that NGF-OE mice exhibit a profound increase in voiding frequency and pelvic sensitivity (53), suggesting that these putative compensatory mechanisms are insufficient.
This study also demonstrates differential changes in BDNF, TrkA, TrkB, and p75NTR transcript expression between urothelium and detrusor smooth muscle in NGF-OE mice. Previous studies from our laboratory (17) have demonstrated additional pleiotropic effects in NGF-OE mice that involve changes in VIP/pituitary adenylate cyclase activating polypeptide (PACAP) and associated receptor (VPAC1, VPAC2, and PAC1) transcript expression. Similar to the results of the present study, differential transcript effects were detected in detrusor smooth muscle and urothelium of NGF-OE mice (17). Changes in transcript expression in detrusor in mice with chronic overexpression of NGF in the urothelium may suggest urothelial-mesenchymal interactions whereby urothelial NGF expression may influence detrusor smooth muscle properties as previously demonstrated for development and differentiation of detrusor smooth muscle (5, 17). The reasons underlying differential expression of transcripts between urothelium and detrusor smooth muscle of NGF-OE mice are not known but may reflect different functional roles for BDNF and TrkA in the urothelium compared with the detrusor smooth muscle.
The changes in BDNF and TrkA protein expression in urinary bladder of NGF-OE mice paralleled changes in transcript expression in the urothelium, whereas changes in TrkB and p75NTR protein expression paralleled changes in transcript expression in the detrusor smooth muscle. No changes in TrkB or p75NTR transcript expression were observed in urothelium of NGF-OE mice, so it is expected that changes in respective protein expression would be driven by changes in detrusor expression of TrkB and p75NTR mRNA in NGF-OE mice. For BDNF and TrkA, both the urothelium and detrusor smooth muscle of NGF-OE mice exhibited significant changes in transcript expression; however, changes in BDNF and TrkA protein expression mirrored that of transcript expression in the urothelium of NGF-OE. This is most likely due to the overall expression of TrkA and BDNF transcripts being greater in urothelium compared with detrusor in NGF-OE mice. Such comparisons can be made because the reverse transcriptions of samples, standard curves, and all cDNA were run simultaneously under identical Q-PCR conditions. In the present study, the lack of correlation between BDNF and TrkA transcripts and protein expression in detrusor of NGF-OE mice may reflect (23, 37, 54, 55) 1) mRNA transcript or protein instability, 2) decreased translation efficiency, 3) aberrant posttranslational mechanisms, and/or 4) a change in the half-life of the protein.
Increased urinary bladder NGF content may underlie many of the sensory changes that occur in patients with OAB symptoms or IC/BPS, including irritative voiding symptoms and pain in the case of IC/BPS. Thus the morphological and functional features of NGF-OE transgenic mice described here and previously (53) reflect many of the changes observed in micturition reflex pathways in patients with OAB symptoms or IC/BPS. The phenotype of these mice reflects many of the known roles of NGF in modulating the growth/maintenance of sensory and sympathetic nerve fibers, local mast cell responses, increased voiding frequency, and referred hyperalgesia (3, 34, 53). The NGF-OE transgenic mice represent a novel animal model of NGF-mediated urinary bladder dysfunction caused by chronic urothelium-specific overexpression of NGF. The value of the mouse model is that it offers a genetically stable model of chronic NGF overexpression from early postnatal development. This is also one limitation of this transgenic mouse model, because NGF-OE in the urothelium from early postnatal development may not reflect the etiology of OAB or IC/BPS. To date, most rodent studies have focused on transient, exogenous administration of NGF into the bladder or immunoneutralization of NGF or NGF receptor blockade in models of chemical/irritant-induced bladder dysfunction. As previously described (53), the NGF-OE transgenic mouse model should provide additional opportunities for testing hypotheses regarding the role of NGF in urinary bladder and visceral-somatic sensory function.
In summary, the present study examined pleiotropic changes in urinary bladder induced by NGF-OE in the urothelium, focusing on BDNF, TrkA, TrkB, and p75NTR given the documented neuroplastic and functional changes in micturition reflex pathways induced by these growth factor/receptor interactions. Our findings of decreased BDNF, TrkA, and TrkB protein expression and increased p75NTR protein expression in the urinary bladder suggest that these changes are compensatory, yet insufficient, to reduce voiding frequency in NGF-OE mice (53). It remains to be determined whether NGF-OE causes additional phenotypic or excitability changes (TRPV1, P2X3, P2X2/3, or Nav1.8) in urinary bladder and bladder sensory afferents that might contribute to the increased voiding frequency and pelvic hypersensitivity in NGF-OE mice (2, 49, 53). Studies investigating the transcriptional plasticity of additional sensory mediators in micturition reflex pathways in NGF-OE mice are in progress.
GRANTS
This work was funded by National Institutes of Health (NIH) Grants DK-051369, DK-060481, and DK-065989. NIH Grant Number P20-RR-16435 from the Centers of Biomedical Research Excellence (COBRE) Program of the National Center for Research Resources also supported the project for research resources.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Dr. Debra Cockayne, Roche Palo Alto, for the generous gift of NGF-OE mouse breeders used in the present study. The authors gratefully acknowledge the technical expertise and support provided by the Vermont Cancer Center DNA Analysis Facility. The authors thank Drs. Rod Parsons and John Tompkins for critical comments on the manuscript.
REFERENCES
- 1. Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int 11: 36–42, 2004 [Google Scholar]
- 2. Allen SJ, Dawbarn D. Clinical relevance of the neurotrophins and their receptors. Clin Sci (Lond) 110: 175–191, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Aloe L. The effect of nerve growth factor and its antibody on mast cells in vivo. J Neuroimmunol 18: 1–12, 1988 [DOI] [PubMed] [Google Scholar]
- 4. Arms L, Girard BM, Vizzard MA. Expression and function of CXCL12/CXCR4 in rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 298: F589–F600, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baskin L, DiSandro M, Li Y, Li W, Hayward S, Cunha G. Mesenchymal-epithelial interactions in bladder smooth muscle development: effects of the local tissue environment. J Urol 165: 1283–1288, 2001 [PubMed] [Google Scholar]
- 6. Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, deGroat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci 5: 856–860, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Braas KM, May V, Zvara P, Nausch B, Kliment J, Dunleavy JD, Nelson MT, Vizzard MA. Role for pituitary adenylate cyclase activating polypeptide in cystitis-induced plasticity of micturition reflexes. Am J Physiol Regul Integr Comp Physiol 290: R951–R962, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brady CM, Apostolidis AN, Harper M, Yiangou Y, Beckett A, Jacques TS, Freeman A, Scaravilli F, Fowler CJ, Anand P. Parallel changes in bladder suburothelial vanilloid receptor TRPV1 and pan-neuronal marker PGP9.5 immunoreactivity in patients with neurogenic detrusor overactivity after intravesical resiniferatoxin treatment. BJU Int 93: 770–776, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Cheppudira BP, Girard BM, Malley SE, Schutz KC, May V, Vizzard MA. Upregulation of vascular endothelial growth factor isoform VEGF-164 and receptors (VEGFR-2, Npn-1, and Npn-2) in rats with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 295: F826–F836, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chuang YC, Fraser MO, Yu YB, Chancellor MB, deGroat WC, Yoshimura N. The role of bladder afferent pathways in bladder hyperactivity induced by the intravesical administration of nerve growth factor. J Urol 165: 975–979, 2001 [PubMed] [Google Scholar]
- 11. Clemow DB, Steers WD, McCarty R, Tuttle JB. Altered regulation of bladder nerve growth factor and neurally mediated hyperactive voiding. Am J Physiol Regul Integr Comp Physiol 275: R1279–R1286, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Corrow KA, Vizzard MA. Phosphorylation of extracellular signal-regulated kinases in urinary bladder in rats with cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol 293: R125–R134, 2007 [DOI] [PubMed] [Google Scholar]
- 13. deGroat WC, Yoshimura N. Afferent nerve regulation of bladder function in health and disease. Handb Exp Pharmacol 194: 91–138, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dmitrieva N, Shelton D, Rice ASC, McMahon SB. The role of nerve growth factor in a model of visceral inflammation. Neuroscience 78: 449–459, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Elitt CM, Malin SA, Koerber HR, Davis BM, Albers KM. Overexpression of artemin in the tongue increases expression of TRPV1 and TRPA1 in trigeminal afferents and causes oral sensitivity to capsaicin and mustard oil. Brain Res 1230: 80–90, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elitt CM, McIlwrath SL, Lawson JJ, Malin SA, Molliver DC, Cornuet PK, Koerber HR, Davis BM, Albers KM. Artemin overexpression in skin enhances expression of TRPV1 and TRPA1 in cutaneous sensory neurons and leads to behavioral sensitivity to heat and cold. J Neurosci 26: 8578–8587, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Girard BM, Malley SE, Braas KM, May V, Vizzard MA. PACAP/VIP and receptor characterization in micturition pathways in mice with overexpression of NGF in urothelium. J Mol Neurosci 42: 378–389, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Girard BM, May V, Bora SH, Fina F, Braas KM. Regulation of neurotrophic peptide expression in sympathetic neurons: quantitative analysis using radioimmunoassay and real-time quantitative polymerase chain reaction. Regul Pept 109: 89–101, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Goodness TP, Albers KM, Davis FE, Davis BM. Overexpression of nerve growth factor in skin increases sensory neuron size and modulates Trk receptor expression. Eur J Neurosci 9: 1574–1585, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Guerios SD, Wang ZY, Bjorling DE. Cyclophosphamide-induced peripheral hypersensitivity is mediated by nerve growth factor (Abstract). Urothelial Cell Physiology in Normal and Disease States 52, 2005 [Google Scholar]
- 21. Guerios SD, Wang ZY, Bjorling DE. Nerve growth factor mediates peripheral mechanical hypersensitivity that accompanies experimental cystitis in mice. Neurosci Lett 392: 193–197, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Guerios SD, Wang ZY, Boldon K, Bushman W, Bjorling DE. Blockade of NGF and trk receptors inhibits increased peripheral mechanical sensitivity accompanying cystitis in rats. Am J Physiol Regul Integr Comp Physiol 295: R111–R122, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heumann R, Korsching S, Scott J, Thoenen H. Relationship between levels of nerve growth factor (NGF) and its messenger RNA in sympathetic ganglia and peripheral target tissues. EMBO J 5: 3183–3189, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu VY, Zvara P, Dattilio A, Redman TL, Allen SJ, Dawbarn D, Stroemer RP, Vizzard MA. Decrease in bladder overactivity with REN1820 in rats with cyclophosphamide induced cystitis. J Urol 173: 1016–1021, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24: 677–736, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jaggar SI, Scott HCF, Rice ASC. Inflammation of the rat urinary bladder is associated with a referred thermal hyperalgesia which is nerve growth factor dependent. Br J Anaesth 83: 442–448, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Kitzman PH, Perrone TN, Le Master AM, Davis BM, Albers KM. Level of p75 receptor expression in sensory ganglia is modulated by NGF level in the target tissue. J Neurobiol 35: 258–270, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Klinger MB, Dattilio A, Vizzard MA. Expression of cyclooxygenase-2 in urinary bladder in rats with cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol 293: R677–R685, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Klinger MB, Girard B, Vizzard MA. p75NTR expression in rat urinary bladder sensory neurons and spinal cord with cyclophosphamide-induced cystitis. J Comp Neurol 507: 1379–1392, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Klinger MB, Vizzard MA. Role of p75NTR in female rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 295: F1778–F1789, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krimm RF, Davis BM, Noel T, Albers KM. Overexpression of neurotrophin 4 in skin enhances myelinated sensory endings but does not influence sensory neuron number. J Comp Neurol 498: 455–465, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lamb K, Gebhart GF, Bielefeldt K. Increased nerve growth factor expression triggers bladder overactivity. J Pain 5: 150–156, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Le Master AM, Krimm RF, Davis BM, Noel T, Forbes ME, Johnson JE, Albers KM. Overexpression of brain-derived neurotrophic factor enhances sensory innervation and selectively increases neuron number. J Neurosci 19: 5919–5931, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Levi-Montalcini R, Skaper SD, Dal Toso R, Petrelli L, Leon A. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci 19: 514–520, 1996 [DOI] [PubMed] [Google Scholar]
- 35. Liang FX, Bosland MC, Huang H, Romih R, Baptiste S, Deng FM, Wu XR, Shapiro E, Sun TT. Cellular basis of urothelial squamous metaplasia: roles of lineage heterogeneity and cell replacement. J Cell Biol 171: 835–844, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin JH, Zhao H, Sun TT. A tissue-specific promoter that can drive a foreign gene to express in the suprabasal urothelial cells of transgenic mice. Proc Natl Acad Sci USA 92: 679–683, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lindsay RM, Harmar AJ. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature 337: 362–367, 1989 [DOI] [PubMed] [Google Scholar]
- 38. Liu HT, Chancellor MB, Kuo HC. Urinary nerve growth factor level could be a biomarker in the differential diagnosis of mixed urinary incontinence in women. BJU Int 102: 1440–1444, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Liu HT, Chancellor MB, Kuo HC. Urinary nerve growth factor levels are elevated in patients with detrusor overactivity and decreased in responders to detrusor botulinum toxin-A injection. Eur Urol 56: 700–706, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Liu HT, Kuo HC. Intravesical botulinum toxin A injections plus hydrodistension can reduce nerve growth factor production and control bladder pain in interstitial cystitis. Urology 70: 463–468, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Liu HT, Kuo HC. Urinary nerve growth factor level could be a potential biomarker for diagnosis of overactive bladder. J Urol 179: 2270–2274, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Liu HT, Kuo HC. Urinary nerve growth factor levels are increased in patients with bladder outlet obstruction with overactive bladder symptoms and reduced after successful medical treatment. Urology 72: 104–108, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Lowe EM, Anand P, Terenghi G, Williams-Chestnut RE, Sinicropi DV, Osborne JL. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol 79: 572–577, 1997 [DOI] [PubMed] [Google Scholar]
- 44. May V, Vizzard MA. Urinary bladder dysfunction and altered somatic sensitivity in pituitary adenylate cyclase activating polypeptide knockout (PACAP−/−) mice. J Urol 183: 772–779, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McMahon SB, Armanini MP, Ling LH, Phillips HS. Expression and coexpression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron 12: 1161–1171, 1994 [DOI] [PubMed] [Google Scholar]
- 46. Murray E, Malley SE, Qiao LY, Hu VY, Vizzard MA. Cyclophosphamide induced cystitis alters neurotrophin and receptor tyrosine kinase expression in pelvic ganglia and bladder. J Urol 172: 2434–2439, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Nicol GD, Vasko MR. Unraveling the story of NGF-mediated sensitization of nociceptive sensory neurons: ON or OFF the Trks? Mol Interv 7: 26–41, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Okragly AJ, Niles AL, Saban R, Schmidt D, Hoffman RL, Warner TF, Moon TD, Uehling DT, Haak-Frendscho M. Elevated tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. J Urol 161: 438–442, 1999 [PubMed] [Google Scholar]
- 49. Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci 29: 507–538, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Pinto R, Frias B, Allen S, Dawbarn D, McMahon SB, Cruz F, Cruz CD. Sequestration of brain derived nerve factor by intravenous delivery of TrkB-Ig2 reduces bladder overactivity and noxious input in animals with chronic cystitis. Neuroscience 166: 907–916, 2010 [DOI] [PubMed] [Google Scholar]
- 51. Qiao LY, Vizzard MA. Cystitis-induced upregulation of tyrosine kinase (TrkA, TrkB) receptor expression and phosphorylation in rat micturition pathways. J Comp Neurol 454: 200–211, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Qiao LY, Vizzard MA. Up-regulation of tyrosine kinase (TrkA, TrkB) receptor expression and phosphorylation in lumbosacral dorsal root ganglia after chronic spinal cord (T8–T10) injury. J Comp Neurol 449: 217–230, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford AP, Vizzard MA, Cockayne DA. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol 298: R534–R547, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sherer TB, Neff PS, Hankins GR, Tuttle JB. Mechanisms of increased NGF production in vascular smooth muscle of the spontaneously hypertensive rat. Exp Cell Res 241: 186–193, 1998 [DOI] [PubMed] [Google Scholar]
- 55. Sherer TB, Neff PS, Tuttle JB. Increased nerve growth factor mRNA stability may underlie elevated nerve growth factor secretion from hypertensive vascular smooth muscle cells. Mol Brain Res 62: 167–174, 1998 [DOI] [PubMed] [Google Scholar]
- 56. Studeny S, Cheppudira BP, Meyers S, Balestreire EM, Apodaca G, Birder LA, Braas KM, Waschek JA, May V, Vizzard MA. Urinary bladder function and somatic sensitivity in vasoactive intestinal polypeptide (VIP)−/− mice. J Mol Neurosci 36: 175–187, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vizzard MA. Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat 21: 125–138, 2001 [DOI] [PubMed] [Google Scholar]
- 58. Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol 161: 273–284, 2000 [DOI] [PubMed] [Google Scholar]
- 59. Vizzard MA. Up-regulation of pituitary adenylate cyclase-activating polypeptide in urinary bladder pathways after chronic cystitis. J Comp Neurol 420: 335–348, 2000 [PubMed] [Google Scholar]
- 60. Vizzard MA, Girard B, Klinger MB. Neurotrophins and visceral pain. In: Visceral Pain, edited by Bjorling DE. Kerala, India: Transworld Research Network, p. 71–106, 2010 [Google Scholar]
- 61. Wright DE, Snyder WD. Neurotrophin receptor mRNA expression defines distinct populations of neurons in rat dorsal root ganglia. J Comp Neurol 351: 329–338, 1995 [DOI] [PubMed] [Google Scholar]
- 62. Yokoyama T, Kumon H, Nagai A. Correlation of urinary nerve growth factor level with pathogenesis of overactive bladder. Neurourol Urodyn 27: 417–420, 2008 [DOI] [PubMed] [Google Scholar]
- 63. Zvara P, Vizzard MA. Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiol 7: 9, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zvarova K, Vizzard MA. Cocaine- and amphetamine-regulated transcript peptide (CARTp) expressing cells in the urinary bladder: a developmental study. J Comp Neurol 489: 501–517, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]