Abstract
The present study examined the effect of transfer of portions of chromosome 1 that includes (FHH.1BN AR+ strain) or excludes (control FHH.1BN AR− strain) a 4.3-Mb region from the Brown Norway (BN) rat that restores the autoregulation (AR) of renal blood flow (RBF) on the development of hypertension and renal injury in congenic strains of Fawn Hooded Hypertensive (FHH) rats. FHH and control AR− rats exhibited poor autoregulation of RBF, and glomerular capillary pressure (Pgc) rose by 19 ± 2 mmHg in FHH rats when renal perfusion pressure (RPP) was increased from 100 to 150 mmHg. In contrast, RBF was well autoregulated in the AR+ strain, and Pgc only increased by 3 ± 1 mmHg when RPP was increased over this range. Baseline mean arterial pressure (MAP) at 12 wk of age was similar in all strains and averaged 122 mmHg. MAP increased significantly in FHH rats and was significantly higher by 12 mmHg in 21-wk-old FHH rats than in the FHH.1BN congenic strains. Protein excretion rose from 5 ± 1 to 397 ± 29 mg/day in 6- vs. 21-wk-old FHH rats. In contrast, protein excretion only increased to 139 ± 21 mg/day in the control AR− strain, and it did not increase significantly in the AR+ strain. Glomerular permeability to albumin was similar in all strains at 6 wk of age. It increased significantly in 9-wk-old FHH and control AR− rats, but not in the AR+ strain. The levels of matrix metalloproteinase (MMP)-2 and transforming growth factor (TGF)-β2 protein were significantly higher in the renal cortex of 9-wk-old FHH rats compared with the levels seen in the AR+ strain. These data indicate that transfer of a 4.3-Mb region of BN chromosome 1 into the FHH genetic background improves autoregulation of RBF, normalizes Pgc, and slows the progression of renal disease.
Keywords: Fawn Hooded Hypertensive rats, renal hemodynamics, renal blood flow, renal injury, transforming growth factor-β2, matrix metalloproteinase-2
the fawn hooded hypertensive (FHH) rat is a renin-dependent (15, 34) model of renal disease that develops progressive proteinuria, focal glomerulosclerosis, and end-stage renal disease (12, 15–16, 25, 28–31, 34). We have reported that FHH rats exhibit impaired autoregulation of RBF that is associated with a defect in the myogenic response of preglomerular renal arterioles (31). More recently, we found that transfer of a 12.9-Mb region of chromosome 1 from the Brown Norway (BN) rat onto the FHH genetic background improves autoregulation of renal blood flow (RBF) and attenuates the development of proteinuria in a FHH.1BN congenic strain (12). However, the mechanism by which restoration of RBF autoregulation alters the development proteinuria and renal disease in this FHH.1BN congenic strain is unclear. Moreover, it remains to be determined whether changes in arterial pressure precede the development of renal injury in FHH rats. In the present study, we examined the hypothesis that impaired autoregulation of RBF promotes renal injury by elevating glomerular capillary pressure (Pgc), leading to upregulation of the expression of transforming growth factor (TGF)-β and matrix metalloproteinase (MMP)-2. The rise in TGF-β and MMP-2 levels then damages the glomerular permeability barrier and promotes epithelial to mesenchymal transition (EMT) and stimulates renal interstitial fibrosis. To test this hypothesis, the time course of changes in arterial pressure and proteinuria, permeability to albumin (Palb), renal TGF-β, and MMP-2 levels was compared in FHH rats and in two genetically similar FHH.1BN congenic strains that differ by the inclusion (FHH.1BN AR+ strain) or exclusion (control FHH.1BN AR− strain) of a 4.3-Mb region of BN chromosome 1 that restored autoregulation of RBF.
METHODS
General.
Experiments were performed on 124, 6- to 21-wk-old male FHH and FHH.1BN congenic rats that were obtained from inbred colonies maintained at the Medical College of Wisconsin. All of the rats were housed in the Animal Care Facility at the Medical College of Wisconsin, which is approved by the American Association for the Accreditation of Laboratory Animal Care. The rats had free access to food and water throughout the study. All protocols received approval by the Animal Care Committee of the Medical College of Wisconsin. The breeding colonies were maintained on a rodent diet purchased from LabDiet (PMI Nutrition International, Brentwood, MO) containing 0.28% NaCl. After weaning, the pups were switched to a purified AIN-76 rodent diet containing 0.4% NaCl (Dyets, Bethlehem, PA).
Protocol 1. Comparison of autoregulation of RBF in FHH rats and FHH.1BN congenic strains.
These experiments were performed on 9- to 12-wk-old FHH rats and FHH.1BN congenic strains. The control AR− congenic strain is genetically identical to the AR+ strain except that they lack a 4.3-Mb AR+ region of chromosome 1 from the BN rat. The rats were anesthetized with ketamine (30 mg/kg im; Phoenix Pharmaceutical, St. Joseph, MO) and inactin (50 mg/kg ip; Sigma, St. Louis, MO), and catheters were placed in the femoral artery for the measurement of mean arterial pressure (MAP) and femoral vein for an intravenous infusion of 6% BSA in a 0.9% NaCl solution at a rate of 100 μl/min. After surgery, MAP was increased to 150 mmHg by tying of the celiac and mesenteric arteries. Next, RBF was measured using a ultrasound flow probe (Transonic System, Ithaca, NY) on the renal artery as renal perfusion pressure (RPP) was varied from 150 to 80 mmHg in steps of 10 mmHg by tightening a clamp on the abdominal aorta above the left renal artery as previously described (12). RBF autoregulatory indexes over the range of pressures from 100 to 140 mmHg were calculated by the method of Semple and De Wardener (21).
Protocol 2. Comparison of RBF, glomerular filtration rate, and Pgc in FHH and FHH.1BN AR+ congenic rats.
These experiments were performed on 9- to 12-wk-old FHH and FHH.1BN AR+ congenic rats. The rats were surgically prepared for the measurement of RBF, glomerular filtration rate (GFR), and Pgc as described above and infused with 2% BSA in 0.9% NaCl solution at a rate of 100 μl/min. A catheter was inserted in the left ureter for the collection of urine, and FITC-labeled inulin (2 mg/ml; Sigma) was added to the intravenous infusion solution for the measurement of GFR. After surgery, RPP was lowered to 100 mmHg by tightening the aortic clamp. After a 30-min equilibration period, urine and plasma samples were collected during a 20-min control period. RPP was then increased to ∼150 mmHg by releasing the aortic clamp and tying off the celiac and mesenteric arteries, and, after a 10-min equilibration period, urine and plasma samples were collected during a 20-min experimental period. At the end of each experiment, the left kidney was removed and weighed, and the concentration of inulin in urine and plasma samples was determined using a plate reader fluorometer.
Additional experiments were performed to estimate autoregulation of Pgc by micropuncture. The left kidney was placed in a stainless steel holder and surrounded with 2.5% agar (Sigma). The surface of the kidney was constantly bathed with a saline solution. Pgc was estimated indirectly in the absence of tubuloglomerular feedback by measuring plasma oncotic pressure and stop flow pressures in three to five early proximal tubules after blocking the tubular lumen with high-vacuum grease as previously described (31).
Protocol 3. Comparison of the time course of the development of hypertension, proteinuria, and Palb in FHH and FHH.1BN congenic rats.
These experiments were performed on 11-wk-old FHH rats and the FHH.1BN congenic strains. Telemetry transmitters (model TA11PA-C40; Data Sciences International, St. Paul, MN) were implanted in the femoral artery, and the transmitters were placed under the skin. After a 1-wk recovery period, MAP was recorded between 1:00 and 4:00 P.M. when the rats were 12, 15, 18, and 21 wk of age. At each time point, the rats were placed in metabolic cages for 24 h for the determination of protein excretion. The concentration of protein in the urine samples was measured using the Bradford method using BSA as the standard (Bio-Rad Laboratories, Hercules, CA). At the end of the protocol, rats were anesthetized, and a catheter was placed in the aorta below the kidneys. The kidneys were flushed with 10 ml of saline, collected, weighed, and fixed in a 10% buffered formalin solution. Paraffin sections (3 μm) were cut and stained with Mason's trichrome to determine the degree of glomerular injury. The degree of injury was assessed on 30 glomeruli/section as the percentage of the glomerular capillary area filled with matrix material on a 0–4 scale with 0 representing no injury, 2 indicating loss of 50% of glomerular capillary area, and 4 representing the complete obstruction of glomerular capillaries (18). Additional analysis was performed to determine the degree of renal interstitial fibrosis. Images were captured using a Nikon Eclipse 55i microscope equipped with a Nikon DS-Fi1 color camera (Nikon, Melville, NY) and analyzed for the percentage of the image stained blue using the NIS-Elements D 3.0 software. Ten to twenty representative fields were analyzed per kidney. The assessment of glomerular injury and renal interstitial fibrosis was done blinded and was performed by the same observer.
Measurement of glomerular Palb.
These experiments determined whether Palb differs in 12- and 21-wk-old FHH rats vs. the corresponding values seen in the FHH.1BN congenic strains. The rats were anesthetized with pentobarbital sodium (60 mg/kg), and the kidneys were collected. Glomeruli were isolated using the sieving method in a 5% albumin solution. Palb was determined by measuring the change in glomerular volume (ΔV) after exchange of the bath with fresh medium containing 1% albumin as previously described (6, 20, 24, 35). Palb was calculated as 1 − (ΔVexperimental/ΔVcontrol), where ΔVcontrol was taken as the mean change in volume measured in all glomeruli isolated from 6-wk-old control Sprague-Dawley rats, which are assumed to have no preexisting renal damage. A minimum of 10 glomeruli were studied from each rat, and these experiments were performed using a minimum of four rats per strain.
Protocol 4. Filtration of FITC-labeled albumin in the kidneys of FHH and FHH.1BN congenic rats.
Experiments were performed in FHH and FHH.1BN congenic strains to confirm our Palb measurements indicating that transfer of the AR+ region attenuates filtration of protein by glomerular capillaries in vivo. A catheter was placed in the femoral vein for the infusion of FITC-labeled albumin (20 mg/kg; Sigma) at a rate of 100 μl/min for 15 min. At the end of this period, kidneys were collected and fixed in a 10% buffered formalin solution. Paraffin sections (3 μm) were prepared and counterstained with Alcian blue. Images were obtained using a fluorescence microscope equipped with a high-sensitivity camera (Q-Imaging digital camera; W. Nuhsbaum, McHenry, IL), and the overlaid images were created using Imaging Pro Plus software.
Protocol 5. Comparison of excretion of protein, Palb, and renal TGF-β2 and MMP-2 levels in 6- to 9-wk-old FHH and FHH.1BN congenic rats.
These experiments were performed in younger animals to determine when differences in protein excretion and Palb first appear in FHH and FHH.1BN congenic rats. The rats were placed in metabolic cages for 24 h at 6 and 9 wk of age for the determination of protein excretion. When the rats were 9 wk of age, they were anesthetized with isoflurane, and the kidneys were harvested. Glomeruli were isolated from one kidney to measure Palb, and the other kidney was collected for biochemical analysis. The renal cortex was separated and homogenized in 1 ml of RIPA buffer (Sigma) for measurement of the expression of TGF-β and MMP-2 protein levels. The homogenates were centrifuged at 5,000 g for 5 min and 11,000 g for 15 min, and the supernatant was collected and frozen in liquid N2 and stored at −80°C. Western blot analysis for TGF-β2 was performed using a 1:500 dilution of TGF-β2 primary antibody (sc90; Santa Cruz Biotechnology, Santa Cruz, CA) and a 1:4,000 dilution of a horseradish peroxidase-coupled secondary antibody (sc2004; Santa Cruz Biotechnology) for 1 h. This antibody recognizes the active form of TGF-β2 (∼12 kDa) and the mature form associated with a latent protein (TGF-β2/LAP, ∼75 kDa). Equal loading of homogenate protein was confirmed by stripping and reprobing the membranes using a 1:8,000 dilution of a β-actin primary antibody (Sigma) and 1:8,000 dilution of horseradish peroxidase-coupled secondary antibody (Santa Cruz Biotechnology) for 1 h. Levels of MMP-2 in the renal cortical homogenates were measured by using a MMP-2 ELISA (R&D Systems, Minneapolis, MN).
Statistical analysis.
Data are presented as means ± SE. Significance of differences in mean values was determined using a one- or two-way repeated-measures ANOVA and the Holm-Sidak test for preplanned comparisons. A P < 0.05 was considered to be significant.
RESULTS
Protocol 1. Comparison of autoregulation of RBF in FHH and FHH.1BN congenic strains.
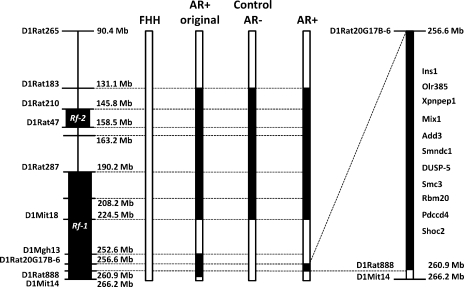
We previously reported that autoregulation of RBF is impaired in FHH rats and that transfer of a 12.9-Mb region of chromosome 1 from the BN rat onto FHH genetic background in a congenic strain (AR+ original; Fig. 1) restores autoregulation of RBF and reduces proteinuria (12). In the present study, we characterized renal function in two additional FHH.1BN congenic strains that were derived from the original congenic strain and are genetically identical to each other, except for the inclusion (FHH.1BN AR+ strain) or exclusion (control FHH.1BN AR− strain) of a 4.3-Mbp region of interest located between the genetic makers D1Rat20G17B-6 and D1Rat888 of BN chromosome 1 (Fig. 1). These two strains also carry a common segment of BN chromosome 1 from markers D1Rat183 to D1Mit18 that are shared with the original congenic strain that encompasses the renal failure 2 (Rf-2) quantitative trait loci (QTL) associated with a knockout of the Rab38 gene in the FHH rat that influences coat color, a bleeding disorder, and the reuptake of filtered protein (8, 11, 17, 19) but not autoregulation of RBF.
Fig. 1.
Genetic map showing the introgressed regions in control FHH.1BN AR− and FHH.1BN AR+ congenic strains. Left: location of genetic markers used to genotype the animals and the location of the previously identified renal failure 1 (Rf-1) and renal failure 2 (Rf-2) quantitative trait loci (QTLs) on chromosome 1 in Fawn Hooded Hypertensive (FHH) rats. Open bars, FHH genome; filled bars, Brown Norway (BN) genomes. Right, the candidate genes in the 4.3-Mb region of interest.
In the present study, autoregulation of RBF in response to an elevation in RPP from 100 to 140 mmHg was nearly absent in FHH rats after acute volume expansion to blunt tubuloglomerular feedback responsiveness (Fig. 2A), and the autoregulatory index averaged 0.8 (Fig. 2B). Similar results were obtained in the control AR− strain (Fig. 2A). In contrast, autoregulation of RBF was restored in the AR+ strain, and RBF remained relatively constant when RPP was increased over this range. The RBF autoregulatory index was significantly lower in the AR+ strain than the corresponding values seen in the FHH rats and the control AR− congenic strain.
Fig. 2.
Relationship between renal perfusion pressure (RPP) and renal blood flow (RBF) in volume-expanded FHH and FHH.1BN congenic strains (A) and RBF autoregulation indexes (B). Nos. in parentheses indicate the no. of rats studied/group. *Significant difference (P < 0.05) from the corresponding RBF value at RPP of 100 mmHg within the same strain. †Significant difference (P < 0.05) from the corresponding value in FHH rats.
Protocol 2. Comparison of RBF, GFR, and Pgc in FHH rats and the AR+ strain.
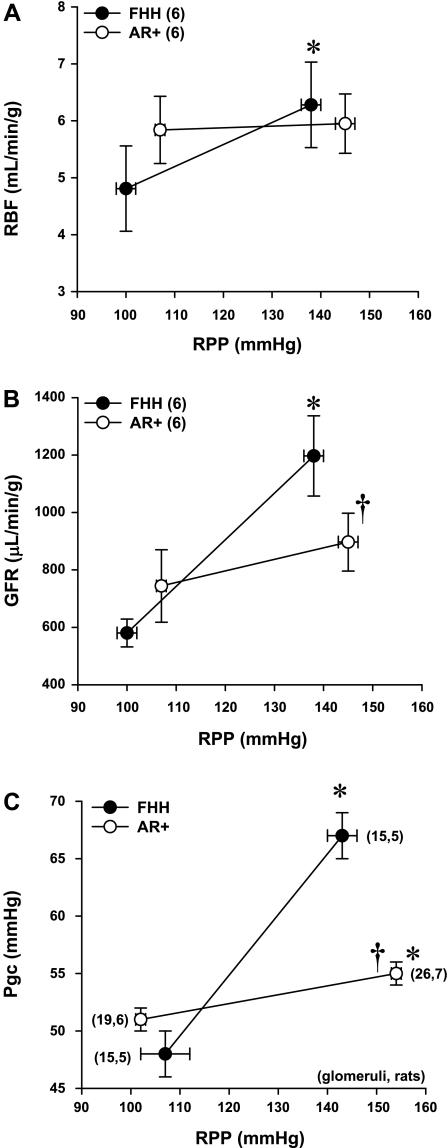
The relationships between RBF, GFR, and Pgc in response to an elevation in RPP from 100 to 150 mmHg are presented in Fig. 3. RBF increased significantly in FHH rats, whereas RBF was not significantly altered in the AR+ strain (Fig. 3A). GFR doubled in FHH rats when RPP was increased from 100 to 150 mmHg, whereas no significant change was observed in the AR+ strain (Fig. 3B). Pgc increased significantly by 19 ± 2 mmHg in FHH rats in response to elevations in RPP from 100 to 150 mmHg (Fig. 3C). In contrast, Pgc only increased by 3 ± 1 mmHg in the AR+ strain.
Fig. 3.
Comparison of the effect of elevations of RPP on RBF (A), glomerular filtration rate (GFR; B), and glomerular capillary pressure (Pgc; C) in FHH and FHH.1BN AR+ congenic strains in which autoregulation of RBF is restored. Nos. in parentheses indicate the no. of rats studied/group. *Significant difference (P < 0.05) from the corresponding RBF value at RPP of 100 mmHg within the same strain. †Significant difference (P < 0.05) from the corresponding value in FHH rats.
Protocol 3. Comparison of the time course of the development of hypertension and proteinuria and renal injury in FHH rats and FHH.1BN congenic strains.
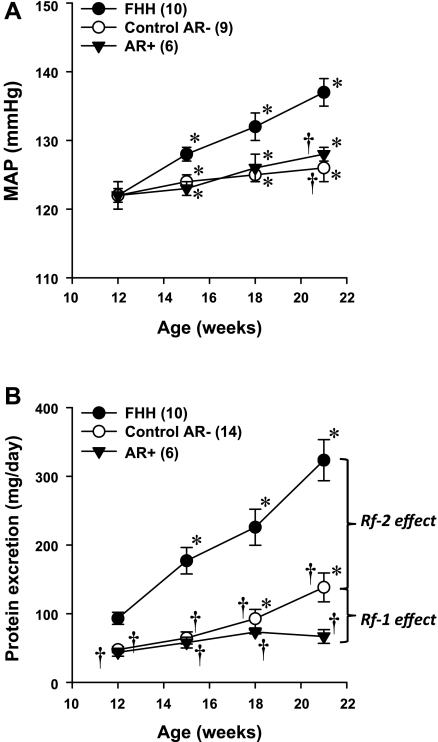
The results of these experiments are presented in Fig. 4. Baseline MAP was not significantly different in 12-wk-old FHH rats and the FHH.1BN congenic strains (Fig. 4A). MAP increased significantly by ∼15 mmHg in the FHH rats over the 9-wk course of the study and was significantly higher by 12 mmHg in 21-wk-old FHH rats than the corresponding values observed in the FHH.1BN congenic strains.
Fig. 4.
Time course of the development of hypertension (A) and proteinuria (B) in 12- to 21-wk-old FHH and FHH.1BN congenic rats. Nos. in parentheses indicate the no. of rats studied/group. *Significant difference (P < 0.05) from the corresponding value at 12 wk of age within the same strain. †Significant difference (P < 0.05) from the corresponding value in FHH rats.
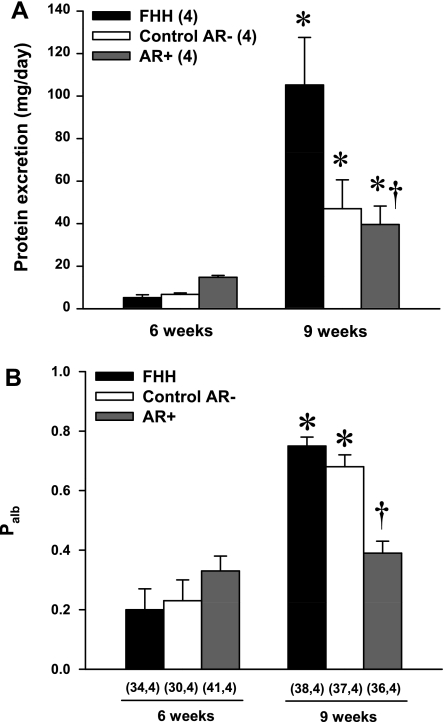
Baseline proteinuria was already twice as high in 12-wk-old FHH rats as that measured in either of the FHH.1BN congenic strains (Fig. 4B). The degree of proteinuria increased significantly to 397 ± 29 mg/day in 21-wk-old FHH rats. In contrast, proteinuria increased significantly less to only 139 ± 21 mg/day in the control AR− strain, and it did not increase significantly over the 9-wk course of the study in the AR+ strain.
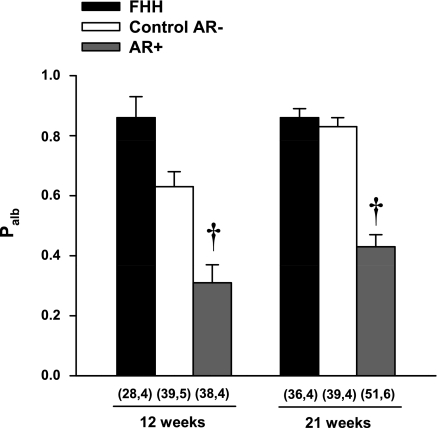
A comparison of changes in Palb in FHH rats and the FHH.1BN congenic strains is presented in Fig. 5. Palb was significantly lower in both the 12- and 21-wk-old AR+ congenic strain compared with the corresponding values seen in FHH rats and the control AR− congenic strain.
Fig. 5.
Comparison of glomerular permeability to albumin (Palb) measured in FHH and FHH.1BN congenic strains at 12 and 21 wk of age. Nos. in parentheses indicate the no. of glomeruli and rats studied/group. †Significant difference (P < 0.05) from the corresponding value in FHH rats.
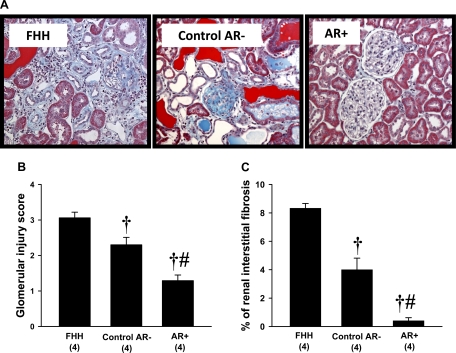
A comparison of the degree of renal injury in 21-wk-old FHH rats and the FHH.1BN congenic strains is presented in Fig. 6. The kidneys from FHH rats and the control AR− strain exhibited severe renal injury with glomerulosclerosis, renal interstitial fibrosis, tubular necrosis, inflammation, and the formation of protein casts. The degree of renal injury was markedly reduced in the AR+ strain (Fig. 6A). The glomerular injury score (Fig. 6B) and the percentage of renal fibrosis (Fig. 6C) was significantly higher in FHH rats than in both FHH.1BN congenic strains, and the degree of glomerular injury and renal fibrosis was significantly greater in the control AR− strain than in the AR+ strain.
Fig. 6.
Comparison of the renal histopathology (A), glomerular injury scores (B), and percentage renal interstitial fibrosis (C) in FHH and FHH.1BN congenic rats at 21 wk of age. Nos. in parentheses indicate the no. of rats studied/group. †Significant difference (P < 0.05) from the corresponding value in FHH rats. #Significant difference (P < 0.05) from the corresponding value in the control AR− congenic strain.
Protocol 4. Filtration of FITC-labeled albumin in the kidneys of FHH rats and the AR+ strain.
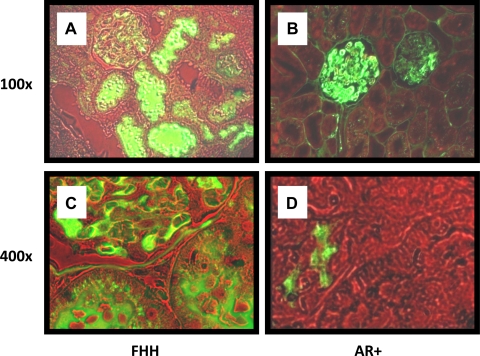
The results of these experiments are presented in Fig. 7. FITC-labeled albumin was present in glomerular capillaries of FHH rats and the AR+ strain. High levels of albumin were filtered and found in the lumen of many proximal tubules in the kidneys of FHH rats (Fig. 7, A-D). In contrast, high levels of albumin were not readily apparent in the lumen of proximal tubules in the AR+ strain.
Fig. 7.
Representation example showing the filtration of FITC-labeled albumin in the kidney of FHH and FHH.1BN AR+ congenic strains between 15 and 21 wk of age. Magnification equals ×100 in A and B and ×400 in C and D.
Protocol 5. Comparison of protein excretion, Palb, and levels of TGF-β1 and MMP-2 in 6- to 9-wk-old FHH rats and FHH.1BN congenic strains.
The results of these experiments are presented in Fig. 8. The excretion of protein was similar in 6-wk-old FHH rats and the FHH.1BN congenic strains (Fig. 8A). Protein excretion increased significantly in all three strains over the course of the study, but it was twofold higher in 9-wk-old FHH rats compared with the corresponding values seen in either of the FHH.1BN congenic strains. Consistent with these findings, Palb was similar in 6-wk-old FHH rats and the FHH.1BN congenic strains (Fig. 8B). Palb increased significantly in both the FHH and the control AR− strain as these rats aged from 6 to 9 wk old. In contrast, Palb did not increase in the AR+ strain in which autoregulation is intact, and it remained significantly lower than the corresponding values seen in either FHH rats or the control AR− strain.
Fig. 8.
Comparison of protein excretion and glomerular Palb in 6- and 9-wk-old FHH and FHH.1BN congenic strains. Nos. in parentheses indicate the no. of rats studied/group at each time period. *Significant difference (P < 0.05) from the corresponding value at 6 wk of age within a strain. †Significant difference (P < 0.05) from the corresponding value in FHH rats.
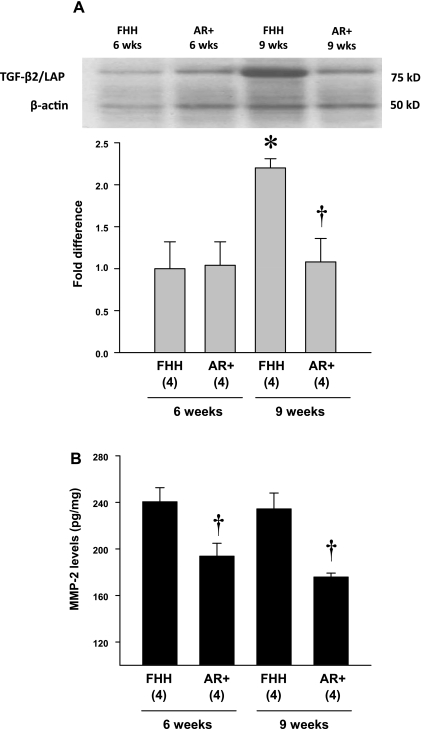
A comparison of the expression of TGF-β2 and MMP-2 protein in the renal cortex of 6- and 9-wk-old FHH and the AR+ strain is presented in Fig. 9. The expression of TGF-β2 protein was not different in the renal cortex of 6-wk-old FHH rats and the AR+ strain (Fig. 9A). However, the expression of TGF-β2 protein increased and was significantly elevated in 9-wk-old FHH rats compared with values seen in the AR+ strain. Similarly, the levels of MMP-2 protein in the renal cortex were also significantly greater in 6- and 9-wk-old FHH rats than the levels seen in the AR+ strain (Fig. 9B).
Fig. 9.
Comparison of the expression of transforming growth factor (TGF)-β2 protein (A) and the levels of matrix metalloproteinase (MMP)-2 (B) in the renal cortex of 6- and 9-wk-old FHH and FHH.1BN AR+ congenic strains. Nos. in parentheses indicate the no. of rats studied/group. *Significant difference (P < 0.05) from the corresponding value in 6-wk-old rats within a strain. †Significant difference (P < 0.05) from the corresponding value in the FHH rats.
DISCUSSION
Previous studies have indicated that FHH rats develop focal glomerulosclerosis and progressive proteinuria that leads to end-stage renal disease (12, 15–16, 25, 28–31, 34). However, the genes and mechanisms involved remain to be determined. Recently, we reported that autoregulation of RBF was impaired in FHH rats and that transfer of a 12.9-Mb region of chromosome 1 containing 83 genes from the BN rat onto the FHH genetic background in a congenic strain restored autoregulation of RBF and attenuated the development of proteinuria and renal injury (12). In the present study, we phenotyped a new FHH.1BN congenic strain that narrows the region of interest for autoregulation of RBF from 12.9 to 4.3 Mb. Transfer of the region of chromosome 1 from markers D1Rat20G17B-6 to D1Rat888 from BN into the FHH genetic background restored autoregulation of RBF in the AR+ strain relative to the impaired autoregulation of RBF seen in both FHH rats and the control AR− strain, which is genetically identical to the AR+ strain except for the region of interest. This finding decreases the number of genes in the candidate region for autoregulation of RBF from 83 to 23 genes, of which only 11 have any known function. There are only three candidate genes in this region that have been reported to affect cardiovascular function. They are soluble aminopeptidase 1 (Xpnpep1), adducin 3 (Add3), and dual-specificity phosphatase (Dusp5). However, further sequencing, expression, and transgenic studies will be needed to determine which of these potential positional candidate genes contributes to the vascular dysfunction in FHH rats.
The purpose of the present study was to determine what role, if any, alterations in RBF autoregulation play in the development of proteinuria and renal disease in FHH rats by comparing the time course changes in MAP, protein excretion, and Palb in FHH rats and two genetically similar FHH.1BN congenic strains that differ only by the inclusion (AR+ strain) or exclusion (control AR− strain) of a 4.3-Mbp region of BN chromosome 1 that restores autoregulation of RBF. We found that Pgc increased in proportion to the rise in RBF in FHH rats as RPP increased from 100 to 150 mmHg. In contrast, Pgc increased by only 3 mmHg in the AR+ strain. This finding is consistent with the view that the control of preglomerular vascular resistance is impaired in FHH rats (31). GFR more than doubled when RPP was increased from 100 to 150 mmHg in FHH rats. This finding is consistent with a marked increase in net filtration pressure, which was estimated to increase from 5 to 35 mmHg when RPP was increased. In addition, it is likely a rise in that glomerular capillary filtration coefficient also rises and contributes to the elevation in GFR in FHH rats when RPP is elevated due to an increase in the length of the glomerular capillaries available for filtration before filtration pressure equilibrium is achieved.
Proteinuria and Palb were similar in all the strains at 6 wk of age. However, Palb increased significantly in both the FHH rats and the control AR− strain by the time the rats were 9 wk of age. In contrast, Palb did not increase in the AR+ strain throughout the 15-wk study in which autoregulation of RBF and Pgc is intact. Damage to the glomerular permeability barrier in the FHH rats was confirmed in vivo by showing that the filtration of FITC-labeled protein was greater in 12-wk-old FHH rats than that seen in the kidneys of the AR+ congenic strain. This indicates that the increase in Palb and proteinuria is linked to the lack of RBF autoregulation, and the critical time window for the onset of renal injury in FHH rats is between 6 and 9 wk of age, which clearly precedes the development of hypertension. Both the FHH and control AR− rats developed proteinuria, glomerulosclerosis, and renal interstitial fibrosis. The degree of renal injury was significantly less in the 21-wk-old AR+ strain relative to the levels seen in FHH rats or the genetically similar control AR− strain that does not autoregulate RBF.
Interestingly, protein excretion was significantly lower in the control AR− strain compared with FHH rats. This is not due to alterations in renal hemodynamics, since this strain failed to autoregulate RBF, and Palb was elevated to the same extent in 9-, 12-, and 21-wk-old FHH rats and the control AR− strain. Moreover, the control AR− strain still exhibited about the same degree of glomerulosclerosis as FHH rats although the degree of renal interstitial fibrosis was not as severe. The reason as to why the protein excretion in the FHH rats may be higher than that seen in the control AR− strain is that the control AR− strain carries a region of BN chromosome 1 from D1Rat210 to D1Rat47, which contains the Rf-2 QTL. In this regard, Rangel-Filho et al. (19) reported that there is an A to G mutation at the start site of transcription of the Rab38 gene in FHH rats that knockout the expression of this protein. This gene is thought to regulate the reuptake of filtered protein in the proximal tubule. Thus protein excretion may be lower in the control AR− strain relative to FHH rats due to increased reuptake and processing of filtered protein by the proximal tubule.
The mechanism by which elevations in Pgc contribute to elevations in Palb and glomerular disease remains to be determined. However, previous studies have indicated the renal levels of TGF-β and MMP are elevated in several models of glomerular disease (1–3, 6–7, 9–10, 13, 22–23). Both TGF-β and MMP are associated with the development of EMT, glomerulosclerosis, and renal interstitial fibrosis (2, 4–5, 26, 33, 36–37). Indeed, TGF-β promotes the synthesis of extracellular matrix proteins such as fibronectin and collagen in fibroblasts, podocytes, and renal tubular and mesangial cells. Previous studies have also linked TGF-β to the disruption of the glomerular permeability barrier causing glomeruli to become leaky to protein (6, 24). In the current study, we found that the levels of TGF-β in the renal cortex were elevated in 9-wk-old FHH rats compared with the levels seen in the AR+ strain. Interestingly, MMP-2 levels were also increased as early as 6 wk of age in FHH rats. MMP-2 is thought to promote the release of TGF-β from the large latent associated protein bound to extracellular matrix (4, 14, 27, 32). Recent studies have also indicated that TGF-β and MMP-2 are involved in the development of EMT leading to the development of chronic renal injury (2, 4–5, 26, 33, 36–37). Collectively, these data suggest that the lack of autoregulation in FHH rats, which increases transmission of systemic pressure to the glomerulus, may trigger elevated production of MMP-2 that increases the release of the active form of TGF-β in the glomerulus. The upregulation of the expression of TGF-β then disrupts the glomerular filtration barrier allowing glomeruli to leak protein into the tubular lumen stimulating the production of MMP-2 and TGF-β in the renal tubules. Over time, this process could promote EMT and accelerate the development of glomerulosclerosis and renal interstitial fibrosis in FHH rats and the control AR− strain that exhibit impaired autoregulation of RBF.
Perspectives
The results from the present study indicate that transfer of a 4.3-Mb region of BN chromosome 1 containing just 13 genes restores the autoregulation of RBF and delays the development of renal injury in FHH rats. It also indicates that the lack of autoregulation of RBF in FHH rats may contribute to the early onset of renal disease by causing glomerular hyperfiltration and increasing Pgc. The rise in Pgc is associated with the upregulation of MMP-2 levels, which increase the expression of TGF-β. TGF-β increases glomerular permeability and eventually leads to glomerulosclerosis and renal interstitial fibrosis. The current data also suggest that this cascade of events happens before the development of hypertension in FHH rats.
GRANTS
This work was supported, in part, by National Institutes of Health Grants HL-36279, HL-29587, HL-2958727S1, and DK-79306 and a United Negro College Fund/Merck Postdoctoral Science Research Fellowship awarded to J. Williams.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Bolbrinker J, Markovic S, Wehland M, Melenhorst WB, van Goor H, Kreutz R. Expression and response to angiotensin-converting enzyme inhibition of matrix metalloproteinases 2 and 9 in renal glomerular damage in young transgenic rats with renin-dependent hypertension. J Pharmacol Exp Ther 316: 8–16, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Bottinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol 13: 2600–2610, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Camp TM, Smiley LM, Hayden MR, Tyagi SC. Mechanism of matrix accumulation and glomerulosclerosis in spontaneously hypertensive rats. J Hypertens 21: 1719–1727, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Cheng S, Lovett DH. Gelatinase A (MMP-2) is necessary and sufficient for renal tubular cell epithelial-mesenchymal transformation. Am J Pathol 162: 1937–1949, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng S, Pollock AS, Mahimkar R, Olson JL, Lovett DH. Matrix metalloproteinase 2 and basement membrane integrity: a unifying mechanism for progressive renal injury. Faseb J 20: 1898–1900, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Dahly-Vernon AJ, Sharma M, McCarthy ET, Savin VJ, Ledbetter SR, Roman RJ. Transforming growth factor-beta, 20-HETE interaction, and glomerular injury in Dahl salt-sensitive rats. Hypertension 45: 643–648, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Dahly AJ, Hoagland KM, Flasch AK, Jha S, Ledbetter SR, Roman RJ. Antihypertensive effects of chronic anti-TGFβ antibody therapy in Dahl S rats. Am J Physiol Regul Integr Comp Physiol 283: R757–R767, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Datta YH, Wu FC, Dumas PC, Rangel-Filho A, Datta MW, Ning G, Cooley BC, Majewski RR, Provoost AP, Jacob HJ. Genetic mapping and characterization of the bleeding disorder in the fawn-hooded hypertensive rat. Thromb Haemost 89: 1031–1042, 2003 [PubMed] [Google Scholar]
- 9. Harendza S, Schneider A, Helmchen U, Stahl RA. Extracellular matrix deposition and cell proliferation in a model of chronic glomerulonephritis in the rat. Nephrol Dial Transplant 14: 2873–2879, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Kuroda T, Yoshida Y, Kamiie J, Kovalenko P, Nameta M, Fujinaka H, Yaoita E, Endo T, Ishizuka S, Nakabayashi K, Yamada A, Nagasawa T, Yamamoto T. Expression of MMP-9 in mesangial cells and its changes in anti-GBM glomerulonephritis in WKY rats. Clin Exp Nephrol 8: 206–215, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Loftus SK, Larson DM, Baxter LL, Antonellis A, Chen Y, Wu X, Jiang Y, Bittner M, Hammer JA, 3rd, Pavan WJ. Mutation of melanosome protein RAB38 in chocolate mice. Proc Natl Acad Sci USA 99: 4471–4476, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lopez B, Ryan RP, Moreno C, Sarkis A, Lazar J, Provoost AP, Jacob HJ, Roman RJ. Identification of a QTL on chromosome 1 for impaired autoregulation of RBF in fawn-hooded hypertensive rats. Am J Physiol Renal Physiol 290: F1213–F1221, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Luft FC. Transforming growth factor beta-angiotensin II interaction: implications for cardiac and renal disease. J Mol Med 77: 517–518, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Maeda S, Dean DD, Gomez R, Schwartz Z, Boyan BD. The first stage of transforming growth factor beta1 activation is release of the large latent complex from the extracellular matrix of growth plate chondrocytes by matrix vesicle stromelysin-1 (MMP-3). Calcif Tissue Int 70: 54–65, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Mattson DL, Dwinell MR, Greene AS, Kwitek AE, Roman RJ, Cowley AW, Jr, Jacob HJ. Chromosomal mapping of the genetic basis of hypertension and renal disease in FHH rats. Am J Physiol Renal Physiol 293: F1905–F1914, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Mattson DL, Kunert MP, Roman RJ, Jacob HJ, Cowley AW., Jr Substitution of chromosome 1 ameliorates l-NAME hypertension and renal disease in the fawn-hooded hypertensive rat. Am J Physiol Renal Physiol 288: F1015–F1022, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Oiso N, Riddle SR, Serikawa T, Kuramoto T, Spritz RA. The rat Ruby (R) locus is Rab38: identical mutations in Fawn-hooded and Tester-Moriyama rats derived from an ancestral Long Evans rat sub-strain. Mamm Genome 15: 307–314, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Raij L, Chiou XC, Owens R, Wrigley B. Therapeutic implications of hypertension-induced glomerular injury. Comparison of enalapril and a combination of hydralazine, reserpine, and hydrochlorothiazide in an experimental model. Am J Med 79: 37–41, 1985 [DOI] [PubMed] [Google Scholar]
- 19. Rangel-Filho A, Sharma M, Datta YH, Moreno C, Roman RJ, Iwamoto Y, Provoost AP, Lazar J, Jacob HJ. RF-2 gene modulates proteinuria and albuminuria independently of changes in glomerular permeability in the fawn-hooded hypertensive rat. J Am Soc Nephrol 16: 852–856, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Savin VJ, Sharma R, Lovell HB, Welling DJ. Measurement of albumin reflection coefficient with isolated rat glomeruli. J Am Soc Nephrol 3: 1260–1269, 1992 [DOI] [PubMed] [Google Scholar]
- 21. Semple SJ, De Wardener HE. Effect of increased renal venous pressure on circulatory autoregulation of isolated dog kidneys. Circ Res 7: 643–648, 1959 [DOI] [PubMed] [Google Scholar]
- 22. Sharma K, McGowan TA. TGF-beta in diabetic kidney disease: role of novel signaling pathways. Cytokine Growth Factor Rev 11: 115–123, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Sharma K, Ziyadeh FN. Renal hypertrophy is associated with upregulation of TGF-β1 gene expression in diabetic BB rat and NOD mouse. Am J Physiol Renal Fluid Electrolyte Physiol 267: F1094–F1101, 1994 [DOI] [PubMed] [Google Scholar]
- 24. Sharma R, Khanna A, Sharma M, Savin VJ. Transforming growth factor-beta1 increases albumin permeability of isolated rat glomeruli via hydroxyl radicals. Kidney Int 58: 131–136, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Simons JL, Provoost AP, Anderson S, Troy JL, Rennke HG, Sandstrom DJ, Brenner BM. Pathogenesis of glomerular injury in the fawn-hooded rat: early glomerular capillary hypertension predicts glomerular sclerosis. J Am Soc Nephrol 3: 1775–1782, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Stahl PJ, Felsen D. Transforming growth factor-beta, basement membrane, and epithelial-mesenchymal transdifferentiation: implications for fibrosis in kidney disease. Am J Pathol 159: 1187–1192, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sun SZ, Wang Y, Li Q, Tian YJ, Liu MH, Yu YH. Effects of benazepril on renal function and kidney expression of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 in diabetic rats. Chin Med J (Engl) 119: 814–821, 2006 [PubMed] [Google Scholar]
- 28. van Dokkum RP, Jacob HJ, Provoost AP. Blood pressure and the susceptibility to renal damage after unilateral nephrectomy and l-NAME-induced hypertension in rats. Nephrol Dial Transplant 15: 1337–1343, 2000 [DOI] [PubMed] [Google Scholar]
- 29. van Dokkum RP, Jacob HJ, Provoost AP. Difference in susceptibility of developing renal damage in normotensive fawn-hooded (FHL) and August × Copenhagen Irish (ACI) rats after N(omega)-nitro-l-arginine methyl ester induced hypertension. Am J Hypertens 10: 1109–1116, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Van Dokkum RP, Jacob HJ, Provoost AP. Genetic differences define severity of renal damage after l-NAME-induced hypertension in rats. J Am Soc Nephrol 9: 363–371, 1998 [DOI] [PubMed] [Google Scholar]
- 31. van Dokkum RP, Sun CW, Provoost AP, Jacob HJ, Roman RJ. Altered renal hemodynamics and impaired myogenic responses in the fawn-hooded rat. Am J Physiol Regul Integr Comp Physiol 276: R855–R863, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Wang M, Zhao D, Spinetti G, Zhang J, Jiang LQ, Pintus G, Monticone R, Lakatta EG. Matrix metalloproteinase 2 activation of transforming growth factor-beta1 (TGF-beta1) and TGF-beta1-type II receptor signaling within the aged arterial wall. Arterioscler Thromb Vasc Biol 26: 1503–1509, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Wang SN, LaPage J, Hirschberg R. Role of glomerular ultrafiltration of growth factors in progressive interstitial fibrosis in diabetic nephropathy. Kidney Int 57: 1002–1014, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Weichert W, Paliege A, Provoost AP, Bachmann S. Upregulation of juxtaglomerular NOS1 and COX-2 precedes glomerulosclerosis in fawn-hooded hypertensive rats. Am J Physiol Renal Physiol 280: F706–F714, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Williams JM, Sharma M, Anjaiahh S, Falck JR, Roman RJ. Role of endogenous CYP450 metabolites of arachidonic acid in maintaining the glomerular protein permeability barrier. Am J Physiol Renal Physiol 293: F501–F505, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yard BA, Chorianopoulos E, Herr D, van der Woude FJ. Regulation of endothelin-1 and transforming growth factor-beta1 production in cultured proximal tubular cells by albumin and heparan sulphate glycosaminoglycans. Nephrol Dial Transplant 16: 1769–1775, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Zeisberg M, Bonner G, Maeshima Y, Colorado P, Muller GA, Strutz F, Kalluri R. Renal fibrosis: collagen composition and assembly regulates epithelial-mesenchymal transdifferentiation. Am J Pathol 159: 1313–1321, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]