Abstract
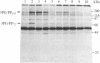
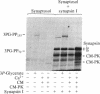
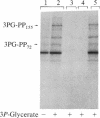
The importance of protein phosphorylation in biological regulation is widely recognized; thus, a number of extracellular signal- and second messenger-linked protein kinases have been well characterized, and various substrates for these kinases have been identified. We present evidence that the glycolytic intermediate 3-phosphoglycerate can cause a marked stimulation of phosphorylation of specific proteins in mammalian tissues. In the brain, there are at least two types of 3-phosphoglycerate-dependent protein phosphorylation systems, which differ in activator specificity, substrate specificity, and sensitivity to the activator. Both of these phosphorylation systems are, however, insensitive to cAMP, cGMP, Ca2+, calmodulin, and protein kinase C activators and have characteristics different from those of casein kinase II-catalyzed protein phosphorylation. The most prominent endogenous substrates in the bovine brain are rather minor proteins with minimal molecular masses of 72 and 155 kDa, which were not detected in "nonnervous" tissues such as heart, liver, lung, and kidney. In nonnervous tissues, 3-phosphoglycerate affected the state of phosphorylation of other proteins. Evidence provided suggests the occurrence of an as yet undescribed type of protein kinase, which may be designated 3-phosphoglycerate-dependent protein kinase, in mammalian tissues.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J. Glucoreceptor mechanisms and the control of insulin release and biosynthesis. Diabetologia. 1980 Jan;18(1):5–15. doi: 10.1007/BF01228295. [DOI] [PubMed] [Google Scholar]
- Avruch J., Nemenoff R. A., Blackshear P. J., Pierce M. W., Osathanondh R. Insulin-stimulated tyrosine phosphorylation of the insulin receptor in detergent extracts of human placental membranes. Comparison to epidermal growth factor-stimulated phosphorylation. J Biol Chem. 1982 Dec 25;257(24):15162–15166. [PubMed] [Google Scholar]
- Bennett M. K., Erondu N. E., Kennedy M. B. Purification and characterization of a calmodulin-dependent protein kinase that is highly concentrated in brain. J Biol Chem. 1983 Oct 25;258(20):12735–12744. [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982 Apr 15;296(5858):613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- Cohen S., Fava R. A., Sawyer S. T. Purification and characterization of epidermal growth factor receptor/protein kinase from normal mouse liver. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6237–6241. doi: 10.1073/pnas.79.20.6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D. W., Bachelard H. S. Attenuation of evoked field potentials from dentate granule cells by low glucose, pyruvate + malate, and sodium fluoride. Brain Res. 1982 May 13;239(2):527–534. doi: 10.1016/0006-8993(82)90527-3. [DOI] [PubMed] [Google Scholar]
- Dirks B., Hanke J., Krieglstein J., Stock R., Wickop G. Studies on the linkage of energy metabolism and neuronal activity in the isolated perfused rat brain. J Neurochem. 1980 Aug;35(2):311–317. doi: 10.1111/j.1471-4159.1980.tb06266.x. [DOI] [PubMed] [Google Scholar]
- Ghajar J. B., Plum F., Duffy T. E. Cerebral oxidative metabolism and blood flow during acute hypoglycemia and recovery in unanesthetized rats. J Neurochem. 1982 Feb;38(2):397–409. doi: 10.1111/j.1471-4159.1982.tb08643.x. [DOI] [PubMed] [Google Scholar]
- Glass D. B., Krebs E. G. Protein phosphorylation catalyzed by cyclic AMP-dependent and cyclic GMP-dependent protein kinases. Annu Rev Pharmacol Toxicol. 1980;20:363–388. doi: 10.1146/annurev.pa.20.040180.002051. [DOI] [PubMed] [Google Scholar]
- Hathaway G. M., Lundak T. S., Tahara S. M., Traugh J. A. Isolation of protein kinases from reticulocytes and phosphorylation of initiation factors. Methods Enzymol. 1979;60:495–511. doi: 10.1016/s0076-6879(79)60047-2. [DOI] [PubMed] [Google Scholar]
- Hathaway G. M., Traugh J. A. Cyclic nucleotide-independent protein kinases from rabbit reticulocytes. Purification of casein kinases. J Biol Chem. 1979 Feb 10;254(3):762–768. [PubMed] [Google Scholar]
- Hathaway G. M., Traugh J. A. Regulation of casein kinase II by 2,3-bisphosphoglycerate in erythroid cells. J Biol Chem. 1984 Mar 10;259(5):2850–2855. [PubMed] [Google Scholar]
- Huttner W. B., DeGennaro L. J., Greengard P. Differential phosphorylation of multiple sites in purified protein I by cyclic AMP-dependent and calcium-dependent protein kinases. J Biol Chem. 1981 Feb 10;256(3):1482–1488. [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blith D. L., Karlsson F. A., Häring H. U., Kahn C. R. Insulin stimulation of phosphorylation of the beta subunit of the insulin receptor. Formation of both phosphoserine and phosphotyrosine. J Biol Chem. 1982 Sep 10;257(17):9891–9894. [PubMed] [Google Scholar]
- Kennedy M. B., McGuinness T., Greengard P. A calcium/calmodulin-dependent protein kinase from mammalian brain that phosphorylates Synapsin I: partial purification and characterization. J Neurosci. 1983 Apr;3(4):818–831. doi: 10.1523/JNEUROSCI.03-04-00818.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. VI. Isolation and partial purification of a protein kinase activated by guanosine 3',5'-monophosphate. J Biol Chem. 1970 May 25;245(10):2493–2498. [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaPorte D. C., Koshland D. E., Jr Phosphorylation of isocitrate dehydrogenase as a demonstration of enhanced sensitivity in covalent regulation. Nature. 1983 Sep 22;305(5932):286–290. doi: 10.1038/305286a0. [DOI] [PubMed] [Google Scholar]
- Lipton P., Robacker K. Glycolysis and brain function: [K+]o stimulation of protein synthesis and K+ uptake require glycolysis. Fed Proc. 1983 Sep;42(12):2875–2880. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Schulman H., Greengard P. Stimulation of brain membrane protein phosphorylation by calcium and an endogenous heat-stable protein. Nature. 1978 Feb 2;271(5644):478–479. doi: 10.1038/271478a0. [DOI] [PubMed] [Google Scholar]
- Schulman H. Phosphorylation of microtubule-associated proteins by a Ca2+/calmodulin-dependent protein kinase. J Cell Biol. 1984 Jul;99(1 Pt 1):11–19. doi: 10.1083/jcb.99.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley D. R., Lefkowitz R. J. Molecular mechanisms of receptor desensitization using the beta-adrenergic receptor-coupled adenylate cyclase system as a model. Nature. 1985 Sep 12;317(6033):124–129. doi: 10.1038/317124a0. [DOI] [PubMed] [Google Scholar]
- Stratford C. A., Fisher S. K., Ueda T. Ergopeptine-sensitive calcium-dependent protein phosphorylation system in the brain. J Neurochem. 1984 Mar;42(3):842–855. doi: 10.1111/j.1471-4159.1984.tb02758.x. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Iwasa Y., Kawahara Y., Mori T., Nishizuka Y. Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem. 1979 May 25;254(10):3692–3695. [PubMed] [Google Scholar]
- Ueda T., Greengard P. Adenosine 3':5'-monophosphate-regulated phosphoprotein system of neuronal membranes. I. Solubilization, purification, and some properties of an endogenous phosphoprotein. J Biol Chem. 1977 Jul 25;252(14):5155–5163. [PubMed] [Google Scholar]
- Ueda T., Greengard P., Berzins K., Cohen R. S., Blomberg F., Grab D. J., Siekevitz P. Subcellular distribution in cerebral cortex of two proteins phosphorylated by a cAMP-dependent protein kinase. J Cell Biol. 1979 Nov;83(2 Pt 1):308–319. doi: 10.1083/jcb.83.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T., Maeno H., Greengard P. Regulation of endogenous phosphorylation of specific proteins in synaptic membrane fractions from rat brain by adenosine 3':5'-monophosphate. J Biol Chem. 1973 Dec 10;248(23):8295–8305. [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Krebs E. G. An adenosine 3',5'-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968 Jul 10;243(13):3763–3765. [PubMed] [Google Scholar]
- Winkler B. S. Glycolytic and oxidative metabolism in relation to retinal function. J Gen Physiol. 1981 Jun;77(6):667–692. doi: 10.1085/jgp.77.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W. C., Walaas S. I., Nairn A. C., Greengard P. Calcium/phospholipid regulates phosphorylation of a Mr "87k" substrate protein in brain synaptosomes. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5249–5253. doi: 10.1073/pnas.79.17.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]