Abstract
The renal vasopressin V2 receptor (V2R) plays a critical role in physiological and pathophysiological processes associated with arginine vasopressin (AVP)-induced antidiuresis. Because clinical data suggests that females may be more prone to hyponatremia from AVP-mediated antidiuresis, we investigated whether there are sex differences in the expression and function of the renal V2R. In normal Sprague-Dawley rat kidneys, V2R mRNA and protein expression was 2.6- and 1.7-fold higher, respectively, in females compared with males. To investigate the potential physiological implications of this sex difference, we studied changes in urine osmolality induced by the AVP V2R agonist desmopressin. In response to different doses of desmopressin, there was a graded increase in urine osmolality and decrease in urine volume during a 24-h infusion. Females showed greater mean increases in urine osmolality and greater mean decreases in urine volume at 0.5 and 5.0 ng/h infusion rates. We also studied renal escape from antidiuresis produced by water loading in rats infused with desmopressin (5.0 ng/h). After 5 days of water loading, urine osmolality of both female and male rats escaped to the same degree physiologically, but V2R mRNA and protein in female kidneys was reduced to a greater degree (−63% and −73%, respectively) than in males (−32% and −48%, respectively). By the end of the 5-day escape period, renal V2R mRNA and protein expression were reduced to the same relative levels in males and females, thereby abolishing the sex differences in V2R expression seen in the basal state. Our results demonstrate that female rats express significantly more V2R mRNA and protein in kidneys than males, and that this results physiologically in a greater sensitivity to V2R agonist administration. The potential pathophysiological implications of these results are that females may be more susceptible to the development of dilutional hyponatremia because of a greater sensitivity to endogenously secreted AVP.
Keywords: renal escape, desmopressin, hyponatremia
the prime determinant of water homeostasis in animals and man is the regulation of urinary free water excretion by circulating plasma levels of the hormone arginine vasopressin (AVP). AVP is a nine-amino acid peptide that is synthesized in magnocellular neural cells located in the hypothalamus. The synthesized peptide is enzymatically cleaved from its prohormone and is transported to the posterior pituitary where it is stored within neurosecretory granules until specific stimuli cause secretion of AVP into the bloodstream (23). Antidiuresis then occurs via interaction of the circulating hormone with AVP V2 receptors (V2Rs) in the kidney, which results in increased water permeability of the collecting duct through the insertion aquaporin-2 (AQP2) water channels into the apical membranes of renal collecting duct principal cells (19). The importance of AVP in water homeostasis is underscored by the pathophysiology that occurs when AVP, or AVP-mediated receptor activation, is either deficient or excessive (27).
The most common disorder of AVP dysregulation encountered in clinical medicine is hypoosmolar hyponatremia. Studies of hyponatremia in hospitalized patients have suggested incidences as high as 15–30% in both acutely (5, 9, 17) and chronically (18) hospitalized patients using a serum [Na+] < 135 mmol/l to define hyponatremia. While most studies of hyponatremia have been conducted in men, studies suggest that women may have more adverse complications from hyponatremia than men (2), raising the possibility that important sex differences exist in the mechanisms underlying this disorder and its adverse consequences. For example, females are more prone to water imbalance disorders such as exercise-induced hyponatremia (1). Such considerations underscore the importance of elucidating the mechanisms underlying the pathogenesis of hypoosmolality in both males and females.
Although available clinical data suggests that females may be more prone to hyponatremia from AVP-mediated antidiuresis, studies of AVP secretion in females and males have not shown very large differences in basal or stimulated plasma AVP levels between the sexes (10, 30). Because the renal V2R plays a critical role in the urinary concentrating process (19), we hypothesized that renal V2R expression might be higher in female compared with male animals, and that the higher levels of V2R expression in females than in males may have physiological consequences. To evaluate this hypothesis, we measured V2R mRNA (measured by real-time PCR) and protein (measured by Western blot analysis) expression in normal female and male Sprague-Dawley rat kidneys. To investigate the potential physiological implications of this sex difference, we compared urine volume and urine osmolality in males and females under basal conditions and during infusion of graded doses of the AVP V2R agonist desmopressin.
Renal epithelial cells undergo pronounced volume regulation in response to changes in extracellular osmolality using the same mechanisms of electrolyte and organic osmolyte fluxes as found in the brain (13). The kidney, however, employs additional mechanisms to adapt to induced antidiuresis and water retention. Chief among these is the phenomenon of renal escape from antidiuresis. In animal models of sustained AVP administration and in patients with the syndrome of inappropriate antidiuretic hormone secretion (SIADH), water loading typically results in free water retention and progressive hyponatremia for several days, which is then followed by escape from the AVP-induced antidiuresis (4, 16). With the onset of vasopressin escape, water excretion increases despite sustained administration of AVP, allowing water balance to be reestablished and the serum [Na+] to be stabilized at a steady, albeit decreased level. Kidney water channels have been shown to play a crucial role in AVP-induced antidiuresis (19). AQP2 is the major water channel expressed in the apical membrane of collecting duct principal cells (12) and is highly regulated by AVP. States of inappropriate antidiuresis are associated with increased kidney AQP2 expression, including animal models of SIADH (11) and congestive heart failure (31). In addition, AQP2 protein and mRNA levels are markedly downregulated during renal escape from antidiuresis in a temporal pattern that correlates closely with the physiological parameters of escape (7).
Experimental data has suggested that the renal AVP V2R plays a key role in the physiological and pathophysiological processes underlying renal escape from AVP-induced antidiuresis in male rats (21). In contrast, much less information is known about the mechanisms underlying renal escape in females. We therefore performed additional studies of renal escape from AVP-induced antidiuresis in female and male rats to evaluate whether changes in basal V2R expression affected the escape process by changing the time course or magnitude of downregulation of V2R mRNA and protein expression, as well as subsequent effects on urine osmolality and volume.
MATERIALS AND METHODS
Animals and desmopressin dose response experiment.
Sprague-Dawley male and female rats (Taconic Farms, Germantown, NY) weighing 225–250 g were acclimated individually in metabolic cages (Lab Products, Seaford, DE) and fed ad libitum a liquid diet (AIN-76A; Bioserv, Frenchtown, NJ) prepared in water with 0.2% saccharin (Sigma-Aldrich, St. Louis, MO) and tap water for 2 days. After this period, the rats (n = 6 males and 6 females for each dose tested) were anesthetized using Aerrane (isoflurane; Baxter Healthcare, Deerfield, IL) and were implanted subcutaneously with osmotic minipumps (model 2001, Alzet; Durect, Cupertino, CA) that delivered dosages of 0, 0.1, 0.5, and 5.0 ng/h of 1-deamino-[8-d-arginine]-vasopressin (desmopressin; Ferring Pharmaceuticals, Parsippany, NJ). Following osmotic minipump insertion, all animals continued to receive the AIN-76 liquid diet and water ad libitum and were maintained in metabolic cages. Daily urine collections and water intake were recorded throughout the duration of the experiment. All rats were euthanized 24 h after osmotic minipump implantation by cardiac puncture under Aerrane anesthesia, and plasma was collected for osmolality and sodium levels. The animals were then perfused with PBS and left and right kidneys were dissected and stored at −80°C. Plasma and urine sodium levels were measured using a Medica EasyLyte sodium/potassium analyzer (Medica, Newtonville, MA), and plasma and urine osmolalities were measured using an Advanced Osmometer (model 3900; Advanced Instruments, Norwood, MA). All procedures were approved by the Georgetown University Animal Care and Use Committee.
Renal escape model.
Male and female Sprague-Dawley rats were maintained under controlled conditions (24°C, lights on 0600–1800 h) for 3–5 days prior to experimentation. Under light isoflurane anesthesia, osmotic minipumps were implanted subcutaneously to deliver 5 ng/h of desmopressin. After 2 days of desmopressin administration, during which time all rats received ad libitum pelleted chow and water, the experimental groups were water loaded by substituting daily feedings of a liquid formula (AIN-76) in a volume of 100 ml. This amount of liquid diet provides sufficient calories for weight maintenance in adult rats. Thus, to maintain their caloric intake, the rats were forced to consume substantial quantities of water as well. The rats were maintained in metabolic cages, allowing quantitative urine collections. Urine volume and osmolality were measured daily. On day 5 after water loading, rats were euthanized by decapitation and the left kidneys were rapidly dissected and homogenized for immunoblotting.
Immunoblotting.
Left whole kidneys were homogenized in chilled membrane-isolation solution containing 250 mM sucrose, 10 mM triethanolamine, 1 μg/ml leupeptin (Sigma-Aldrich), and 0.1 mg/ml PMSF (United States Biochemical, Cleveland, OH) adjusted to pH 7.6. Protein concentration was measured by using Protein Assay Dye Reagent (Bio-Rad Laboratories, Hercules, CA). Samples were then diluted to 2 μg/μl with 5× sample buffer (7.5% SDS, 30% glycerol, 50 mM Tris pH6.8, 30 mg/ml DTT). SDS-PAGE was carried out on 10% Tris-glycine polyacrylamide gels using a Bio-Rad Criterion System. The proteins were transferred from the gels to polyvinylidene fluoride membranes electrophoretically using the Bio-Rad Criterion System. The polyvinylidene fluoride membranes were probed overnight at 4°C with rabbit polyclonal anti-vasopressin V2R (H-80) antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) or polyclonal anti-AQP2 antibodies [gift from Dr. Carolyn Ecelbarger (6)]. The secondary antibody was goat anti-rabbit IgG conjugated to horseradish peroxidase (KPL, Gaithersburg, MD) used at a concentration of 0.1 μg/ml. To visualize sites of antibody-antigen reaction, blots were exposed to a luminol-based enhanced chemiluminescent reagent (LumiGLO; KPL) or Supersignal West Dura horseradish peroxidase detection kit (Pierce, Rockford, IL) before exposure to X-ray film (FUJI Film, Kanagawa, Japan). Image densitometric analysis of the protein bands was performed using the OptiQuant image analysis software (PerkinElmer Life Sciences). For all studies, equal numbers of both male and female kidney extracts were run on the same gels to allow quantitative comparisons of the densitometry results.
Real-time PCR.
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA). First-strand cDNA was prepared from total RNA (2 μg) using the iScript cDNA synthesis kit (Bio-Rad) with Moloney murine leukemia virus RNase H+ reverse transcriptase, oligo(dT), and random hexamers. Quantitation of V2R mRNA and 18S ribosomal RNA (for control) was performed by real-time PCR using an ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA). The PCR reaction mixture for rat vasopressin V2R detection consisted of RNase-free water, TaqMan Universal PCR Master Mix (Applied Biosystems), and 300 nM specific primers and 200 nM probe [forward primer (800F): 5′-CCA AGA CCG TGA GGA TGA CAC T-3′; reverse primer (944R): 5′-CTA GCC AGC AGC ATG AGC AA-3′; probe (891T): 6FAM-TCC GGA AGC TCC TCT GGA AAG ACC C-TAMRA], and cDNA samples. PCR reactions without reverse transcription were included to control for contamination by genomic DNA. Results obtained for V2R mRNA levels were normalized to 18S ribosomal RNA.
Statistics.
Data are presented as means ± SE. Urine data were statistically analyzed by two-way, repeated-measures ANOVA followed by Bonferroni multiple comparisons. Results from immunoblot densitometry were analyzed by one-way ANOVA followed by Student Newman-Keuls multiple comparison. Differences were considered statistically significant at P < 0.05.
RESULTS
Basal V2R gene expression in female and male rat kidney cortex.
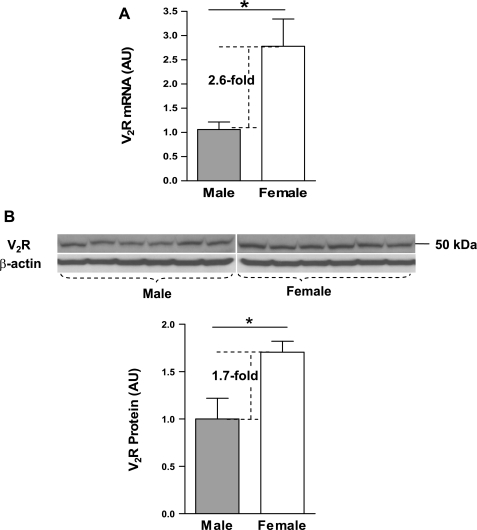
To detect V2R gene expression levels, mRNA and protein were isolated from the kidneys of adult female (n = 6) and male (n = 6) Sprague-Dawley rats. Both real-time PCR and immunoblotting demonstrated that female rats expressed higher levels of V2R mRNA [arbitrary units (AU): female, 2.8 ± 0.57; male, 1.1 ± 0.15; P < 0.05] and protein [AU: female, 1.7 ± 0.12; male, 1.0 ± 0.22; P < 0.05] than males. V2R mRNA (Fig. 1A) and protein (Fig. 1B) were 2.6- fold and 1.7-fold higher in the females compared with the males, respectively.
Fig. 1.
Expression levels of arginine vasopressin (AVP) V2 receptor (V2R) in the kidneys of Sprague-Dawley rats. A: real-time PCR results of V2R mRNA expression in female and male rat kidneys. B: results of Western blot analysis of V2R protein expression in female and male rat kidneys. AU, Arbitrary units. Values are expressed as means ± SE; *P < 0.05 compared with males.
Desmopressin dose response.
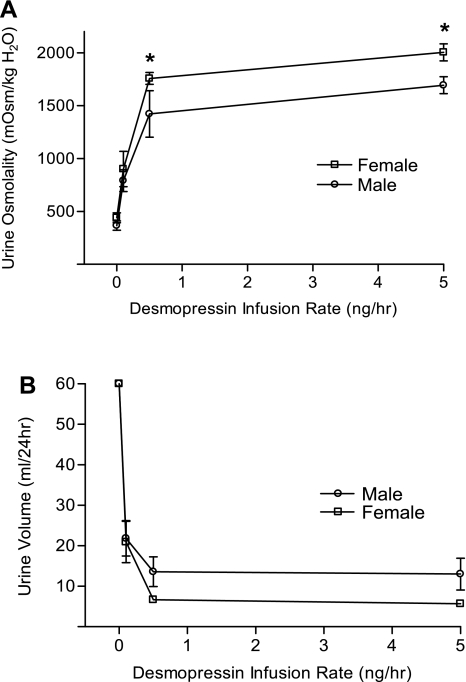
Female (n = 6) and male (n = 6) rats were water loaded with liquid diet. All rats excreted > 60 ml/24 with a urine osmolality <500 mosmol/kgH2O, and there were no significant differences between the females and males at baseline (0 ng/h desmopressin dose). In response to desmopressin, there was a graded increase in urine osmolality (Fig. 2A) and decrease in urine volume (Fig. 2B) during the 24-h desmopressin infusion. Females showed greater mean increases in urine osmolality and greater mean decreases in urine volume only at the two highest infusion rates (0.5 and 5.0 ng/h). Analysis by two-way ANOVA showed a significant effect of dose on urine osmolality (F = 31.63, P = 0.001) and urine volume (F = 7.29, P = 0.003). A significant effect of sex was also found for urine osmolality (F = 5.56, P = 0.025), but the effect of sex on urine volume did not achieve statistical significance (F = 3.08, P = 0.089).
Fig. 2.
Response of urine volume and urine osmolality to desmopressin in female and male rats implanted subcutaneously with osmotic minipumps that delivered desmopressin dosages of 0, 0.1, 0.5, and 5.0 ng/h. Daily urine collections and water intake were recorded. All rats were euthanized 24 h after minipump implantation, and plasma was collected for osmolality and sodium levels. In response to desmopressin, there was a graded increase in urine osmolality (A) and decrease in urine volume (B) during the 24-h desmopressin infusion. Values are expressed as means ± SE; *P < 0.05 compared with males.
Renal escape from desmopressin-induced antidiuresis in female and male rats.
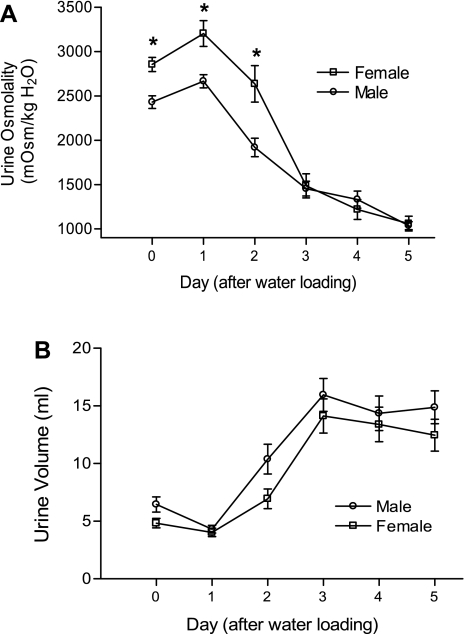
Figure 3 shows the time course of changes in daily urine osmolality (Fig. 3A) and urine volume (Fig. 3B) in female (n = 24) and male (n = 21) rats undergoing escape from desmopressin-induced antidiuresis. Urine osmolality in response to the desmopressin infusion was significantly higher in female rats at baseline and during the first 2 days of water loading; two-way ANOVA analysis showed significant differences in urine osmolality between male and female rats during the 5 days of renal escape (F = 16.66, P < 0.0001) (Fig. 3A). Analysis by two-way ANOVA also showed a significant effect of sex on urine volume after the onset of water loading (F = 7.03, P = 0.0085) (Fig. 3B). As reported previously using this model (21), urine volume began to increase and urine osmolality began to decrease by day 2 of water loading in both females and males, indicating the onset of renal escape from antidiuresis. Urine osmolality remained higher in the female rats on the second day after water loading, but by day 3 onward there were no significant differences in urine osmolality (Fig. 3A) or urine volume (Fig. 3B) between the female and male rats.
Fig. 3.
Physiological parameters of renal function during escape from antidiuresis in female and male rats. A: 24-h urine osmolality. B: urine volumes in female and male rats during renal escape from desmopressin-induced antidiuresis. All rats were implanted with desmopressin osmotic minipumps at day −2, and all had access to pelleted rat chow and water ad libitum. Beginning on day 0, all rats were water loaded by substituting daily feedings of a liquid formula for the pelleted chow. Values are expressed as means ± SE; *P < 0.05 compared with males.
Changes in V2R gene expression in female and male rat kidney after renal escape.
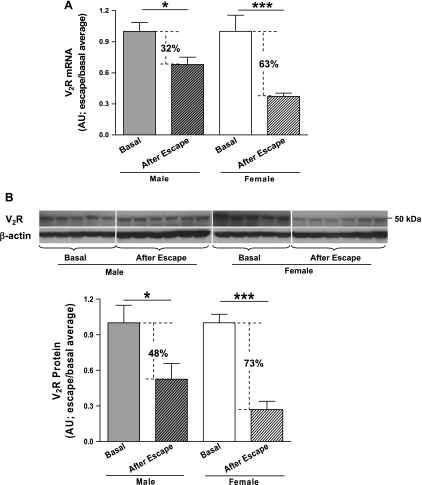
At day 5 after the onset of water loading, mRNA and protein were isolated from kidneys of desmopressin-treated male and female rats. V2R mRNA was significantly reduced in both sexes compared with basal V2R mRNA levels [AU: female (basal), 1.0 ± 0.16, n = 9 vs. female (after escape), 0.37 ± 0.030, n = 24; P < 0.0001; male (basal), 1.0 ± 0.085, n = 10 vs. male (after escape), 0.68 ± 0.071, n = 21; P < 0.05], but the magnitude of the fall was substantially greater in females (63%) compared with males (32%) (Fig. 4A). A similar pattern of change was observed for V2R protein expression at day 5 of escape compared with basal levels measured in a subset of the females and males [AU: female (basal), 1.0 ± 0.071, n = 8 vs. female (after escape), 0.27 ± 0.068, n = 9; P < 0.0001; male (basal), 1.0 ± 0.15, n = 8 vs. male (after escape), 0.52 ± 0.13, n = 9; P < 0.05]. Again similar to V2R mRNA, the relative decrease in kidney V2R protein level was greater in females (73%) compared with males (48%) (Fig. 4B).
Fig. 4.
Effect of renal escape from antidiuresis after 5 days of water loading on AVP V2R expression in female and male rat kidneys by real-time PCR for V2R mRNA (A) and Western blotting for V2R protein (B). The female basal (before water loading) and escape groups (after 5 days of water loading) are normalized to the female basal average; the male basal (before water loading) and escape groups (after 5 days of water loading) are normalized to the male basal average. Values are expressed as means ± SE; *P < 0.05 compared with basal levels; ***P < 0.0001 compared with basal levels.
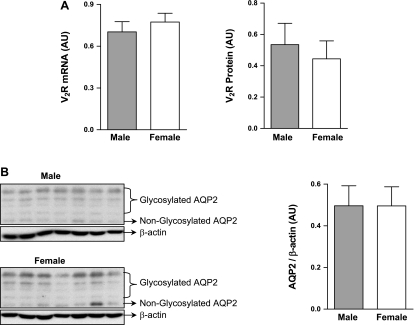
Comparison of V2R and AQP2 expression in female and male rats after escape from antidiuresis.
Real-time PCR result showed that following escape from antidiuresis, female (n = 24) and male (n = 21) V2R mRNA reached the same levels of expression [AU: female (after escape), 0.77 ± 0.063; male (after escape), 0.70 ± 0.074; not significant (NS)] (Fig. 5A), despite marked differences in basal V2R expression (Fig. 1A). Expression of V2R protein also reached the same level in female (n = 9) and male (n = 9) rats after escape from antidiuresis [AU: female (after escape), 0.44 ± 0.11; male (after escape), 0.54 ± 0.16; NS] (Fig. 5A), despite marked differences in basal V2R protein (Fig. 1B). Similarly, kidney AQP2 protein levels (measured as the sum of the glycosylated and nonglycosylated bands by Western blot analysis) also showed the same relative levels of expression between female (n = 14) and male (n = 14) rats after escape from antidiuresis [AU: female (after escape), 0.50 ± 0.092; male (after escape), 0.50 ± 0.096; NS] (Fig. 5B). However, under basal conditions, AQP2 expression was not significantly different between male and female rats (data not shown). These results are consistent with the physiological results indicating identical parameters of renal escape (i.e., urine osmolality and volume) in the female and the male rats after 5 days of water loading (Fig. 3).
Fig. 5.
Expression of kidney V2R and aquaporin-2 (AQP2) after completion of renal escape from antidiuresis after 5 days of water loading. A: V2R mRNA by real-time PCR and V2R protein by Western blot analysis in female and male rats after completion of escape from antidiuresis. B: AQP2 protein by Western blot analysis in female and male rats after completion of escape from antidiuresis. Values are means ± SE.
DISCUSSION
These studies demonstrate the presence of marked sex differences in renal V2R expression in female compared with male rats. The 2.6-fold greater V2R mRNA and 1.7-fold greater V2R protein levels in female rats are consistent with the hypothesis that renal V2R gene expression is higher in female compared with male animals under basal conditions.
The higher levels of renal V2R expression in females compared with males was manifested physiologically by a higher desmopressin-stimulated urine osmolality in female rats compared with males (Fig. 2). Although a significant sex difference was not found in desmopressin-stimulated decreases in urine output, this is not surprising given the relative insensitivity of urine volume to changes in urine osmolality at high levels of AVP stimulation. Interestingly, the higher urine osmolality in female rats compared with males was observed predominantly at higher doses of desmopressin administration more so than at lower doses, or under basal conditions where neither the baseline urine osmolality nor renal AQP2 expression was significantly different between female and male rats. This suggests that the increased V2R receptor expression in females may be of greater functional significance during situations of high ligand concentrations. This may indicate that under basal conditions of submaximal AVP stimulation the limiting factor in urine concentration is not V2R receptor number, but rather circulating AVP levels. Another possibility to explain these findings is that under basal or low AVP concentrations, much of the increased V2R in females may be in the form of “spare receptors” that are not functionally coupled to G protein signaling proteins (8, 15), whereas at higher AVP concentrations increased G protein coupling of the receptors occurs. In either case, the result would be that female rats manifest greater maximal responses to high AVP concentrations.
Because of these initial findings, we undertook additional studies of escape from AVP-induced antidiuresis, since previous studies from our laboratory have indicated that renal escape is accompanied by a marked downregulation of V2R expression (26), with decreased AVP-stimulated signal transduction in collecting tubule principal cells and resultant downregulation of AQP2 expression (7). We hypothesized that because females had higher basal levels of V2R expression, they would undergo escape from antidiuresis more slowly and to a lesser absolute degree relative to the males. Although to some extent this was found to be true, i.e., urine osmolality remained significantly higher in the female rats on days 1 and 2 of water loading (Fig. 3A), these differences were relatively small and did not reach statistical significance on all subsequent days of escape. More importantly, both the female and male rats reached the same level of escape by completion of the escape process, as reflected by the day 5 urine osmolalities and volumes. Interestingly, the relative changes in kidney V2R expression mirrored the physiological results in that the female rats experienced a greater downregulation of V2R mRNA and protein compared with the males. The magnitude of this effect can be best appreciated by the observation that after 5 days of escape, the profound sex differences in basal V2R expression were completely abolished (Fig. 5A).
The most reliable correlate of renal escape from antidiuresis has been decreased renal expression of AQP2 protein, which is the final mediator of AVP-stimulated antidiuresis (7). Any decrease in AVP-mediated signal transduction is capable of causing decreased water reabsorption by the kidney, as demonstrated by the effect of AVP V2R receptor antagonists to produce an aquaresis in animals (22, 25, 29) and humans (14, 29). Thus, decreased AVP-stimulated signal transduction as a result of decreased V2R expression should also be accompanied by decreased kidney AQP2 expression, which was found to an equivalent degree in both the female and male rats in this study (Fig. 5B).
Although sexually dimorphic differences in V2R and AQP2 expression in response to continuous desmopressin administration were not observed in this study, the escape process represents an adaptation to chronic AVP administration that takes days to occur. The more acute desmopressin stimulation studies (Fig. 2) indicate that the increased V2R expression in females does alter the renal responses to shorter-term changes in AVP levels and/or V2R activation.
In normal XX female mammals, the second X chromosome is silenced in each cell by a mechanism called “X inactivation”; however, some genes escape X inactivation and are expressed on both the active and inactive X chromosome in the same cell. Genes that escape X inactivation are potential contributors to sexually dimorphic traits and to the phenotypic variability among females heterozygous for X-linked conditions, as well as to clinical abnormalities in patients with abnormal X chromosomes. In 2005, Carrel and Willard (3) published the X inactivation status of 95% of the assayable genes on the X chromosome. The V2R gene scored 9 out of 9 in the X-inactivation tests in heterozygous human fibroblasts, suggesting that this gene has a high probability of escaping X inactivation. Thus, one potential explanation for our findings is that the V2R gene might escape X-inactivation in females, thereby allowing expression from both X chromosomes in females compared with expression from a single X chromosome in males. However, this hypothesized sex chromosomal effect will require further studies for definitive proof, since intact females and males manifest different levels of gonadal hormones, which could also contribute to the sex differences in V2R expression.
The results of these studies have several important implications both molecularly and physiologically. First, our results demonstrate physiologically significant ramifications of sex differences in renal V2R expression. Understanding the underlying mechanisms responsible for increased V2R expression in females may in turn provide insights into observed sex differences in other diseases such as hypertension and cardiovascular disease (20). Second, these results add additional support to V2R regulation as the predominant mechanism that regulates escape from antidiuresis (28), since the changes in V2R expression in females and males in this study accurately predicted the physiological manifestations of escape. Finally, our results suggest that the molecular mechanisms underlying V2R, and possibly other G protein-coupled receptor, regulation act in a manner capable of downregulating mRNA and protein expression to an absolute degree, rather than simply to a relative degree; if the latter were true, we would have expected to find proportional decreases in V2R mRNA and protein during escape from antidiuresis in both males and females rather than a greater degree of downregulation in females. All of these findings will require further studies to fully understand the significance of sex differences in AVP V2R expression, and the mechanisms leading to the loss of this sexual dimorphism during escape from antidiuresis.
Clinically, increased V2R expression in females may cause greater sensitivity to nonosmotically stimulated AVP thereby leading to hyponatremia from SIADH more frequently, which has not been carefully studied to date. Similarly, increased V2R expression and function in females might cause a relatively greater resistance to the aquaretic effect of acutely administered AVP V2R antagonists, which are now in clinical use (14, 24). Finally, our results raise the possibility that females may be more sensitive to the renal effects of exogenously administered AVP or desmopressin, in which case lower doses may achieve acceptable antidiuretic effects in females with less risk of hyponatremia. These and other potential clinical implications await further study.
GRANTS
This work was supported by National Heart, Lung and Blood Institute Grant HL-083428 (to J. G. Verbalis), National Institute of Aging Grant AG-19291 (to K. Sandberg), and National Kidney Foundation, Washington, DC, Grant-in-Aid (to J. Liu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Almond CS, Shin AY, Fortescue EB, Mannix RC, Wypij D, Binstadt BA, Duncan CN, Olson DP, Salerno AE, Newburger JW, Greenes DS. Hyponatremia among runners in the Boston Marathon.N Engl J Med 352:1550–1556, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Ayus JC, Wheeler JM, Arieff AI. Postoperative hyponatremic encephalopathy in menstruant women.Ann Intern Med 117:891–897, 1992 [DOI] [PubMed] [Google Scholar]
- 3. Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females.Nature 434:400–404, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Chan WY. A study on the mechanism of vasopressin escape: effects of chronic vasopressin and overhydration on renal tissue osmolality and electrolytes in dogs.J Pharmacol Exp Ther 184:244–251, 1973 [PubMed] [Google Scholar]
- 5. DeVita MV, Gardenswartz MH, Konecky A, Zabetakis PM. Incidence and etiology of hyponatremia in an intensive care unit.Clin Nephrol 34:163–166, 1990 [PubMed] [Google Scholar]
- 6. Ecelbarger CA, Kim GH, Terris J, Masilamani S, Mitchell C, Reyes I, Verbalis JG, Knepper MA. Vasopressin-mediated regulation of epithelial sodium channel abundance in rat kidney.Am J Physiol Renal Physiol 279:F46–F53, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Ecelbarger CA, Nielsen S, Olson BR, Murase T, Baker EA, Knepper MA, Verbalis JG. Role of renal aquaporins in escape from vasopressin-induced antidiuresis in rat.J Clin Invest 99:1852–1863, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Finkel MS, Mendelsohn FA, Quirion R, Zamir N, Keiser HR. Physiologic regulation and distribution of the renal vasopressin receptor.Pharmacology 39:165–175, 1989 [DOI] [PubMed] [Google Scholar]
- 9. Flear CT, Gill GV, Burn J. Hyponatraemia: mechanisms and management.Lancet 2:26–31, 1981 [DOI] [PubMed] [Google Scholar]
- 10. Forsling ML, Anderson CH, Wheeler MJ, Raju KS. The effect of oophorectomy and hormone replacement on neurohypophyseal hormone secretion in women.Clin Endocrinol (Oxf) 44:39–44, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Fujita N, Ishikawa SE, Sasaki S, Fujisawa G, Fushimi K, Marumo F, Saito T. Role of water channel AQP-CD in water retention in SIADH and cirrhotic rats.Am J Physiol Renal Fluid Electrolyte Physiol 269:F926–F931, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Fushimi K, Uchida S, Hara Y, Hirata Y, Marumo F, Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule.Nature 361:549–552, 1993 [DOI] [PubMed] [Google Scholar]
- 13. Garcia-Perez A, Burg MB. Renal medullary organic osmolytes.Physiol Rev 71:1081–1115, 1991 [DOI] [PubMed] [Google Scholar]
- 14. Greenberg A, Verbalis JG. Vasopressin receptor antagonists.Kidney Int 69:2124–2130, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Guillon G, Trueba M, Joubert D, Grazzini E, Chouinard L, Cote M, Payet MD, Manzoni O, Barberis C, Robert M. Vasopressin stimulates steroid secretion in human adrenal glands: comparison with angiotensin-II effect.Endocrinology 136:1285–1295, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Hall JE, Montani JP, Woods LL, Mizelle HL. Renal escape from vasopressin: role of pressure diuresis.Am J Physiol Renal Fluid Electrolyte Physiol 250:F907–F916, 1986 [DOI] [PubMed] [Google Scholar]
- 17. Hawkins RC. Age and gender as risk factors for hyponatremia and hypernatremia.Clin Chim Acta 337:169–172, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Kleinfeld M, Casimir M, Borra S. Hyponatremia as observed in a chronic disease facility.J Am Geriatr Soc 27:156–161, 1979 [DOI] [PubMed] [Google Scholar]
- 19. Knepper MA. Molecular physiology of urinary concentrating mechanism: regulation of aquaporin water channels by vasopressin.Am J Physiol Renal Physiol 272:F3–F12, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Maric C. Sex differences in cardiovascular disease and hypertension: involvement of the renin-angiotensin system.Hypertension 46:475–476, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Murase T, Tian Y, Fang XY, Verbalis JG. Synergistic effects of nitric oxide and prostaglandins on renal escape from vasopressin-induced antidiuresis.Am J Physiol Regul Integr Comp Physiol 284:R354–R362, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Naitoh M, Suzuki H, Murakami M, Matsumoto A, Arakawa K, Ichihara A, Nakamoto H, Oka K, Yamamura Y, Saruta T. Effects of oral AVP receptor antagonists OPC-21268 and OPC-31260 on congestive heart failure in conscious dogs.Am J Physiol Heart Circ Physiol 267:H2245–H2254, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Robertson GL. Posterior pituitary.In: Endocrinology and Metabolism, edited by Felig P, Baxter JD, Frohman LA. New York: McGraw-Hill, 1995, p. 385–432 [Google Scholar]
- 24. Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 355:2099–2112, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Tahara A, Tomura Y, Wada KI, Kusayama T, Tsukada J, Takanashi M, Yatsu T, Uchida W, Tanaka A. Pharmacological profile of YM087, a novel potent nonpeptide vasopressin V1A and V2 receptor antagonist, in vitro and in vivo.J Pharmacol Exp Ther 282:301–308, 1997 [PubMed] [Google Scholar]
- 26. Tian Y, Sandberg K, Murase T, Baker EA, Speth RC, Verbalis JG. Vasopressin V2 receptor binding is down-regulated during renal escape from vasopressin-induced antidiuresis.Endocrinology 141:307–314, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Verbalis JG. Disorders of body water homeostasis.Best Pract Res Clin Endocrinol Metab 17:471–503, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Verbalis JG. Escape from antidiuresis: a good story.Kidney Int 60:1608–1610, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Verbalis JG. Vasopressin V2 receptor antagonists.J Mol Endocrinol 29:1–9, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Vokes TJ, Weiss NM, Schreiber J, Gaskill MB, Robertson GL. Osmoregulation of thirst and vasopressin during normal menstrual cycle.Am J Physiol Regul Integr Comp Physiol 254:R641–R647, 1988 [DOI] [PubMed] [Google Scholar]
- 31. Xu DL, Martin PY, Ohara M, St John J, Pattison T, Meng X, Morris K, Kim JK, Schrier RW. Upregulation of aquaporin-2 water channel expression in chronic heart failure rat.J Clin Invest 99:1500–1505, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]