Abstract
Tubuloglomerular feedback (TGF), the change of afferent arteriolar resistance initiated by changes of luminal NaCl concentration, is thought to be related to NaCl-dependent release of ATP by macula densa cells. In the present study, we have explored the possibility that the released ATP may directly interact with vasoconstrictor P2 purinergic receptors in the vicinity of the glomerular vascular pole. In two different strains of wild-type mice (SWR/J and FVB), TGF responses were determined in vivo by measuring the stop flow pressure (PSF) change caused by a saturating increase in loop of Henle flow rate before and during the administration of the P2 receptor inhibitors PPADS (12 mg/kg + 35 mg·kg−1·h−1 iv) or suramin (50 mg/kg + 150 mg·kg−1·h−1). Both agents significantly reduced the blood pressure response to the P2X agonist α,β-methylene ATP. In SWR/J and FVB mice, elevating flow to 30 nl/min reduced PSF by 16.4 ± 2.2 and 17.1 ± 1.8%. During infusion of PPADS, PSF fell by 18.8 ± 2 (P = 0.4) and 16.5 ± 1.5% (P = 0.82) in the two strains of mice. During suramin infusion, PSF decreased by 14.7 ± 2.4 (P = 0.62) and 15 ± 1.3% (P = 0.4) in SWR/J and FVB mice, respectively. Including PPADS (10−4 M) in the loop perfusate did not significantly alter the PSF response (18.9 ± 1.8%; P = 0.54). Arterial blood pressure was not systematically affected by the P2 inhibitors. As measured by free-flow micropuncture, PPADS significantly reduced proximal tubular fluid reabsorption in both fractional and absolute terms. These results indicate that the direct activation of P2 purinergic receptors by ATP is not a major cause of TGF-induced vasoconstriction in vivo.
Keywords: PPADS, suramin, stop-flow pressure, proximal reabsorption, micropuncture
the tubuloglomerular feedback (TGF) response is defined as the change in glomerular filtration rate or glomerular capillary pressure when the perfusion rate in the loop of Henle of individual nephrons is altered (26). There is general agreement that this response is for the most part the result of a direct relationship between the loop of Henle perfusion rate and afferent arteriolar resistance. In addition, experimental evidence favors the notion that TGF-dependent vasomotion is initiated by purinergic control mechanisms activated by the flow-dependent release of ATP from macula densa cells into the underlying extraglomerular mesangial interstitium (3). It has remained uncertain how any released ATP may affect the tone of the afferent arterioles.
One proposal has been that ATP activates P2 receptors on extraglomerular mesangial cells and that the resulting increase in cytosolic Ca is transmitted to the afferent arteriole through gap junctional coupling (14, 19). ATP may also directly activate P2 receptors on the afferent arteriole, with P2X1 receptors being the most likely receptor subtype since its presence in afferent arterioles has been well documented (8). Alternatively, ATP may be dephosphorylated to AMP and adenosine by ecto-ATPases and ecto-5′ nucleotidase, a paradigm that has been shown to underlie the effects of ATP in a number of tissues (10, 18, 34). Such a mode of action of ATP is supported by the consistent and complete inhibition of TGF responses in vivo by inhibitors of A1 adenosine receptors (A1AR) and by the absence of TGF responses in mice with targeted deletion of A1AR (5, 6, 29, 32). In addition, interference with the dephosphorylation pathway has been found to reduce the TGF response magnitude (7, 20).
The present studies were performed to assess the effect of acute inhibition of P2 purinergic receptors by pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid tetrasodium salt (PPADS) or suramin on TGF responses in vivo. We believe that these studies add new evidence to the existing literature since the direct effect of broad-spectrum P2 inhibition on in vivo TGF responses has not been studied previously. Our experiments show that both agents were efficacious in reducing the blood pressure elevation caused by the P2X agonist α,β-methylene ATP, but that they did not significantly reduce the TGF response magnitude in two different strains of wild-type mice. These results do not support the notion that direct activation of P2 receptors on juxtaglomerular target cells plays a major role in the TGF response elicited by in vivo loop of Henle microperfusion in mice.
METHODS
Animals.
Experiments were performed in male mice of the SWR/J and FVB wild-type strains bred and reared at the National Institutes of Health (NIH) animal facility. Animals ranged from 20 to 31 g in weight and from 3 to 5 mo in age. Animals were kept on a standard diet (0.2% NaCl content) and tap water. Animal care and experimentation were approved and carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Animal preparation.
Mice were anesthetized with 100 mg/kg thiobutabarbital (inactin) intraperitoneally and 100 mg/kg ketamine subcutaneously. Body temperature was maintained at 37.5°C by placing the animals on an operating table with a servo-controlled heating plate. The trachea was cannulated, and a stream of 100% oxygen was blown toward the tracheal tube throughout the experiment. The left carotid artery was catheterized with hand-drawn polyethylene tubing for continuous measurement of arterial blood pressure and blood withdrawal. Two catheters connected to infusion pumps were inserted into the right jugular vein. One catheter was used for an intravenous maintenance infusion of saline at 300 μl/h; in the experiments in which single-nephron glomerular filtration rate (SNGFR) was measured, saline infused through this line contained [125I]iothalamate. The other catheter was used to infuse saline vehicle at 100 μl/h that was exchanged for saline containing PPADS or suramin in the experimental period. PPADS was given as a 12 mg/kg bolus and 36 mg·kg−1·h−1 infusion, while suramin was given as a 50 mg/kg bolus and a 150 mg·kg−1·h−1 infusion. The bladder was catheterized for urine collections.
Micropuncture experiments.
A control period during which TGF responses were measured in one or two tubules was followed by an experimental period in which either PPADS or suramin was infused. Measurements of stop-flow pressure (PSF) during perfusion of the loop of Henle were done as described previously (30, 33). When PSF had stabilized, the perfusion rate of the loop of Henle was increased to 30 nl/min and maximum PSF responses were determined. The perfusion rate was then reduced to 0 nl/min and maintained until steady states were achieved at each flow rate. Two such responses were determined successively in each nephron. An example of an original recording showing TGF responses in the same nephron in control and after initiation of PPADS administration is shown in Fig. 1. In three experiments, timed collections of tubular fluid were made in the early proximal tubule at a flow rate of 30 nl/min and after perfusion was stopped to assess TGF responses of SNGFR as described earlier (32). Tubular fluid volumes were determined in a constant-bore capillary. The perfusion fluid contained (in mM) 136 NaCl, 4 NaHCO3, 4 KCl, 2 CaCl2, and 7.5 urea, as well as 100 mg/100 ml FD&C green (Keystone, Bellefonte, PA). In some experiments, the perfusate in addition contained PPADS at a concentration of 10−4 M.
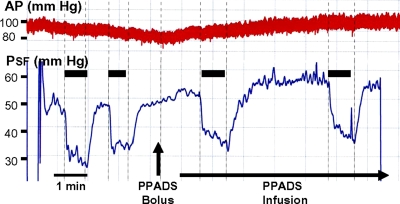
Fig. 1.
Original recording of stop-flow pressure (PSF, bottom trace) and arterial blood pressure (AP; top trace) before and after the intravenous (iv) administration of PPADS as a bolus (12 mg/kg) followed by an infusion at 36 mg·kg−1·h−1. Periods of loop of Henle flow elevation from 0 to 30 nl/min are indicated by black bars and vertical lines.
To determine nephron filtration and absorption rates, FVB mice were infused with [125I]iothalamate (Glofil-125, Iso-Tex Diagnostics, Friendswood, TX) at ∼40 μCi/h. A control period of ∼30 min was followed by an experimental period of 30–40 min with PPADS infusion. Free-flow micropuncture was performed according to techniques previously described (12). Briefly, end-proximal segments were identified by injecting a bolus of artificial tubular fluid stained with FD&C green from a 3- to 4-mm tip pipette connected to a pressure manometer. This pipette remained in place during the collections to permit control of intratubular pressure. All proximal collections were done in the last surface segment (collection times 2.5–3.5 min) using oil-filled pipettes. Fluid volume was determined from the column length in a constant-bore capillary. Samples were transferred into a counting vial, and radioactivity was determined in a gamma counter. Three blood samples were collected in heparinized 5-μl microcaps at the beginning and end of the control period and at the end of the experiment. [125I]iothalamate radioactivity was measured in duplicate using 500-nl samples of plasma and urine.
Statistics.
Statistical comparisons were performed by a paired or nonpaired t-test with a significance level of P < 0.05. Group comparisons between control and the two treatment groups (PPADS and suramin) were done by one-way ANOVA with a Bonferroni post hoc test.
RESULTS
Efficacy of inhibitor treatment.
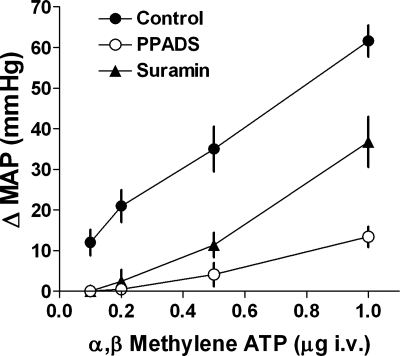
To assess the efficacy of the PPADS and suramin infusion protocols, the blood pressure response to the P2X receptor agonist α,β-methylene ATP was determined before the start of the micropuncture control period and at the end of the experimental periods with PPADS or suramin infusion. Data are summarized in Fig. 2. It can be seen that under control conditions intravenous bolus administration of α,β-methylene-ATP caused a dose-dependent blood pressure increase in the dose range between 0.1 and 1 μg. Both PPADS and suramin markedly reduced the blood pressure increment caused by α,β-methylene ATP, especially at the lower doses. All mean arterial pressure (MAP) responses during PPADS or suramin administration were significantly smaller than control responses at P < 0.05 (ANOVA).
Fig. 2.
Relationship between the increase in mean arterial blood pressure (MAP; ±SE) and the amount of injected α,β-methylene ATP during control (●; n = 10) and during administration of PPADS (○; n = 7) or suramin (▴, n = 7). Data from FVB and SWR/J mice are pooled as there was no discernible difference between strains.
TGF responsiveness.
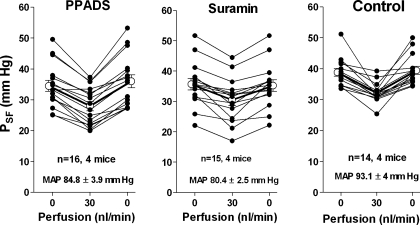
Responses of stop-flow pressure (PSF) to an increase in loop of Henle perfusion rate were determined in eight male SWR/J mice and in six male FVB mice. In SWR/J mice, PSF in the control period averaged 38.8 ± 1.2 mmHg at 0 loop flow, fell to 32.3 ± 0.9 mmHg when the flow rate was increased to 30 nl/min, and returned to 39.5 ± 1.3 mmHg with a return to 0 nl/min. Measurements performed during the administration of PPADS averaged 34.5 ± 1.8, 28 ± 1.5, and 36 ± 2.1 mmHg at perfusion rates of 0, 30, and 0 nl/min, respectively. During suramin infusion, PSF averaged 35.7 ± 1.9, 30.5 ± 1.9, and 35.2 ± 1.9 mmHg at 0, 30, and 0 loop perfusion. The reduction of PSF caused by the elevation of loop perfusion was significant in all three groups (P < 0.001). Data from individual tubules are shown in Fig. 3. Mean arterial blood pressure (MAP) was significantly higher in the control period compared with the suramin period (93.1 ± 4 vs. 80.4 ± 2.5 mmHg; P = 0.012), but not compared with the PPADS period (84.8 ± 3.9 mmHg; P = 0.18). In a small series of three experiments in SWR/J mice, we also determined the response of early proximal flow rate (EPFR), a close correlate of SNGFR, to an increase in loop flow rate during the administration of PPADS. In 10 tubules EPFR fell from 7.51 ± 0.9 to 3.75 ± 0.5 nl/min (P = 0.004). This fall in EPFR of 45.2% is similar to that observed in several previous studies under control conditions (7, 11, 32).
Fig. 3.
Tubuloglomerular feedback (TGF) responses in SWR/J mice. Data are shown for the first of 2 TGF tests assessing the response of PSF to increases in loop perfusion rate from 0 to 30 nl/min during iv infusion of either PPADS or suramin compared with control. Lines connect values from the same tubule.
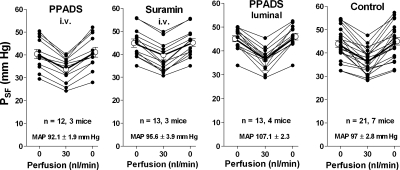
Control data obtained in seven FVB mice averaged 43.9 ± 1.5 mmHg at 0, 36.2 ± 1.15 mmHg at 30, and 45 ± 1.6 mmHg at 0 nl/min loop flow. During PPADS infusion PSF averaged 40.4 ± 2, 33.6 ± 1.6, and 41.2 ± 2.2 mmHg at 0, 30, and 0 nl/min. In the suramin periods mean PSF was 44.8 ± 1.8, 39.6 ± 1.75, and 45.1 ± 1.7 mmHg at the three flow rates. MAP averaged 97 ± 2.8, 92.1 ± 1.9, and 95.6 ± 3.9 mmHg in the control, PPADS, and suramin periods, values not significantly different from each other. Data from individual tubules are shown in Fig. 4. Using FVB mice, we also examined whether the luminal administration of PPADS may affect TGF responses. When PPADS at a concentration of 10−4 M was included in the luminal perfusate, mean PSF fell from 45.1 ± 1.3 to 36.5 ± 1.3 mmHg when flow was increased from 0 to 30 nl/min, and it returned to 46 ± 1.4 when flow was stopped. Data from individual tubules are included in Fig. 4. PSF values and PSF changes were not significantly different between tubules with or without PPADS.
Fig. 4.
TGF responses in FVB mice. Data are shown for the first of 2 TGF tests assessing the response of PSF to increases in loop perfusion rate from 0 to 30 nl/min during iv infusion of either PPADS or suramin and during luminal perfusion with PPADS (10−4 M) compared with control. Lines connect values from the same tubule.
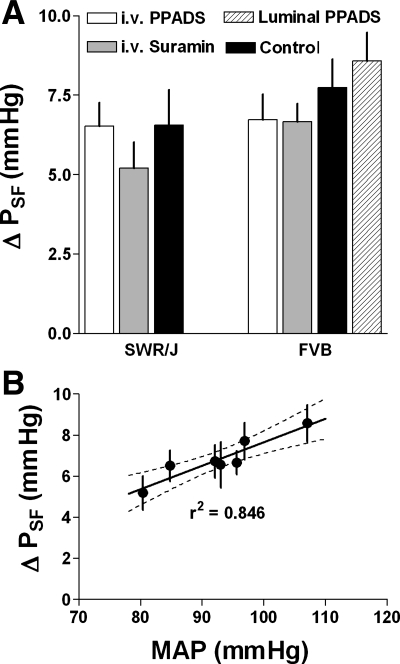
Mean PSF responses expressed as the difference between PSF at zero and at 30 nl/min are summarized in Fig. 5A. As can be seen, there is a tendency for PSF responses to be smaller than in control in both SWR/J and FVB mice when suramin was administered and in FVB mice when PPADS was given, but none of these differences reached statistical significance (P values between 0.33 and 0.87). When all treatment groups (iv suramin and iv PPADS in both SWR/J and FVB mice) and all control data from the two strains were combined to increase statistical power, PSF responses averaged 7.3 ± 0.7 mmHg in control (n = 35) and 6.2 ± 0.4 mmHg during P2 receptor inhibition (n = 56), a difference that again did not reach statistical significance (P = 0.16). In Fig. 5B, mean PSF responses of both FVB and SWR/J mice are related to MAP. The high correlation suggests that the observed response differences can largely be accounted for by uncontrolled blood pressure differences.
Fig. 5.
A: mean TGF responses (±SE) in SWR/J (left) and FVB mice (right) expressed as the difference between PSF at 0 and 30 nl/min during iv administration of PPADS (white bars) or suramin (gray bars), during luminal perfusion with PPADS (10−4 M; in FVB only; hatched) and during control (black bars). B: relationship between MAP and mean TGF responses as shown in A (data from both SWR/J and FVB mice) with linear regression and 95% confidence intervals.
Proximal tubule function.
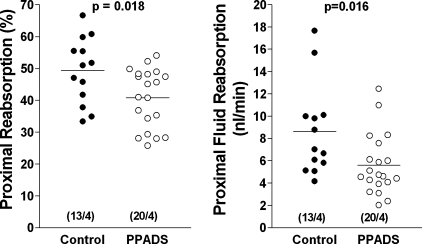
The effect of P2 receptor inhibition by PPADS on nephron hemodynamics and proximal reabsorption was examined by micropuncture in four FVB mice. SNGFR was lower in the PPADS period (13.8 ± 1.15 nl/min) compared with the control period (17.4 ± 1.7 nl/min), but this difference was only of borderline statistical significance (P = 0.079). It was however, paralleled by a significantly lower kidney GFR in the PPADS compared with the control period (339.5 ± 54 vs. 418.8 ± 60.1 μl/min; P = 0.013). As shown in Fig. 6, absolute proximal tubular fluid reabsorption was significantly lower during PPADS infusion than in control (5.6 ± 0.6 vs. 8.6 ± 1.14 nl/min; P = 0.016), a difference that is in part due to the lower SNGFR in the PPADS period. However, there was also a significantly lower fractional reabsorption of fluid during PPADS infusion than in control (40.8 ± 2.07 vs. 49.4 ± 2.9%; P = 0.018; Fig. 6). MAP averaged 88.2 ± 2.7 mmHg during control and 90.5 ± 1.7 mmHg during PPADS infusion (P > 0.05).
Fig. 6.
Proximal tubular fluid reabsorption in % of filtered load (left) and in absolute terms (right) during control and during the administration of PPADS. Numbers in brackets are individual tubules/mice.
DISCUSSION
The results of the current study show that the intravenous administration of PPADS or suramin, two widely used broad-spectrum inhibitors of P2 purinergic receptors, does not significantly alter the TGF response of PSF in mice. Furthermore, PPADS reduced the rate and fraction of proximal tubular fluid reabsorption as determined by free-flow micropuncture. These results suggest that direct activation of P2 purinergic receptors by nucleotides such as ATP does not contribute in a major way to the afferent arteriolar vasoconstriction elicited by standard in vivo microperfusion of the loop of Henle. The absence of significant differences in the TGF response magnitude between wild-type and P2X1 receptor-deficient mice as published previously in preliminary form are in line with this conclusion (28).
To our knowledge, neither PPADS nor suramin have previously been used to study TGF responsiveness in vivo. Both agents have been shown in numerous studies to inhibit the majority of both P2X and P2Y receptors (2). While this property is disadvantageous when one is trying to identify the role of specific receptor subtypes, the ability of these agents to affect a multitude of receptors would seem to be decidedly advantageous at the current understanding of P2 receptor participation in TGF responsiveness. The critical question of drug efficacy was assessed in our studies by verifying that PPADS and suramin reduced the hypertensive response to the P2X agonist α,β-methylene ATP. A similar efficacy test has recently been used successfully in studies designed to determine the role of P2 receptors in renal blood flow autoregulation (21). We do not think that the nearly exclusive reliance on PSF measurements to assess TGF responsiveness limits the strength of our conclusions to a major extent, although a dissociation between PSF and SNGFR responses over the entire relevant range of tubular flows has been observed under conditions of simultaneous cyclooxygenase and ACE inhibition (22). In this rare and singular case, PSF responses were found to be absent while SNGFR responses were partially maintained. However, the finding of the converse, the absence of SNGFR responses despite normal declines of PSF, would be unprecedented and hemodynamically highly unlikely. In addition, a TGF response of early proximal flow rate during PPADS administration was observed in a small number of tubules.
We realize that the existence of a small contribution of P2 receptor activation to TGF responsiveness is not impossible since responses during PPADS and suramin treatment as well as in P2X1−/− mice were numerically smaller than control responses. Due to the intrinsic variability of the micropuncture methodology, the significance of small differences is notoriously difficult to establish, enhancing the probability of not finding an effect when there really may be one (type II error). On the other hand, differences in the TGF response magnitude may be related to factors unrelated to the drug treatment such as random or strain differences of mice in tolerating anesthesia and laparotomy, which could be associated with random or strain-specific blood pressure variations. Specifically, our data suggest that the significantly lower blood pressure during suramin treatment in SWR/J mice is probably not a direct drug effect since a similar observation was not made in the FVB strain. In fact, as shown in Fig. 5B, PSF responses appeared to correlate well with MAP in the different mouse strains and treatment protocols. Thus blood pressure could be the underlying cause for any observed TGF response differences, a causality supported by earlier observations in the rat (4, 27).
In contrast to the in vivo assessment of TGF, suramin has been utilized to assess P2 receptor participation in the only other direct way to determine TGF responses, the double-perfused afferent arteriole/thick ascending limb preparation (17). Conclusions from such studies are contradictory. In an earlier study in rabbit tissue, suramin was found to be without effect on the response of afferent arteriolar diameter to an increase in luminal NaCl (25). Since acceleration of ATP breakdown enhanced and inhibition of 5′-ecto nucleotidase and of A1ARs blocked the arteriolar diameter response, these results are consistent with the ATP dephosphorylation/adenosine paradigm (24, 25). More recently, however, almost complete abolition of afferent arteriolar Ca and constrictor responses has been reported during exposure of a similar preparation from the mouse to suramin (23, 31). TGF response characteristics in these recent studies differed in a number of ways from the in vivo pattern, most notably in its dependence on flow rate as a stimulus, its independence of NaCl, and the noneffectiveness of an A1AR blockade (31). The reasons for this divergence are presently unclear, but it is to be noted that a highly novel approach was used in at least part of the latter study, in that only the macula densa cells of the perfused nephron were maintained while all thick ascending limb cells were removed by microdissection (31). One may therefore entertain the possibility that macula densa cells can elicit a flow-dependent and NaCl-independent effect through direct P2 receptor activation (31), whereas the presence of thick ascending limb cells is required for the “conventional” TGF response that is flow independent, NaCl dependent, and affects vascular tone through A1AR activation (26). Nevertheless, given the wide differences in experimental approaches the possibility of technical reasons for the obvious discrepancies cannot be excluded. Inferences about TGF responsiveness have also been drawn from in vitro and in vivo studies of the autoregulatory response to changes in perfusion pressure. Specifically, autoregulation has been found to be impaired in the blood-perfused juxtaglomerular nephron population of P2X1-deficient mice or during PPADS and suramin administration (15, 16). Furthermore, renal blood flow autoregulation of rats in vivo was reported to be relatively inhibited during PPADS-induced blockade of P2 receptors (21). In view of the present observations, one would have to conclude that inhibition of P2 purinergic receptors may affect autoregulation through a largely non-TGF-dependent mechanism, perhaps through interference with the myogenic component of this complex response.
We also examined whether TGF under normal closed-loop conditions may be indirectly affected by P2 receptors through their effect on proximal tubule reabsorption. Expression of P2Y receptors in the proximal tubule has been established, and an inhibitory effect of P2Y1 receptor activation on HCO3 reabsorption has been observed in stationary microperfusion studies (1). On the other hand, addition of ATP to the peritubular capillary blood increased HCO3 reabsorption, suggesting that the overall effect of P2 receptors on proximal reabsorption is complex (9). The current measurements under free-flow conditions show that PPADS administration caused a small, but significant reduction in fractional proximal fluid reabsorption consistent with opposing effects of ATP depending upon the sidedness of P2 receptor inhibition. Thus whether ATP stimulates or inhibits fluid reabsorption would depend upon the exact site of release and reflect the local action of the nucleotide. Whether inhibition of proximal fluid reabsorption and an increased distal NaCl delivery could be the cause for the reduction of GFR during PPADS administration is not entirely clear. It is also conceivable that systemic inhibitor administration causes net renal vasoconstriction because the effect of blockade of P2 receptors on endothelial cells and the reduction of endothelial vasodilators such as nitric oxide or prostacyclin may be dominant over the diminished constrictor effect of P2X activation in smooth muscle cells (19). However, in a recent study in rats no effect of PPADS on baseline renal blood flow or blood pressure was observed (21). Since the PPADS period always followed control, a time-dependent decline of renal function in the present experiments cannot be excluded although no such reduction was seen over a comparable time span in a previous study (13).
In summary, the intravenous administration of inhibitors of P2 purinergic receptors at doses that significantly reduced the blood pressure response to α,β-methylene ATP as well as luminal inhibitor perfusion did not significantly affect the TGF response to a saturating increase in loop perfusion rate. Direct activation of P2 receptors by nucleotides released from the macula densa does not appear to play a major role in the vasoconstriction elicited by elevated loop flows in vivo.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
ACKNOWLEDGMENTS
This work was supported by the intramural research program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD.
REFERENCES
- 1. Bailey MA. Inhibition of bicarbonate reabsorption in the rat proximal tubule by activation of luminal P2Y1 receptors. Am J Physiol Renal Physiol 287: F789–F796, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Bailey MA, Shirley DG, King BF, Burnstock G, Unwin RJ. Extracellular nucleotides and renal function. In: The Kidney: Physiology and Pathophysiology, edited by Alpern RJ, Hebert SC. London: Elsevier-Academic, 2008, p. 425–442 [Google Scholar]
- 3. Bell PD, Lapointe JY, Sabirov R, Hayashi S, Peti-Peterdi J, Manabe K, Kovacs G, Okada Y. Macula densa cell signaling involves ATP release through a maxi anion channel. Proc Natl Acad Sci USA 100: 4322–4327, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bell PD, Navar LG. Stop-flow pressure feedback responses during reduced renal vascular resistance in the dog. Am J Physiol Renal Fluid Electrolyte Physiol 237: F204–F209, 1979 [DOI] [PubMed] [Google Scholar]
- 5. Brown R, Ollerstam A, Johansson B, Skøtt O, Gebre-Medhin S, Fredholm B, Persson AE. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol 281: R1362–R1367, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Carlstrom M, Wilcox CS, Welch WJ. Adenosine A2 receptors modulate tubuloglomerular feedback. Am J Physiol Renal Physiol 299: F412–F417, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Castrop H, Huang Y, Hashimoto S, Mizel D, Hansen P, Theilig F, Bachmann S, Deng C, Briggs J, Schnermann J. Impairment of tubuloglomerular feedback regulation of GFR in ecto-5′-nucleotidase/CD73-deficient mice. J Clin Invest 114: 634–642, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chan CM, Unwin RJ, Bardini M, Oglesby IB, Ford AP, Townsend-Nicholson A, Burnstock G. Localization of P2X1 purinoceptors by autoradiography and immunohistochemistry in rat kidneys. Am J Physiol Renal Physiol 274: F799–F804, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Diaz-Sylvester P, Mac Laughlin M, Amorena C. Peritubular fluid viscosity modulates H+ flux in proximal tubules through NO release. Am J Physiol Renal Physiol 280: F239–F243, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Eltzschig HK, Weissmuller T, Mager A, Eckle T. Nucleotide metabolism and cell-cell interactions. Methods Mol Biol 341: 73–87, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Faulhaber-Walter R, Chen L, Oppermann M, Kim SM, Huang Y, Hiramatsu N, Mizel D, Kajiyama H, Zerfas P, Briggs JP, Kopp JB, Schnermann J. Lack of A1 adenosine receptors augments diabetic hyperfiltration and glomerular injury. J Am Soc Nephrol 19: 722–730, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hashimoto S, Adams JW, Bernstein KE, Schnermann J. Micropuncture determination of nephron function in mice without tissue angiotensin-converting enzyme. Am J Physiol Renal Physiol 288: F445–F452, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Hashimoto S, Huang Y, Briggs J, Schnermann J. Reduced autoregulatory effectiveness in adenosine 1 receptor-deficient mice. Am J Physiol Renal Physiol 290: F888–F891, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Inscho EW. P2 receptors in regulation of renal microvascular function. Am J Physiol Renal Physiol 280: F927–F944, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Inscho EW. Purinoceptor-mediated regulation of the renal microvasculature. J Auton Pharmacol 16: 385–388, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. J Clin Invest 112: 1895–1905, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ito S, Carretero OA. An in vitro approach to the study of macula densa-mediated glomerular hemodynamics. Kidney Int 38: 1206–1210, 1990 [DOI] [PubMed] [Google Scholar]
- 18. Matsuoka I, Ohkubo S. ATP- and adenosine-mediated signaling in the central nervous system: adenosine receptor activation by ATP through rapid and localized generation of adenosine by ecto-nucleotidases. J Pharmacol Sci 94: 95–99, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Navar LG, Inscho EW, Majid SA, Imig JD, Harrison-Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev 76: 425–536, 1996 [DOI] [PubMed] [Google Scholar]
- 20. Oppermann M, Friedman DJ, Faulhaber-Walter R, Mizel D, Castrop H, Enjyoji K, Robson SC, Schnermann J. Tubuloglomerular feedback and renin secretion in NTPDase1/CD39-deficient mice. Am J Physiol Renal Physiol 294: F965–F970, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Osmond DA, Inscho EW. P2X1 receptor blockade inhibits whole kidney autoregulation of renal blood flow in vivo. Am J Physiol Renal Physiol 298: F1360–F1368, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Persson AE, Gushwa LC, Blantz RC. Feedback pressure-flow responses in normal and angiotensin-prostaglandin-blocked rats. Am J Physiol Renal Fluid Electrolyte Physiol 247: F925–F931, 1984 [DOI] [PubMed] [Google Scholar]
- 23. Peti-Peterdi J. Calcium wave of tubuloglomerular feedback. Am J Physiol Renal Physiol 291: F473–F480, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Ren Y, Arima S, Carretero OA, Ito S. Possible role of adenosine in macula densa control of glomerular hemodynamics. Kidney Int 61: 169–176, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Ren Y, Garvin JL, Liu R, Carretero OA. Role of macula densa adenosine triphosphate (ATP) in tubuloglomerular feedback. Kidney Int 66: 1479–1485, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Schnermann J, Briggs JP. Function of the juxtaglomerular apparatus: control of glomerular hemodynamics and renin secretion. In: The Kidney: Physiology and Pathophysiology, edited by Alpern RJ, Hebert SC.London: Elsevier-Academic, 2008, p. 589–626 [Google Scholar]
- 27. Schnermann J, Briggs JP. Interaction between loop of Henle flow and arterial pressure as determinants of glomerular pressure. Am J Physiol Renal Fluid Electrolyte Physiol 256: F421–F429, 1989 [DOI] [PubMed] [Google Scholar]
- 28. Schnermann J, Briggs JP. Tubuloglomerular feedback: mechanistic insights from gene-manipulated mice. Kidney Int 74: 418–426, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schnermann J, Weihprecht H, Briggs JP. Inhibition of tubuloglomerular feedback during adenosine 1 receptor blockade. Am J Physiol Renal Fluid Electrolyte Physiol 258: F553–F561, 1990 [DOI] [PubMed] [Google Scholar]
- 30. Schnermann JB, Traynor T, Yang T, Huang YG, Oliverio MI, Coffman T, Briggs JP. Absence of tubuloglomerular feedback responses in AT1A receptor-deficient mice. Am J Physiol Renal Physiol 273: F315–F320, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Sipos A, Vargas SL, Peti-Peterdi J. Direct demonstration of tubular fluid flow sensing by macula densa cells. Am J Physiol Renal Physiol 299: F1087–F1093, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci USA 98: 9983–9988, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wright FS, Schnermann J. Interference with feedback control of glomerular filtration rate by furosemide, triflocin, and cyanide. J Clin Invest 53: 1695–1708, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zuo P, Picher M, Okada SF, Lazarowski ER, Button B, Boucher RC, Elston TC. Mathematical model of nucleotide regulation on airway epithelia. Implications for airway homeostasis. J Biol Chem 283: 26805–26819, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]