Abstract
To determine whether in the transgenic rat model [TGR(Cyp1a1Ren2)] with inducible ANG II-dependent malignant hypertension changes in the activation of intrarenal renin-angiotensin system may contribute to the pathogenesis of hypertension, we examined the gene expression of angiotensinogen (AGT) in renal cortical tissues and renin and prorenin receptor [(P)RR] in the collecting duct (CD) of the kidneys from Cyp1a1Ren2 rats (n = 6) fed a normal diet containing 0.3% indole-3-carbinol (I3C) for 10 days and noninduced rats maintained on a normal diet (0.6% NaCl diet; n = 6). Rats induced with I3C developed malignant hypertension and exhibited alterations in the expression of renin and (P)RR expressed by the CD cells. In the renal medullary tissues of the Cyp1a1Ren2 transgenic rats with malignant hypertension, renin protein levels in CD cells were associated with maintained renin content and lack of suppression of the endogenous Ren1c gene expression. Furthermore, these tissues exhibited increased levels of (P)RR transcript, as well as of the protein levels of the soluble form of this receptor, the s(P)RR. Intriguingly, although previous findings demonstrated that urinary AGT excretion is augmented in Cyp1a1Ren2 transgenic rats with malignant hypertension, in the present study we did not find changes in the gene expression of AGT in renal cortical tissues of these rats. The data suggest that upregulation of renin and the s(P)RR in the CD, especially in the renal medullary tissues of Cyp1a1Ren2 transgenic rats with malignant hypertension, along with the previously demonstrated increased availability of AGT in the urine of these rats, may constitute a leading mechanism to explain elevated formation of kidney ANG II levels in this model of ANG II-dependent hypertension.
Keywords: intrarenal renin-angiotensin system, ANG II-dependent hypertension, distal nephron renin, tubular renin, soluble (P)RR, gene expression
in angiotensin ii (ANG II)-dependent hypertension, the mechanisms involved in the augmentation of intrarenal ANG II content are not completely understood. High intrarenal ANG II augmentation is due to ANG II sequestration from the systemic circulation mediated by the ANG II type 1 receptor (AT1R) (29, 49, 51) and to de novo intrarenal ANG II formation driven by the activation of a local renin-angiotensin system (RAS) (11, 30, 31, 51). In several experimental animal models of ANG II-dependent hypertension, different possible mechanisms emerged as being responsible for the enhanced intrarenal ANG II formation: 1) the presence of all components of the RAS in the kidney, 2) the generation of angiotensin peptides from locally formed angiotensinogen (AGT) in proximal tubule cells, 3) the amplification effect via activation of AT1R that ANG II exerts on the local expression of its own precursor, AGT mRNA and protein, 4) increases in proximal tubule-derived AGT secretion into the lumen with augmented excretion in the urine, 5) augmented expression of renin in the collecting duct (CD) despite the coexistent suppression of renin at its primary source, the juxtaglomerular (JG) cells, and 6) the presence of angiotensin-converting enzyme (ACE) along the nephron. The increases in kidney tissue AGT synthesis, secretion, and urinary excretion, as well as renin in the principal cells of the CD in several animal models of ANG II-dependent hypertension have been associated with the increased capability of the kidney to form de novo ANG II (7, 16–18, 24, 40, 42).
The (pro)renin receptor, (P)RR, recently cloned is expressed in mesangial cells, cortical renal arteries, and distal tubules of the kidney, specifically in the luminal aspect of the intercalated cells (1, 32). The (P)RR increases ANG II tissue generation by binding either renin or (pro)renin and increasing the catalytic AGT cleavage efficiency (5, 23, 32). In addition, there is demonstrated evidence that ACE activity is present in the CD (3, 22, 43) and that chronic ANG II infusions upregulate ACE (21) and downregulate ACE2 in vitro and in vivo (6, 13). These findings highlight the importance that upregulation of AGT from proximal nephron segments and renin at the distal nephron may serve as a suitable pathway to explain the increased de novo intrarenal ANG II formation and the impaired ability to suppress the activity of the intrarenal RAS during ANG II-dependent hypertension states.
In the transgenic rat model [TGR(Cyp1a1Ren2)] with inducible activation of the extrarenal Ren2 renin gene expression, the induction of ANG II-dependent malignant hypertension has been associated with increased plasma renin activity (PRA) and high circulating and intrarenal ANG II levels. In this model, the induction of Cyp1a1 promoter is mediated by the dietary administration of the aryl hydrocarbon indole-3-carbinol (I3C), which drives the expression of the Ren2 renin gene primarily in the liver and grades the severity of high blood pressure (BP) in a dose-dependent manner (15, 25, 27). Indeed, this model is considered to be the genetic equivalent of different fixed amounts of renin infusion that escape from homeostatic regulation. Thereby, in contrast to the chronic ANG II-infused rat model, in which the kidneys are exposed to elevated circulating ANG II levels and marked suppression of PRA and decreased kidney renin content (47, 51), the kidneys of hypertensive TGR(Cyp1a1Ren2) rats are exposed to elevated circulating levels of both (pro)renin/renin and ANG II (25, 38). In the TGR (Cyp1a1Ren2) rats even though renin is not constitutively expressed in the kidney (15), the renal tissue ANG II levels are enhanced and renal hemodynamic function is reduced (25, 27). Thus, it has been suggested that the elevated BP in the TGR(Cyp1a1Ren2) rats is at least partially due to increased intrarenal ANG II levels (25, 28).
Consistent with our previous findings demonstrating that total kidney ANG II content is elevated in Cyp1a1Ren2 rats with malignant hypertension (25, 36), the increases in the ANG II content in the kidney are prevented by AT1R blockade (25, 48). Interestingly, the intrarenal ANG II content measured in the Cyp1a1Ren2 rats induced with I3C is high in the cortex and even greater in the kidney medulla of these rats (48). Nevertheless, the inappropriate activation of AT1R by ANG II that contributes to the augmented BP and to increased kidney tissue ANG II levels, particularly in the renal medulla of Cyp1a1Ren2 rats with malignant hypertension, is due to mechanisms still uncertain (25). Thus, in the present study, we aimed to determine whether alterations in the expression of other components of the intrarenal RAS may help to explain the increases in intrarenal ANG II formation in this model. We used dissected renal cortical and inner medullary tissues from Cyp1a1Ren2 transgenic rats fed a diet with high content of I3C (0.3%) to induce ANG II-dependent malignant hypertension to determine whether changes in activation of intrarenal AGT and renin and (P)RR in the CD of the kidney contribute to the pathogenesis of hypertension in this model of ANG II-induced hypertension.
METHODS
Animals and Experimental Design
All experiments were performed using adult male transgenic TGR(Cyp1a1Ren2) rats with inducible expression of the Ren2 renin gene (15) with protocols approved by the Tulane Institutional Animal Care and Use Committee. All the transgenic rats used in this study were bred at Tulane University School of Medicine from stock animals supplied by Harlan UK Limited (Bicester, UK). Animals were divided into two groups as follows: group 1 (noninduced; n = 6) rats maintained on a normal diet [0.6% NaCl diet (diet TD 99414); Harlan-Teklad, Madison, WI] and group 2 (0.3% I3C; n = 6) Cyp1a1Ren2 rats fed a normal diet containing a I3C at a dose of 0.3% wt/wt (diet TD 05381; Harlan-Teklad) for 10 days to induce ANG II-dependent malignant hypertension, as described previously (8, 25, 27, 36, 37, 48). In all rats, body weight was measured every day. At the completion of the experimental protocol, the rats were anesthetized with pentobarbital sodium (IP, Nembutal Sodium Solution; Ovation, Deerfield, IL), and the abdominal cavity was opened to excise the left kidney immediately after unilateral renal ligature. This kidney was decapsulated under sterile RNAse-free conditions, renal inner medulla and renal cortex were dissected under stereomicroscopy, and samples were divided to be either snap-frozen in liquid nitrogen and stored at −80°C for eventual determinations of renin content and Western blot analysis or were included in RNAse later (Ambion) and stored at −80°C until be processed for total RNA extractions. The right kidney was used for immunohistochemistry studies after sequentially perfusion for 10 min with saline solution (0.9% NaCl at room temperature) and 20 min with cold 4% paraformaldehyde (PFH) by placement of a needle (18-gauge) into the right cardiac ventricle and opening a notch on the wall of the right atrium.
Intrarenal RAS Expression Studies
qRT-PCR studies.
Twenty nanograms per well of total RNA were extracted from rat kidney cortex samples to amplify AGT mRNA and from the medulla samples to amplify Ren1c, Ren2, and (P)RR genes. The quantifications were performed using similar approaches as previously described (40). Following sequences were used for: 1) AGT: primers [sense 5′-GAAGATGAACTTGCCACTA GA-3′; antisense 5′-AAGTGA ACG TAGGTGTTGAAA-3′] and probe [5′-6-FAM-CAGCACGGACAGCACCCTATT-BHQ1a-3′], 2) Renin 1c: primers [sense 5′-AGTACTATGGTGAGATCGGCATT-3′; antisense 5′-AGATTCACAACCTCTATGACTCCTC-3′] and probe [5′-6-FAM-TTCAAAGTCATCTTTGACCACGGGTTCAG-BHQ1a-3′], 3) Ren2: primers [sense 5′-ACAGTATCCCAACAGGAGAAGACAAG-3′; antisense 5′-GCACCCAGGACCCAGACA-3′] and probe [5′-(6-FAM)TGGCTCTCCATGCCATGGACATCC-(BHQ1a-6FAM)-3′], and 4) (P)RR: primers (sense 5′-ATCCTTGAGACGAAACAAGA-3′; antisense 5′-AGCCAGTCATAATCCACAGT-3′) and probe [5′-6-FAM-ACACCCAAAGTCCCTACAACCTTG-BHQ1a-3′]. All samples were assayed in triplicate in addition to coamplification of the β-actin gene labeled with 5′-6-HEX and 3“-black hole quencher-2.
Western blot analysis.
AGT protein expression levels were determined in 20 μg/well of protein extracted from renal cortexes, as previously described (19). We used a primary rabbit anti-rat/mouse AGT antibody (Code no. 28101, Immuno-Biological Laboratories; IBL, Japan; Dr. Hiroyuki Kobori, Tulane Univ., New Orleans, LA) at a 1:20 dilution and a mouse β-actin antibody at a 1:2,000 dilution as a loading control. For renin and prorenin protein expression in the CD, 40 μg of protein extracts from inner renal medullary tissues were assayed with a polyclonal rat renin/prorenin antibody raised in rabbit (Santa Cruz Biotechnology, Santa Cruz, CA; cat. ID scH-105) at a 1:100 dilution normalized against β-actin, as previously described (40). For (P)RR, the protein expression levels were separately quantified in tissue samples from renal cortex and medulla using a polyclonal rabbit anti-(P)RR (ATP6AP2, cat. ID HPA003156; Sigma, St. Louis, MO) at a 1:400 dilution. Results were expressed as fold of change of the analyzed specific bands of the gene of interest normalized against β-actin (Santa Cruz Biotechnology).
Immunohistochemistry.
Three-micrometer paraffin-embedded, PFH-perfused kidney sections were immunostained using a peroxidase technique and either a polyclonal rat renin antibody raised in rabbit (generously provided by Dr. T. Inagami, Vanderbilt University) at 1:4,000 dilution or a rabbit anti-(P)RR (N. 1623; Dr. G. Nguyen, College of France, France) at a 1:4,000 dilution and detection with the specific horseradish peroxidase-Polymer kit (Biocare Medical). Peroxidase activity was visualized by 3,3′-diaminobenzidine tetrahydrochloride (Biocare Medical). For imaging capture and analysis, a Nikon digital camera (DS-U2/L2 USB) attached to a Nikon Eclipse 50i microscope was used.
Statistical Analyses
Results are expressed as means ± SE. Data were evaluated by Grubb's test followed when appropriate by paired and unpaired Student's t-test or by one-way ANOVA with Tukey post hoc test. The significance of differences among groups was defined at a value of P < 0.05.
RESULTS
The rats induced with 0.3% I3C demonstrated severe lethargy, assumption of a hunched posture, and piloerection, which are manifestations of malignant hypertension in the rat (15, 25, 37). After 10 days of being on a normal diet containing a 0.3% I3C to induce ANG II-dependent malignant hypertension, the induced rats exhibited a substantial decrease in body weight compared with noninduced rats (248 ± 2 vs. 349 ± 1 g; P < 0.01).
The renin content was similar in the Cyp1a1Ren2 hypertensive rats and in noninduced rats in both tissues, the renal cortex and renal medulla; however, the renin content in inner medullary tissues was markedly higher than in the cortex [hypertensive rats cortex: 659 ± 36; medulla: 3,067 ± 1,013 μg ANG I·h−1·g−1 vs. noninduced (cortex: 470 ± 35; medulla: 3,398 ± 420 μg ANG I·h−1·g−1); P < 0.05].
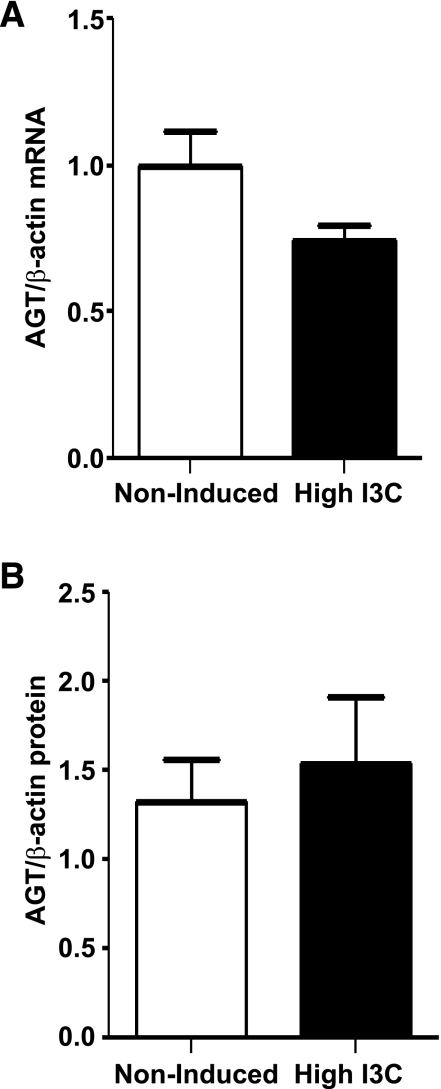
The expression levels of AGT mRNA and protein quantified in renal cortical tissues are shown in Fig. 1. AGT transcript and protein levels (52-kDa band) measured by qRT-PCR and Western blot were similar in both groups of rats [AGT mRNA: (0.8 ± 0.2 vs. 1.0 ± 0.1 AU); AGT protein (1.5 ± 0.4 vs. 1.3 ± 0.2-fold change); Fig. 1].
Fig. 1.
Angiotensinogen (AGT) mRNA and protein levels measured in microdissected renal cortex samples from Cyp1a1Ren2 rats with inducible ANG II-dependent malignant hypertension. The AGT mRNA (A) and protein (B) levels measured, respectively, by real-time qRT-PCR and Western blot were no different between Cyp1a1Ren2 rats (n = 6) with inducible ANG II-dependent malignant hypertension and noninduced normotensive (n = 6) rats.
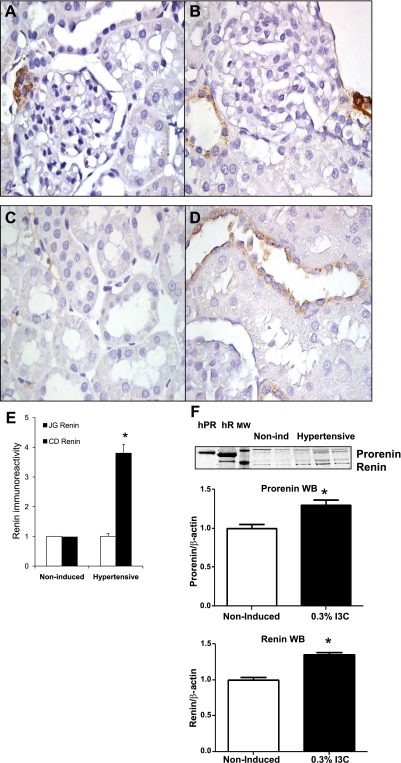
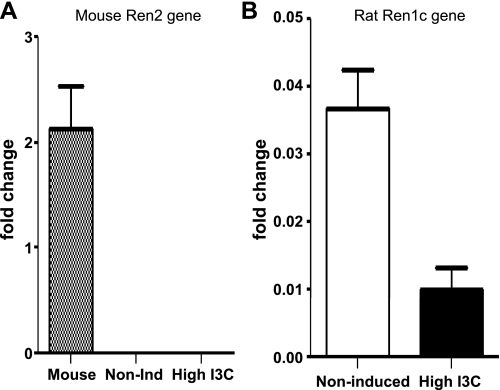
Renin immunostaining using a polyclonal rabbit anti-renin antibody showed no significant decrease in the positive immunoreactivity in JG cells of induced rats (Fig. 2, A and B). In contrast, renin immunoreactivity in CD cells was 3.8-fold higher in the induced Cyp1a1Ren2 transgenic rats compared with noninduced normotensive control rats (Fig. 2, C–E). Renin protein levels quantified in renal inner medullary tissues by Western blot analysis showed augmentation of prorenin and renin in hypertensive Cyp1a1Ren2 transgenic rats compared with noninduced normotensive control rats [prorenin: (1.29 ± 0.1 vs. 1.0 ± 0.0 AU; P < 0.05); renin: (1.34 ± 0.1 vs. 1.0 ± 0.1; P < 0.01); Fig. 2F]. To further assess the origin of prorenin/renin present in the CD of the Cyp1a1Ren2 transgenic rats, we also quantified the mRNA levels of the transgene, the mouse Ren2 renin gene, as well as the endogenous rat Ren1c renin gene in the renal medulla to avoid contribution of JG renin component. We did not find amplification whatsoever of the mouse Ren2 renin gene using as a control RNA extracted from a mouse strain 129 kidney, indicating that prorenin/renin is not derived from renal expression of the transgene (Fig. 3A). However, we found detectable levels of the transcript for the Ren1c renin gene in the renal medullary tissues of both groups (0.3 ± 0.1 vs. 1.0 ± 0.1; P < 0.05; Fig. 3B), suggesting that the endogenous Ren1c gene is not completely suppressed in induced Cyp1a1Ren2 rats.
Fig. 2.
Renin immunoreactivity in juxtaglomerular (JG) cells and collecting duct (CD) cells of paraffin-embedded kidney sections of Cyp1a1Ren2 rats with inducible ANG II-dependent malignant hypertension and noninduced normotensive rats. Top panels show similar renin-positive immunoreactivity (3,3′-diaminobenzidine tetrahydrochloride, brown chromogen; arrow) in the JG cells of a noninduced rat (A) and a Cyp1a1Ren2 rat (B) kidney sections (3 μm) stained by immunoperoxidase technique. Likewise, C (noninduced rat) and D (Cyp1a1Ren2 rat) show renin immunoreactivity in CD cells. Semiquantitation of renin immunoreactivity (E) in JG cells is not different between noninduced and Cyp1a1Ren2-hypertensive rats; however, renin immunoreactivity in CD cells is 3.8-fold higher in hypertensive rats than in noninduced rats. F: representative renin and prorenin Western blot and densitometry analysis using loads of 40 μg/lane of protein extracted from renal medullary tissue samples from noninduced and Cyp1a1Ren2-hypertensive rats induced with 0.3% high indole-3-carbinol (I3C). As positive controls, 1 μg of human recombinant prorenin (hPR) and human recombinant renin (hR) was used. MW, molecular weight. Values are means ± SE. *P < 0.05 vs. noninduced rats.
Fig. 3.
Renin mRNA levels quantified by real-time PCR (qRT-PCR) in inner renal medulla of Cyp1a1Ren2-hypertensive rats. A: inner medullary tissues from Cyp1a1Ren2 rats, noninduced (n = 6) and induced (n = 6) with a high-I3C diet, have no detectable mRNA levels of the mouse Ren2 gene (transgene). However, the mRNA levels of the endogenous rat renin 1c gene (Ren1c) are not completely suppressed in the renal medulla of Cyp1a1Ren2 rats subjected to a similar scheme of diet (B). RNA extracted from kidney tissues from a mouse 129 strain was used as control of Ren2 gene expression.
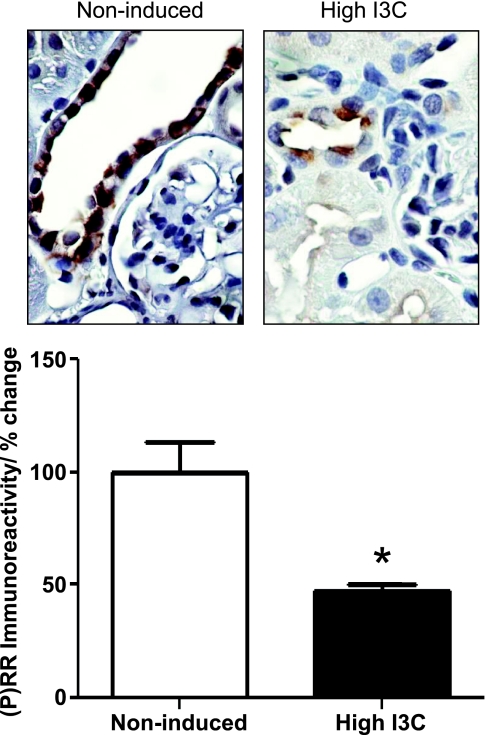
Intense (P)RR immunoreactivity was observed primarily in epithelial cells of the connecting tubules and CDs throughout the cortex, the outer medulla, and extending into the inner medulla (Fig. 4, top). Proximal tubules and thick ascending limbs of Henle's loop were negative. In sections in which the primary antibody was substituted with buffer only, no immunoreactivity was observed (data not shown). Cells of the CD exhibited decreased apical (P)RR immunoreactivity in the hypertensive rats induced with high I3C compared with noninduced rats (47.6 ± 2.6 vs. 100 ± 23.2%; P < 0.01; Fig. 4, bottom). Rarely, cells were observed that had diffused basolateral label in addition to apical label. In the CDs at the renal cortex, most of the cells expressed apical (P)RR immunoreactivity as well. In both, the outer and the inner stripe of the outer and inner medullary CDs, positive apical (P)RR immunoreactivity was predominantly observed in those cells with a rounded outline and bulging apical surface suggesting that they were intercalated cells as has been described previously (1).
Fig. 4.
Semiquantitation of prorenin receptor [(P)RR] immunoreactivity in CD of paraffin-embedded kidney sections of Cyp1a1Ren2 rats. Densitometric analysis of the (P)RR intensity of Cyp1a1Ren2 rats shows reduced immunoreactivity in CD of Cyp1a1Ren2-hypertensive rats induced with I3C diet compared with noninduced normotensive rats. Values are means ± SE. *P < 0.05 vs. noninduced rats.
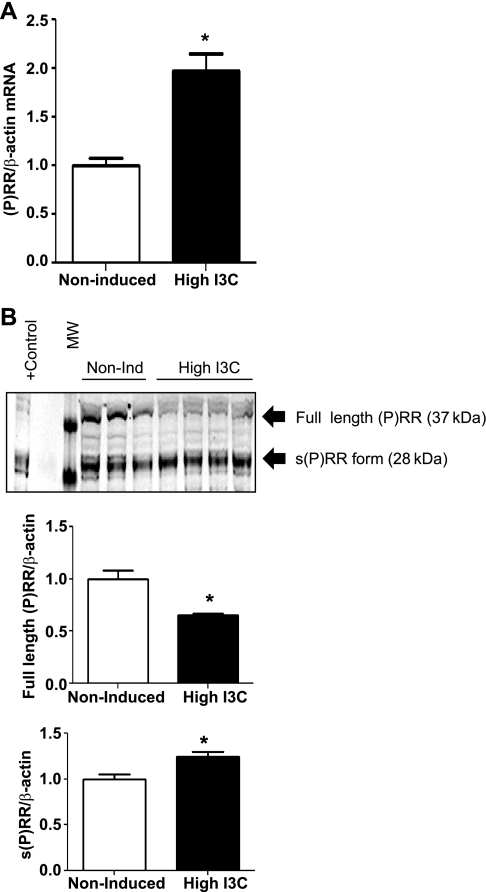
The (P)RR transcript levels were significantly greater in the renal medulla than in the cortex, regardless of the group (data not shown). (P)RR mRNA levels were significantly augmented in the renal medullary tissues of hypertensive rats compared with the noninduced controls (1.97 ± 0.2 vs. 1.0 ± 0.1-fold increase; P < 0.01; Fig. 5A). In these tissues, the (P)RR immunoblot using a purified rabbit IgG against an epitope of the ectodomain and small portion of the cytoplasmic domain (cat. ID HPA003156; Sigma) showed that the Cyp1aRen2 rats induced with a high dose of I3C and that exhibit malignant hypertension presented a slightly decreased expression of the full-length (P)RR (37-kDa band; Cyp1aRen2: 0.7 ± 0.1 vs. noninduced: 1.0 ± 0.3-fold change; P < 0.05; Fig. 5B). However, the soluble form of the (P)RR (28-kDa band) was significantly augmented in the renal medulla of these rats (Cyp1aRen2: 1.3 ± 0.1 vs. noninduced: 1.0 ± 0.1-fold change; P < 0.05; Fig. 5B). In this experiment, protein extracted from lysate of neuronal cells overexpressing (P)RR generously given by Dr. Y. Feng (Tulane Univ., New Orleans, LA) was used as a positive control.
Fig. 5.
Prorenin receptor mRNA and protein levels quantified by qRT-PCR and Western blot in inner medullary tissues of Cyp1a1Ren2 rats. A: (P)RR mRNA levels in Cyp1a1Ren2 rats are significantly higher in hypertensive rats induced with high-I3C diet. B: in the renal inner medullary tissues of Cyp1a1Ren2 rats are detected 2 forms of the (P)RR, the full-length (37-kDa band) and the soluble form [s(P)RR; 28-kDa band]. In these tissues, using 40 μg of protein loads/lane and an anti-(P)RR primary antibody (1:400 dilution) against an epitope of the ectodomain (ATP6AP2, cat. ID HPA003156; Sigma), both forms of the (P)RR were detected, the full-length and the soluble forms. The protein levels of the s(P)RR were higher in hypertensive rats induced with I3C; however, the full length of the (P)RR was slightly decreased compared with noninduced normotensive rats. Values are means ± SE. *P < 0.05 vs. noninduced rats.
DISCUSSION
Induction of the Ren2 renin gene by dietary administration of 0.3% I3C for 10 days resulted in a marked decrease in body weight, extreme lethargy, hunched posture, and piloerection, which are clinical manifestations of malignant hypertension previously described in these model rat (15, 25, 37). Most importantly, the results of the present study demonstrate that in Cyp1a1Ren2 transgenic rats fed a diet containing 0.3% I3C to induce ANG II-dependent malignant hypertension, there are alterations in the expression of renin and (P)RR expressed by the CD cells. These alterations may contribute to increased intrarenal ANG II de novo formation and thus, to the pathogenesis of hypertension in this model. In the renal medullary tissues of the Cyp1a1Ren2 transgenic rats with malignant hypertension, we found increased renin protein levels in CD cells associated with maintained renin content and lack of suppression of the endogenous Ren 1c gene expression. Furthermore, these tissues exhibited increased levels of (P)RR transcript, as well as of the protein levels of the soluble form of this receptor, the s(P)RR. Intriguingly, although previous findings demonstrated that urinary AGT excretion is augmented in Cyp1a1Ren2 transgenic rats fed a diet with high content of I3C(24), in the present study we did not find changes in the gene expression of AGT in renal cortical tissues of these rats.
Previous studies demonstrated that despite the marked elevation of arterial pressure, the renal tissues of rats with ANG II-dependent hypertension such as the nonclipped kidney of the 2K1C Goldblatt hypertensive rat and the kidneys of ANG II chronically infused rats, TGR(mRen2)27 transgenic rats, and Cyp1a1Ren2 transgenic rats exhibit a marked augmentation of intrarenal ANG II content beyond its levels in the circulation (9, 26, 52). The increases in intrarenal ANG II content in the Cyp1a1Ren2 rats with malignant hypertension may be secondary to AT1R-mediated stimulation uptake of circulating ANG II (25, 52) given that AT1R are located on the luminal and the basolateral membranes of the proximal tubule and CD cells (10). However, de novo generation of ANG II may also occur in this model such as has been reported in the chronic ANG II-infused rat model (20, 52). Augmented tissue ANG II formation in the kidneys of chronic ANG II-infused rats and in the nonclipped kidney of 2K1C Goldblatt rats has been associated with upregulation of tubular renin in the CD (40–42). Renin synthesis in principal cells of the CD is stimulated by ANG II (40) in ANG II-dependent hypertension via an AT1R mechanism (41). In the present study, the demonstration that JG renin expression is maintained in Cyp1a1Ren2 rats with malignant hypertension together with the findings that the renal medullary tissues of these rats, which are devoid of JG renin, exhibit augmented renin immunoreactivity in the CD cells, increased renin protein levels, and proven amplification of the endogenous Ren1c, indicates that the kidneys of Cyp1a1Ren2-hypertensive rats are not depleted of renin. In contrast to the kidneys of chronic ANG II-infused rats, in which the kidneys are exposed to elevated systemic ANG II levels and marked suppression of plasma renin activity (47, 50, 51); the kidneys of hypertensive Cyp1a1Ren 2 rats are subjected to elevated systemic levels of both renin and ANG II due to the extrarenal induction of the Ren2 transgene. Therefore, although it cannot be ruled out that part of renin found in the kidneys of Cyp1a1Ren2 rats is from systemic origin, the presence of the transcript of the endogenous renin gene (Ren1c) indicates that renin synthesis is still able to occur in the kidneys of Cyp1a1Ren2 rats regardless of the marked elevation of arterial BP. The findings that renin immunoreactivity is augmented in the principal cells of I3C-induced hypertensive rats and that the renin activity in renal medullary tissues of these rats is similar to those values quantified in noninduced rats also indicate that the activity of renin in the CDs of these rats is not suppressed even in conditions of severe hypertension. This observation is consistent with our previous findings demonstrating that the ANG II contents in the kidney medulla and cortex are elevated in Cyp1a1Ren2 rats with malignant hypertension (48). Accordingly, it is apparent that the kidneys of Cyp1a1Ren2 transgenic rats exhibit an impaired ability to suppress intrarenal JG renin, and most importantly, display stimulation of renin in the CD, which may contribute to the increased intrarenal ANG II contents which are actually greater in the renal medulla than in the cortex (48).
To investigate further the mechanisms underlying the maintained capability on the kidneys to continue forming intrarenal ANG II in the Cyp1a1Ren2 transgenic rats with malignant hypertension, we also examined the gene expression of (P)RR in the renal tissues of these rats. Nguyen and associates (32) cloned the (P)RR that equally binds renin and prorenin. Receptor-bound renin exhibits fivefold increased catalytic activity and receptor-bound prorenin exhibits full enzymatic activity comparable to that of active renin (32). We found that the (P)RR is predominantly immunoexpressed on the apical aspects of the bulging CD cells in kidneys from induced Cyp1a1Ren2 rats. The presence of (P)RR on the cell apical surface of the intercalated cells (1) in the CD might be of great relevance in the light of new findings of renin synthesis and secretion from the principal cells of the CD (14, 40, 42, 44). We proposed that (P)RR located on the surface of the intercalated cells may play a pivotal role to increase the efficiency of intratubular angiotensin peptide generation in the CD by anchoring renin or prorenin synthesized and released by principal cells (39). (Pro)renin receptor expression appears to be upregulated under pathological conditions, e.g., in the heart of spontaneously hypertensive rats on a high-salt diet (12). In this study, we also found upregulated levels of (P)RR transcript in the kidney medulla of Cyp1a1Ren2 transgenic rats with malignant hypertension. This finding contrasts with the decreased (P)RR immunoreactivity in cortical, outer, and inner medullary CDs, as well as of protein levels of the full-length (P)RR form (37-kDa band) and it is consistent with the existence of a short negative feedback loop proposed by Schefe et al. (45), by which high prorenin levels suppress the (P)RR gene expression. According to the concept of Schefe et al. (45), it is possible that high prorenin circulating levels such as occur in the hypertensive Cyp1a1Ren2 rats lead to diminished renal (P)RR expression. This seems to be the case in the Cyp1a1Ren2 rats in which high circulating and intrarenal prorenin/renin levels downregulate the (P)RR immunoreactivity in the medullary CD cells and the protein levels of the full-length (P)RR but not the s(P)RR in these tissues. Nevertheless, the mechanisms underlying the augmented expression of the soluble form of the (P)RR (28-kDa band) in the same renal tissues of Cyp1a1Ren2 rats are an issue that remains to be clarified. Recently, it has been proposed that a few aminoacids outside of the transmembrane domain serve as a putative cutting site for furin, a protease that participates in (P)RR processing and that might liberate the extracellular (pro)renin binding part (2, 4) into the circulation or other compartments where it may play a functional role (4, 33). In ANG II-dependent hypertension, the renal interstitial ANG II levels are greater than can be explained on the basis of equilibration with the plasma concentrations (34, 35, 46), suggesting that local regulation of ANG II formation in the renal interstitial compartment and enhanced production of interstitial ANG II might be secondary to specialized ANG II-forming pathways or accumulation mechanisms (34). The (P)RR immunoexpression on the basolateral side of distal tubular cells may be particularly significant in regulating interstitial ANG II levels (32). Given that the presence of (P)RR has also been localized to the basolateral side of distal tubular segments cells, it is likely that the secreted soluble form could contribute to the pool of ANG II in the interstitial renal compartment in Cyp1a1Ren2 rats with malignant hypertension by binding (pro)renin in the interstitium. Nonetheless, at the present, whether there is secretion of the s(P)RR into the renal interstitium or the tubular lumen or whether the s(P)RR plays a functional role in these compartments is an issue that remains uncertain.
Finally, we determined the renal expression of AGT in the Cyp1a1Ren2 transgenic rats with malignant hypertension. In these rats, neither renal cortical AGT mRNA nor protein expression was increased. However, we recently reported that Cyp1a1Ren2 transgenic rats with inducible malignant hypertension exhibit enhanced urinary AGT excretion (24). It is possible that the increased urinary levels of AGT may reflect glomerular damage and subsequent filtration of systemic AGT. In this regard, we previously showed that during the development of malignant hypertension, the kidneys of hypertensive Cyp1a1Ren2 transgenic rats exhibit renal pathological changes characterized by increased inflammation and cellular proliferation in cortical vessels and tubulointerstitium and higher glomerulosclerosis than normotensive rats (8). These changes may help to explain the increases in the urinary excretion of AGT in Cyp1a1Ren2 transgenic rats and, thus, abundant substrate availability in the lumen of the CD.
In summary, the present findings demonstrate that Cyp1a1Ren2 rats with ANG II-dependent malignant hypertension exhibit upregulation of renin and (P)RR gene expression in the CD and maintained gene expression of AGT in the renal cortex. Evidence demonstrating that renin/prorenin can be secreted by the principal cells of the distal nephron segment (14, 44) along with the observations that Cyp1a1Ren2 rats exhibit increased urinary AGT excretion (24) and upregulation of renin and (P)RR gene expression in the CDs may constitute a leading mechanism for the elevation of kidney ANG II levels that occurs in Cyp1a1Ren2 rats with inducible ANG II-dependent malignant hypertension.
GRANTS
M. C. Prieto is a BIRCWH scholar supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (K12-HD-043451) and the American Heart Association (09BGIA2280440). This grant was supported by the Institutional Award (IdeA) program of NCRR (P20RR-017659-06) and National Heart, Lung, and Blood Institute Grant HL-26371.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank V. L. Martin, C. Luffman, P. Davis, and D. M. Seth for excellent technical assistance. We also want to express thanks to Dr. T. Inagami (Vanderbilt University), Dr. G. Nguyen (College of France, France), and Dr. H. Kobri (Tulane Univ.) for generously providing, respectively, the rabbit anti-renin, rabbit anti-(P)RR, and rabbit anti-AGT antibodies; to Dr. B. Mickelson (Harlan-Teklad) for helping with the design and production of the I3C-containing diet, and to Dr. Y. Feng (Tulane Univ.) for providing a sample of lysate of neuronal cells overexpressing (P)RR used as a positive control in this study. The qRT-PCR studies were performed at the Molecular Core Facility of the Tulane Hypertension and Renal Center of Excellence at Tulane University Health Sciences Center.
REFERENCES
- 1. Advani A, Kelly DJ, Cox AJ, White KE, Advani SL, Thai K, Connelly KA, Yuen D, Trogadis J, Herzenberg AM, Kuliszewski MA, Leong-Poi H, Gilbert RE. The (Pro)renin receptor: site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension 54: 261–269, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Bader M. The second life of the (pro)renin receptor. J Renin Angiotensin Aldosterone Syst 8: 205–208, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Casarini DE, Boim MA, Stella RCR, Krieger-Azzolini MH, Krieger JE, Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol Renal Physiol 272: F405–F409, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Cousin C, Bracquart D, Contrepas A, Corvol P, Muller L, Nguyen G. Soluble form of the (pro)renin receptor generated by intracellular cleavage by furin is secreted in plasma. Hypertension 53: 1077–1082, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Danser AHJ, van Kats JP, Admiraal PJJ, Derkx FHM, Lamers JMJ, Verdouw PD, Saxena PR, Schalekamp MADH. Cardiac renin and angiotensins. Uptake from plasma versus in situ synthesis. Hypertension 24: 37–48, 1994 [DOI] [PubMed] [Google Scholar]
- 6. Gallagher PE, Chappell MC, Ferrario CM, Tallant EA. Distinct roles for ANG II and ANG-(1–7) in the regulation of angiotensin-converting enzyme 2 in rat astrocytes. Am J Physiol Cell Physiol 290: C420–C426, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Gonzalez-Villalobos RA, Seth DM, Satou R, Horton H, Ohashi N, Miyata K, Katsurada A, Tran DV, Kobori H, Navar LG. Intrarenal angiotensin II and angiotensinogen augmentation in chronic angiotensin II-infused mice. Am J Physiol Renal Physiol 295: F772–F779, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Graciano ML, Mouton CR, Patterson ME, Seth DM, Mullins JJ, Mitchell KD. Renal vascular and tubulointerstitial inflammation and proliferation in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension. Am J Physiol Renal Physiol 292: F1858–F1866, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Guan S, Fox J, Mitchell KD, Navar LG. Angiotensin and angiotensin converting enzyme tissue levels in two-kidney, one clip hypertensive rats. Hypertension 20: 763–767, 1992 [DOI] [PubMed] [Google Scholar]
- 10. Harrison-Bernard LM, Navar LG, Ho MM, Vinson GP, El-Dahr SS. Immunohistochemical localization of ANG II AT1 receptor in adult rat kidney using a monoclonal antibody. Am J Physiol Renal Physiol 273: F170–F177, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Harrison-Bernard LM, Zhuo J, Kobori H, Ohishi M, Navar LG. Intrarenal AT(1) receptor and ACE binding in ANG II-induced hypertensive rats. Am J Physiol Renal Physiol 282: F19–F25, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ichihara A, Kaneshiro Y, Suzuki F. Prorenin receptor blockers: effects on cardiovascular complications of diabetes and hypertension. Expert Opin Investig Drugs 15: 1137–1139, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension 43: 970–976, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Kang JJ, Toma I, Sipos A, Meer EJ, Vargas SL, Peti-Peterdi J. The CD is the major source of prorenin in diabetes. Hypertension 51: 1597–1604, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kantachuvesiri S, Fleming S, Peters J, Peters B, Brooker G, Lammie AG, McGrath I, Kotelevtsev Y, Mullins JJ. Controlled hypertension, a transgenic toggle switch reveals differential mechanisms underlying vascular disease. J Biol Chem 276: 36727–36733, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int 61: 579–585, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension 37: 1329–1335, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension 41: 592–597, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary angiotensinogen as an indicator of intrarenal angiotensin status in hypertension. Hypertension 41: 42–49, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kobori H, Prieto-Carrasquero MC, Ozawa Y, Navar LG. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension 43: 1126–1132, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koka V, Huang XR, Chung ACK, Wang W, Truong LD, Lan HY. Angiotensin II upregulates angiotensin I-converting enzyme (ACE), but downregulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am J Pathol 172: 1174–1183, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Komlosi P, Fuson AL, Fintha A, Peti-Peterdi J, Rosivall L, Warnock DG, Bell PD. Angiotensin I conversion to angiotensin II stimulates cortical CD sodium transport. Hypertension 42: 195–199, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Krebs C, Weber M, Steinmetz O, Meyer-Schwesinger C, Stahl R, Danser AH, Garrelds I, van GH, Nguyen G, Muller D, Wenzel U. Effect of (pro)renin receptor inhibition by a decoy peptide on renal damage in the clipped kidney of Goldblatt rats. Kidney Int 74: 823–824, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Milani CJ, Kobori H, Mullins JJ, Mitchell KD. Enhanced urinary angiotensinogen excretion in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension. Am J Med Sci 340: 389–394, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mitchell KD, Bagatell SJ, Miller CS, Mouton CR, Seth DM, Mullins JJ. Genetic clamping of renin gene expression induces hypertension and elevation of intrarenal ANG II levels of graded severity in Cyp1a1-Ren2 transgenic rats. J Renin Angiotensin Aldosterone Syst 7: 74–86, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Mitchell KD, Jacinto SM, Mullins JJ. Proximal tubular fluid, kidney, and plasma levels of angiotensin II in hypertensive ren-2 transgenic rats. Am J Physiol Renal Physiol 273: F246–F253, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Mitchell KD, Mullins JJ. Enhanced tubuloglomerular feedback in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension. Am J Physiol Renal Physiol 289: F1210–F1216, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Mullins LJ, Bailey MA, Mullins JJ. Hypertension, kidney, and transgenics: a fresh perspective. Physiol Rev 86: 709–746, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Navar LG, Harrison-Bernard LM, Imig JD, Wang CT, Cervenka L, Mitchell KD. Intrarenal angiotensin II generation and renal effects of AT1 receptor blockade. J Am Soc Nephrol 10: S266–S272, 1999 [PubMed] [Google Scholar]
- 30. Navar LG, Imig JD, Zou L, Wang CT. Intrarenal production of angiotensin II. Semin Nephrol 17: 412–422, 1997 [PubMed] [Google Scholar]
- 31. Navar LG, Kobori H, Prieto-Carrasquero M. Intrarenal angiotensin II and hypertension. Curr Hypertens Rep 5: 135–143, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nguyen G, Muller DN. The biology of the (pro)renin receptor. J Am Soc Nephrol 21: 18–23, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Nishiyama A, Seth DM, Navar LG. Angiotensin II type 1 receptor-mediated augmentation of renal interstitial fluid angiotensin II in angiotensin II-induced hypertension. J Hypertens 21: 1897–1903, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Nishiyama A, Seth DM, Navar LG. Renal interstitial fluid angiotensin I and angiotensin II concentrations during local angiotensin-converting enzyme inhibition. J Am Soc Nephrol 13: 2207–2212, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Ortiz RM, Graciano ML, Mullins JJ, Mitchell KD. Aldosterone receptor antagonism alleviates proteinuria, but not malignant hypertension, in Cyp1a1-Ren2 transgenic rats. Am J Physiol Renal Physiol 293: F1584–F1591, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Patterson ME, Mullins JJ, Mitchell KD. Renoprotective effects of neuronal NOS-derived nitric oxide and cyclooxygenase-2 metabolites in transgenic rats with inducible malignant hypertension. Am J Physiol Renal Physiol 294: F205–F211, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Peters B, Grisk O, Becher B, Wanka H, Kuttler B, Ludemann J, Lorenz G, Rettig R, Mullins JJ, Peters J. Dose-dependent titration of prorenin and blood pressure in Cyp1a1ren-2 transgenic rats: absence of prorenin-induced glomerulosclerosis. J Hypertens 26: 102–109, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Prieto-Carrasquero MC, Botros FT, Kobori H, Navar LG. CD renin: a major player in angiotensin II-dependent hypertension. J Am Soc Hypertens 3: 96–104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of CD renin in angiotensin II-dependent hypertensive rats. Hypertension 44: 223–229, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutierrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of CD renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol 289: F632–F637, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Prieto-Carrasquero MC, Botros FT, Pagan J, Kobori H, Seth DM, Casarini DE, Navar LG. CD renin is upregulated in both kidneys of 2-kidney, 1-clip Goldblatt hypertensive rats. Hypertension 51: 1590–1596, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Redublo Quinto BM, Camargo de Andrade MC, Ronchi FA, Santos EL, ves Correa SA, Shimuta SI, Pesquero JB, Mortara RA, Casarini DE. Expression of angiotensin I-converting enzymes and bradykinin B2 receptors in mouse inner medullary CD cells. Int Immunopharmacol 8: 254–260, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension 34: 1265–1274, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Schefe JH, Menk M, Reinemund J, Effertz K, Hobbs RM, Pandolfi PP, Ruiz P, Unger T, Funke-Kaiser H. A novel signal transduction cascade involving direct physical interaction of the renin/prorenin receptor with the transcription factor promyelocytic zinc finger protein. Circ Res 99: 1355–1366, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Siragy HM, Carey RM. Protective role of the angiotensin AT2 receptor in a renal wrap hypertension model. Hypertension 33: 1237–1242, 1999 [DOI] [PubMed] [Google Scholar]
- 47. Von Thun AM, Vari RC, El-Dahr SS, Navar LG. Augmentation of intrarenal angiotensin II levels by chronic angiotensin II infusion. Am J Physiol Renal Fluid Electrolyte Physiol 266: F120–F128, 1994 [DOI] [PubMed] [Google Scholar]
- 48. Williams DE, Prieto MC, Mullins JJ, Navar LG, Mitchell KD. AT1 receptor blockade prevents the increase in blood pressure and the augmentation of intrarenal ANG II levels in hypertensive Cyp1a1-Ren2 transgenic rats fed with a high-salt diet. Am J Med Sci 339: 356–361, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. ANG II accumulation in rat renal endosomes during ANG II-induced hypertension: role of AT(1) receptor. Hypertension 39: 116–121, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Zou L, Hymel A, Imig JD, Navar LG. Renal accumulation of circulating angiotensin II in angiotensin II-infused rats. Hypertension 27: 658–662, 1996 [DOI] [PubMed] [Google Scholar]
- 51. Zou L, Imig JD, Hymel A, Navar LG. Renal uptake of circulating angiotensin II in Val5-angiotensin II infused rats is mediated by AT1 receptor. Am J Hypertens 11: 570–578, 1998 [DOI] [PubMed] [Google Scholar]
- 52. Zou L, Imig JD, Von Thun AM, Hymel A, Ono H, Navar LG. Receptor-mediated intrarenal ANG II augmentation in ANG II-infused rats. Hypertension 28: 669–677, 1996 [DOI] [PubMed] [Google Scholar]