Abstract
Inhibition of bladder activity by tibial nerve stimulation was investigated in α-chloralose-anesthetized cats with an intact spinal cord. Short-duration (3–5 min) tibial nerve stimulation at both low (5 Hz) and high (30 Hz) frequencies applied repeatedly during rhythmic isovolumetric bladder contractions was effective in inhibiting reflex bladder activity. Both frequencies of stimulation were also effective in inducing inhibition that persisted after the termination of the stimulation. The poststimulation inhibitory effect induced by the short-duration stimulation significantly increased bladder capacity to 181.6 ± 24.36% of the control capacity measured before applying the stimulation. Thirty-minute continuous stimulation induced prolonged poststimulation inhibition of bladder activity, which lasted for more than 2 h and significantly increased bladder capacity to 161.1 ± 2.9% of the control capacity. During the poststimulation periods, 5-Hz stimulation applied during the cystometrogram elicited a further increase (∼30% on average) in bladder capacity, but 30-Hz stimulation was ineffective. These results in cats support the clinical observation that tibial nerve neuromodulation induces a long-lasting poststimulation inhibitory effect that is useful in treating overactive bladder symptoms.
Keywords: electrical stimulation, neuromodulation, detrusor overactivity, urology
overactive bladder symptoms including detrusor overactivity, urinary frequency, urgency, and incontinence are very difficult to manage with medications (3). However, percutaneous tibial nerve stimulation is effective in treating patients with overactive bladder symptoms (1, 2, 4, 9, 10, 12–14, 16, 18–20, 23, 28–31). The standard treatment includes 20-Hz stimulation for 30 min once per week for 12 consecutive wk through a needle electrode inserted percutaneously cephalad to the medial malleolus (Urgent PC stimulator, Uroplasty) (28). If the initial 12-wk treatment is effective, a maintenance treatment (once every 2–3 wk) is usually required (1, 18). These clinical reports (1, 2, 4, 9, 10, 12–14, 16, 18–20, 23, 28–31) indicate that tibial nerve stimulation could have a prolonged poststimulation inhibitory effect on bladder activity lasting for days or weeks.
Only a few animal studies (15, 25) investigated the inhibitory effect of tibial/peroneal nerve stimulation on bladder activity. These studies (15, 25) showed that stimulation frequencies of 0.5–10 Hz acutely suppressed reflex bladder activity. However, the prolonged poststimulation inhibitory effect detected following 20-Hz stimulation in clinical studies (1, 2, 4, 9, 10, 12–14, 16, 18–20, 23, 28–31) has not been confirmed in animal studies. There is also a report indicating that tibial nerve stimulation has no acute inhibitory effect on bladder activity in humans (7). Due to the lack of basic science evidence supporting the clinical treatment of bladder overactivity by tibial nerve stimulation, the efficacy of this treatment has been questioned by many clinicians (7, 11).
This animal study on cats focused on the following questions: 1) Is tibial nerve stimulation effective in inhibiting reflex bladder activity? 2) Which stimulation frequencies are effective? 3) Does tibial nerve stimulation have a long-lasting poststimulation effect? Another purpose of this study was to establish an animal model of tibial nerve inhibition of bladder activity that could be used to analyze the neurophysiology and neuropharmacology of this type of neuromodulation. The results of our study indicate that tibial nerve stimulation elicits immediate as well as prolonged bladder inhibition that persists following the stimulation, and thus provide support for the use of tibial nerve stimulation in the treatment of overactive bladder symptoms.
MATERIALS AND METHODS
All protocols involving the use of animals in this study were approved by the Animal Care and Use Committee at the University of Pittsburgh.
Experimental setup.
The experiments were conducted on 18 female cats (2.2 to 3.3 kg) under α-chloralose anesthesia (65 mg/kg iv supplemented as necessary) after induction with isoflurane (2–3% in O2). Systemic blood pressure was monitored throughout the experiment via a catheter inserted in the right carotid artery. A tracheotomy was performed and a tube was inserted to keep the airway patent. A catheter for intravenous infusion was introduced into the right ulnar vein. The ureters were cut and drained externally. A double lumen catheter was inserted through the urethra into the bladder and secured by a ligature around the urethra. One lumen of the catheter was connected to a pump to infuse the bladder with saline at a rate of 0.5–2 ml/min and the other lumen was connected to a pressure transducer to measure the pressure change in the bladder. The tibial nerve was exposed on the medial side of left hindlimb above the ankle. After a tripolar cuff electrode (NC223pt, MicroProbe, Gaithersburg, MD) was implanted on the tibial nerve for stimulation, the skin was closed by sutures.
Stimulation protocol.
Uniphasic rectangular pulses (0.2-ms pulse width) at low (5 Hz) or high (30 Hz) frequency were delivered to the tibial nerve via the cuff electrode. The intensity threshold for inducing toe movement was determined at 5 Hz by gradually increasing the stimulation intensity. Then, multiples of the threshold intensity were used for tibial nerve stimulation.
In the first group of experiments, the bladder was infused with saline to a volume ∼100–110% of the threshold volume (i.e., bladder capacity) for inducing large amplitude (>30 cmH2O), rhythmic reflex bladder contractions (see Fig. 1), and then maintained under isovolumetric conditions. At this bladder volume, the tibial nerve was stimulated at different frequencies (5 or 30 Hz) to determine the effective frequencies for inhibiting bladder activity. The stimulation duration (3–5 min) was always longer than the period of at least two bladder contractions to clearly demonstrate an inhibitory effect. To determine whether the stimulation increased bladder capacity, we performed several cystometrograms (CMGs) before and after the recording of rhythmic isovolumetric bladder contractions during which short-duration tibial nerve stimulation was applied multiple times. The CMGs consisted of a slow infusion of saline (0.5–2 ml/min) starting with an empty bladder.
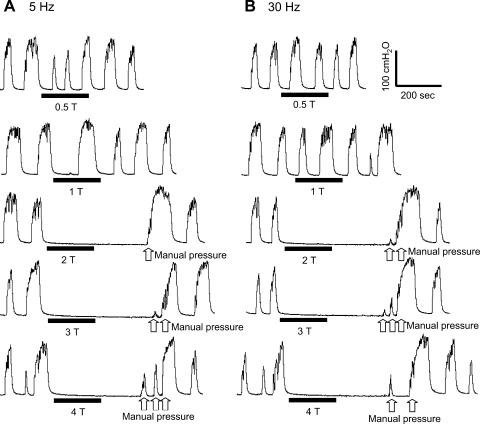
Fig. 1.
Tibial nerve stimulation at both 5 Hz (A) and 30 Hz (B) and at different stimulus intensities inhibits rhythmic isovolumetric bladder contractions. The black bars under pressure traces indicate the stimulation duration. T, stimulation intensity threshold for inducing toe movement. Stimulation pulse width = 0.2 ms. T = 3.5 V. The white arrows indicate bladder pressure increases induced by transient manual pressure on the lower abdomen.
In the second group of experiments, the poststimulation effect of prolonged (30 min) tibial nerve stimulation was examined by performing repeated CMGs. Initially, two or three CMGs were preformed without stimulation to obtain the control bladder capacity and evaluate reproducibility. Then, two groups of experiments were conducted: 1) control group without stimulation and 2) treatment group with tibial nerve stimulation. In the treatment group, the bladder volume was maintained at a volume slightly above the bladder capacity to induce rhythmic isovolumetric bladder contractions. Then, tibial nerve stimulation (frequency: 5 or 30 Hz; intensity: 2–4 times threshold for inducing toe movement) was applied for 30 min to inhibit the isovolumetric contractions. After the 30-min stimulation, five CMGs were performed within a 1.5- to 2-h period to examine the change of bladder capacity. At the end of the fifth CMG, the bladder volume was again maintained at a volume slightly above the bladder capacity to induce rhythmic isovolumetric contractions, during which a second 30-min tibial nerve stimulation was applied to inhibit the contractions. The poststimulation effect on bladder capacity induced by the second 30-min stimulation was evaluated by another five CMGs repeated within 1.5–2 h after the termination of the second stimulation treatment. The stimulation frequency (5 or 30 Hz) was randomized between the first and second 30-min treatment. In the control group, the procedures similar to those used in the treatment group were performed, but the tibial nerve stimulation was not applied during either the first or the second 30-min treatment period. Instead, the rhythmic isovolumetric bladder contractions were allowed to continue during each 30-min period. The bladder was emptied after each CMG and a 5- to 10-min rest period was inserted between CMGs to allow the distended detrusor to recover. The 30-min stimulation duration was chosen to mimic the clinical application of 30-min tibial nerve stimulation (1, 2, 4, 9, 10, 12–14, 16, 18–20, 23, 28–31). At the end of the second group of experiments after examining the poststimulation effect of tibial nerve stimulation, the nerve stimulation was applied again during the CMGs to determine whether the bladder capacity could be further increased.
Animals used in the first group of experiments, in which repeated short periods of tibial nerve stimulation were applied during rhythmic isovolumetric bladder contractions, also received 30-min stimulation treatments later in the experiments to compare the effects with those elicited in the second group of experiments where short periods of stimulation were not applied before the 30-min stimulation.
Data analysis.
For the analysis of rhythmic isovolumetric bladder contractions, the area under bladder pressure curve was measured during tibial nerve stimulation and was normalized to the measurement during the same time period before the stimulation (see Fig. 3A, inset). For the repeated CMG recordings, the bladder capacities were measured and normalized to the measurement of the first control CMG in each experimental group. Repeated measurements in the same animal during the same experiment were averaged. The normalized data from different animals are presented as means ± SE. ANOVA followed by Bonferroni posttests and Student's t-test was used to detect statistical significance (P < 0.05).
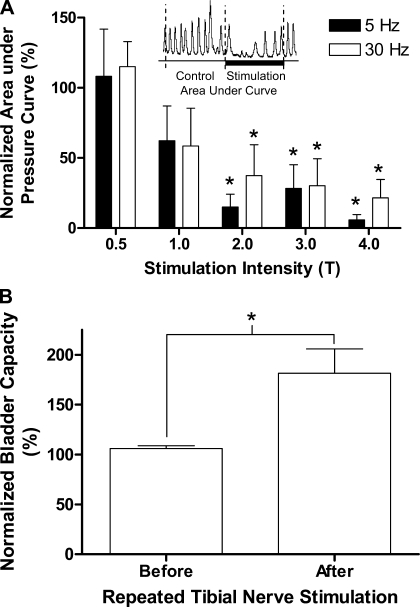
Fig. 3.
A: short-duration tibial nerve stimulation at intensities at or above 2 times T intensity for inducing toe movement significantly inhibited rhythmic isovolumetric bladder contractions. B: bladder capacity is also significantly increased after repeated tibial nerve stimulation applied during the rhythmic isovolumetric bladder contractions. Stimulation: pulse width = 0.2 ms; intensity 0.5–4 T (1.5–21 V). *Significant difference from 100% in A. *Significant difference between groups in B; n = 6.
RESULTS
Tibial nerve stimulation inhibits rhythmic isovolumetric reflex bladder contractions.
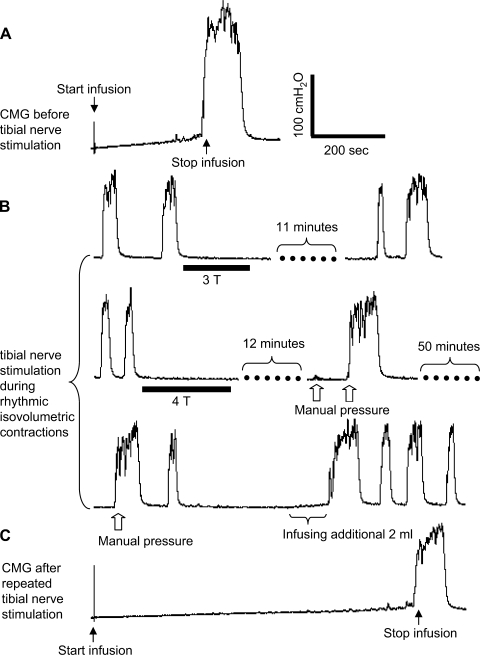
When the bladder was filled with saline to a volume exceeding the threshold for eliciting a large-amplitude micturition reflex contraction and then maintained under constant volume conditions, large-amplitude rhythmic contractions (40–100 cmH2O) occurred at regular intervals (1–2 per min) over the course of many hours (Fig. 1). Short periods (3- to 5-min duration) of tibial nerve stimulation at both 5 and 30 Hz significantly (P < 0.05) inhibited the rhythmic bladder contractions when the stimulation intensity was stronger than two times the intensity threshold (T) for inducing toe movement (Figs. 1, 2, 3). There was no significant difference between the magnitude of the inhibition elicited by 5- and 30-Hz stimulation (Figs. 1 and 3A; n = 6). It is noteworthy that the inhibition of the rhythmic bladder contractions was often complete and commonly lasted longer than the duration of stimulation, indicating a poststimulation effect (Fig. 1; n = 5). Partial inhibition with reduced contraction amplitude during the stimulation was observed in one cat. Manually pressing on the lower abdomen to generate a transient rise in bladder pressure was effective in reinitiating rhythmic bladder contractions during the period of complete inhibition induced by tibial nerve stimulation (Figs. 1 and 2B). Subsequent application of additional short periods of tibial nerve stimulation at the same or at higher intensities elicited a similar inhibitory effect (Fig. 1). At a stimulation intensity below 3 T, the rhythmic bladder contractions could also reappear spontaneously after a relatively long interval (longer than 10 min as shown in Fig. 2B, first trace). However, when the stimulation intensity was 4 T (n = 5), the rhythmic contractions did not recover within a 10- to 15-min period. In one experiment, repeated pressure on the abdominal area during a 1-h period failed to reinitiate the isovolumetric contractions (Fig. 2B, second and third traces). However, infusion of an additional 2 ml of saline into the bladder was effective in eliciting rhythmic bladder contractions (Fig. 2B, third trace).
Fig. 2.
Bladder capacity was significantly increased by repeated tibial nerve stimulation applied during rhythmic isovolumetric contractions. A: cystometrogram (CMG) performed before repeated short-duration tibial nerve stimulation was applied during rhythmic isovolumetric contractions. B: selected bladder pressure traces during rhythmic isovolumetric contractions showing 3 sequential recordings spread over approximately a 90-min period showing inhibition of rhythmic bladder contractions by short-duration tibial nerve stimulation and spontaneous recovery from inhibition in the top recording. Bottom 2 recordings show failure of spontaneous recovery after more intense stimulation even with manual pressure but recovery after infusing additional fluid into the bladder. C: CMG after repeated short-duration tibial nerve stimulation shows increased bladder capacity. All records are from the same cat and the record in C was obtained 10 min after the last record in B. The black bars under pressure traces indicate the stimulation duration. Stimulation: frequency = 5 Hz; pulse width = 0.2 ms; T = 3 V. The white arrows indicate a bladder pressure increase induced by transient manual pressure on the lower abdomen. Infusion rate = 1 ml/min.
CMGs performed before (Fig. 2A) and after (Fig. 2C) the inhibition of rhythmic bladder contractions induced by repeated, short-duration, tibial nerve stimulation at intensities from 2–4 T applied over periods ranging from 1 to 2 h revealed that the stimulation elicited a significant increase (P < 0.05) in bladder capacity to 181.6 ± 24.36% of control capacity (Fig. 3B) further indicating a poststimulation inhibitory effect.
Prolonged tibial nerve stimulation elicits a persistent poststimulation increase in bladder capacity.
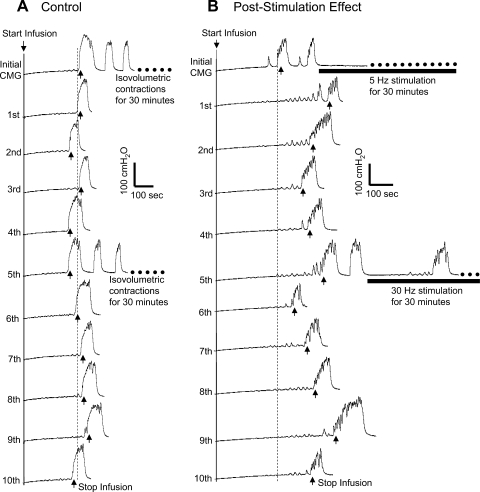
As shown in Fig. 4A, the bladder capacity in control experiments remained relatively constant during repeated CMGs performed over 3–4 h which included two 30-min periods of bladder distension to induce rhythmic bladder contractions. However, when 5-Hz tibial nerve stimulation was applied during the first 30-min period at intensities that inhibited the rhythmic bladder contractions (Fig. 4B), the bladder capacity was significantly increased following the stimulation and the increased bladder capacity was maintained for up to 2 h after the stimulation (Fig. 4B). A second 30-min tibial nerve stimulation did not further increase the bladder capacity (Fig. 4B). Results similar to Fig. 4B were also observed when 30-Hz stimulation was applied in the first 30-min treatment (n = 2). In a series of nine cats, complete inhibition occurred in eight cats during the first 30-min stimulation, while it occurred in only four cats during the second 30-min stimulation. This was probably due to the larger bladder volume during the second 30-min stimulation than during the first 30-min stimulation (Fig. 4B).
Fig. 4.
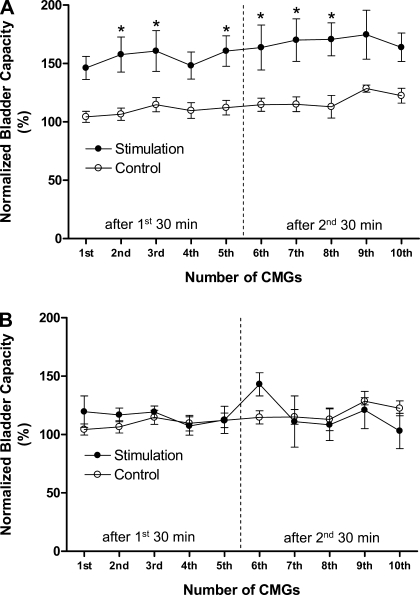
Poststimulation inhibitory effect of tibial nerve stimulation is evident during repeated CMG testing. A: bladder capacity was not significantly changed during repeated CMGs in the absence of nerve stimulation. Note: after the initial and 5th CMG, the bladder was maintained for 30 min in a distended condition and exhibited rhythmic contractions. B: bladder capacity was significantly increased after a 30-min tibial nerve stimulation following the initial CMG, but was not further increased after the second 30-min tibial nerve stimulation. Five repeated CMGs (1st-5th and 6th-10th) were performed within 1.5–2 h after each 30-min period of stimulation. The vertical dashed line indicates the control bladder capacity. Infusion rate: 1 ml/min. The horizontal black bar indicates the 30-min tibial nerve stimulation. Stimulation: frequency 5 or 30 Hz; pulse width = 0.2 ms; intensity 9 V (3 T).
The poststimulation inhibitory effect of 30-min tibial nerve stimulation is summarized in Fig. 5A. In the control unstimulated animals, bladder capacity did not significantly (P > 0.05) change during 10 repeated CMGs performed over a 3- to 4-h period. On the other hand, bladder capacity was significantly (P < 0.05) increased after the first 30-min tibial nerve stimulation, but did not increase further after the second 30-min stimulation. The increase of bladder capacity was not observed in experiments where the 30-min stimulation treatment was preceded by repeated short-duration tibial nerve stimulations (Fig. 5B). However, in these experiments, bladder capacity was already significantly increased by the repeated, short-duration tibial nerve stimulation applied during rhythmic bladder contractions (Figs. 2, A and C, and 3B) and this presumably occluded a further increase of bladder capacity in response to the 30-min stimulation.
Fig. 5.
Prolonged poststimulation inhibition induced by 30-min tibial nerve stimulation. A: bladder capacity was significantly increased after the first 30-min tibial nerve stimulation, but was not further increased after the second 30-min stimulation. The poststimulation inhibitory effect lasted more than 1.5–2 h. B: increase of bladder capacity did not occur if the 30-min stimulation was preceded by repeated short-duration tibial nerve stimulation during rhythmic isovolumetric contractions. However, note that the repeated short-duration stimulation also increases bladder capacity as shown in Fig. 3B. Stimulation: pulse width = 0.2 ms; intensity 7.5–14 V (2–4 T). *Statistically significant difference between the control and treatment groups. Vertical dashed line indicates the time of the second 30-min stimulation. Control: n = 12. Stimulation: n = 9 in A, n = 5 in B.
Tibial nerve stimulation during CMGs further increases bladder capacity.
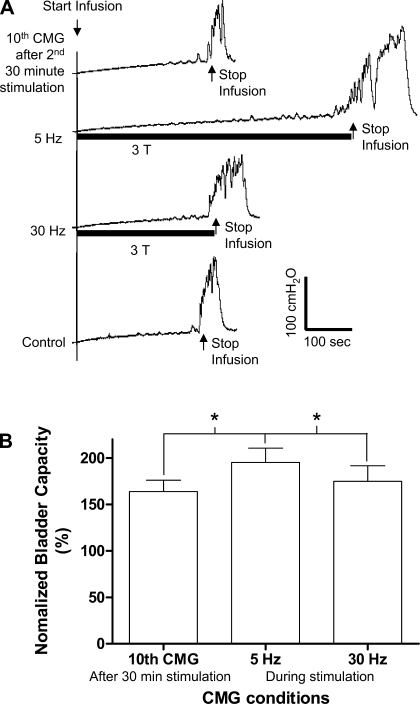
After the second 30-min stimulation treatment in the second group of experiments, tibial nerve stimulation at 5 Hz but not at 30 Hz applied during the CMG increased bladder capacity above the level (average 30% increase) that occurred after the two periods of 30-min stimulation (Fig. 6).
Fig. 6.
A: after the second 30-min tibial nerve stimulation, 5-Hz tibial nerve stimulation applied during the CMG further increased bladder capacity during the 11th CMG, but subsequent 30-Hz stimulation during the 12th CMG did not increase the bladder capacity. Infusion rate: 1 ml/min. Stimulation: pulse width = 0.2 ms; T = 3 V. B: summarized results (n = 9). Stimulation: pulse width = 0.2 ms; intensity 2–4 T. *Statistically significant difference.
DISCUSSION
This study showed in cats that short-duration (3–5 min) tibial nerve stimulation at low (5 Hz) and high (30 Hz) frequencies applied repeatedly during rhythmic isovolumetric bladder contractions is effective in inhibiting reflex bladder activity (Figs. 1–3). The stimulation also induced a poststimulation inhibitory effect (Figs. 1–2) and a persistent increase in bladder capacity (Figs. 2, A and C, and 3B). A longer period of tibial nerve stimulation (30-min duration) elicited prolonged poststimulation inhibition lasting at least 1.5–2 h (Figs. 4–5). During the poststimulation inhibition, tibial nerve stimulation at 5 Hz could further increase bladder capacity when it was applied during the CMG, but 30-Hz stimulation was ineffective (Fig. 6). These results provide experimental evidence supporting the clinical application of tibial nerve stimulation for treatment of overactive bladder symptoms.
Both 5- and 30-Hz tibial nerve stimulation inhibited bladder activity, which is very different from the effect of pudendal nerve stimulation (5, 27) that induces an inhibitory effect at a low (5 Hz) frequency but an excitatory effect at a high (20–30 Hz) frequency. This difference indicates that the underlying mechanisms for pudendal nerve and tibial nerve neuromodulation are significantly different. Furthermore, long-lasting poststimulation inhibition was not detected following pudendal nerve neuromodulation in either animal (5, 27) or clinical (21, 22) studies, providing evidence for different mechanisms underlying these two types of neuromodulations.
Repeated short-duration stimulation that produced a transient complete inhibition of rhythmic isovolumetric bladder contractions (Figs. 1–2) also induced a persistent poststimulation inhibitory effect evident as an increase in bladder capacity that was comparable in magnitude to the effect of long-duration (30 min) continuous stimulation (Figs. 3B and 5A). These persistent increases in bladder capacity induced by either repeated short-duration or long-duration stimulation were not enhanced by a subsequent 30-min stimulation (Fig. 5, A and B), suggesting that either type of stimulation produces the maximal inhibition. Thus, other stimulation patterns in addition to continuous 30-min tibial nerve stimulation that is currently used clinically for the treatment of overactive bladder symptoms should be evaluated to determine the optimal parameters for tibial nerve neuromodulation. It is also worth noting that statistical significance was not detected in every CMG during the poststimulation period (see Fig. 5A), which is probably due to the small sample size (n = 9). This study only tested the poststimulation effect within a 1.5- to 2-h period. A study with a longer testing period is warranted to examine the duration of the poststimulation effect.
Although it is clear that both the transient and persistent inhibitory effects of tibial nerve stimulation on bladder function are dependent on central neural pathways, the synaptic mechanisms underlying the inhibition are uncertain. Since the tibial afferent nerves enter the lumbosacral spinal cord at the similar levels as the pelvic afferent nerves (24); and it is known that somatic and bladder afferent inputs converge on certain populations of spinal interneurons, it is possible that interactions between the tibial and bladder sensory pathways in the lumbosacral spinal cord could contribute to tibial nerve modulation of bladder capacity. However, a previous study in cats (17) showed that the acute inhibitory effect on bladder activity elicited by electrical stimulation of the nerves from hindlimb muscles was lost after chronic spinal cord transection at the thoracic level, indicating a possible role of the supraspinal mechanisms in somato-vesical inhibition. Furthermore, the neural switching circuit for controlling bladder capacity is located in the pontine micturition center (PMC) (8). Thus, it is reasonable to assume that the increased bladder capacity induced by tibial nerve stimulation is elicited by direct modulation of the PMC gating circuit or suppression of afferent input to that circuit. It is well-known that extensive viscero-somatic convergence occurs in the thalamus (6) raising the possibility that this region as well as other sites in the forebrain that regulate micturition have a role in the long-lasting modulation of bladder capacity induced by stimulation of tibial nerve afferents.
Our recent study in cats (26) showed that electrical stimulation applied to the skin surface of the foot produced an immediate and rapidly reversible inhibition of reflex bladder activity. Similar to the results of current experiments, foot stimulation at both low (5 Hz) and high (20 Hz) frequencies was effective. However, a long-lasting poststimulation inhibitory effect was not reported after foot stimulation (26). The inhibitory effect was only investigated when the foot stimulation was applied during CMGs similar to the experiment shown in Fig. 6. Therefore, the poststimulation inhibitory effect induced by the initial foot stimulation was not investigated. Since the foot is innervated by both tibial and peroneal nerves (24), electrical stimulation of the foot might induce very different responses than direct stimulation of the tibial nerve. More studies on foot stimulation to determine its poststimulation effect are warranted.
The 30-min tibial nerve stimulation was applied when the bladder was full and contracting rhythmically (Fig. 4B) to observe its immediate inhibitory effect. It will be important in future experiments to determine whether the condition of the bladder is a factor in producing the long-lasting inhibition. For example, tibial nerve stimulation could be applied when the bladder was empty so that any effect caused by the 30-min bladder distention and tonic bladder afferent input to the spinal cord during the stimulation could be eliminated. This question is partially addressed by the control experiments (Figs. 4A and 5), which showed that 30-min bladder distention with rhythmic isovolumetric contractions did not change the bladder capacity in the following 3- to 4-h period. Furthermore, during the second 30-min stimulation when the bladder volume was larger than the volume during the first 30-min stimulation, the bladder capacity was not further increased in the following 1.5- to 2-h period after the second 30-min stimulation (Figs. 4B and 5). Therefore, the 30-min bladder distension is not likely to be a major factor influencing the results of this study. However, it still needs to be investigated by conducting similar stimulation experiments with the bladder empty.
Stimulation intensity of two to four times threshold for inducing toe movement was required to achieve bladder inhibition (Figs. 1 and 3A). At this intensity, the stimulation probably only activated the large afferent nerve fibers rather than the small Aδ- and C-fiber afferents that can induce painful sensations. These intensities are similar to those used clinically. On the other hand, there are major differences between the current study in cats and the tests of tibial nerve stimulation in humans including the use of anesthetized animals and the short duration of the experiments. These preclude direct comparisons between animal and human data. In anesthetized cat model, we investigated the effect of tibial nerve stimulation on reflex bladder activity rather than the effects on urgency sensations or frequency of voiding that are commonly studied in humans. However, human overactive bladder symptoms also include detrusor overactivity and involuntary loss of urine (i.e., urge incontinence), which are reflexive in nature. Thus, the animal model established in this study could be used to investigate the mechanisms underlying tibial nerve inhibition of reflex bladder activity. Also, uninhibited bladder contractions in human occur at varying bladder volumes, often well below bladder capacity. This study only investigated tibial inhibition of reflex bladder activity induced at a volume above bladder capacity. Different neural pathways may be involved at different bladder volumes. Nevertheless, our experimental results provide the first basic science evidence consistent with clinical reports (1, 2, 4, 9, 10, 12–14, 16, 18–20, 23, 28–31) that tibial nerve stimulation can elicit an inhibitory effect on reflex bladder function that persists for hours after the termination of stimulation. Although many questions about tibial nerve neuromodulation still need to be answered, this animal study and a recent multicenter, double-blind, randomized, controlled clinical trial (20) indicate that tibial nerve neuromodulation could be a very promising clinical treatment for overactive bladder symptoms.
GRANTS
This study is supported by the National Institutes of Health under Grants DK-068566, DK-090006, and DK-077783 and by the Christopher and Dana Reeve Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Agro EF, Campagna A, Sciobica F, Petta F, Germani S, Zuccala A, Miano R. Posterior tibial nerve stimulation: is the once-a-week protocol the best option? Minerva Urol Nefrol 57: 119–123, 2005 [PubMed] [Google Scholar]
- 2. Amarenco G, Ismael SS, Even-Schneider A, Raibaut P, Demaille-Wlodyka S, Parratte B, Kerdraon J. Urodynamic effect of acute transcutaneous posterior tibial nerve stimulation in overactive bladder. J Urol 169: 2210–2215, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev 56: 581–631, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Andrews BJ, Reynard JM. Transcutaneous posterior tibial nerve stimulation for treatment of detrusor hyperreflexia in spinal cord injury. J Urol 170: 926, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Frequency-dependent selection of reflexes by pudendal afferents in the cat. J Physiol 577: 115–126, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bruggemann J, Shi T, Apkarian AV. Squirrel monkey lateral thalamus. II. Viscerosomatic convergent representation of urinary bladder, colon, and esophagus. J Neurosci 14: 6796–6814, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fjorback MV, van Rey FS, van der Pal F, Rijkhoff NJM, Petersen T, Heesakkers JP. Acute urodynamic effects of posterior tibial nerve stimulation on neurogenic detrusor overactivity in patients with MS. Eur Urol 51: 464–472, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Fowler CJ, Griffths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci 9: 453–465, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gennaro MD, Capitanucci ML, Mastracci P, Silveri M, Gatti C, Mosiello G. Percutaneous tibial nerve neuromodulation is well tolerated in children and effective for treating refractory vesical dysfunction. J Urol 171: 1911–1913, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Govier FE, Litwiller S, Nitti V, Kreder KJ, Rosenblatt P. Percutaneous afferent neuromodulation for the refractory overactive bladder: results of a multicenter study. J Urol 165: 1193–1198, 2001 [PubMed] [Google Scholar]
- 11. Hasan ST, Robson WA, Pridie AK, Neal DE. Transcutaneous electrical nerve stimulation, and temporary S3 neuromodulation in idiopathic detrusor instability. J Urol 155: 2005–2011, 1996 [PubMed] [Google Scholar]
- 12. Kabay SC, Yucel M, Kabay S. Acute effect of posterior tibial nerve stimulation on neurogenic detrusor overactivity in patients with multiple sclerosis: urodynamic study. Urology 71: 641–645, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Klingler HC, Pycha A, Schmidbauer J, Marberger M. Use of peripheral neuromodulation of the S3 region for treatment of detrusor overactivity: a urodynamic-based study. Urology 56: 766–771, 2000 [DOI] [PubMed] [Google Scholar]
- 14. MacDiarmid SA, Peters KM, Shobeiri SA, Wooldridge LS, Rovner ES, Leong FC, Siegel SW, Tate SB, Feagins BA. Long-term durability of percutaneous tibial nerve stimulation for the treatment of overactive bladder. J Urol 183: 234–240, 2010 [DOI] [PubMed] [Google Scholar]
- 15. McGuire E, Morrissey S, Zhang S, Horwinski E. Control of reflex detrusor activity in normal and spinal injured nonhuman primates. J Urol 129: 197–199, 1983 [DOI] [PubMed] [Google Scholar]
- 16. McGuire E, Zhang SC, Horwinski ER, Lytton B. Treatment of motor and sensory detrusor instability by electrical stimulation. J Urol 129: 78–79, 1983 [DOI] [PubMed] [Google Scholar]
- 17. McPherson A. The effects of somatic stimuli on the bladder in the cat. J Physiol 185: 185–196, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pal FVD, van Balken MR, Heesakkers JPFA, Debruyne FMJ, Bemelmans BLH. Percutaneous tibial nerve stimulation in the treatment of refractory overactive bladder syndrome: is maintenance treatment necessary? BJU Int 97: 547–550, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Pal FVD, van Balken MR, Heesakkers JPFA, Debruyne FMJ, Bemelmans BLH. Implant-drive tibial nerve stimulation in the treatment of refractory overactive bladder syndrome: 12-month follow-up. Neuromodulation 9: 163–171, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Peters KM, Carrico DJ, Perez-Marrero RA, Khan AU, Wooldridge LS, Davis GL, MacDiarmid SA. Randomized trial of percutaneous tibial nerve stimulation vs. sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trial. J Urol 183: 1438–1443, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Peters KM, Feber KM, Bennett RC. Sacral vs. pudendal nerve stimulation for voiding dysfunction: a prospective, single-blinded, randomized, crossover trial. Neurourol Urodyn 24: 643–647, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Peters KM, Feber KM, Bennett RC. A prospective, single-blind, randomized crossover trial of sacral vs. pudendal nerve stimulation for interstitial cystitis. BJU Int 100: 835–839, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Peters KM, MacDiarmid SA, Wooldridge LS, Leong FC, Shobeiri SA, Rovner ES, Siegel SW, Tate SB, Jarnagin BK, Rosenblatt PL, Feagins BA. Randomized trial of percutaneous tibial nerve stimulation vs. extended-release tolterodine: results from the overactive bladder innovative therapy trial. J Urol 182: 1055–1060, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Reighard J, Jennings HS. Anatomy of the Cat. New York, NY: Holt, Rinehart and Winston, 1935 [Google Scholar]
- 25. Sato A, Sato Y, Schmidt RF. Reflex bladder activity induced by electrical stimulation of hind limb somatic afferents in the cat. J Auton Nerv Syst 1: 229–241, 1980 [DOI] [PubMed] [Google Scholar]
- 26. Tai C, Shen B, Chen M, Wang J, Liu H, Roppolo JR, de Groat WC. Suppression of bladder overactivity by activation of somatic afferent nerves in the foot. BJU Int In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tai C, Smerin SE, de Groat WC, Roppolo JR. Pudendal-to-bladder reflex in chronic spinal cord injured cat. Exp Neurol 197: 225–234, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Van Balken MR. Percutaneous tibial nerve stimulation: the Urgent PC device. Expert Rev Med Dev 4: 693–698, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Van Balken MR, Vandoninck V, Gisolf KWH, Vergunst H, Kiemeney LALM, Debruyne FMJ, Bemelmans BLH. Posterior tibial nerve stimulation as neuromodulative treatment of lower urinary tract dysfunction. J Urol 166: 914–918, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Vandoninck V, van Balken MR, Agro EF, Petta F, Caltagirone C, Heesakkers JPFA, Kiemeney LALM, Debruyne FMJ, Bemelmans BLH. Posterior tibial nerve stimulation in the treatment of urge incontinence. Neurourol Urodynam 22: 17–23, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Vandoninck V, van Balken MR, Agro EF, Petta F, Micali F, Heesakkers JPFA, Debruyne FMJ, Kiemeney LALM, Bemelmans BLH. Percutaneous tibial nerve stimulation in the treatment of overactive bladder: urodynamic data. Neurourol Urodynam 22: 227–232, 2003 [DOI] [PubMed] [Google Scholar]