Abstract
Coronary artery disease is a leading cause of death. Hypertension (HT) increases the incidence of cardiac events, but its effect on cardiac adaptation to coexisting coronary artery stenosis (CAS) is unclear. We hypothesized that concurrent HT modulates microvascular function in chronic CAS and aggravates microvascular remodeling and myocardial injury. Four groups of pigs (n = 6 each) were studied: normal, CAS, HT, and CAS+HT. CAS and HT were induced by placing local irritant coils in the left circumflex coronary artery and renal artery, respectively. Six weeks later multidetector computerized tomography (CT) was used to assess systolic and diastolic function, microvascular permeability, myocardial perfusion, and responses to adenosine in the “area at risk.” Microvascular architecture, inflammation, and fibrosis were then explored in cardiac tissue. Basal myocardial perfusion was similarly decreased in CAS and CAS+HT, but its response to adenosine was significantly more attenuated in CAS. Microvascular permeability in CAS+HT was greater than in CAS and was accompanied by amplified myocardial inflammation, fibrosis, and microvascular remodeling, as well as cardiac systolic and diastolic dysfunction. On the other hand, compared with normal, micro-CT-derived microvascular (20–200 μm) transmural density decreased in CAS but not in HT or CAS+HT. We conclude that the coexistence of early renovascular HT exacerbated myocardial fibrosis and vascular remodeling distal to CAS. These changes were not mediated by loss of myocardial microvessels, which were relatively preserved, but possibly by exacerbated myocardial inflammation and fibrosis. HT modulates cardiac adaptive responses to CAS and bears cardiac functional consequences.
Keywords: coronary artery stenosis, microvascular dysfunction, remodeling
cardiovascular disease remains the main cause of death in the United States, despite substantial advances in diagnostic and therapeutic tools. One in three American adults have cardiovascular disease, and more than half of these subjects are younger than 60 yr (30). Although the clinical focus has been on the large coronary arterial system, coronary artery stenosis (CAS) only becomes hemodynamically significant at rest when the stenosis is >70% of the arterial lumen (14). In fact, the myocardial microvasculature is responsible for >70% of the coronary resistance under normal conditions and plays a pivotal role in regulating myocardial vascular resistance and flow (4).
Hypertension (HT) is a major risk factor for coronary heart disease and impairs microvascular function (24) and myocardial perfusion, especially in patients with left ventricular (LV) hypertrophy (15). It is increasingly recognized that the initial site of cardiac injury in early HT is often at the level of the myocardial microcirculation. We have previously shown (29, 41) that early experimental HT is characterized by microvascular dysfunction and impaired myocardial perfusion responses, which in turn may be accompanied by episodes of ischemia and consequently stimulate vascular growth. Indeed, myocardial microvascular proliferation and dysfunction observed in HT is associated with an increase in the expression of vascular endothelial growth factor (VEGF) (42). We have recently demonstrated (42) that these new microvessels can function as coronary collaterals and potentially play a role in protecting the myocardium from acute ischemic insults at an early stage of HT. However, newly formed microvessels are often thin-walled and abnormal, provide entry points for inflammatory cells (32), and might thus eventually lead to tissue damage. Therefore, whether such microvessels confer protection when HT is superimposed on chronic CAS remains to be elucidated.
Microvascular permeability (MP) (2, 10, 23, 25), an index of endothelial barrier function, and myocardial perfusion (27, 28, 42) can both be assessed noninvasively by fast computerized tomography (CT). The present study addressed the hypothesis that HT modulates myocardial microvascular adaptive responses to chronic CAS. The hypothesis was tested in a unique pig model of renovascular HT and chronic CAS with the use of in vivo and in vitro imaging tools to assess microvascular function and architecture.
METHODS
Animal experiments.
All animal procedures followed the Guide for the Care and Use of Laboratory Animals (National Research Council, Washington, DC: National Academy Press, 1996) and were approved by the Institutional Animal Care and Use Committee. Twenty-four female domestic pigs (initially weighing 25–35 kg) were randomized into four groups (n = 6 each): normal, CAS, renovascular HT, and CAS+HT. CAS and renovascular HT were induced at baseline by placing a local irritant coil in the left circumflex coronary artery (LCX; Fig. 1A) and the main renal artery, respectively, via vascular catheterization, as previously described (8, 28, 29, 36, 40). This approach leads to renovascular HT within 5–7 days, as we have shown before (7, 11). Mean arterial pressure (MAP) was measured by a PhysioTel telemetry system (Data Sciences) implanted at baseline in the left femoral artery. Aspirin (650 mg/day) and amiodarone (200 mg/day) were given orally 2 days before coronary coil placement to prevent complications (e.g., acute thrombosis and arrhythmia). Amiodarone (150 mg in 100 ml saline) was also infused intravenously during the procedure.
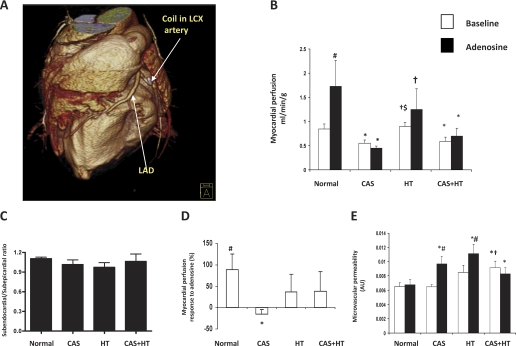
Fig. 1.
A: 3-dimensional fast computerized tomography (CT) image illustrating coronary artery stenosis (CAS) in the swine left circumflex artery (LCX). LAD, left anterior descending coronary artery. B: myocardial perfusion obtained before (open bars) and after (filled bars) adenosine infusion by fast CT in the lateral wall “area at risk” in normal, CAS, renovascular hypertension (HT), and CAS+HT pigs. Basal myocardial perfusion decreased in CAS and CAS+HT. C: subendocardial-to-subepicardial myocardial perfusion ratio. D: myocardial perfusion response to adenosine. Adenosine disclosed impaired responses in CAS, which were relatively preserved in HT and CAS+HT. E: myocardial microvascular permeability [MP, arbitrary units (AU)]. Basal MP increased in CAS+HT, while adenosine increased MP in CAS and HT. *P < 0.05 vs. normal; #P < 0.05 vs. baseline; †P < 0.05 vs. CAS, $P < 0.05 vs. CAS+HT.
After 6 wk of observation, pigs were anesthetized (ketamine 15.7 mg·kg−1·h−1 and xylazine 2.3 mg·kg−1·h−1 in saline) and ventilated for CT study. Catheters were then placed fluoroscopically through carotid vascular sheaths in the right atrium for injection of contrast media; a sidearm was used for subsequent administration of adenosine (5). Fast CT studies were performed to assess cardiac function and structure in vivo, myocardial perfusion and MP (before and after adenosine), LV muscle mass (LVMM), and systolic and diastolic cardiac function (10, 29, 40). Oxygen consumption was assessed by the double product of systolic blood pressure and heart rate during the study. Systemic venous blood was drawn to measure plasma renin activity (PRA). A few days after completion of the in vivo studies, the pigs were euthanized with pentobarbital (100 mg/kg) and lateral wall LV tissue from the area at risk distal to the coil was harvested for in vitro studies. LV myocardial segments were fresh frozen or preserved in formalin, and another segment was prepared for micro-CT studies. Microvascular architecture was assessed by evaluation of microvascular density and wall thickness, inflammation by the infiltration of T lymphocytes and macrophages, and myocardial fibrosis by the expression of collagen I and matrix metalloproteinase (MMP)-9 and by trichrome staining. Vascular integrity was evaluated by the expression of anti-zonula occludens-1 (ZO-1), a tight junction protein that regulates endothelial barrier function and overexpresses in response to strain (9). To assess angiogenic activity, VEGF, its receptor (FLK-1), basic fibroblast growth factor (bFGF), Notch-1, and its receptor delta-like ligand 4 (DLL4) were also evaluated.
In vivo CT studies.
To evaluate cardiac and microvascular function in vivo, pigs were scanned by 64-slice multidetector CT (MDCT, Somatom Sensation-64, Siemens Medical Solution, Forchheim, Germany) as previously described (10, 18).
Briefly, mid-LV levels were selected for measurement of microvascular perfusion and function. A 50-s flow study during respiratory suspension at end expiration immediately followed a bolus injection of nonionic, low osmolar contrast medium (Isovue-370, 0.33 ml/kg over 2 s) into the right atrium. Fifteen minutes later the functional study was repeated during a 5-min intravenous infusion of adenosine (400 μg·kg−1·min−1). Two parallel 6-mm-thick cardiac sections were studied throughout the cardiac cycle with a full-scan reconstruction (330 ms) with a 50-ms scan reconstruction increment. For cardiac systolic and diastolic functions and LVMM, the entire LV was scanned 20 times throughout the cardiac cycle to obtain multilevel images.
CT data analysis.
All images were analyzed with the Analyze software package (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN). For LV size and systolic function, the LV endocardial and epicardial surfaces were traced at end diastole and end systole. LV ejection fraction, stroke volume, cardiac output, and LVMM were calculated as previously described (26, 40). For diastolic function, the LV cavity volume was calculated throughout the cardiac cycle and plotted as a function of time. Early (E) and late (A) LV filling rates were calculated from the positive slopes of the curve, as we described previously (18, 40). Systemic vascular resistance was calculated as MAP × 80/(cardiac output).
For microvascular function, regions of interest were traced at end diastole in the LV lateral wall myocardium (“area at risk”) as well as in its subepicardial and subendocardial regions. The time-density data were then used to assess MP by the Patlak method (10, 18) (arbitrary units) and myocardial perfusion with the indicator dilution technique (27, 28, 40, 42), as we previously described.
Angiography and stenosis assessment.
A guide catheter was positioned under fluoroscopic guidance (Siemens Siremobil Compact) in the left main coronary artery for selective injection of contrast media. The images were recorded, and the degree of CAS was assessed off-line (with a quantitative coronary angiography system) as the decrease in luminal diameter of the LCX at the most stenotic point compared with a stenosis-free segment (6, 42).
Micro-CT study.
Micro-CT was used to evaluate myocardial microvascular architecture, as previously shown (18, 42). Briefly, the heart was excised and perfused under physiological pressure with Microfil (MV-122, Flow Tech) through a branch of the LCX distal to the CAS, and a myocardial segment (≈2 × 1 × 1 cm) was dissected from the area at risk and scanned. Three-dimensional volume images were analyzed with Analyze in seven slices obtained at equal intervals in the subepicardium or subendocardium to calculate the spatial density and average diameters of myocardial microvessels (diameters 20–500 μm). Additionally, one to three intramyocardial arterioles and their branches were tomographically isolated in each pig and vessel tortuousity was determined, as previously shown (43).
Immunohistochemistry.
To assess indexes of inflammation and vascular remodeling, the primary antibodies mouse anti-CD8 (1:20, Novocastra), anti-macrophage CD163 (1:20, Serotec), and anti-human α-smooth muscle actin (SMA; DakoCytomation) were incubated with deparaffined and rehydrated slides and detected by the avidin-biotin complex technique with the diaminobenzidine (DAB) chromogen (Vector) and hematoxylin counterstain. Quantitative analysis utilized a computer-aided image analysis program (MetaMorph, Meta-Imaging Series 6.3.2, Molecular Devices, Sunnyvale, CA) to calculate the fraction of positive stain for three random fields per slide. Inflammatory cell numbers were also normalized to the number of myocyte nuclei in the same section. Microvascular wall thickness and lumen diameter were measured and their ratio calculated (18). In addition, capillary density was assessed in hematoxylin and eosin-stained sections under ×1,000 magnification as the ratio of capillaries to muscle fibers, as previously described (35, 40).
Western blotting.
Total proteins from heart lyses (100 μg) were loaded onto 4–15% SDS-polyacrylamide gel (Bio-Rad) and transferred onto polyvinylidene difluoride (PVDF) membrane (Millipore). The primary antibodies (rabbit IgG) used for blotting were anti-ZO-1 (1:1,000, Zymed), collagen I (1:1,000, Cosmo Bio), active and pro-MMP-9 (1:500, Millipore), notch1 (1:500, Novus), DLL4 (1:500, Novus), VEGF (1:200, Santa Cruz), FLK-1 (1:200, Santa Cruz), and bFGF (1:500, Millipore). GAPDH was blotted to confirm equal loading, except for active MMP-9 expression that was quantified relative to the pro-form.
Statistical analysis.
All results are presented as means ± SE. Analysis of variance (ANOVA) was used to evaluate differences among the groups, followed by an unpaired t-test with Bonferroni correction, while paired Student's t-tests were used to detect changes within groups (adenosine vs. baseline). P ≤ 0.05 was considered significant.
RESULTS
After a 6-wk observation, the corresponding pigs developed significant degree of coronary stenosis (Table 1), while no visible collaterals were observed and MAP was similarly increased in HT and CAS+HT compared with normal and CAS groups. PRA was similar among the groups (Table 1). Heart rate in CAS+HT pigs was significantly increased compared with all other groups (P < 0.01). In response to adenosine, MAP significantly and similarly decreased in all groups (P < 0.05; Table 1) and heart rate increased significantly in all groups except for CAS+HT. LVMM was increased in HT and CAS+HT compared with normal (P < 0.01 and P = 0.02, respectively; Table 1).
Table 1.
Systemic and cardiac hemodynamics in normal, chronic CAS, HT, and CAS+HT pigs
| Normal | CAS | HT | CAS+HT | |
|---|---|---|---|---|
| Body weight, kg | 46.4 ± 0.9 | 48.5 ± 0.5 | 51.0 ± 2.2 | 44.0 ± 3.2 |
| Degree of CAS, % | 91.7 ± 3.8 | 90 ± 2.7 | ||
| Mean arterial pressure, mmHg | 100 ± 5 | 102 ± 5 | 118 ± 7*‡ | 122 ± 6*‡ |
| Change after adenosine, % | −13 ± 3† | −10 ± 2† | −14 ± 4† | −8 ± 2† |
| Heart rate (baseline), bpm | 77 ± 9 | 72 ± 5 | 70 ± 5 | 104 ± 9*‡§ |
| Change after adenosine, % | 16 ± 7† | 22 ± 5† | 15 ± 6† | 5 ± 3 |
| Ejection fraction, % | 59 ± 3 | 52 ± 3 | 55 ± 4 | 49 ± 3* |
| Stroke volume, ml | 50 ± 6 | 44 ± 3 | 46 ± 3 | 34 ± 2*‡§ |
| E/A | 1.6 ± 0.2 | 1.1 ± 0.2* | 1.0 ± 0.2* | 0.6 ± 0.2*‡§ |
| LV muscle mass, g/kg body wt | 1.9 ± 0.10 | 2.0 ± 0.03 | 2.5 ± 0.10*‡ | 2.4 ± 0.02* |
| SVR, mmHg·l−1·min | 2,060 ± 220 | 2,590 ± 210 | 2,680 ± 240* | 2,840 ± 280* |
| Double product, mmHg × bpm | 8,500 ± 760 | 9,887 ± 799 | 9,600 ± 1,630 | 14,654 ± 1,015*‡§ |
| PRA, pg·ml−1·h−1 | 0.21 ± 0.05 | 0.30 ± 0.04 | 0.25 ± 0.05 | 0.35 ± 0.08 |
Values are means ± SE for n = 6 pigs/group. CAS, coronary artery stenosis; HT, renovascular hypertension; bpm, beats/minute; E/A, early (E)-to-late (A) left ventricle (LV) filling rate ratio; SVR, systemic vascular resistance; PRA, plasma renal activity.
P ≤ 0.05 vs. normal;
P < 0.05 vs. baseline;
P < 0.05 vs. CAS;
P < 0.05 vs. HT.
The ejection fraction that tended to be 12% lower in CAS compared with normal (P = 0.06) was significantly lower in CAS+HT (by 17%, P = 0.03 vs. normal; Table 1). Stroke volume decreased only in CAS+HT (Table 1). E-to-A ratio was significantly lower in CAS and HT compared with normal control animals (−31% and −37.5%, P = 0.04 and P = 0.008, respectively), while in CAS+HT the E/A was further lower than all other groups (−62.5% lower than normal, P < 0.03 for all groups), suggesting that diastolic dysfunction in CAS was aggravated by HT. Furthermore, systemic vascular resistance was significantly elevated in HT and CAS+HT (P = 0.048 and P = 0.033, respectively; Table 1), while in CAS the increase did not reach statistical significance (P = 0.06; Table 1). In addition, the double product increased only in CAS+HT compared with normal, CAS, and HT (Table 1), suggesting that coexistence of HT increased oxygen consumption and energy demand.
Coronary microvascular function.
Myocardial perfusion in the lateral wall area at risk was similarly decreased in CAS and CAS+HT compared with normal (0.55 ± 0.06, 0.58 ± 0.08, and 0.84 ± 0.10 ml·min−1·g−1, P = 0.02 and P = 0.04, respectively; Fig. 1B) as well as compared with HT (0.90 ± 0.08 ml·min−1·g−1, P = 0.005 and P = 0.01, respectively; Fig. 1B). The ratio of subendocardial to subepicardial perfusion was not significantly different among the groups (P = 0.2; Fig. 1C). Adenosine induced a significant increase in myocardial perfusion in normal pigs (to 1.73 ± 0.53 ml·min−1·g−1, P ≤ 0.05 vs. baseline), which was blunted in CAS, HT, and CAS+HT (to 0.45 ± 0.04, 1.25 ± 0.42, and 0.70 ± 0.16 ml·min−1·g−1, respectively, P < 0.05 vs. normal; Fig. 1B). Furthermore, the degree of response to adenosine was significantly different compared with normal only in CAS (P = 0.01, Fig. 1D) but not in HT and CAS+HT. Therefore, HT attenuated a decrease in myocardial perfusion in CAS in response to adenosine.
Myocardial MP in this region was significantly increased in CAS+HT at baseline compared with normal and CAS (Fig. 1E) but was not different from HT. Adenosine induced a significant increase in MP compared with baseline in both the CAS (+49.3 ± 14.7%, P = 0.02) and HT (+33.4 ± 8.3%, P = 0.004) groups, and these were also greater compared with the response in normal pigs (+5.4 ± 14.0%, both P ≤ 0.03). In contrast, CAS+HT MP remained elevated and did not change any further (Fig. 1E).
Microvascular density.
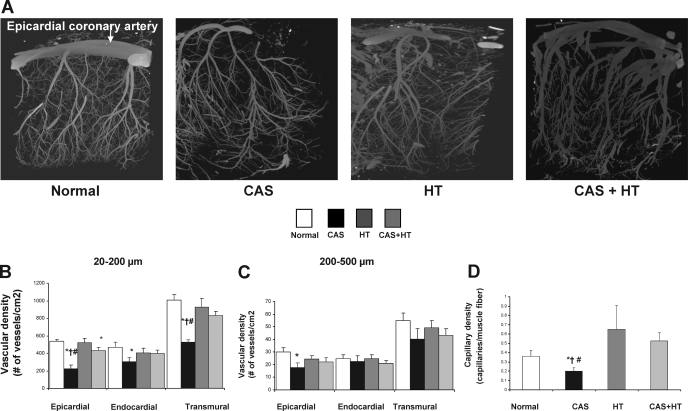
The epicardial density of microvessels (20–200 μm in diameter) in the area at risk in CAS (225 ± 89 vessels/cm2) significantly decreased compared with all other groups (P < 0.05; Fig. 2), while endocardial microvascular density decreased only compared with normal (to 303 ± 89 vessels/cm2, P = 0.03; Fig. 2B). In CAS+HT epicardial density also slightly decreased (430 ± 37 vessels/cm2, P = 0.04 compared with normal), but endocardial and transmural density remained similar to normal and higher than CAS (P = 0.005 and P = 0.01, respectively). The epicardial density of larger microvessels (200–500 μm) was slightly lower only in CAS compared with normal (P = 0.04; Fig. 2C). These observations were supported by a decrease in histological capillary density in CAS compared with normal, HT, and CAS+HT (Fig. 2D).
Fig. 2.
Myocardial microvascular density assessed by micro-CT in normal, CAS, HT, and CAS+HT. A: representative micro-CT images of myocardial microvessels. B and C: quantitation of microvascular density. The transmural density of small microvessels (20–200 μm in diameter) in normal decreased in CAS and was preserved in HT and CAS+HT. D: capillary density expressed as ratio of capillaries to cardiac muscle fibers. *P < 0.05 vs. normal; †P < 0.05 vs. HT; #P < 0.05 vs. CAS+HT.
In addition, microvascular tortuousity (dimensionless), which characterizes angiogenic vessels, was increased in CAS, HT, and CAS+HT compared with normal (1.8 ± 0.3, 1.6 ± 0.1, 1.5 ± 0.1 and 1.1 ± 0.02, respectively, P < 0.05 for all), suggesting new vessel formation. The average diameters of these microvessels were similar in normal, CAS, HT, and CAS+HT animals (95 ± 8, 96 ± 2.3, 99 ± 3.5, and 112 ± 13 μm, respectively).
Inflammation, fibrosis, and remodeling.
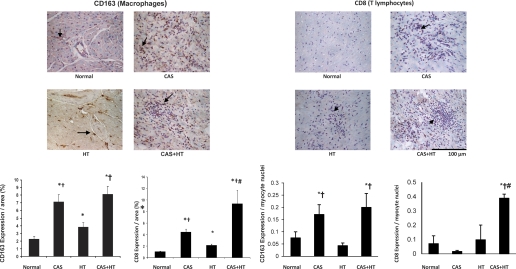
HT increased myocardial macrophage (CD163+) and CD8+ T-cell infiltration (P = 0.03 and P = 0.003 vs. normal, respectively; Fig. 3). In the area at risk of CAS and CAS+HT, macrophages and T cells were also observed in myocardium and the perivascular area. Macrophage infiltration was significantly greater in both CAS and CAS+HT compared with normal (P = 0.007 and P = 0.0007, respectively; Fig. 3) and with HT (P = 0.03 and P = 0.009, respectively). Similarly, when normalized by the number of myocyte nuclei macrophage infiltration was increased in CAS and CAS+HT compared with normal and with HT (Fig. 3). Moreover, CD8+ T-cell infiltration in CAS+HT pigs was greater than in either CAS or HT alone (P = 0.01 and P < 0.001, respectively; Fig. 3), but when normalized by the number of myocyte nuclei CD8+ T-cell infiltration was increased only in CAS+HT compared with all other groups (Fig. 3).
Fig. 3.
Myocardial macrophage (CD163+, left) and T lymphocyte (CD8+, right) infiltration in normal, CAS, HT, and CAS+HT pigs detected by immunohistochemistry (top, ×40) and quantified (bottom). Inflammatory infiltration increased in CAS, HT, and CAS+HT compared with normal, but CD8+ cells significantly increased in CAS+HT compared with normal, HT, and CAS. *P < 0.05 vs. normal; †P < 0.05 vs. HT, #P < 0.05 vs. CAS.
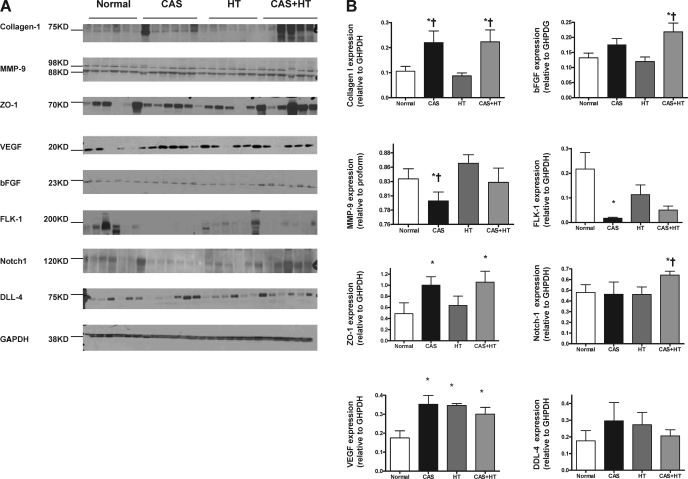
Myocardial expression of collagen I increased in CAS and CAS+HT compared with normal and HT (P ≤ 0.02 for all; Fig. 4), while MMP-9 expression was decreased only in CAS compared with normal (P = 0.04). Trichrome staining showed that HT induced mild fibrosis (P = 0.01 vs. normal; Fig. 5), while in CAS the percentage of fibrotic tissue at the risk area was higher than in normal and HT (P < 0.001 for both). Moreover, CAS+HT significantly increased lateral wall fibrosis compared with all other groups (Fig. 5). Microvascular media-to-lumen ratio, an index of wall thickening, increased in HT compared with normal (P = 0.002) and in CAS+HT compared with all groups (P ≤ 0.001 for all; Fig. 5). The expression of ZO-1, the tight junction protein that regulates endothelial barrier function, was similarly upregulated in CAS and CAS+HT compared with normal (P = 0.04 for both; Fig. 4) and tended to be higher than in HT (P = 0.07 for both).
Fig. 4.
A: expression of myocardial collagen I, matrix metalloproteinase (MMP)-9, zonula occludens-1 (ZO-1), vascular endothelial growth factor (VEGF), basic fibroblastic growth factor (bFGF), VEGF receptor FLK-1, Notch-1, and its receptor Delta-like receptor 4 (DLL-4). B: respective quantitation showing increased fibrosis and angiogenic factors in CAS and in CAS+HT compared with normal and increased bFGF and Notch-1 only in CAS+HT. *P < 0.05 vs. normal; †P < 0.05 vs. HT.
Fig. 5.
Myocardial vessel wall-to-lumen ratio quantified in α-smooth muscle actin (SMA)-stained slides (right, ×40) and myocardial fibrosis in trichrome-stained slides (left, ×40). Myocardial fibrosis and microvascular media-to-lumen ratio increased in HT and CAS+HT compared with normal. CAS increased fibrosis compared with HT. *P < 0.05 vs. normal; †P < 0.05 vs. HT; #P < 0.05 vs. CAS.
Vascular density regulation.
VEGF expression similarly increased among the groups compared with normal, while bFGF, which tended to increase in CAS (P = 0.06 vs. normal), was significantly upregulated in CAS+HT (P = 0.02 vs. normal and P = 0.01 vs. HT; Fig. 4). In contrast, FLK-1 expression decreased only in CAS (P = 0.04), while in HT and CAS+HT a slight decrease did not reach statistical significance. Notch-1 was upregulated in CAS+HT compared with normal (P = 0.03) and HT (P = 0.02; Fig. 4), but the expression of its receptor DLL4 was similar among the groups.
DISCUSSION
The present study shows that a chronic CAS impairs myocardial microvascular function and endothelial barrier integrity, but these microvascular functional attributes are not intensified by experimental renovascular HT, despite aggravated tissue remodeling (microvascular remodeling, perivascular inflammation, and myocardial fibrosis) in HT. The relatively minor incremental effect of HT on myocardial microvascular function in CAS might be due to relative preservation of microvascular density in the area at risk. Nevertheless, cardiac systolic and diastolic function declined compared with normotensive CAS, likely because of greater inflammation, fibrosis, systemic vascular resistance, and myocardial energy demands. These data suggest that coexistence of early HT and CAS aggravates cardiac functional and structural consequences of CAS, which the partial preservation of the myocardial microvasculature fails to prevent.
Chronic HT induces microvascular pathophysiological changes like resistance artery remodeling and peripheral capillary rarefaction that may contribute to HT and amplify its hemodynamic consequences. Myocardial microvascular dysfunction (26) and loss of structural integrity (18) are also frequently associated with HT, as well as with an upstream CAS (4). On the other hand, being a proinflammatory condition, early HT may elicit transient proliferation of microvessels (30, 41), which we have recently shown (40) can serve as recruitable collaterals and partly protect the myocardium from ischemia during acute coronary obstruction. However, because such microvessels are often anomalous (32), it remained unclear how myocardial microcirculatory alterations in HT affect cardiac adaptation to superimposed chronic CAS. This study extends our previous observation (42) and shows that while concurrent HT preserved microvascular density and partly sustained myocardial microvascular function (e.g., myocardial perfusion response to adenosine) in chronic CAS, these were ineffective in preserving cardiac function because of the pronounced inflammation and interstitial fibrosis elicited by HT.
Endothelial barrier function is important for maintaining vascular integrity and regulation of vascular tone. Interestingly, we observed that the LV lateral wall in CAS+HT increased basal MP that characterizes disruption of endothelial integrity (27). Inflammatory mediators can increase barrier permeability by regulating the expression of and rearranging endothelial tight junction proteins (33), resulting in increased leakage (16, 32). In this study, we found in CAS and CAS+HT increased expression of the endothelial tight junction component ZO-1 that links the actin cytoskeleton and tight junctions (38), which may imply tight junction reassembly reflecting disruption of endothelial barrier integrity (9, 18). Furthermore, the increase in MP may account for the chronic inflammatory infiltration observed in both groups (32). We found that HT exacerbated chronic inflammatory cell infiltration in CAS, particularly T cells. The slight disagreement between the cell counts when normalized to myocardial area versus number of myocyte nuclei might be secondary to interstitial expansion due to fibrosis or inadvertent inclusion of macrophages in the total nuclei count. Furthermore, we did not account for polyploidy, which may be present in hypertrophic myocytes, albeit rarely. Inflammatory cells in turn may produce cytokines, chemokines, and growth factors that further increase MP and perpetuate the vicious cycle of inflammation, microvascular dysfunction, and interstitial fibrosis. It is also likely that increased myocardial flow in CAS+HT increased shear stress, and thus might be in part responsible for disruption of endothelial barrier integrity and inflammatory cell filtration. However, subtle changes in barrier function may be disclosed only during challenges like increased cardiac demand (27), as we observed in HT and distal to CAS during adenosine infusion.
As per the experimental model of 90% CAS, basal myocardial perfusion was lower than normal in CAS and CAS+HT, although the subendocardial-to-subepicardial perfusion ratio was unchanged, as we previously observed during early disease (26–28). Nevertheless, while myocardial perfusion was not redistributed away from the endocardium, the increases in systemic vascular resistance and myocardial energy demands in CAS+HT compared with CAS might result in greater relative ischemia. In normal pigs adenosine also induces an increase in myocardial perfusion, which is blunted in HT (29), as we observed in the present study, in line with abnormal coronary flow reserve demonstrated in hypertensive patients (3, 15). In both CAS and CAS+HT the area at risk myocardial perfusion did not respond to challenge. However, in CAS alone the degree of the response was significantly lower than normal (Fig. 1D). These observations suggest that at its early stage HT superimposed on CAS offsets a decline in coronary flow reserve, which may partly protect microvascular function during chronic CAS and facilitate delivery of blood to the area at risk.
The combination of anatomic assessment by micro-CT and histology with functional assessment of myocardial vascular function by fast CT allowed us to study the relation between microvascular anatomy and function. Microvascular rarefaction is commonly observed in advanced HT (1), while at its early phase myocardial microvascular density may initially be preserved (29, 40, 42) to support myocardial remodeling and evolving hypertrophy (26, 29, 42). In the present study we observed in CAS a decrease in microvascular density, particularly of the resistance microvessels probably secondary to myocardial fibrosis and microvascular regression. In contrast, in CAS+HT microvascular density remained similar to normal, probably because of the coexisting HT, which tends to preserve it during development of LV hypertrophy. These microvessels may contribute to myocardial preconditioning during the progression of HT and partly protect the myocardium during acute insults (12, 42).
The mechanisms responsible for preservation of microvascular density in HT and CAS+HT may be angiogenic. Coexistence of both CAS and HT increased not only VEGF but also bFGF, which is linked with HT (39) and has a synergistic effect with VEGF on angiogenesis (22). In contrast, in normotensive CAS, decreased myocardial expression of the VEGF receptor Flk-1 may lead to altered or insufficient angiogenesis, as described in diabetic patients (31). Interestingly, CAS+HT also increased expression of Notch-1, which is induced by VEGF and acts downstream to negatively regulate vessel growth (19, 34). MMP-9 is also an important factor in angiogenesis (17, 37), and its downregulation in CAS can interfere with angiogenesis. Nevertheless, rather than direct modulation of microvascular density, the increased coronary perfusion pressure secondary to HT might have also partly overcome the impedance of the obstruction and prevented microvascular regression.
We also observed that the increased media-to-lumen ratio in HT was exacerbated in the lateral wall of CAS+HT, and may have been intensified by leukocyte infiltration (20). Since microvascular diameter remained unchanged, outward remodeling and thickening of the media likely preceded luminal narrowing (29). Furthermore, impaired vascular smooth muscle cell responses to cardiac challenge in HT can worsen microvascular dysfunction (15). Indeed, while myocardial perfusion responses to challenge were slightly superior in HT and CAS+HT compared with CAS alone, they were blunted compared with baseline.
The present study shows that CAS and CAS+HT induced myocardial interstitial fibrosis, accompanied by amplified myocardial expression of collagen I, and in CAS also decreased myocardial expression of MMP-9, as observed in chronic diseases (13, 21). This mechanism in CAS may exacerbate cardiac stiffness that leads to impaired diastolic and systolic function. Notably, the relative preservation of the microcirculation in the poststenotic territory in CAS+HT, which may serve to decrease the likelihood of acute ischemic events, did not prevent a decline in global cardiac function in this group. This might be due to microvascular dysfunction or cytokine activity exacerbated by HT in adjacent territories. In addition, the presence of HT imposes a significant increase in energy demand (estimated by the double product, a crude measure of oxygen consumption), which may subsequently aggravate deterioration of global cardiac function, despite preservation of microvascular density. The renin-angiotensin system might also participate in cardiac remodeling in HT, although the similarity of systemic PRA levels in the four groups argues against a major contribution of this system to the alterations we observed. Nevertheless, additional studies will be needed to interrogate activation of tissue components of the renin-angiotensin system in CAS+HT.
Limitations.
In the present study we used relatively young animals, which may show greater potential for new vessel formation than adults. The female pigs were premenstrual, so that hormonal changes were unlikely to affect our results. CAS and HT were induced simultaneously, and their duration was shorter than that observed in patients, who often have chronic HT and multivessel coronary artery disease, limiting the clinical translation power of our observations. Alas, this exposure sufficed to illustrate that their coexistence has significant pathophysiological implications regarding the evolution of cardiac injury.
Summary.
We observed that HT exacerbated myocardial inflammation, fibrosis, and vascular remodeling distal to CAS. These changes might modulate cardiac adaptive responses to superimposed chronic CAS and bear functional consequences. Interestingly, early HT also preserved microvascular density and function, which may partly and temporarily protect the myocardium at risk from acute ischemic insults. Nevertheless, global cardiac function in the setting of chronic CAS exhibited a greater decline in the presence of HT, indicating that microvascular preservation is not an effective long-term mechanism. Moreover, subsequent microvascular loss associated with long-term HT and cardiac hypertrophy might further amplify the deleterious effects of HT on myocardial fibrosis, microvascular function, and cardiac diastolic and systolic function.
GRANTS
This study was partly supported by National Institutes of Health Grants DK-73608, DK-77013, HL-77131, and PO1-HL-085307.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Present address of F. D. Favreau: INSERM U927 and Université de Poitiers, Faculté de Médecine et de Pharmacie, Poitiers, F-86000, France.
REFERENCES
- 1. Antonios TF. Microvascular rarefaction in hypertension—reversal or over-correction by treatment? Am J Hypertens 19: 484–485, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Birukova AA, Adyshev D, Gorshkov B, Bokoch GM, Birukov KG, Verin AD. GEF-H1 is involved in agonist-induced human pulmonary endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 290: L540–L548, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Brush JE, Jr, Cannon RO, 3rd, Schenke WH, Bonow RO, Leon MB, Maron BJ, Epstein SE. Angina due to coronary microvascular disease in hypertensive patients without left ventricular hypertrophy. N Engl J Med 319: 1302–1307, 1988 [DOI] [PubMed] [Google Scholar]
- 4. Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 356: 830–840, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Chade AR, Herrmann J, Zhu X, Krier JD, Lerman A, Lerman LO. Effects of proteasome inhibition on the kidney in experimental hypercholesterolemia. J Am Soc Nephrol 16: 1005–1012, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Chade AR, Rodriguez-Porcel M, Herrmann J, Zhu X, Grande JP, Napoli C, Lerman A, Lerman LO. Antioxidant intervention blunts renal injury in experimental renovascular disease. J Am Soc Nephrol 15: 958–966, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, Napoli C, Lerman A, Lerman LO. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation 119: 547–557, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chareonthaitawee P, Christenson SD, Anderson JL, Kemp BJ, Hodge DO, Ritman EL, Gibbons RJ. Reproducibility of measurements of regional myocardial blood flow in a model of coronary artery disease: comparison of H215O and 13NH3 PET techniques. J Nucl Med 47: 1193–1201, 2006 [PubMed] [Google Scholar]
- 9. Collins NT, Cummins PM, Colgan OC, Ferguson G, Birney YA, Murphy RP, Meade G, Cahill PA. Cyclic strain-mediated regulation of vascular endothelial occludin and ZO-1: influence on intercellular tight junction assembly and function. Arterioscler Thromb Vasc Biol 26: 62–68, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Daghini E, Primak AN, Chade AR, Zhu X, Ritman EL, McCollough CH, Lerman LO. Evaluation of porcine myocardial microvascular permeability and fractional vascular volume using 64-slice helical computed tomography (CT). Invest Radiol 42: 274–282, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Favreau F, Zhu XY, Krier JD, Lin J, Warner L, Textor SC, Lerman LO. Revascularization of swine renal artery stenosis improves renal function but not the changes in vascular structure. Kidney Int 78: 1110–1118, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fukuda S, Kaga S, Sasaki H, Zhan L, Zhu L, Otani H, Kalfin R, Das DK, Maulik N. Angiogenic signal triggered by ischemic stress induces myocardial repair in rat during chronic infarction. J Mol Cell Cardiol 36: 547–559, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Gonzalez-Avila G, Iturria C, Vadillo-Ortega F, Ovalle C, Montano M. Changes in matrix metalloproteinases during the evolution of interstitial renal fibrosis in a rat experimental model. Pathobiology 66: 196–204, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Gould KL, Lipscomb K. Effects of coronary stenoses on coronary flow reserve and resistance. Am J Cardiol 34: 48–55, 1974 [DOI] [PubMed] [Google Scholar]
- 15. Hamasaki S, Al Suwaidi J, Higano ST, Miyauchi K, Holmes DR, Jr, Lerman A. Attenuated coronary flow reserve and vascular remodeling in patients with hypertension and left ventricular hypertrophy. J Am Coll Cardiol 35: 1654–1660, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, Jain RK, McDonald DM. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol 156: 1363–1380, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herrmann J, Saguner AM, Versari D, Peterson TE, Chade A, Olson M, Lerman LO, Lerman A. Chronic proteasome inhibition contributes to coronary atherosclerosis. Circ Res 101: 865–874, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Lin J, Zhu X, Chade AR, Jordan KL, Lavi R, Daghini E, Gibson ME, Guglielmotti A, Lerman A, Lerman LO. Monocyte chemoattractant proteins mediate myocardial microvascular dysfunction in swine renovascular hypertension. Arterioscler Thromb Vasc Biol 29: 1810–1816, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci USA 104: 3219–3224, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marchesi C, Ebrahimian T, Angulo O, Paradis P, Schiffrin EL. Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension 54: 1384–1392, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Maric C, Sandberg K, Hinojosa-Laborde C. Glomerulosclerosis and tubulointerstitial fibrosis are attenuated with 17beta-estradiol in the aging Dahl salt sensitive rat. J Am Soc Nephrol 15: 1546–1556, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Mattern J, Koomagi R, Volm M. Coexpression of VEGF and bFGF in human epidermoid lung carcinoma is associated with increased vessel density. Anticancer Res 17: 2249–2252, 1997 [PubMed] [Google Scholar]
- 23. Mohlenkamp S, Lerman LO, Lerman A, Behrenbeck TR, Katusic ZS, Sheedy PF, 2nd, Ritman EL. Minimally invasive evaluation of coronary microvascular function by electron beam computed tomography. Circulation 102: 2411–2416, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Panza JA, Quyyumi AA, Brush JE, Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med 323: 22–27, 1990 [DOI] [PubMed] [Google Scholar]
- 25. Reddy HK, Campbell SE, Janicki JS, Zhou G, Weber KT. Coronary microvascular fluid flux and permeability: influence of angiotensin II, aldosterone, and acute arterial hypertension. J Lab Clin Med 121: 510–521, 1993 [PubMed] [Google Scholar]
- 26. Rodriguez-Porcel M, Herrman J, Chade AR, Krier JD, Breen JF, Lerman A, Lerman LO. Long-term antioxidant intervention improves myocardial microvascular function in experimental hypertension. Hypertension 43: 493–498, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Rodriguez-Porcel M, Lerman A, Best PJ, Krier JD, Napoli C, Lerman LO. Hypercholesterolemia impairs myocardial perfusion and permeability: role of oxidative stress and endogenous scavenging activity. J Am Coll Cardiol 37: 608–615, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Rodriguez-Porcel M, Lerman A, Herrmann J, Schwartz RS, Sawamura T, Condorelli M, Napoli C, Lerman LO. Hypertension exacerbates the effect of hypercholesterolemia on the myocardial microvasculature. Cardiovasc Res 58: 213–221, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Rodriguez-Porcel M, Zhu XY, Chade AR, Amores-Arriaga B, Caplice NM, Ritman EL, Lerman A, Lerman LO. Functional and structural remodeling of the myocardial microvasculature in early experimental hypertension. Am J Physiol Heart Circ Physiol 290: H978–H984, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 117: e25–e146, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Sasso FC, Torella D, Carbonara O, Ellison GM, Torella M, Scardone M, Marra C, Nasti R, Marfella R, Cozzolino D, Indolfi C, Cotrufo M, Torella R, Salvatore T. Increased vascular endothelial growth factor expression but impaired vascular endothelial growth factor receptor signaling in the myocardium of type 2 diabetic patients with chronic coronary heart disease. J Am Coll Cardiol 46: 827–834, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Sluimer JC, Kolodgie FD, Bijnens AP, Maxfield K, Pacheco E, Kutys B, Duimel H, Frederik PM, van Hinsbergh VW, Virmani R, Daemen MJ. Thin-walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions. Relevance of compromised structural integrity for intraplaque microvascular leakage. J Am Coll Cardiol 53: 1517–1527, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Song L, Pachter JS. Monocyte chemoattractant protein-1 alters expression of tight junction-associated proteins in brain microvascular endothelial cells. Microvasc Res 67: 78–89, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, Eichmann A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci USA 104: 3225–3230, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suuronen EJ, Veinot JP, Wong S, Kapila V, Price J, Griffith M, Mesana TG, Ruel M. Tissue-engineered injectable collagen-based matrices for improved cell delivery and vascularization of ischemic tissue using CD133+ progenitors expanded from the peripheral blood. Circulation 114: I138–I144, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Urbieta Caceres VH, Lavi R, Zhu XY, Crane JA, Textor SC, Lerman A, Lerman LO. Early atherosclerosis aggravates the effect of renal artery stenosis on the swine kidney. Am J Physiol Renal Physiol 299: F135–F140, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 93: 411–422, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Willott E, Balda MS, Heintzelman M, Jameson B, Anderson JM. Localization and differential expression of two isoforms of the tight junction protein ZO-1. Am J Physiol Cell Physiol 262: C1119–C1124, 1992 [DOI] [PubMed] [Google Scholar]
- 39. Zacharieva S, Atanassova I, Orbetzova M, Kirilov G, Nachev E, Kalinov K, Shigarminova R. Vascular endothelial growth factor (VEGF), prostaglandin E2 (PGE2) and active renin in hypertension of adrenal origin. J Endocrinol Invest 27: 742–746, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Zhu XY, Daghini E, Chade AR, Napoli C, Ritman EL, Lerman A, Lerman LO. Simvastatin prevents coronary microvascular remodeling in renovascular hypertensive pigs. J Am Soc Nephrol 18: 1209–1217, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Zhu XY, Daghini E, Chade AR, Rodriguez-Porcel M, Napoli C, Lerman A, Lerman LO. Role of oxidative stress in remodeling of the myocardial microcirculation in hypertension. Arterioscler Thromb Vasc Biol 26: 1746–1752, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Zhu XY, Daghini E, Chade AR, Versari D, Krier JD, Textor KB, Lerman A, Lerman LO. Myocardial microvascular function during acute coronary artery stenosis: effect of hypertension and hypercholesterolaemia. Cardiovasc Res 83: 371–380, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhu XY, Rodriguez-Porcel M, Bentley MD, Chade AR, Sica V, Napoli C, Caplice N, Ritman EL, Lerman A, Lerman LO. Antioxidant intervention attenuates myocardial neovascularization in hypercholesterolemia. Circulation 109: 2109–2115, 2004 [DOI] [PubMed] [Google Scholar]