Abstract
Ventricular tachycardia (VT) in Brugada Syndrome patients often originates in the right ventricular outflow tract (RVOT). We explore the physiological basis for this observation using murine whole heart preparations. Ventricular bipolar electrograms and monophasic action potentials were recorded from seven epicardial positions in Langendorff-perfused wild-type and Scn5a+/− hearts. VT first appeared in the RVOT, implicating it as an arrhythmogenic focus in Scn5a+/− hearts. RVOTs showed the greatest heterogeneity in refractory periods, response latencies, and action potential durations, and the most fractionated electrograms. However, incidences of concordant alternans in dynamic pacing protocol recordings were unaffected by the Scn5a+/− mutation or pharmacological intervention. Conversely, particularly at the RVOT, Scn5a+/− hearts showed earlier and more frequent transitions into discordant alternans. This was accentuated by flecainide, but reduced by quinidine, in parallel with their respective pro- and anti-arrhythmic effects. Discordant alternans preceded all episodes of VT. The RVOT of Scn5a+/− hearts also showed steeper restitution curves, with the diastolic interval at which the gradient equaled one strongly correlating with the diastolic interval at which discordant alternans commenced. We attribute the arrhythmic tendency within the RVOT to the greater spatial heterogeneities in baseline electrophysiological properties. These, in turn, give rise to a tendency to drive concordant alternans phenomena into an arrhythmogenic discordant alternans. Our findings may contribute to future work investigating possible pharmacological treatments for a disease in which the current mainstay of treatment is implantable cardioverter defibrillator implantation.
Keywords: sodium channel, transgenic mouse, arrhythmia
brugada syndrome (brs) is associated with a loss of Na+ channel function and increased risks of polymorphic ventricular tachycardia (VT) and ventricular fibrillation. BrS is thought to be primarily a right ventricular (RV) disease, as reflected in an electrocardiographic ST elevation in the right precordial leads, right bundle branch block, and changes specific to RV epicardial action potential (AP) waveforms (24). Previous studies have implicated either slowed RV AP conduction (25, 36) or alterations in AP repolarization (3, 24) in the arrhythmogenesis associated with BrS. Thus, on the one hand, reduced Na+ currents in BrS would be expected to compromise AP conduction. On the other, their reduction in relationship to transient outward K+ currents (Ito) in BrS would shorten the RV epicardial AP duration (APD) and consequently permit reentrant waves of excitation.
Ventricular fibrillation in patients with BrS is often induced at the RV outflow tract (RVOT) (33), and some clinical studies have, therefore, attempted to localize electrophysiological abnormalities seen in BrS specifically to the RVOT. However, these studies have been limited by their use of human subjects. Many thus relied on noninvasive methods of assessing RVOT conduction delay, either through body surface mapping (22), signal-averaged ECGs (21), or tissue Doppler echocardiography (55). A limited number of invasive studies have shown endocardial RVOT activation delays (23), and Lambiase et al. (25) demonstrated electrogram prolongation and steeper activation recovery interval (ARI) restitution curves in the RVOT in endocardial recordings. However, clinical studies investigating the epicardial heterogeneity of depolarization and repolarization in BrS are challenging to perform. One study recorded epicardial electrograms from the conus branch of the right coronary artery, which runs over the RVOT surface, showing activation delays not found endocardially (36). RVOT ARIs recorded using an epicardial catheter in the great cardiac vein shortened during ST elevation. Another study was performed during open chest surgery to demonstrate spike-and-dome AP waveforms in the epicardium, but not endocardium (24). Finally, an explanted BrS heart showed activation slowing without a transmural repolarization gradient in the RVOT (14).
Experimental models of BrS have often used the RV pharmacological wedge preparation, which precludes localization of arrhythmias to the RVOT. However, an in vivo, closed chest canine study caused the signature ECG changes of BrS following cooling of a small epicardial RVOT region, and produced a “spike and dome” appearance in the monophasic AP (MAP) in the epicardium, but this model did not show eventual loss of the AP dome (37).
Experimental systems that contain genetic modifications directly replicating those known to exist in BrS may provide more specific models to clarify physiological abnormalities associated with the disease condition, while permitting invasive studies impractical in human subjects. BrS is a genetically heterogeneous condition. Nevertheless, up to 30% of patients have mutations in the SCN5A gene, which encodes the cardiac voltage-gated Na+ channel 1.5 (Nav1.5) α-subunit (16). Thus far, it is the only gene that has been extensively studied in connection with BrS. Our group has produced a heterozygotic Scn5a+/− mouse, which shows a 50% reduction in the transmembrane Na+ current (40). While loss of function mutations in the SCN5A gene both clinically (59) and in our mouse model (40) may lead to a complex range of phenotypes, incorporating sick sinus syndrome and progressive conduction disorders, the Scn5a+/− mouse has been shown to closely reproduce many of the key human features of BrS. Thus it demonstrates ST elevation (28) and an enhanced ventricular arrhythmogenesis that is exacerbated by flecainide and relieved by quinidine (28, 29). Its MAP recordings have shown delayed response latencies and increased transmural gradients of repolarization across the RV (29). Furthermore, the Scn5a+/− mice have been shown to exhibit fibrosis specific to the RV, which worsens with age (56), in line with similar clinical findings (14).
The present studies extend these experiments by systematically analyzing a range of electrophysiological parameters at several locations in the RV and left ventricular (LV) epicardium. We used Langendorff whole heart preparations to examine ventricular effective refractory periods (VERPs), response latencies, electrogram durations (EGDs), APDs, alternans, and restitution curves, to show increased arrhythmogenic potential specifically in the RVOT of murine Scn5a+/− hearts for the first time. We use the cardiotropic drugs flecainide and quinidine to illustrate these differences. Flecainide is used as an established test to unmask the arrhythmogenic phenotype in BrS patients (46), while quinidine has been shown to have therapeutic effects in BrS (2). Our findings may contribute to future work investigating possible pharmacological treatments for a disease in which the current mainstay of treatment is implantable cardioverter defibrillator implantation. This is particularly important in a relatively young patient population, where implantable cardioverter defibrillator implantation leads to a high level of inappropriate shocks and a significant risk of device-related complications (50).
METHODS
Langendorff preparation.
Mice aged 6–8 mo were obtained from breeding pairs of heterozygote Scn5a+/− and wild-type (WT) inbred 129/sv mice initially supplied by Harlan (UK). All procedures conformed to the UK Animals (Scientific Procedures) Act 1986 and were performed under licenses approved by local ethical committees and issued by the UK Home Office.
Experiments used a Langendorff-perfused preparation adapted for the murine heart, as described previously (5). Following the start of perfusion, viable hearts suitable for subsequent experimentation regained a pink coloration and spontaneous rhythmic contraction with warming. Where used, 10 μM flecainide and 5 μM quinidine (Sigma-Aldrich, Poole, UK) dissolved in buffer solution were perfused for 15 min before and throughout data acquisition. Concentrations were within the same range as clinical therapeutic levels used in BrS (flecainide: 0.2–0.9 mg/l; quinidine: 1.0–3.0 mg/l) (7, 45).
Bipolar electrogram recording.
Bipolar electrograms (BEGs) were recorded from the epicardial surface using paired platinum recording electrodes (1-mm interpole spacing). Cycles of a decremental, paced electrogram fractionation sequence comprising an eight-beat stimulus (S1) drive train, followed by an extra stimulus (S2), were applied at 8 Hz. The S1-S2 interval was reduced by 1 ms between successive drive trains until the preparation became refractory. The resulting BEGs were amplified, band-pass filtered (30 Hz to 1 kHz) using a Gould 2400S amplifier (Gould-Nicolet Technologies, Ilford, Essex, UK), and digitized using an analog-to-digital converter at a sampling frequency of 5 kHz (CED 1401plus, Cambridge Electronic Design, Cambridge, UK). Conduction curves were constructed using Spike2 software (Cambridge Electronic Design) and used to measure the EGD and VERP.
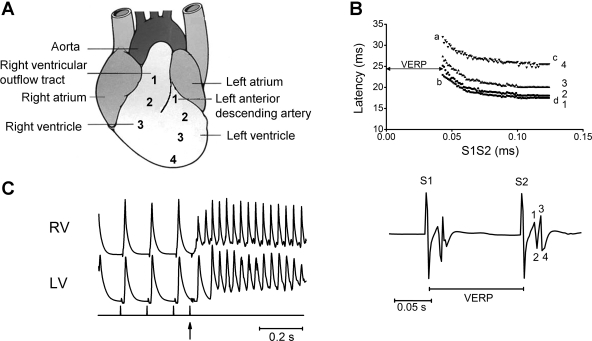
Measurements were made from three positions on the RV and four positions on the LV, evenly spaced at 10-mm intervals from base to apex, with the stimulating electrode at 10 mm from each. These positions were kept constant through all experiments. This distance allowed the hearts to be placed into the rig, with the stimulating electrode coming into contact with the septum and the recording electrodes coming into contact with the LV and RV. As the clamp was secure throughout experiments, this distance was constant to ∼0.5 mm, i.e., 5% of the distances between stimulating and recording electrodes. Figure 1A illustrates a typical mouse heart, showing the seven recording regions, three in the RV and four in the LV. Region 1 in the RV was at its base, as close as possible on the epicardial surface to the underlying RVOT. Region 3 in the RV and region 4 in the LV were positioned as apically as possible. While the direct absolute distance between electrodes would not necessarily reflect the path through which the electrical signal would be conducted in the spherical whole heart, the fact that the distance was maintained between experiments allowed consistent measurements of response latencies. For each run, two simultaneous recordings were made from adjacent regions of the heart. For example, recordings from regions RV1 and RV2 were made concurrently, and then recordings made from regions RV2 and RV3. In addition, some recordings were made simultaneously from the RVOT and base of the LV.
Fig. 1.
A: typical mouse heart, showing the 7 recording regions: 3 in the right ventricle (RV) and 4 in the left ventricle (LV). B: typical peak and trough bipolar electrogram (BEG) data obtained in the course of a programmed electrical stimulation (PES) procedure (bottom) and resulting conduction curve constructed using an experimental paced electrogram fractionation analysis (PEFA) procedure (top) (see methods for details). C: typical trace showing simultaneous recording from the RV outflow tract (RVOT) and LV outflow tract (LVOT) at the initiation of ventricular tachycardia (VT), showing earlier onset in the RVOT. Vertical markings denote timings of the pacing (S1) and the extra-systolic (S2) stimuli (arrowed). VERP, ventricular effective refractory period.
Figure 1B, bottom, illustrates typical peak and trough BEG data obtained in the course of a programmed electrical stimulation procedure. Conduction curves (top panel) were next constructed using an experimental paced electrogram fractionation analysis (PEFA) procedure translated from clinical practice (52). The response latencies (ms) of each BEG deflection, defined as the time differences between the extra stimulus (S2) and the peaks and troughs of the resulting BEG, are plotted against the corresponding S1-S2 interval (ms).
The VERP, defined as the shortest S1-S2 interval that elicits an electrogram response in the absence of absolute refractoriness, is given by the abscissa of point a. In addition, the effect of heterogeneous refractoriness on the difference between cardiac regions was expressed as the difference between neighboring VERP values. The EGD describes the degree of spread of conduction velocities at any given S1-S2 interval and represents the time difference between the first and last BEG peaks. EGD ratios were computed by normalizing the EGD obtained at the shortest S1-S2 interval (a − b) at which the S2 extra stimulus resulted in a BEG, with the EGD obtained at the longest S1-S2 interval (c − d) to give the expression (a − b)/(c − d).
MAP recording.
MAPs were recorded using electrodes constructed from two pieces of galvanically chlorided, Teflon-coated, 0.25-mm-diameter silver wire twisted together to form one contact electrode and one reference electrode. This was either placed against the cardiac surface for epicardial recordings or introduced into the ventricular cavity through a small access window created in the ventricular wall for endocardial recordings. MAP signals were amplified and band-pass filtered between 0.1 and 300 Hz (Neurolog AC amplifiers and filters models NL104 and NL125/6, respectively; Digitimer, Welwyn Garden City, Herts, UK), then digitized using a 1401plus interface (Cambridge Electronic Design). Paired platinum stimulating electrodes paced the heart on the interventricular septum at twice threshold voltage. Again, measurements were made at the same three positions on the RV and four positions on the LV, with the stimulating and recording electrodes clamped 10 mm apart from one another.
The presence or absence of VT, each episode defined as lasting at least 1 s, was noted for each run. For measurement of APDs, hearts were paced at 8 Hz for 5 min, and recordings were made at the seven epicardial sites. MAP waveforms were analyzed using Spike2 software (Cambridge Electronic Design). The point of maximum positive deflection was considered the point of 0% repolarization; that of full return to baseline of 100% repolarization, allowing the time to 90% repolarization, the APD90, to be measured. The effect of heterogeneous repolarization on the difference between cardiac regions was expressed empirically as the difference between neighboring APD values. Response latencies were calculated from the stimulus time to time of maximum depolarization.
For investigation of restitution properties, hearts underwent a dynamic pacing protocol. This comprised cycles each consisting of 100 stimuli delivered over a range of basic cycle lengths (BCLs). Steady states were consistently reached over the first 50 responses in each cycle, and thus mean epicardial and endocardial APD90 values and diastolic intervals (DIs), given by the difference between the BCL and the preceding APD90, were calculated from the final 50 APs of each cycle. With each successive cycle, BCL was decremented in 5-ms steps from an initial value of 175 ms. Cycles were continued until a reproducible sequence of consistently shaped waveforms was no longer obtained. Traces were analyzed for the presence or absence of alternans, as reflected in alternating short-long-short sequences in APD at the shortest BCLs. Alternans was judged to have occurred when alternate APDs differed by >5% over at least 10 beats. Alternans was compared in the recordings made simultaneously from two adjacent cardiac regions and labeled as either concordant, if the APs with the larger duration were synchronized, or discordant, if the APs with the larger duration in one region were synchronized with the APs with the shorter duration in a neighboring area. The BCL and DI at which the recording progressed from normal APs, through concordant and discordant alternans, to VT, were noted. For each run, the APD90 was then plotted against the preceding DI to form a restitution curve. These graphs were fitted with exponential functions of the type:
where y represents APD, x represents DI and y0, and A and τ are constants obtained by least squares fitting to the experimental values of APD and DI in each case. From them, the DIs at which the gradients equaled unity (the “critical DI” or DIcrit) were calculated from the equation:
Statistical procedures.
A set of 16 WT and 16 Scn5a+/− mice were used for MAP and BEG measurements, with one-half of each group exposed to flecainide and one-half to quinidine. All results were expressed as mean ± SE values. The significance of differences in APD, VERP, EGD ratio, response latency, and the gradients of APDs and VERPs were analyzed using ANOVA with post-hoc Tukey's honestly significant difference tests. Differences were analyzed at a significance level of P < 0.05. The differences in incidence of arrhythmia and in incidence of alternans were analyzed by Fisher exact tests. Correlation between DIs at which alternans occurred and at which restitution slopes equaled unity were analyzed by Pearson's correlation.
RESULTS
VT occurs earlier in the RVOT than at the base of the LV.
Scn5a+/− hearts showed higher incidences of VT than WT (25 vs. 6%, Fisher exact test: P = 0.0003, n = 80). Simultaneous MAP recordings using pairs of electrodes applied to the base of the RV and LV demonstrated that, although they eventually involved the entire heart, ventricular arrhythmias in Scn5a+/− hearts appeared to begin at earlier times in the RVOT than at the base of the LV (mean time difference = 54.2 ± 8.3 ms, one sample t-test: P = 0.0073, n = 4), as shown in the example in Fig. 1C. There was only one episode of VT in the WT hearts, which did not significantly differ in its time of onset between LV and RV. In addition, when the electrode pairs were applied to different positions in the RV, these further suggested earlier onsets of such arrhythmias at position RV1 (RVOT) than RV2, with a mean time difference of 10.3 ± 2.5 ms (one sample t-test: P = 0.0259, n = 4). These preliminary findings were thus consistent with clinical reports of arrhythmia onset at the RVOT.
Electrograms are more fractionated in the RVOT in Scn5a+/− hearts.
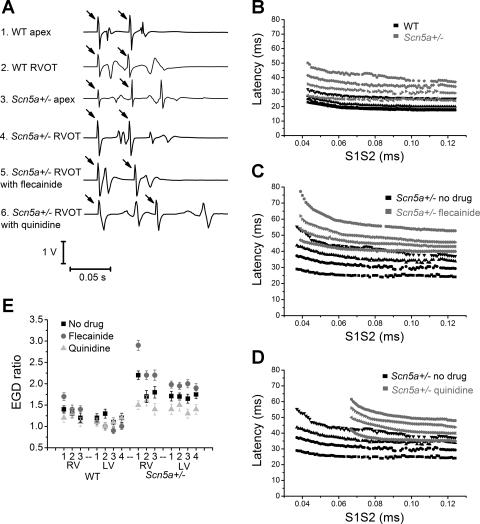
Figure 2A compares typical BEG traces from the RV apex or RVOT of WT and Scn5a+/− hearts, either before or after treatment with either flecainide or quinidine. It displays BEG deflections obtained in response to the last S2 stimulus that elicited APs and the immediately preceding S1 stimulus. Each trace contains deflections arising successively from the S1 stimulus artifact (arrowed), its BEG response, and the S2 stimulus artifact (arrowed), and its BEG response.
Fig. 2.
A: typical BEG traces from RV apex or RVOT of wild-type (WT) and Scn5a+/− hearts, either before drug or after treatment with either flecainide or quinidine. The arrows denote stimulus artifacts, the first in each trace from an S1 stimulus and the second from an S2 stimulus. B–D: comparison of conduction curves from the base of the RV in a WT heart and in an Scn5a+/− heart (B), and at the base of an Scn5a+/− heart before and after flecainide (C) or quinidine (D). E: electrogram duration (EGD) ratios calculated from WT and Scn5a+/− hearts in 7 positions across the heart, before and after the addition of flecainide or quinidine.
From the traces, calculations of the latencies of each of the responses were first made. EGDs were then determined from the time difference between the initial and final BEG deflections. The EGD ratio was calculated by normalizing the EGD obtained at the shortest S1S2 interval to one following the S2 stimuli imposed at the longest S1S2 intervals in the stimulus cycle. (The latter is indistinguishable from that following the S1 stimulus in Fig. 2A.) Traces of this kind obtained over the full range of S1S2 intervals can be quantified using conduction curves. Figure 2, B–D, compares typical conduction curves obtained from the base of the RV in a WT heart and in an Scn5a+/− heart (B), and in an Scn5a+/− heart before and after flecainide (C) or quinidine (D) administration.
Figure 2E displays EGD ratios derived from such conduction curves graphically from the seven regions of WT and Scn5a+/− ventricles before and after drug treatment. A larger EGD ratio has previously been associated with arrhythmogenesis in Scn5a+/− mice. We similarly found that, if EGD ratio values are pooled, they are significantly larger in the Scn5a+/− hearts (1.75 ± 0.08 vs. 1.24 ± 0.10, P value 0.0004, n = 112). EGD ratios are also larger in the RVOT of Scn5a+/− hearts than WT (2.2 ± 0.10 vs. 1.4 ± 0.08, P value < 0.0001, n = 16). However, on further analysis, we found that while the ratios are not significantly different between cardiac regions in WT hearts, they were significantly larger in the base of the RV than in either the base of the LV (2.2 ± 0.10 vs. 1.7 ± 0.11, P value = 0.002, n = 16), or than in the rest of the RV (2.2 ± 0.10 vs. 1.7 ± 0.10, P = 0.001, and 1.8 ± 0.10, P = 0.008, at positions RV2 and RV3, respectively, n = 16). The ratios were not significantly changed by quinidine in either WT or Scn5a+/− hearts. However, the ratios were increased by flecainide at the RVOT in WT hearts and Scn5a+/− hearts (from 1.4 ± 0.1 to 1.7 ± 0.1 in WT hearts, P = 0.05, n = 8, and from 2.2 ± 0.1 to 2.9 ± 0.1 in Scn5a+/− hearts, P = 0.0002, n = 8) and also in the rest of the RV in Scn5a+/− hearts (P < 0.05).
VERP heterogeneity is greatest at the RVOT in Scn5a+/− hearts.
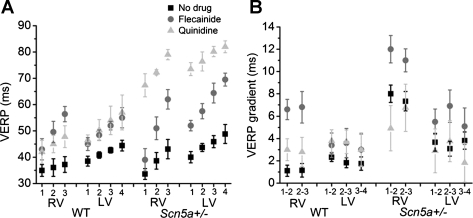
Data of the kind illustrated in Fig. 2A also permitted comparisons of VERP recordings obtained from the S1S2 intervals in stimulus cycles that last triggered APs following the S2 stimulus. These demonstrated that, in both the WT and Scn5a+/− hearts, there was a gradient of refractory period existing along the long axis of both the RVs and the LVs, such that the VERP was larger at the apex than the base (Fig. 3A) (e.g., 37.2 ± 3.0 ms at apex compared with 34.9 ± 2.3 ms at the base for RV in WT hearts, P = 0.05, n = 16). These VERP gradients were slightly, but not significantly, larger in the LV than the RV in WT hearts. However, this pattern was reversed in the Scn5a+/− hearts, where the gradient was significantly larger in the RV than the LV (Fig. 3B) (e.g., 3.7 ± 0.8 ms in LV1–2 compared with 8.0 ± 0.8 ms in RV1–2, P = 0.0007, n = 16). Thus VERP gradients were larger in Scn5a+/− hearts than WT only in the RV.
Fig. 3.
VERP values (A) and VERP gradients (B) between 7 recording regions of the heart, in both WT and Scn5a+/− hearts, before and after the addition of flecainide and quinidine. In B, for example, “1–2” denotes the difference in VERP between recording positions 1 and 2.
Flecainide significantly increased the VERP values in both the RV and LV in both WT and Scn5a+/− hearts (e.g., from 33.3 ± 2.1 to 39.0 ± 4.3 ms in RV1 in Scn5a+/−, P = 0.01, n = 8). It also significantly increased the VERP gradients in the RV and the LV of Scn5a+/− hearts and in the RV of WT hearts. It also led to a larger gradient in the RV than the LV in both WT and Scn5a+/− hearts (5.5 ± 1.1 ms in LV1–2 compared with 11.0 ± 1.2 ms in RV1–2 in Scn5a+/−, P = 0.0045, n = 8). Quinidine significantly increased the VERP values in both RV and LV in WT and Scn5a+/− hearts, but did not significantly alter the gradients either in WT or in Scn5a+/− hearts.
Response latencies are more heterogeneous at the RVOT.
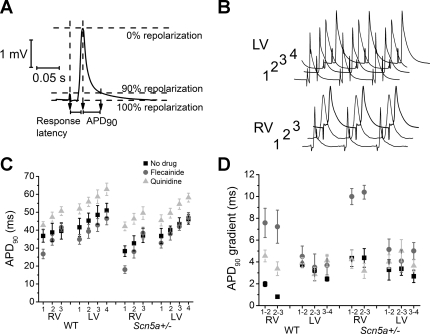
Figure 4A shows a typical MAP recording, demonstrating APD and latency measurements. Response latencies were larger in Scn5a+/− than WT hearts (32.1 ± 2.9 vs. 15.6 ± 2.1 ms, P < 0.0001, n = 112 as a total of recordings from all regions). They were increased by flecainide in WT (to 26.2 ± 2.0 ms, P = 0.0019, n = 56) and Scn5a+/− (to 44.1 ± 4.0 ms, P = 0.025, n = 56), and by quinidine in WT hearts (to 23.3 ± 3.2 ms, P = 0.04, n = 56), but not Scn5a+/−. There was no obvious difference in mean response latency between different regions of the RV and LV; however, our method would be unlikely to detect subtle changes in latency, as it measures the entire conduction path between stimulating and recording electrode. It is impossible to keep this distance constant to each recording electrode, as the detailed three-dimensional conduction pathway in the heart is likely to be different in different regions. However, on closer examination of traces recorded from the RV1 position in Scn5a+/− hearts, i.e., at the base of the RV closest to the RVOT, while the overall mean response latency was not significantly different from other regions, some traces had noticeably longer response latencies than the surrounding tissue. This was reflected in the larger standard deviation of values (14.3 in RVOT vs. 9.8 in rest of RV for Scn5a+/− hearts). This effect was accentuated in the presence of flecainide (SD = 16.9).
Fig. 4.
A: typical monophasic action potential (MAP) recording from the RV of an Scn5a+/− heart, showing action potential duration (APD) and latency measurements. B: typical MAP recordings taken from the 7 recording positions in the RV and LV of a Scn5a+/− heart. C and D: APD to 90% repolarization (APD90) values (C) and APD90 gradients (D) between 7 recording regions of the heart, in both WT and Scn5a+/− hearts, before and after the addition of flecainide and quinidine. In D, for example, “1–2” denotes the difference in VERP between recording positions 1 and 2.
Repolarization heterogeneity is greatest in Scn5a+/− hearts at the RVOT.
Figure 4B shows typical traces of MAPs from the RV and LV of a WT heart. APD values (Fig. 4C) and their gradients (D) are then shown in a graphical format.
APD values were generally significantly smaller in the Scn5a+/− hearts than WT in the RV, while gradients were larger (e.g., 4.3 ± 0.8 vs. 2.0 ± 0.2 ms in RV1–2, P = 0.0091, n = 16). However, gradients of APD existed along the long axis of both WT and Scn5a+/− hearts. The largest values were observed at the apex of the heart and the smallest at the base (e.g., 28.3 ± 3.0 ms at base vs. 37.0 ± 3.9 ms at apex, P = 0.036, n = 16 for RV of Scn5a+/− hearts). This was true in both the RV and LV. However, the gradient was larger in the RV than the LV in both WT and Scn5a+/− (e.g., 4.4 ± 0.8 ms in RV1–2 vs. 3.3 ± 0.9 ms in LV1–2, P = 0.048, n = 16 in Scn5a+/−).
The addition of flecainide reduced the APD in both WT and Scn5a+/− hearts, and particularly so in the RV (e.g., from 28.3 ± 3.0 to 18.0 ± 2.1 ms, P = 0.0082, n = 16 in position RV1 in Scn5a+/− hearts, P < 0.01), and so a larger difference between RV and LV emerged. The gradient of APD values across the ventricle was increased by flecainide, most prominently in the RV. This was particularly true in the case of the Scn5a+/− hearts (e.g., from 4.3 ± 0.8 to 10.0 ± 0.7 ms, P = 0.0034, n = 16) in RV1–2 in Scn5a+/− hearts (P < 0.01). Quinidine increased APD values in both WT and Scn5a+/− hearts. It increased the APD gradients in the WT hearts, but left the Scn5a+/− hearts unchanged, both in the LV and RV.
The RVOT of Scn5a+/− hearts shows increased incidences of discordant alternans.
During dynamic pacing protocols, a proportion of the experimental hearts displayed transitions from regular APs into alternans, in the form of alternating AP waveforms with reductions in BCL.
Through the use of simultaneous recordings using two electrodes in adjacent areas of myocardium, the present experiments permitted us to distinguish these into concordant and discordant alternans. Such episodes always began with a concordant alternans between adjacent areas. However, in a proportion of cases, these would progress to a discordant pattern. Thus the longer APD in one trace would coincide with the shorter APD in the trace from an adjacent region. In a proportion of cases, such discordant alternans would then degenerate into VT. The incidence of discordant alternans in a particular ventricular region could not be directly correlated with the initiation of VT, as our restriction to two recording sites precluded accurate mapping.
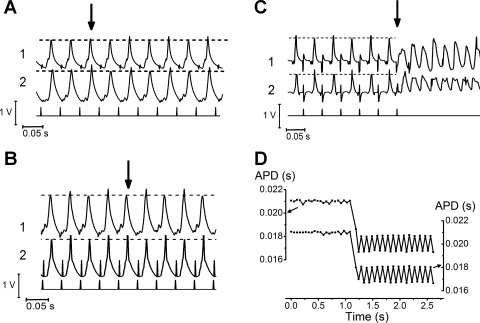
Figure 5, A–C shows typical traces from positions RV1 and RV2 of an Scn5a+/− heart first transitioning from regular APs to concordant alternans (A), from concordant alternans to discordant alternans (B), and from discordant alternans into VT (C). Figure 5D displays graphically the change from normal APs to alternans on changing to a shorter BCL. Table 1 shows the proportion of runs in adjacent cardiac regions in WT and Scn5a+/− hearts that resulted in either concordant alternans or discordant alternans. Figure 6, A–C, shows the mean values of BCL at which hearts transitioned from normal APs to concordant alternans, and then to discordant alternans both before drug (A) and after flecainide (B) or quinidine (C). Results from two simultaneous recordings of each of the adjacent regions in WT (solid squares) and Scn5a+/− (shaded squares) hearts are shown.
Fig. 5.
A–C: traces from the position RV1 and RV2 (labeled 1 and 2) of an Scn5a+/− heart first transitioning from regular action potentials (APs) to concordant alternans (A), from concordant alternans to discordant alternans (B), and from discordant alternans into VT (C). Transition points are arrowed. Dotted lines along the AP peaks are included to emphasize the change in morphology, although transition points were quantified in the form of APDs and not AP amplitudes. D: graph showing the transition from normal APs to alternans on changing to a shorter basic cycle length (BCL).
Table 1.
Incidence of concordant and discordant alternans
| RV1-2 | RV2-3 | LV1-2 | LV2-3 | LV3-4 | ||
|---|---|---|---|---|---|---|
| WT | ||||||
| No drug | Concordant | 8/16 | 0/16 | 0/16 | 8/16 | 0/16 |
| Discordant | 0/16 | 0/16 | 0/16 | 0/16 | 0/16 | |
| Flecainide 10 μM | Concordant | 4/8 | 5/8 | 5/8 | 4/8 | 4/8 |
| Discordant | 2/8 | 1/8 | 1/8 | 0/8 | 0/8 | |
| Quinidine 5 μM | Concordant | 5/8 | 0/8 | 0/8 | 5/8 | 5/8 |
| Discordant | 1/8 | 0/8 | 0/8 | 0/8 | 0/8 | |
| Scn5a+/− | ||||||
| No drug | Concordant | 6/16 | 4/16 | 4/16 | 6/16 | 5/16 |
| Discordant | 6/16 | 4/16 | 3/16 | 4/16 | 3/16 | |
| Flecainide 10 μM | Concordant | 7/8 | 5/8 | 4/8 | 5/8 | 4/8 |
| Discordant | 6/8 | 4/8 | 2/8 | 3/8 | 3/8 | |
| Quinidine 5 μM | Concordant | 6/8 | 3/8 | 2/8 | 4/8 | 3/8 |
| Discordant | 2/8 | 1/8 | 0/8 | 1/8 | 0/8 |
Number of runs in adjacent cardiac regions in wild-type (WT) and Scn5a+/− hearts that resulted in either concordant alternans or discordant alternans. The denominator is the total number of runs at each region.
RV, right ventricle; LV, left ventricle. 1, 2, 3, and 4: regions 1, 2, 3, and 4.
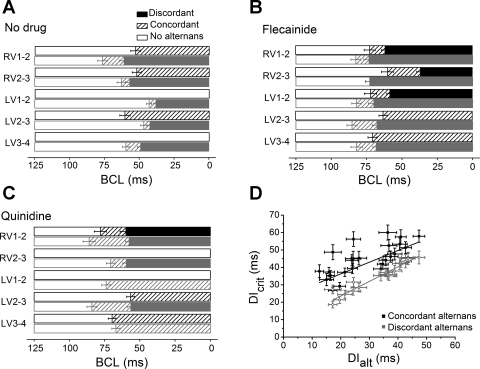
Fig. 6.
A–C: mean values of BCL at which hearts transitioned from normal APs (open bars) to concordant alternans (hatched bars), and then to discordant alternans (solid and shaded bars) both before drug (A) and after flecainide (B) or quinidine (C). Results from two simultaneous recordings of each of the adjacent regions in WT (solid bars) and Scn5a+/− (shaded bars) hearts are shown. D: critical diastolic interval (DIcrit) plotted against DI at which alternans commenced (DIalt), showing a relatively poor correlation between DIcrit and the initiation of concordant alternans (Pearson's correlation 0.70, P < 0.01), but a strong correlation between DIcrit and the initiation of discordant alternans (Pearson's correlation = 0.97, P < 0.01).
Overall, the incidence of concordant alternans was not distinguishable between Scn5a+/− and WT hearts, or before or after drug. However, Scn5a+/− but not WT hearts showed significant incidences of discordant alternans (25% compared with 0%, respectively, P < 0.001, n = 80). This incidence was greater in the RV compared with the LV (31% compared with 21%, P = 0.048, n = 48 for LV and 32 for RV). There were also significantly greater incidences of discordant alternans between positions RV1 and RV2, i.e., near the base of the RV, close to the RVOT, compared with the rest of the RV in Scn5a+/− hearts (from 22 to 38%, P = 0.020, n = 16). Flecainide increased incidences of discordant alternans in WT hearts (from 0 to 10%, P < 0.0001, n = 40) and in Scn5a+/− hearts (from 25 to 40%, P = 0.009, n = 40). Quinidine had no significant impact on discordant alternans in WT hearts, but significantly reduced the incidence of discordant alternans in Scn5a+/− hearts to 10% (P = 0.009, n = 40).
Similarly, if results from all regions are pooled, the BCLs at which hearts transitioned from normal APs to concordant alternans in either WT or Scn5a+/− hearts were not significantly different, before or after drug. However, Scn5a+/− hearts transitioned to discordant alternans at significantly earlier (i.e., larger) BCLs than WT hearts (which, in fact, did not show discordant alternans at all). The base of the RV showed earlier transitions into discordant alternans than other regions of Scn5a+/− hearts (e.g., 76.3 ± 3.4 ms in RV1–2 compared with 62.6 ± 3.2 ms in RV2–3, P = 0.006, n = 16). Both WT and Scn5a+/− hearts transitioned to discordant alternans significantly earlier in the presence of flecainide (e.g., 92.6 ± 3.5 ms, P = 0.005, n = 8 for RV1–2 in Scn5a+/− hearts). Quinidine increased the BCL at which WT hearts transitioned to discordant alternans (from no discordant alternans), but significantly decreased the BCL at which Scn5a+/− hearts did so (e.g., from 37.92 ± 1.9 ms to no discordant alternans, P < 0.0001, n = 8 for LV1–2 in Scn5a+/− hearts).
Scn5a+/− hearts have steeper restitution curves at the RVOT.
Figure 7 shows restitution curves, plotting APD against the preceding DI, from seven regions in WT (A) and Scn5a+/− (C) hearts, and in RV1 before drug and after flecainide or quinidine in WT (B) and Scn5a+/− (D) hearts. The vertical lines represent the DIcrit for each curve, at which the gradient equals unity. The results are shown graphically in the seven regions of the heart (Fig. 7E) and in RV1 before and after flecainide or quinidine (F).
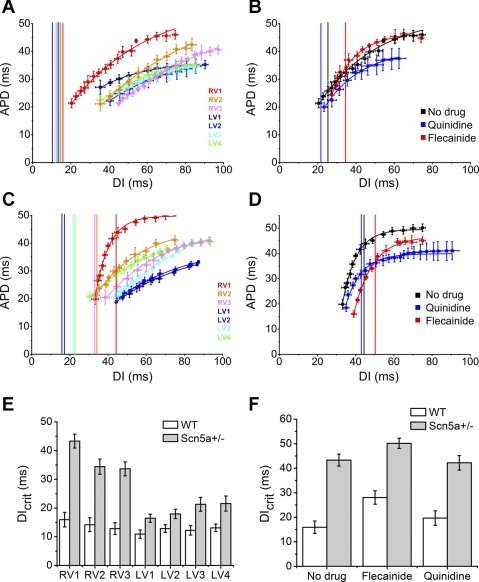
Fig. 7.
A–D: restitution curves, plotting APD against the preceding DI, from 7 regions in WT (A) and Scn5a+/− (C) hearts, and in RV1 before drug and after flecainide or quinidine in WT (B) and Scn5a+/− (D) hearts. The graphs are fitted with exponential functions, and from them the DIs at which the gradients equal unity (the DIcrit) have been calculated and are shown by the vertical lines. The results are shown graphically in the 7 regions of the heart (E) and in RV1 before and after flecainide or quinidine (F).
Values of DIcrit were not significantly different from each other in any of the seven regions of the WT hearts and fell to the left of the restitution curves. However, in Scn5a+/− hearts, DIcrit was significantly larger in the RV than the LV, and significantly larger in position RV1 than in the rest of the RV (e.g., 43.3 ± 2.4 ms in RV1 compared with 34.4 ± 2.6 ms in RV2, P = 0.018, n = 16). Values of DIcrit were significantly larger in Scn5a+/− hearts than in WT in all three regions of the RV and in positions LV3 and LV4 (P < 0.05, n = 16). Upon addition of both quinidine and flecainide, DIcrit was significantly increased in WT hearts (e.g., from 15.9 ± 2.54 to 19.7 ± 2.96 ms, P = 0.03, n = 8 with quinidine and to 28.06 ± 2.74 ms, P = 0.003, n = 8 with flecainide in RV1–2), while, in Scn5a+/− hearts, DIcrit was significantly increased by flecainide (e.g., from 43.3 ± 2.42 to 50.2 ± 2.05 ms, P = 0.02, n = 8 in RV1–2), but unchanged by quinidine.
Previous experiments have failed to establish a strong correlation between the DIcrit and the presence of alternans or VT, finding that alternans may occur at low-restitution slopes, or that steep restitution slopes did not produce alternans or VT. Our experiments were unable to provide evidence for a correlation with VT, as our technique did not allow us to identify the origin of VT, as this spread rapidly through the whole heart. However, we did investigate the relationship between DIcrit and the presence of alternans. Figure 6D plots DIcrit against the DI at which alternans commenced. This shows that there is a relatively poor correlation between DIcrit and the initiation of concordant alternans (Pearson's correlation 0.70, P < 0.01). However, there is a strong correlation between DIcrit and the initiation of discordant alternans (Pearson's correlation = 0.97, P < 0.01).
DISCUSSION
Our murine whole heart preparation provides a specific genetic loss of sodium channel function model without the need for pharmacological manipulation, while also allowing easy access to the epicardial surface of the heart for multiple simultaneous recordings not readily feasible in clinical studies. Our use of a wide range of techniques successfully localized the abnormal electrophysiological properties in our BrS model to the base of the RV, at the RVOT. This area showed increased heterogeneities in APDs, VERPs, EGD ratios, and response latencies. This spatial heterogeneity closely correlated with an increased incidence of discordant alternans, and with steeper restitution slopes. All of the abnormalities demonstrated were exacerbated by flecainide, but not quinidine.
It is known that, in healthy hearts, the LV and RV are structurally heterogeneous, with regional differences in shape, wall thickness, and fiber orientation. They show regional differences in APD, demonstrable both in the intact heart and in myocytes isolated from the different regions. In general, APs are longer at early activation sites and shorter at late activation sites (17), resulting in relatively homogeneous recovery times. Thus a degree of nonuniformity in conduction and refractory period is physiological (9).
There is already a considerable body of work examining roles of increased heterogeneity of electrophysiological properties in arrhythmogenesis. However, much of this has focused on transmural differences rather than base-apex gradients. Thus increased transmural gradients of APD across the RV wall have been found in the canine wedge preparation (61) and in the genetic Scn5a+/− mouse model (29). This is consistent with heterogeneous Ito channel distributions with higher densities in the epicardial tissue than in the endocardium.
However, there are fewer studies examining heterogeneity within the ventricle from base to apex. Available results are conflicting. Larger APDs and smaller K+ currents were found in apex than base in rabbit hearts (12, 51), ferret ventricle (8), and human myocytes (27); smaller APDs in apex than base in pig hearts (15) and canine myocytes (54); gradients in epicardial APD running in an oblique direction from the LV apex to the right atrioventricular groove (26); or indeed no gradient at all in guinea pig hearts (10). Our experiments in murine hearts showed smaller APDs and VERPs at the RVOT than elsewhere and also demonstrated a steeper gradient of values in this region.
Clinical increases in this apex-base dispersion have been associated with arrhythmogenesis in cardiomyopathies and have been linked to an increased incidence of T-wave alternans and VT (11). Although some clinical studies have been unable to demonstrate repolarization abnormalities (43), in animal BrS models, increased dispersion of repolarization within the ventricle, although not necessarily along an apex-base gradient, has been linked to arrhythmogenesis. Thus data from the canine RV wedge preparation implicate phase 2 reentry as a result of epicardial dispersion of repolarization as the trigger for ventricular arrhythmia (1, 34).
As well as demonstrating increased heterogeneities of APDs and VERPs, we also show increased EGDs specifically in the RVOT of Scn5a+/− hearts. The latter were demonstrated using PEFA, which is also used clinically to stratify arrhythmogenic risk in terms of alterations in EGD (52). These parameters are derived from families of ventricular conduction curves, which reflect the presence or otherwise of arrhythmogenic reentrant pathways. PEFA has been applied experimentally on earlier occasions to assess arrhythmic tendency and its physiological basis in KCNE1−/− (5) and Scn5a+/Δ mice (20). Previous studies in Scn5a+/− mice have shown increased fractionation in the LV (53). Our experiments localize particular increases in EGD to the base of the RV, near to the RVOT. Clinical studies have also demonstrated increased fractionation and longer electrograms in BrS patients, although, in this case, clustering of the widest electrograms was seen in a range of RV regions (44).
Our results showed both increased heterogeneity of response latencies, combined with larger APD and VERP gradients in the RVOT of Scn5a+/− hearts. It is possible that increased repolarization dispersion is produced through electrotonic modulation by discontinuous propagation of activation, as proposed in other studies (39, 63). Further work mapping activation and repolarization times in the Scn5a+/− heart could shed light on this mechanism. Nevertheless, our use of MAP measurements did permit direct visualizations of AP waveform, and the presence or absence of either concordant or discordant alternans, which would only indirectly be apparent from ARIs offered by mapping methods. Our present experiments suggest that spatial heterogeneity in the RVOT may then promote the transition of concordant alternans to discordant alternans. Electrical alternans has been associated with arrhythmogenesis in both clinical (48) and experimental (41) studies.
Several hypotheses exist for the cellular mechanism for the AP alternans itself; although there is a likely role for SR calcium cycling, APD restitution is also key. The latter specifically concerns the attenuation of APD that occurs in response to faster heart rates and is thought to be an adaptive mechanism for preserving DI. At a given cycle length, APD prolongation during one beat is necessarily followed by a short DI, which will shorten APD in the following beat, causing repeated short-long cycles.
Alternans phenomena have classically been analyzed by the construction of restitution curves relating APD to the preceding DI as heart rate is varied (38). The “restitution hypothesis” states that APD alternans will occur when the slope of the APD restitution curve exceeds unity. However, this has primarily been based on modeling studies (47, 58), and the corresponding experimental evidence is conflicting. Relationships between restitution curve slopes and arrhythmogenicity have been established both in clinical BrS (19) and in the Scn5a+/− mouse model (49). More recently, a predilection of the RV for alternans has been found in the Scn5a+/− mouse model (30). However, other studies have found a more complex relationship between the onset of alternans and restitution slopes (6, 13).
We here demonstrate that the incidence of concordant alternans is not significantly changed by the Scn5a+/− mutation, or by drug addition. Furthermore, there is only a weak correlation between the presence of concordant alternans and the restitution slope. However, the incidence of discordant alternans and the transition time from concordant to discordant alternans is strongly correlated with restitution slope and is associated specifically with the RVOT in Scn5a+/− hearts.
Thus, although concordant AP alternans itself is not necessarily arrhythmogenic, it is a prerequisite for discordant alternans. The cellular mechanism responsible for the change from concordant to discordant alternans has not yet been fully elucidated. While, in some cases, it is possible that spatial heterogeneities of calcium handling are the cause, in others the change in cycle length acts as a premature stimulus, which can propagate more slowly into partially repolarized myocardium. The conduction slowing prolongs the DI between the next beats in the downstream myocardium. Because of APD restitution, downstream myocytes will undergo prolongation instead of shortening of APD, causing a switch in phase of APD relative to the upstream myocytes (60). In regions with preexisting spatial heterogeneities, a switch to discordant alternans will be more likely.
This transformation from concordant to discordant alternans will, in turn, have significant consequences on the spatial organization of repolarization across the ventricle (60). The discordant alternans can amplify the heterogeneities of repolarization present at baseline into pathophysiological heterogeneities of sufficient magnitude to produce conduction block and reentrant excitation (41). Discordant alternans also produces a substrate by which conduction block and reentrant excitation can be easily initiated by a premature stimulus. Consequently, discordant alternans is a mechanism linking discordant alternans to cardiac arrhythmogenesis and explains why, in our studies, VT never occurs without discordant alternans.
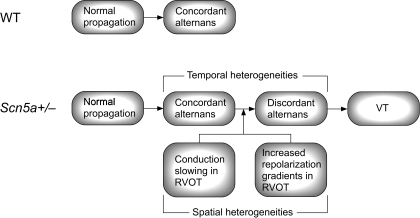
In summary, in the RVOT of Scn5a+/− hearts, there exist spatial heterogeneities of both depolarization and repolarization. Ventricular pacing may induce concordant alternans in both WT and Scn5a+/− hearts. However, while, in WT hearts, concordant alternans will continue as a protective mechanism, in Scn5a+/− hearts, the spatial electrophysiological heterogeneities will act as a substrate to drive the alternans into discordance. This, in turn, can magnify the spatial heterogeneities and lead to reentrant circuits and VTs. This scheme is summarized in Fig. 8.
Fig. 8.
Scheme of arrhythmogenic mechanism. On pacing, both WT and Scn5a+/− hearts may undergo transition from normal propagation to concordant alternans. However, spatial heterogeneities in the RVOT of Scn5a+/− hearts then drive the rhythm into discordance and subsequent arrhythmogenesis.
While other papers have examined transmural differences (29), the small size of the mouse heart makes accurate placement of multiple recording electrodes in the endocardium technically challenging, and thus the focus of this paper was heterogeneities confined to the epicardium. The small size also means that the total number of recording sites was limited to three on the right epicardium and four on the left epicardium. The consequent low spatial resolution makes it more difficult to confidently make inferences about the ability of the demonstrated heterogeneities to lead to reentrant circuits. Finally, strong electrotonic forces may act to minimize the effect of any spatial heterogeneities that are created and reduce their potential to create reentrant substrate. However, despite these limitations, we succeeded in showing clear gradients of APDs, VERPs, and EGDs, and linking these to an increased incidence of discordant alternans and earlier onset of VT at the RVOT.
Previous studies have highlighted the effect of ageing in the Scn5a+/− model on conduction velocities and RV structural changes (56). The mean age of sudden death in BrS patients is 41 yr (4), and thus we chose to use “middle-aged” mice between 6 and 8 mo old to model the disease. It is possible that the heterogeneities in our model are increased by the presence of areas of local fibrosis, as documented both in clinical cases of BrS (14) and in the Scn5a+/− mouse model (56).
It is currently unclear why both the depolarization and repolarization changes seen in BrS are localized to the RVOT. It is possible that there is normally a lower expression of Na+ channels in this area, which exacerbates the effect of Na+ channel loss of function in BrS, at least in the 30% of patients who display a Na+ channel mutation. In a guinea pig study, Veeraraghavan and Poelzing (57) found that, during partial Na+ channel blockade using flecainide, RV conduction velocity decreased more than LV. Furthermore, Western blotting demonstrated lower Nav1.5 expression in RV compared with LV. They concluded that the LV may have an increased depolarization reserve compared with the RV, and, while interventricular inwardly rectifying K+ current (IK1) heterogeneities may underlie conduction heterogeneities observed under control conditions, under conditions in which Na+ current is functionally reduced in disease or during pharmacological Na+ channel blockade, the heterogeneity in Nav1.5 expression may become a significant determinant of conduction heterogeneities. Alternatively, the increased Ito in the RV could influence the reduction of Na+ current to a different extent in the RV than the LV. This could explain the protective effects of quinidine in reducing the spatial and temporal electrophysiological heterogeneities and the incidence of arrhythmias.
An alternative theory proposes the presence of slow conducting tissue in the RVOT, i.e., nodelike tissue whose AP upstroke is L-type Ca2+ current (ICa,L) dependent (31). The RV has a different embryological origin from the LV (62), and the outflow tract derives from the same group of cells that compose the atrioventricular region, thus possessing slow conduction properties (32). While these nodelike cells are essential for peristaltic blood movement in the embryonic heart, which has yet to develop cardiac valves, remnants of these cells may constitute the substrate for arrhythmias originating in the RVOT. This may explain the suppression of ST elevation and arrhythmias by isoproterenol, as isoproteronol induces ICa,L increases. Conversely, smaller ICa,L expression in males than females (42) may explain higher disease prevalence in males. More molecular and immunohistochemical work is needed in this area to determine the underlying reason for this localization of effect.
GRANTS
Funding was provided by the British Heart Foundation, the Medical Research Council, the Wellcome Trust, and the Biotechnology and Biological Research Council, UK. C. A. Martin was supported by a Medical Research Council Clinical Research Fellowship and a Sackler Studentship of the University of Cambridge School of Clinical Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Aiba T, Shimizu W, Hidaka I, Uemura K, Noda T, Zheng C, Kamiya A, Inagaki M, Sugimachi M, Sunagawa K. Cellular basis for trigger and maintenance of ventricular fibrillation in the Brugada syndrome model: high-resolution optical mapping study. J Am Coll Cardiol 47: 2074–2085, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Alings M, Dekker L, Sadee A, Wilde A. Quinidine induced electrocardiographic normalization in two patients with Brugada syndrome. Pacing Clin Electrophysiol 24: 1420–1422, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Antzelevitch C. Role of spatial dispersion of repolarization in inherited and acquired sudden cardiac death syndromes. Am J Physiol Heart Circ Physiol 293: H2024–H2038, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Antzelevitch C, Brugada P, Brugada J, Brugada R. Brugada syndrome: from cell to bedside. Curr Probl Cardiol 30: 9–54, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balasubramaniam R, Grace AA, Saumarez RC, Van denberg JI, Huang CL. Electrogram prolongation and nifedipine-suppressible ventricular arrhythmias in mice following targeted disruption of KCNE1. J Physiol 552: 535–546, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Banville I, Gray RA. Effect of action potential duration and conduction velocity restitution and their spatial dispersion on alternans and the stability of arrhythmias. J Cardiovasc Electrophysiol 13: 1141–1149, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Birkett D. Therapeutic drug monitoring. Aust Prescr 20: 9–11, 1997 [Google Scholar]
- 8. Brahmajothi MV, Morales MJ, Reimer KA, Strauss HC. Regional localization of ERG, the channel protein responsible for the rapid component of the delayed rectifier, K+ current in the ferret heart. Circ Res 81: 128–135, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Burton FL, Cobbe SM. Dispersion of ventricular repolarization and refractory period. Cardiovasc Res 50: 10–23, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Burton FL, Cobbe SM. Effect of sustained stretch on dispersion of ventricular fibrillation intervals in normal rabbit hearts. Cardiovasc Res 39: 351–359, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Chauhan VS, Downar E, Nanthakumar K, Parker JD, Ross HJ, Chan W, Picton P. Increased ventricular repolarization heterogeneity in patients with ventricular arrhythmia vulnerability and cardiomyopathy: a human in vivo study. Am J Physiol Heart Circ Physiol 290: H79–H86, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Cheng J, Kamiya K, Liu W, Tsuji Y, Toyama J, Kodama I. Heterogeneous distribution of the two components of delayed rectifier K+ current: a potential mechanism of the proarrhythmic effects of methanesulfonanilideclass III agents. Cardiovasc Res 43: 135–147, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Cherry EM, Fenton FH. Suppression of alternans and conduction blocks despite steep APD restitution: electrotonic, memory, and conduction velocity restitution effects. Am J Physiol Heart Circ Physiol 286: H2332–H2341, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Coronel R, Casini S, Koopmann TT, Wilms-Schopman FJ, Verkerk AO, de Groot JR, Bhuiyan Z, Bezzina CR, Veldkamp MW, Linnenbank AC, van der Wal AC, Tan HL, Brugada P, Wilde AA, de Bakker JM. Right ventricular fibrosis and conduction delay in a patient with clinical signs of Brugada syndrome: a combined electrophysiological, genetic, histopathologic, and computational study. Circulation 112: 2769–2777, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Dean JW, Lab MJ. Regional changes in ventricular excitability during load manipulation of the in situ pig heart. J Physiol 429: 387–400, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eckardt L, Probst V, Smits JP, Bahr ES, Wolpert C, Schimpf R, Wichter T, Boisseau P, Heinecke A, Breithardt G, Borggrefe M, Le Marec H, Bocker D, Wilde AA. Long-term prognosis of individuals with right precordial ST-segment-elevation Brugada syndrome. Circulation 111: 257–263, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Franz MR, Bargheer K, Rafflenbeul W, Haverich A, Lichtlen PR. Monophasic action potential mapping in human subjects with normal electrocardiograms: direct evidence for the genesis of the T wave. Circulation 75: 379–386, 1987 [DOI] [PubMed] [Google Scholar]
- 18. Frustaci A, Priori SG, Pieroni M, Chimenti C, Napolitano C, Rivolta I, Sanna T, Bellocci F, Russo MA. Cardiac histological substrate in patients with clinical phenotype of Brugada syndrome. Circulation 112: 3680–3687, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Hayashi M, Takatsuki S, Maison-Blanche P, Messali A, Haggui A, Milliez P, Leenhardt A, Extramiana F. Ventricular repolarization restitution properties in patients exhibiting type 1 Brugada electrocardiogram with and without inducible ventricular fibrillation. J Am Coll Cardiol 51: 1162–1168, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Head CE, Balasubramaniam R, Thomas G, Goddard CA, Lei M, Colledge WH, Grace AA, Huang CL. Paced electrogram fractionation analysis of arrhythmogenic tendency in DeltaKPQ Scn5a mice. J Cardiovasc Electrophysiol 16: 1329–1340, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Hisamatsu K, Kusano KF, Morita H, Takenaka S, Nagase S, Nakamura K, Emori T, Matsubara H, Mikouchi H, Nishizaki Y, Ohe T. Relationships between depolarization abnormality and repolarization abnormality in patients with Brugada syndrome: using body surface signal-averaged electrocardiography and body surface maps. J Cardiovasc Electrophysiol 15: 870–876, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Izumida N, Asano Y, Doi S, Wakimoto H, Fukamizu S, Kimura T, Ueyama T, Sakurada H, Kawano S, Sawanobori T, Hiraoka M. Changes in body surface potential distributions induced by isoproterenol and Na channel blockers in patients with the Brugada syndrome. Int J Cardiol 95: 261–268, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Kanda M, Shimizu W, Matsuo K, Nagaya N, Taguchi A, Suyama K, Kurita T, Aihara N, Kamakura S. Electrophysiologic characteristics and implications of induced ventricular fibrillation in symptomatic patients with Brugada syndrome. J Am Coll Cardiol 39: 1799–1805, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Kurita T, Shimizu W, Inagaki M, Suyama K, Taguchi A, Satomi K, Aihara N, Kamakura S, Kobayashi J, Kosakai Y. The electrophysiologic mechanism of ST-segment elevation in Brugada syndrome. J Am Coll Cardiol 40: 330–334, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Lambiase PD, Ahmed AK, Ciaccio EJ, Brugada R, Lizotte E, Chaubey S, Ben-Simon R, Chow AW, Lowe MD, McKenna WJ. High-density substrate mapping in Brugada syndrome: combined role of conduction and repolarization heterogeneities in arrhythmogenesis. Circulation 120: 106–117, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Laurita KR, Girouard SD, Rosenbaum DS. Modulation of ventricular repolarization by a premature stimulus. Role of epicardial dispersion of repolarization kinetics demonstrated by optical mapping of the intact guinea pig heart. Circ Res 79: 493–503, 1996 [DOI] [PubMed] [Google Scholar]
- 27. Li GR, Feng J, Yue L, Carrier M, Nattel S. Evidence for two components of delayed rectifier K+ current in human ventricular myocytes. Circ Res 78: 689–696, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Martin CA, Zhang Y, Grace AA, Huang CL. In vivo studies of Scn5a+/− mice modeling Brugada syndrome demonstrate both conduction and repolarization abnormalities. J Electrocardiol 43: 433–439, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martin CA, Zhang Y, Grace AA, Huang CL. Increased right ventricular repolarization gradients promote arrhythmogenesis in a murine model of Brugada Syndrome. J Cardiovasc Electrophysiol 21: 1153–1159, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matthews GD, Martin CA, Grace AA, Zhang Y, Huang CL. Regional variations in action potential alternans in isolated murine Scn5a(+/−) hearts during dynamic pacing. Acta Physiol (Oxf) 200: 129–146, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Meregalli PG, Wilde AA, Tan HL. Pathophysiological mechanisms of Brugada syndrome: depolarization disorder, repolarization disorder, or more? Cardiovasc Res 67: 367–378, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Moorman AF, Schumacher CA, de Boer PA, Hagoort J, Bezstarosti K, van den Hoff MJ, Wagenaar GT, Lamers JM, Wuytack F, Christoffels VM, Fiolet JW. Presence of functional sarcoplasmic reticulum in the developing heart and its confinement to chamber myocardium. Dev Biol 223: 279–290, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Morita H, Fukushima-Kusano K, Nagase S, Takenaka-Morita S, Nishii N, Kakishita M, Nakamura K, Emori T, Matsubara H, Ohe T. Site-specific arrhythmogenesis in patients with Brugada syndrome. J Cardiovasc Electrophysiol 14: 373–379, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Morita H, Zipes DP, Fukushima-Kusano K, Nagase S, Nakamura K, Morita ST, Ohe T, Wu J. Repolarization heterogeneity in the right ventricular outflow tract: correlation with ventricular arrhythmias in Brugada patients and in an in vitro canine Brugada model. Heart Rhythm 5: 725–733, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Moss AJ, Kass RS. Long QT syndrome: from channels to cardiac arrhythmias. J Clin Invest 115: 2018–2024, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nagase S, Kusano KF, Morita H, Fujimoto Y, Kakishita M, Nakamura K, Emori T, Matsubara H, Ohe T. Epicardial electrogram of the right ventricular outflow tract in patients with the Brugada syndrome: using the epicardial lead. J Am Coll Cardiol 39: 1992–1995, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Nishida K, Fujiki A, Mizumaki K, Sakabe M, Sugao M, Tsuneda T, Inoue H. Canine model of Brugada syndrome using regional epicardial cooling of the right ventricular outflow tract. J Cardiovasc Electrophysiol 15: 936–941, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Nolasco JB, Dahlen RW. A graphic method for the study of alternation in cardiac action potentials. J Appl Physiol 25: 191–196, 1968 [DOI] [PubMed] [Google Scholar]
- 39. Osaka T, Kodama I, Tsuboi N, Toyama J, Yamada K. Effects of activation sequence and anisotropic cellular geometry on the repolarization phase of action potential of dog ventricular muscles. Circulation 76: 226–236, 1987 [DOI] [PubMed] [Google Scholar]
- 40. Papadatos GA, Wallerstein PM, Head CE, Ratcliff R, Brady PA, Benndorf K, Saumarez RC, Trezise AE, Huang CL, Van denberg JI, Colledge WH, Grace AA. Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene Scn5a. Proc Natl Acad Sci U S A 99: 6210–6215, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pastore JM, Girouard SD, Laurita KR, Akar FG, Rosenbaum DS. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation 99: 1385–1394, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Pham TV, Robinson RB, Danilo P, Jr, Rosen MR. Effects of gonadal steroids on gender-related differences in transmural dispersion of L-type calcium current. Cardiovasc Res 53: 752–762, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Postema PG, van Dessel PF, Kors JA, Linnenbank AC, van Herpen G, Ritsema van Eck HJ, van Geloven N, de Bakker JM, Wilde AA, Tan HL. Local depolarization abnormalities are the dominant pathophysiologic mechanism for type 1 electrocardiogram in brugada syndrome a study of electrocardiograms, vectorcardiograms, and body surface potential maps during ajmaline provocation. J Am Coll Cardiol 55: 789–797, 2010 [DOI] [PubMed] [Google Scholar]
- 44. Postema PG, van Dessel PFHM, de Bakker JMT, Dekker LRC, Linnenbank AC, Hoogendijk MG, Coronel R, Tijssen JGP, Wilde AAM, Tan HL. Slow and discontinuous conduction conspire in Brugada Syndrome: a right ventricular mapping and stimulation study. Circ Arrhythm Electrophysiol 1: 379–386, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Postema PG, Wolpert C, Amin AS, Probst V, Borggrefe M, Roden DM, Priori SG, Tan HL, Hiraoka M, Brugada J, Wilde AA. Drugs and Brugada syndrome patients: review of the literature, recommendations, and an up-to-date website (www.brugadadrugs org). Heart Rhythm 6: 1335–1341, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Priori SG, Napolitano C, Schwartz PJ, Bloise R, Crotti L, Ronchetti E. The elusive link between LQT3 and Brugada syndrome: the role of flecainide challenge. Circulation 102: 945–947, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Qu Z, Garfinkel A, Chen PS, Weiss JN. Mechanisms of discordant alternans and induction of reentry in simulated cardiac tissue. Circulation 102: 1664–1670, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Rosenbaum DS, Jackson LE, Smith JM, Garan H, Ruskin JN, Cohen RJ. Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med 330: 235–241, 1994 [DOI] [PubMed] [Google Scholar]
- 49. Sabir IN, Li LM, Jones VJ, Goddard CA, Grace AA, Huang CL. Criteria for arrhythmogenicity in genetically-modified Langendorff-perfused murine hearts modelling the congenital long QT syndrome type 3 and the Brugada syndrome. Pflügers Arch 455: 637–651, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sacher F, Probst V, Iesaka Y, Jacon P, Laborderie J, Mizon-Gerard F, Mabo P, Reuter S, Lamaison D, Takahashi Y, O'Neill MD, Garrigue S, Pierre B, Jais P, Pasquie JL, Hocini M, Salvador-Mazenq M, Nogami A, Amiel A, Defaye P, Bordachar P, Boveda S, Maury P, Klug D, Babuty D, Haissaguerre M, Mansourati J, Clementy J, LeMarec H. Outcome after implantation of a cardioverter-defibrillator in patients with Brugada syndrome: a multicenter study. Circulation 114: 2317–2324, 2006 [DOI] [PubMed] [Google Scholar]
- 51. Salata JJ, Jurkiewicz NK, Jow B, Folander K, Guinosso PJ, Jr, Raynor B, Swanson R, Fermini B. IK of rabbit ventricle is composed of two currents: evidence for IKs. Am J Physiol Heart Circ Physiol 271: H2477–H2489, 1996 [DOI] [PubMed] [Google Scholar]
- 52. Saumarez RC, Grace AA. Paced ventricular electrogram fractionation and sudden death in hypertrophic cardiomyopathy and other non-coronary heart diseases. Cardiovasc Res 47: 11–22, 2000 [DOI] [PubMed] [Google Scholar]
- 53. Stokoe KS, Balasubramaniam R, Goddard CA, Colledge WH, Grace AA, Huang CL. Effects of flecainide and quinidine on arrhythmogenic properties of Scn5a+/− murine hearts modelling the Brugada syndrome. J Physiol 581: 255–275, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Szentadrassy N, Banyasz T, Biro T, Szabo G, Toth BI, Magyar J, Lazar J, Varro A, Kovacs L, Nanasi PP. Apico-basal inhomogeneity in distribution of ion channels in canine and human ventricular myocardium. Cardiovasc Res 65: 851–860, 2005 [DOI] [PubMed] [Google Scholar]
- 55. Tukkie R, Sogaard P, Vleugels J, de Groot IK, Wilde AA, Tan HL. Delay in right ventricular activation contributes to Brugada syndrome. Circulation 109: 1272–1277, 2004 [DOI] [PubMed] [Google Scholar]
- 56. van Veen TA, Stein M, Royer A, Le Quang K, Charpentier F, Colledge WH, Huang CL, Wilders R, Grace AA, Escande D, de Bakker JM, van Rijen HV. Impaired impulse propagation in Scn5a-knockout mice: combined contribution of excitability, connexin expression, and tissue architecture in relation to aging. Circulation 112: 1927–1935, 2005 [DOI] [PubMed] [Google Scholar]
- 57. Veeraraghavan R, Poelzing S. Mechanisms underlying increased right ventricular conduction sensitivity to flecainide challenge. Cardiovasc Res 77: 749–756, 2008 [DOI] [PubMed] [Google Scholar]
- 58. Watanabe MA, Fenton FH, Evans SJ, Hastings HM, Karma A. Mechanisms for discordant alternans. J Cardiovasc Electrophysiol 12: 196–206, 2001 [DOI] [PubMed] [Google Scholar]
- 59. Wilde AA, Coronel R. The complexity of genotype-phenotype relations associated with loss-of-function sodium channel mutations and the role of in silico studies. Am J Physiol Heart Circ Physiol 295: H8–H9, 2008 [DOI] [PubMed] [Google Scholar]
- 60. Wilson LD, Rosenbaum DS. Mechanisms of arrythmogenic cardiac alternans. Europace 9, Suppl 6: vi77–vi82, 2007 [DOI] [PubMed] [Google Scholar]
- 61. Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation 100: 1660–1666, 1999 [DOI] [PubMed] [Google Scholar]
- 62. Zaffran S, Kelly RG, Meilhac SM, Buckingham ME, Brown NA. Right ventricular myocardium derives from the anterior heart field. Circ Res 95: 261–268, 2004 [DOI] [PubMed] [Google Scholar]
- 63. Zubair I, Pollard AE, Spitzer KW, Burgess MJ. Effects of activation sequence on the spatial distribution of repolarization properties. J Electrocardiol 27: 115–127, 1994 [DOI] [PubMed] [Google Scholar]