Abstract
The role of other STAT subtypes in conferring ischemic tolerance is unclear. We hypothesized that in STAT-3 deletion alternative STAT subtypes would protect myocardial function against ischemia-reperfusion injury. Wild-type (WT) male C57BL/6 mice or mice with cardiomyocyte STAT-3 knockout (KO) underwent baseline echocardiography. Langendorff-perfused hearts underwent ischemic preconditioning (IPC) or no IPC before ischemia-reperfusion. Following ex vivo perfusion, hearts were analyzed for STAT-5 and -6 phosphorylation by Western blot analysis of nuclear fractions. Echocardiography and postequilibration cardiac performance revealed no differences in cardiac function between WT and KO hearts. Phosphorylated STAT-5 and -6 expression was similar in WT and KO hearts before perfusion. Contractile function in WT and KO hearts was significantly impaired following ischemia-reperfusion in the absence of IPC. In WT hearts, IPC significantly improved the recovery of the maximum first derivative of developed pressure (+dP/dtmax) compared with that in hearts without IPC. IPC more effectively improved end-reperfusion dP/dtmax in WT hearts compared with KO hearts. Preconditioned and nonpreconditioned KO hearts exhibited increased phosphorylated STAT-5 and -6 expression compared with WT hearts. The increased subtype activation did not improve the efficacy of IPC in KO hearts. In conclusion, baseline cardiac performance is preserved in hearts with cardiac-restricted STAT-3 deletion. STAT-3 deletion attenuates preconditioning and is not associated with a compensatory upregulation of STAT-5 and -6 subtypes. The activation of STAT-5 and -6 in KO hearts following ischemic challenge does not provide functional compensation for the loss of STAT-3. JAK-STAT signaling via STAT-3 is essential for effective IPC.
Keywords: signal transducer and activator of transcription 5 and 6, Janus-activated kinase, ischemia-reperfusion
ischemic preconditioning (IPC) is a phenomenon by which brief ischemic episodes render resistance to subsequent ischemic insults. Preconditioning-induced cardioprotection may include by a reduction in infarct size the preservation of systolic or diastolic function, a decrease in the number or lethality of arrhythmias, the maintenance of intracellular acid-base status, and the attenuation of the myocardial inflammatory response (3, 4). IPC initiates several endogenous strategies of cellular adaptation via the activation of survival kinase pathways including JAK-STAT and phosphatidylinositol 3-kinase-Akt. Moreover, stress-responsive cellular signaling pathways initiate de novo synthesis of cardioprotective proteins, such as nitric oxide and cyclooxygenase-2, during the late phase of preconditioning (3).
The JAK-STAT pathway is comprised of a family of receptor-associated cytosolic tyrosine kinases (JAKs) that activate signal-dependent transcription factors (STATs). We and others have previously shown that this pathway is activated by stresses such as ischemia, mechanical stress, cytotoxic agents, or bacterial inflammation (4, 6, 17, 12). Pre- or postconditioning activates STAT-3 and protects cardiac performance following ischemic insult. Targeted transgenic knockout (KO) of STAT-3 in cardiomyocytes results in a preserved myocardial function but renders these KO hearts more susceptible to ischemic and oxidative cardiac injury and incapable of activating classical IPC (11, 13, 18). Although studies of the mitogen-activated protein kinase and protein kinase C families have demonstrated no compensatory change in isoform expression following targeted KO of a specific subtype, similar investigations have not been performed for the JAK-STAT pathway (1, 17).
The purposes of our study were to investigate the role of JAK-STAT signaling and the efficacy of IPC in the absence of cardiomyocyte STAT-3 and to characterize the response of cardiac-restricted STAT-3-deficient mice to ischemia-reperfusion (I/R) challenge. We hypothesized that the compensatory activation of other STAT subtypes, specifically STAT-5 and -6, in the presence of cardiac-restricted STAT-3 deletion would protect myocardial performance against I/R injury.
MATERIALS AND METHODS
Animals.
All animal protocols conformed to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996) and were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati. Wild-type (WT) male C57BL/6 mice at 10 wk of age (Harlan, Indianapolis, IN) and cardiomyocyte-specific STAT-3-deleted animals were provided a standard mouse chow diet and water ad libitum and acclimatized for 2 to 3 days before experimentation. The hearts from STAT-3 KO mice were subjected to experimental protocols at 10–12 wk of age (breeding pairs provided by Dr. Kerry Russell, Yale University, New Haven, CT). WT (C57BL/6) age-matched animals served as controls.
In vivo echocardiography.
Before isolated heart experimentation, the animals were anesthetized with isoflurane and two-dimensional transthoracic echocardiographic images of the left ventricle were obtained using a 7.5-MHz transducer at the level of the papillary muscles to assess left ventricular function. The percent fractional shortening and ejection fraction were calculated after measuring left ventricular end-diastolic and -systolic dimensions.
Isolated heart perfusion.
At least 24 h after echocardiography, randomly selected animals were anesthetized (ketamine, 90 mg/kg; and xylazine, 10 mg/kg) and heparinized (200 units) via intraperitoneal injection. Hearts were rapidly excised into warmed, oxygenated Krebs-Henseleit buffer, and the aorta was cannulated with a 23-gauge cannula. Langendorff perfusion commenced, as previously described (7), with normothermic (37°C), oxygenated (95% O2-5% CO2) Krebs-Henseleit buffer containing (in mM) 10 glucose, 118 NaCl, 2.5 CaCl2, 4.7 KCl, and 25 NaHCO3 (pH 7.4) at a constant flow of 2 to 3 ml/min. Aortic pressure was maintained at 50–55 mmHg throughout the perfusion protocol. A cannula inserted into the left ventricle via an incision in the left atrium was connected to a pressure transducer (AD Instruments, Milford, MA) and coupled to a PowerLab/400 (AD Instruments) for continuous data recording. Hearts were equilibrated in a warmed (37°C), dry chamber for 10 min before any intervention. The hearts that could not achieve a minimal left ventricular pressure of 85 mmHg and a coronary flow of 2 to 3 ml/min at the end of the equilibration period were discarded. A three-way stopcock above the aortic root was used to create global ischemia, during which the hearts were immersed in a 37°C degassed organ bath. Following equilibration, WT and STAT-3 KO hearts were randomly assigned (coin flip) to undergo I/R with or without preceding IPC (Fig. 1). Nonpreconditioned control hearts (I/R) underwent 30 min of global, normothermic ischemia and 40 min of reperfusion. The hearts in the IPC groups were preconditioned with 5 min of ischemia and 5 min of reperfusion before undergoing I/R. Cardiac function was assessed at the end of the equilibration (baseline) and at 10-min intervals during reperfusion by determining the maximum and minimum first derivative of developed pressure (+dP/dtmax and −dP/dtmin, respectively, in mmHg/s). Coronary flow (in ml/min) was measured by timed collection of pulmonary artery effluent at each time point during reperfusion.
Fig. 1.
Time course of experimental protocols. Wild-type (WT) and knockout (KO) hearts were randomized to undergo ischemia-reperfusion (I/R) with or without preceding preconditioning (n = 6 to 7 for each group). IPC, ischemic preconditioning.
Protein isolation and Western blot analysis.
At the completion of the perfusion protocols, the hearts were rapidly frozen in liquid nitrogen and stored at −80°C until analysis. The preparation of nuclear extracts to identify phosphorylated (activated) and total STAT (pSTAT and tSTAT, respectively) protein was performed using NXTRACT (Sigma-Aldrich Chemical, St. Louis, MO) according to the manufacturer's recommendations with the addition of phosphatase inhibitors consisting of (in mM) 200 imidazole, 100 NaFl, 115 sodium molybdate, 100 sodium orthovandate, and 400 sodium tartrate dehydrate. Total protein concentrations were determined using the BCA Protein Assay Kit (Pierce, Rockford, IL).
Aliquots of nuclear fractions, corresponding to 75 μg of protein, were separated by 10% SDS-PAGE (Gene Mate Express gels, ISC Bioexpress, Salt Lake City, UT), transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA), and blocked with 5% bovine serum albumin-TBS-Tween. STAT-3 expression was analyzed using a primary monoclonal antibody against pSTAT-3705 (1:200, Santa Cruz Biotechnology, Santa Cruz, CA) and tSTAT-3 (1:200, Santa Cruz Biotechnology). Similarly, nuclear fractions were analyzed for pSTAT-5 (1:1,000, Invitrogen, Carlsbad, CA), tSTAT-5 (1:200, Santa Cruz Biotechnology), pSTAT-6 (1:1,000, Millipore, Billerica, MA), and tSTAT-6 (1:200, Santa Cruz Biotechnology). To normalize protein loading, the membranes were analyzed with a primary antibody for calsequestrin (1:2,500, Affinity Bioreagents, Golden, CO). The intensity of protein expression was calculated by arbitrary units derived by dividing the densitometry values (Alpha Ease) determined for each protein by their respective calsequestrin densitometry values. Immunoreactive signals were visualized with enhanced chemiluminescence luminal reagents (ECL; Amersham Pharmacia Biotech, Piscataway, NJ).
Statistical analysis.
Data are expressed as means ± SE. Statistical analysis was performed by two-tailed t-test or one-way ANOVA with Tukey's post hoc analysis for multiple comparisons; P < 0.05 was considered significant.
RESULTS
Baseline cardiac performance.
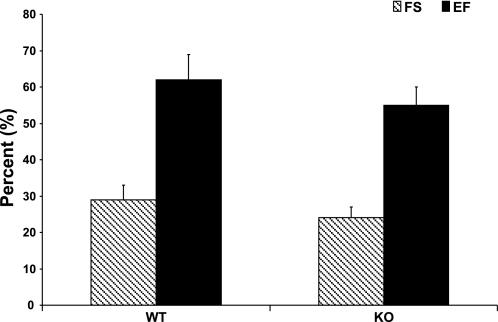
Baseline cardiac performance was determined in vivo using two-dimensional transthoracic echocardiography. There were no significant differences in left ventricular function (percent fractional shortening and ejection fraction) between WT and KO hearts (Fig. 2).
Fig. 2.
Baseline in vivo systolic performance assessed by two-dimensional transthoracic echocardiography. Myocardial performance was similar between WT and KO animals. FS, fractional shortening; EF, ejection fraction.
Baseline ex vivo cardiodynamics were obtained following 10 min of equilibration on the Langendorff apparatus. Coronary flow was significantly higher in the KO hearts; all other cardiodynamic variables were not different. (Table 1).
Table 1.
Baseline ex vivo cardiodynamics
| n | DP, mmHg | LVP, mmHg | EDP, mmHg | CF, ml/min | HR, beats/min | +dP/dtmax, mmHg/s | −dP/dtmin, mmHg/s | |
|---|---|---|---|---|---|---|---|---|
| WT | 12 | 104 ± 3 | 97 ± 2 | −7 ± 1 | 2.2 ± 0.1 | 483 ± 16 | 3,042 ± 105 | −3,206 ± 160 |
| KO | 12 | 106 ± 5 | 99 ± 4 | −7 ± 1 | 2.8 ± 0.1* | 387 ± 21 | 2,752 ± 161 | −2,982 ± 206 |
Values are means ± SE; n, number of mice. DP, developed pressure; LVP, left ventricular pressure; EDP, end-diastolic pressure; CF, coronary flow; HR, heart rate; +dP/dtmax and −dP/dtmin, maximum and minimum first derivative of DP, respectively; WT, wild-type; KO, knockout.
P < 0.05 vs. WT.
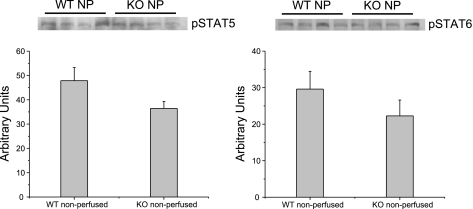
Western blot analysis of nonperfused (baseline) hearts revealed similar levels of pSTAT-5 and pSTAT-6 expression in WT and KO hearts (Fig. 3).
Fig. 3.
Representative immunoblots of protein expression in nonperfused (NP) WT and KO hearts. There was no significant difference in baseline expression of phosphorylated (p)STAT-5 or -6 in WT and KO hearts.
Cardiac-specific STAT-3 deletion and ischemic tolerance.
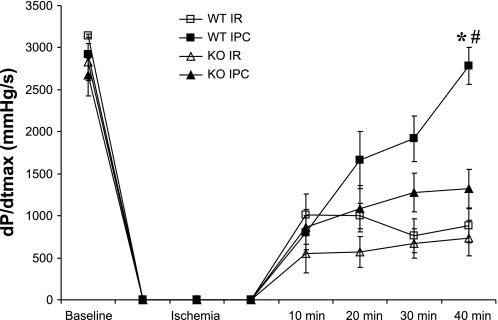
I/R challenge without preconditioning resulted in a considerable impairment in myocardial systolic and diastolic function in both WT and KO hearts. At end-reperfusion, WT hearts recovered only 25% of their baseline developed pressure (end reperfusion vs. baseline developed pressure, 27 ± 6 vs. 105 ± 5 mmHg, P < 0.001). Similarly, KO hearts recovered 24% of their baseline developed pressure (end reperfusion vs. baseline developed pressure, 23 ± 8 vs. 108 ± 9 mmHg, P < 0.001). When compared with WT hearts, KO hearts undergoing I/R alone demonstrated no differences in any of the measured cardiodynamic parameters (Table 2).
Table 2.
End-reperfusion cardiodynamics
| n | CF, ml/min | HR, beats/min | +dP/dtmax, mmHg/s | −dP/dtmin, mmHg/s | |
|---|---|---|---|---|---|
| WT | |||||
| I/R | 6 | 1.9 ± 0.1 | 357 ± 22 | 880 ± 204 | −983 ± 213 |
| IPC | 7 | 2.2 ± 0.1 | 331 ± 9 | 2,783 ± 221* | −2,795 ± 267* |
| KO | |||||
| I/R | 6 | 2.1 ± 0.3 | 406 ± 31 | 732 ± 211 | −721 ± 201 |
| IPC | 6 | 2.3 ± 0.3 | 391 ± 21 | 1,319 ± 229† | −1,358 ± 250† |
Values are means ± SE; n, number of mice. I/R, ischemia-reperfusion; IPC, ischemic preconditioning.
P < 0.05 vs. WT I/R;
P < 0.05 vs. WT IPC.
IPC in the absence of cardiomyocyte STAT-3.
IPC significantly enhanced end-reperfusion myocardial recovery in WT hearts compared with I/R alone. When compared with WT I/R hearts, IPC in WT hearts significantly improved end-reperfusion +dP/dtmax and −dP/dtmin (Fig. 4). No differences in end-reperfusion left ventricular pressure, end-diastolic pressure, coronary flow, or heart rate were identified (Table 2). By contrast, IPC in KO hearts had no significant effect on myocardial functional recovery compared with KO I/R hearts (Table 3). Furthermore, IPC more effectively improved end-reperfusion systolic performance in WT hearts than in KO hearts (Fig. 5). There were no differences in end-reperfusion coronary flow or heart rate between WT and KO hearts following preconditioning (Table 2).
Fig. 4.
Myocardial function during I/R and IPC protocols, as reflected by the maximum positive first derivative of developed pressure (+dP/dtmax). *P < 0.001 vs. WT I/R; #P < 0.001 vs. KO IPC.
Table 3.
Contractile function
| n | Baseline | 10 min | 20 min | 30 min | 40 min | |
|---|---|---|---|---|---|---|
| WT | ||||||
| I/R | 6 | 3,142 ± 20 | 1,013 ± 245 | 997 ± 152 | 764 ± 205 | 880 ± 204 |
| IPC | 7 | 2,920 ± 45 | 797 ± 201 | 1,662 ± 341 | 1,916 ± 271 | 2,783 ± 221* |
| KO | ||||||
| I/R | 6 | 2,829 ± 224 | 550 ± 229 | 571 ± 184 | 673 ± 173 | 732 ± 211 |
| IPC | 6 | 2,676 ± 248 | 868 ± 211 | 1,085 ± 277 | 1,276 ± 233 | 1,319 ± 229† |
Values (+dP/dtmax, mmHg/s) are means ± SE; n, number of mice.
P < 0.05 vs. WT I/R;
P < 0.05 vs. WT IPC.
Fig. 5.
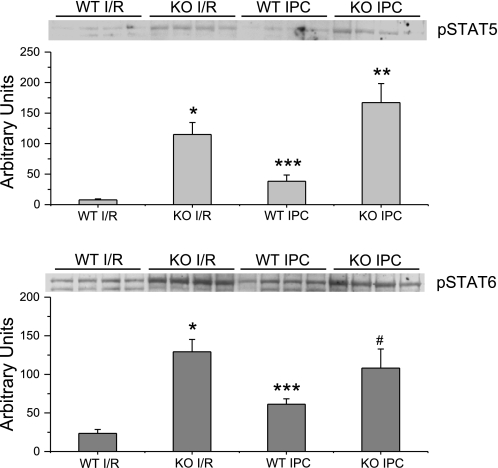
Representative immunoblots of protein expression following I/R in the presence or absence of IPC in WT and KO hearts. Expression of pSTAT-5 was significantly increased in KO hearts compared with WT after I/R and IPC. Expression of pSTAT-6 was significantly increased in KO hearts compared with WT after I/R but not after IPC. Following IPC, expression of pSTAT-5 and -6 were significantly increased in WT but not KO hearts compared with hearts subjected to I/R alone. *P < 0.01 vs. WT I/R; **P < 0.01 vs. WT IPC; #P = 0.12 vs. WT IPC; ***P = 0.03 vs. WT I/R.
Protein expression following I/R.
Following I/R, left ventricular samples from KO hearts demonstrated a significantly higher expression of pSTAT-5 and pSTAT-6 by Western blot analysis compared with WT hearts (Fig. 5). However, IPC in WT hearts increased the expression of pSTAT-5 and pSTAT-6 compared with WT without IPC. There were no significant differences in total STAT-5 or -6 expression following I/R.
Preconditioning in STAT-3 KO hearts induced a significantly higher expression of only pSTAT-5 compared with preconditioned WT hearts. Unlike the changes in phosphorylated protein seen in preconditioned WT hearts, there was no significant change in pSTAT-5 or -6 expression in KO hearts following preconditioning compared with I/R alone (Fig. 5).
DISCUSSION
The results of this study demonstrate for the first time to our knowledge that IPC does not protect myocardial function from I/R injury in the absence of cardiomyocyte STAT-3. Increased activation of STAT-5 and -6 in STAT-3 KO hearts, compared with WT hearts, did not offer protection against I/R-induced cardiac dysfunction, nor did it improve cardiac performance following IPC. Importantly, our results show that STAT-3 activation is essential to IPC-induced cardioprotection and that the activation of STAT-5 and -6 was insufficient for effective preconditioning in the absence of STAT3.
STAT-3 KO and cardiac performance.
The complete knockout of STAT-3 is embryonically lethal. However, animals with cardiomyocyte-restricted STAT-3 deletion using the Cre-loxP system have reduced cardiomyocyte STAT-3 expression by 40% at birth and nearly 100% by 10–12 wk of age. These animals have an increased susceptibility to doxorubicin-induced oxidative injury, enhanced fibrotic and apoptotic response to proinflammatory stimuli, and a propensity to develop cardiac dysfunction with age (13). Moreover, Hilfiker-Kleiner et al. (11) have suggested that the evolution of reduced myocardial capillary density and amplified interstitial fibrosis by 2 to 3 mo of age may contribute to the STAT-3 KO heart vulnerability to injury. As STAT-3 KO mice age, they display increased ventricular dilation, apoptosis, and interstitial fibrosis, resulting in premature mortality.
Despite the genetic depletion of cardiac STAT-3, our data demonstrate that cardiac performance in KO hearts at 2 to 3 mo of age is similar to that of WT hearts. Hilfiker-Kleiner et al. (11) importantly illustrate that it is critical to perform physiological studies on these animals at a young age (2 to 3 mo). Our echocardiography results coupled with the baseline ex vivo cardiac function indicate a preserved myocardial contractile function in KO hearts, supporting this age as an appropriate time frame for investigation.
Role of STAT-3 in IPC.
We and others have previously demonstrated the essential role of STAT-3 activation in ischemic tolerance and IPC (3, 4). Many of these studies have used the administration of AG490, a nonspecific JAK-2 inhibitor, to attenuate STAT-3 activation before the ischemic insult. Few studies, however, have characterized the myocardial response to I/R injury following cardiac deletion of STAT-3. Hilfiker-Keliner et al. (11) demonstrated that STAT-3 KO hearts display a reduced ischemic tolerance to coronary ligation as assessed by increased infarct size, cardiomyocyte apoptosis, and deterioration of ventricular systolic function. Smith et al. (18) additionally showed that IPC failed to improve ischemic tolerance in STAT-3 KO cardiomyocytes or reduce infarct size in ex vivo-perfused KO hearts. Our results complement these studies by demonstrating that cardiomyocyte-restricted STAT-3 deletion maintains a normal cardiac phenotype but abrogates the protective effects of IPC on cardiodynamics. The protective effect of STAT-3 following I/R challenge may involve a rebalancing of pro- and antiapoptotic factors (2). Cardiomyocyte-restricted STAT-3 deletion may allow for STAT-3 activation in other cell types, including endothelial cells, to contribute to postischemic myocardial protection. Wang et al. (19) demonstrated a diminished postischemic functional recovery in hearts lacking endothelial cell STAT-3. In contrast to the present study, however, the in vivo cardiac phenotype was not characterized. A KO heart combining the cardiomyocyte and endothelial cell STAT-3 deletion may permit a more comprehensive study of cardiac function and postischemic recovery in the absence of STAT-3.
Differential STAT signaling following ischemia.
The STATs comprise a family of transcription factors that transmit the interactions of cytokines, hormones, or growth factors from cell surface receptors to responsive elements in the nucleus. The seven subtypes of STATs display a broad range of functions that may be overlapping, redundant, or even opposing. Whereas STAT-3 and STAT-1 engage in conflicting roles with respect to modulation of apoptosis, both STAT-5 and -6 have been shown to be activated following myocardial ischemic challenge in a similar fashion to STAT-3 (14). Initially discovered as a transcription factor integral to prolactin-induced mammary gland development, STAT-5 is currently recognized as playing a role in hematopoiesis, immunity, and inflammation (5). STAT-5 is further divided into STAT-5A and -5B, which are differentially expressed in many cell types, but expressed in equal amounts in the heart (9). While the essential functions of STAT-5A involve prolactin signaling, STAT-5B is located downstream in growth hormone signaling as well as the angiogenic response to hypoxia-induced erythropoeitin release (21). STAT-6 is integral to IL-4/IL-13 signaling and the subsequent polarization of TH2 cells but also demonstrates an increased expression in the intima and media of atherosclerotic coronary arteries (8, 16).
Although cardiac JAK-STAT signaling research has predominantly focused on STAT-3 activation, several studies have investigated the roles of STAT-5 and -6 in cardiac adaptation to stress. Mascareno et al. (14, 21) demonstrated that although both STAT-5 and -6 are activated following cardiac I/R challenge, only STAT-5A was shown to be involved in IPC. Moreover, STAT-3, -5, and -6 are all activated by angiotensin II to participate in mechanisms intrinsic to myocardial hypertrophy (10, 14). To our knowledge, however, this study is the first to address the differential myocardial STAT activation in the absence of cardiomyocyte STAT-3.
Our results demonstrate that although the baseline expression of STAT-5 and -6 in STAT-3 KO hearts is similar to that of WT hearts, KO hearts show an increased expression of both pSTAT-5 and -6 following I/R compared with WT hearts. Following IPC, however, pSTAT-5 and -6 expression is increased in WT but not KO hearts compared with hearts undergoing I/R alone. In addition, our data show that despite the elevated expression of pSTAT-5 and -6 in KO hearts following I/R and IPC, there is no corresponding functional compensation in myocardial recovery as a result of the increased pSTAT-5 and -6. By suggesting that STAT-3 specifically is essential to preconditioning-induced cardioprotection, our results disagree with previous studies that propose a more integral role for STAT-5A. We have demonstrated that STAT-5 activation is insufficient to provide IPC-induced functional cardioprotection in the absence of STAT-3. Yamaura et al. (21) found that murine hearts lacking STAT-5A had no change in infarct size following preconditioning compared with I/R alone, suggesting that STAT-5A plays a role in IPC. This difference may be explained by the specificity of the antibody against the STAT-5A subtype. Interestingly, previous studies that have used STAT-5 and -6 KO models to implicate a role for these transcription factors in I/R and IPC have not characterized the myocardial activation of other STATs, as we have in the present study.
Summary.
Our results support an essential role for STAT-3 signaling in the cardioprotective response induced by preconditioning following an ischemic challenge. The present data suggest that in hearts lacking cardiomyocyte STAT-3, STAT-5 and -6 activation is augmented following I/R challenge but is not further increased by IPC. Importantly, an increased activation of STAT-5 and -6 does not provide functional compensation for the genetic deletion of cardiomyocyte STAT-3. JAK-STAT signaling via STAT-3 therefore remains a critical target to attenuate myocardial injury and dysfunction induced by I/R injury.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants T32-HL-07382 (to Arnie Schwartz) and HL-68867 (to K. L. Butler).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Aouadi M, Benitruy B, Caron L, LeMarchand-Brustel Y, Bost F. Role of MAPKs in development and differentiation: lessons from knockout mice. Biochimie 88: 1091–1098, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Barry SP, Townsend PA, Latchman DS, Stephanou A. Role of the JAK-STAT pathway in myocardial injury. Trends Mol Med 13: 82–89, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Bolli R, Dawn B, Xuan Y. Role of the JAK-STAT pathway in protection against myocardial ischemia/reperfusion injury. Trends Cardiovasc Med 13: 72–79, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Butler KL, Huffman LC, Koch SE, Hahn HS, Gwathmey JK. STAT-3 activation is necessary for ischemic preconditioning in hypertrophied myocardium. Am J Physiol Heart Circ Physiol 291: H797–H803, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Desrivières S, Kunz C, Barash I, Vafaizadeh V, Borghouts C, Groner B. The biological functions of the versatile transcription factors STAT3 and STAT5 and new strategies for their targeted inhibition. J Mammary Gland Biol Neoplasia 11: 75–87, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Fischer P, Hilfiker-Kleiner D. Survival pathways in hypertrophy and heart failure: the gp130-STAT3 axis. Basic Res Cardiol 102: 279–297, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Goodman MD, Koch SE, Fuller-Bicer GA, Butler KL. Regulating RISK: a role for JAK-STAT signaling in postconditioning? Am J Physiol Heart Circ Physiol 295: H1649–H1656, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hebenstreit D, Wirnsberger G, Horejs-Hoeck J, Duschl A. Signaling mechanisms, interaction partners, and target genes of STAT6. Cytokine Growth Factor Rev 17: 173–188, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Hennighausen L, Robinson G. Interpretation of cytokine signaling through the transcription factors STAT5A and STAT5B. Genes Dev 22: 711–721, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hikoso S, Yamaguchi O, Higuchi Y, Hirotani S, Takeda T, Kashiwase K, Watanabe T, Taniike M, Tsujimoto I, Asahi M, Matsumara Y, Nishisa K, Nakajima H, Akira S, Hori M, Otsu K. Pressure overload induces cardiac dysfunction and dilation in signal transducer and activator of transcription 6-deficient mice. Circulation 110: 2631–2637, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Hilfiker-Kleiner D, Hilfiker A, Fuchs M, Kaminski K, Schaefer A, Schieffer B, Hillmer A, Schmiedl A, Ding Z, Podewski E, Podewski E, Poli V, Schneider MD, Schulz R, Park J, Wollert KC, Drexler H. Signal transducer and activator of transcription 3 is required for myocardial capillary growth, control of interstitial matrix deposition, and heart protection from ischemic injury. Circ Res 95: 187–195, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Huffman LC, Koch SE, Butler KL. Coronary effluent from a preconditioned heart activates the JAK-STAT pathway and induces cardioprotection in a donor heart. Am J Physiol Heart Circ Physiol 294: H257–H262, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Jacoby JJ, Kalinowski A, Liu MG, Zhang SS, Gao Q, Chai GX, Ji L, Iwamoto Y, Li E, Schneider M, Russell KS, Fu XY. Cardiomyocyte-restricted knockout of STAT3 results in higher sensitivity to inflammation, cardiac fibrosis, and heart failure with advanced age. Proc Natl Acad Sci USA 100: 12929–12934, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mascareno E, El-Shafei M, Maulik N, Sato M, Guo Y, Das DK, Siddiqui MA. JAK/STAT signaling is associated with cardiac dysfunction during ischemia and reperfusion. Circulation 104: 325–329, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Mascareno E, Siddiqui MA. The role of JAK/STAT signaling in heart tissue renin-angiotensin system. Mol Cell Biochem 212: 171–175, 2000 [PubMed] [Google Scholar]
- 16. Satterthwaite G, Francis SE, Suvarna K, Blakemore S, Ward C, Wallace D, Braddock M, Crossman D. Differential gene expression in coronary arteries from patients presenting with ischemic heart disease: further evidence for the inflammatory basis of atherosclerosis. Am Heart J 150: 488–499, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Saurin AT, Pennington DJ, Raat NJ, Latchman DS, Owen MJ, Marber MS. Targeted disruption of the protein kinase C epsilon gene abolishes the infarct size reduction that follows ischaemic preconditioning of isolated buffer-perfused mouse hearts. Cardiovasc Res 55: 672–680, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Smith RM, Suleman N, Lacerda L, Opie LH, Akira S, Chien KR, Sack MN. Genetic depletion of cardiac myocyte STAT-3 abolishes classical preconditioning. Cardiovasc Res 63: 611–616, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Wang MW, Zhang W, Crisostomo P, Markel T, Meldrum KK, Fu XY, Meldrum DR. Endothelial STAT3 plays a critical role in generalize myocardial proinflammatory and proapoptotic signaling. Am J Physiol Heart Circ Physiol 293: H2101–H2108, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Xuan Y, Guo Y, Han H, Zhu Y, Bolli R. An essential role of the JAK-STAT pathway in ischemic preconditioning. Proc Natl Acad Sci USA 98: 9050–9055, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yamaura G, Turoczi T, Yamamoto F, Siddqui MA, Maulik N, Das DK. STAT signaling in ischemic heart: a role of STAT5A in ischemic preconditioning. Am J Physiol Heart Circ Physiol 285: H476–H482, 2003 [DOI] [PubMed] [Google Scholar]