Abstract
Chronic hypoxia protects the heart against injury caused by acute oxygen deprivation, but its salutary mechanism is poorly understood. The aim was to find out whether cardiomyocytes isolated from chronically hypoxic hearts retain the improved resistance to injury and whether the mitochondrial large-conductance Ca2+-activated K+ (BKCa) channels contribute to the protective effect. Adult male rats were adapted to continuous normobaric hypoxia (inspired O2 fraction 0.10) for 3 wk or kept at room air (normoxic controls). Myocytes, isolated separately from the left ventricle (LVM), septum (SEPM), and right ventricle, were exposed to 25-min metabolic inhibition with sodium cyanide, followed by 30-min reenergization (MI/R). Some LVM were treated with either 30 μM NS-1619 (BKCa opener), or 2 μM paxilline (BKCa blocker), starting 25 min before metabolic inhibition. Cell injury was detected by Trypan blue exclusion and lactate dehydrogenase (LDH) release. Chronic hypoxia doubled the number of rod-shaped LVM and SEPM surviving the MI/R insult and reduced LDH release. While NS-1619 protected cells from normoxic rats, it had no additive salutary effect in the hypoxic group. Paxilline attenuated the improved resistance of cells from hypoxic animals without affecting normoxic controls; it also abolished the protective effect of NS-1619 on LDH release in the normoxic group. While chronic hypoxia did not affect protein abundance of the BKCa channel regulatory β1-subunit, it markedly decreased its glycosylation level. It is concluded that ventricular myocytes isolated from chronically hypoxic rats retain the improved resistance against injury caused by MI/R. Activation of the mitochondrial BKCa channel likely contributes to this protective effect.
Keywords: continuous hypoxia, ventricular myocytes, metabolic inhibition, cell viability, potassium channels
kopecky and daum (19) were the first to demonstrate experimentally that adaptation to chronic hypoxia increases tolerance of the heart against injury caused by acute oxygen deprivation. This observation has been subsequently elaborated in numerous studies that confirmed the protective effect of chronic hypoxia using a variety of experimental models and adaptation protocols. The improved tolerance of chronically hypoxic hearts to ischemia-reperfusion injury manifests itself as a limitation of myocardial infarct size, increased postischemic recovery of cardiac contractile function, and reduced incidence and severity of both ischemic and reperfusion ventricular arrhythmias (25). Probably the most important feature of this adaptive phenomenon is that its salutary effect on myocardial viability persists much longer than any form of preconditioning (22).
Compared with short-lived preconditioning, the molecular mechanism underlying the long-lasting myocardial protection afforded by chronic hypoxia is still poorly understood, yet the experimental evidence available points to an important role of adaptive changes occurring on the level of mitochondria (12, 18). Among mitochondrial components, ATP-sensitive K+ (KATP) channels were most studied, and several reports have suggested their involvement in the protective mechanism of chronic hypoxia against all major endpoints of ischemia-reperfusion injury (3, 11, 21, 38).
Recent studies have revealed that, besides KATP channels, the inner mitochondrial membrane contains large-conductance Ca2+-activated K+ (BKCa) channels (27, 31, 36, 39) that are opened by hypoxia (10) and contribute to myocardial protection (5, 9, 23, 29, 32, 36). The protective effect of BKCa opening has been attributed to increased matrix K+ uptake and volume, improved respiratory control (2), inhibition of mitochondrial Ca2+ overload (17, 33), and prevention of permeability transition pore opening (10). The channel is composed of the pore-forming α-subunits and auxillary β-subunits that regulate its activity. The β1-subunit is highly expressed in cardiomyocytes (4). It has been shown that it has two N-glycosylation sites, and its enzymatic deglycosylation activates the channel by increasing both open probability and mean open time (15).
Thus the primary goal of our study was to find out whether BKCa channels are involved in the cardioprotective mechanism of adaptation to chronic hypoxia using a selective BKCa blocker paxilline and opener NS-1619 (29). As this channel has not been detected in the sarcolemma of cardiac myocytes (27), isolated cardiomyocytes appear to be a suitable model to study the role of channels localized in mitochondria. We hypothesized that cells isolated from chronically hypoxic rats will retain the improved resistance to injury caused by simulated ischemia, and that the BKCa channels will contribute to this effect. Moreover, we analyzed effects of chronic hypoxia on the regulatory β1-subunit protein level and glycosylation status to consider its potential role in BKCa-mediated cardioprotection.
METHODS
Animals.
A total of 24 adult male Wistar rats (250–300 g body wt) were exposed to continuous hypoxia (inspired O2 fraction: 0.10) using a normobaric chamber equipped with hypoxic generators (Everest Summit, Hypoxico, NY) for 3 wk. No reoxygenation occurred during this period. Animals were removed from the hypoxic chamber 24 h before experiments. The control group of 28 rats was kept under normoxic (inspired O2 fraction: 0.21) conditions. All animals were housed in a controlled environment (23°C; 12:12-h light-dark cycle; light from 5:00 AM) with free access to water and standard chow diet. The study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals, published by the US National Institutes of Health (National Institutes of Health publication no. 85–23, revised 1996). Experimental protocols were approved by the Animal Care and Use Committee of the Institute of Physiology, Academy of Sciences of the Czech Republic.
Isolation of cardiomyocytes.
Rats were heparinized (5,000 IU ip) and killed by cervical dislocation. The heart was quickly excised, mounted on a perfusion system, and perfused via the aorta with Tyrode solution under constant flow conditions (10 ml/min) for 5 min, followed by perfusion with nominally Ca2+-free Tyrode for 8 min. Tissue digestion was initiated by adding 14,000 U collagenase and 7 mg proteinase type XIV into 30 ml of Ca2+-free Tyrode containing 50 mg BSA. After 9–12 min, the collagenase-protease cocktail was washed out by 10-min perfusion with Ca2+-free Tyrode. The right ventricle was cut off first and then the interventricular septum and the left ventricle. Separate ventricular parts were dispersed mechanically; myocyte solutions were adjusted to the same cell density and transferred to culture medium.
Experimental protocol.
Isolated cardiomyocytes were cultured in cell culture medium (50% Dulbecco's modified Eagle's medium and 50% Nutrient Mixture F12HAM, containing 0.2% BSA, 100 U/ml penicillin, and 100 μg/ml streptomycin). Myocytes were kept in a CO2 incubator (95% air, 5% CO2, 28°C) for 1-h stabilization period.
In the first series of experiments, myocytes isolated separately from the left ventricle (LVM), the right ventricle (RVM), and the septum (SEPM) of chronically hypoxic and normoxic rats were subjected to metabolic inhibition (MI) and reenergization (MI/R). In the second set of experiments, LVM isolated from chronically hypoxic and normoxic rats were treated with BKCa modulators, starting 25 min before MI and continuing during the whole experiment. LVM from each heart were divided into four groups treated with either 30 μM NS-1619 (BKCa opener), 2 μM paxilline (BKCa blocker), or 0.1% DMSO (vehicle control); the last group served as untreated control. In a separate experiment, an additional group of LVM from normoxic rats was pretreated with paxilline for 25 min before NS-1619.
Myocytes from each ventricular part and/or treatment group were split into two parts of equal volumes. Control cells were incubated in normal Krebs solution and not exposed to MI/R. Experimental cells were subjected to 25 min of MI, followed by 30 min of reenergization. MI was induced by incubation of cells in the modified Krebs solution containing 1.5 mM NaCN and 20 mM 2-deoxyglucose instead of glucose. The reenergization was achieved by removing the metabolic inhibitors and replacing the MI solution with the normal cell culture medium (the same medium was applied to control cells).
Cell viability and LDH release.
Cell viability and LDH release were evaluated at the beginning of the experiments (after stabilization), after MI (LDH release only), and after reenergization. The number of viable and dead (stained) myocytes was determined by Trypan blue exclusion (34). Typically, 50–100 myocytes were counted in duplicates from 6–8 independent experiments. Viable myocytes were divided into two fractions: rod-shaped myocytes with the cell length-to-width ratio > 3:1 and non-rod-shaped myocytes with the ratio < 3:1. Viability after MI/R was expressed as a percentage of rod-shaped cells that survived the MI/R insult, normalized to the appropriate control group not exposed to MI/R.
LDH was determined spectrophotometrically (7) using the LDH Liqui-UV kit (Stanbio, Boerne, TX). LDH released during MI and during reenergization was normalized to total LDH content in the cells and expressed as a percentage of appropriate control values.
Western blot analysis of BKCa channel β1-subunit.
Cardiomyocytes isolated from chronically hypoxic and normoxic rats were frozen in liquid nitrogen and stored at −80°C until use. Samples were prepared as described earlier (16). The cells were pelleted at 500 g for 5 min, the isolation buffer was removed, and 1 ml SDS sample buffer (0.1 M Tris, 4% SDS, 20% glycerol) was added to achieve cells-to-buffer ratio of 1:5 (vol/vol) and vortexed until the cell pellet was completely resolved. The samples were heated to 100°C for 10 min, and 100 μl were removed to measure protein concentration. This volume was replaced by 100 μl β-mercaptoethanol, and 10 μl bromphenol blue were added. The samples were again exposed to 100°C for 10 min, and aliquots of 50 μl were taken and stored at −80°C until use.
Samples of 3–5 μl per lane were loaded on a 10% acrylamide gel. The proteins were separated at constant voltage (100 V) for ∼90 min and transferred to polyvinylidene difluoride membrane at 0.35 A for 75 min. The transferred proteins were blocked overnight in 5% fat-free milk and 1% BSA. The primary antibody against β1 (diluted 1:200 in 5% milk and 1% BSA) was applied for 1 h at room temperature, followed by a three-times washing with PBS. Then the blots were incubated with anti-rabbit horseradish peroxidase-labeled secondary antibody (1:30,000) for 1 h, followed by three more washes. The blots were exposed to the Amersham Hyperfilm ECL for 15 min.
After the films were developed, the blots were washed in PBS, and the antibodies were removed using a common desorption protocol. The blots were then incubated with blocking solution (5% milk, 1% BSA) overnight and probed against GAPDH to verify the amount of protein loaded to the gel. Films were scanned and evaluated by using AIDA software.
Deglycosylation of the BKCa channel β1-subunit.
Cells were centrifuged at 1,000 g for 5 min to a final pellet of 250 μl. The same volume of a solubilization buffer (50 mM sodium-phosphate buffer, 150 mM NaCl, 10 mM KCl, 1.8% SDS, 17.5% glycerol, pH 7.2, and 3.3 μl/ml protease inhibitor cocktail P8340) was added and vortexed to yield a clear yellow-beige solution. The solubilized sample was kept 5 min at room temperature, followed by centrifugation at 12,000 g for 15 min. The final supernatant protein concentration was between 5 and 10 μg/μl. Deglycosylation was performed using the GKE5006 kit (Prozyme, San Leandro, CA) as follows: 35 μl of the solubilized protein, 10 μl reaction buffer (100 mM sodium phosphate, 0.1% sodium azide, pH 7.5), 2.5 μl denaturation buffer (2% sodium dodecyl sulfate, 1 M β-mercaptoethanol), 2.5 μl detergent solution (15% nonyl phenoxylpolyethoxylethanol), and 4 μl N-glycanase (peptide-N-glycosidase F) were added to a tube, vortexed, and incubated at 37°C for 18 h in a thermo block. Control tube contained 4 μl water instead of N-glycanase. After 18 h, 10 μl dithiothreitol (1 M) and 60 μl loading buffer (100 mM Tris·HCl, pH 6.8, 200 mM dithiothreitol, 4% SDS, 0.2% bromphenol blue, 20% glycerol) were added to stop the reaction. The tube was vortexed and incubated at room temperature for 30 min. Samples of 20–30 μl per lane were applied to a 10% gel, and Western blotting was performed as described above.
Drugs and chemicals.
Collagenase was obtained from Yakult (Tokyo, Japan). The antibody against BKCa channel subunit β1 was from Alomone Laboratories (Jerusalem, Israel), the anti-GAPDH antibody from Applied Biosystems (Foster City, CA), the anti-rabbit secondary antibody from Bio-Rad (Hercules, CA), and the PBS tablets from Gibco (Carlsbad, CA). All other chemicals and drugs were purchased from Sigma (Hamburg, Germany).
Statistical analysis.
Data are expressed as means ± SE from the indicated number of experiments. One-way ANOVA with Bonferroni post hoc means comparison was used. Differences were considered statistically significant when P < 0.05.
RESULTS
Isolated cardiomyocytes retain protective effects of chronic hypoxia.
Viability of myocytes ranged between 51 and 66% after 1-h stabilization and was always higher in RVM than in LVM and SEPM (Table 1). Adaptation of rats to chronic hypoxia did not affect starting viability. The numbers of viable myocytes at the end of control experiments were similar to that counted after stabilization within each experimental group. Total LDH content in cell preparations from normoxic animals did not differ among ventricular parts. Chronic hypoxia did not affect total LDH in LVM and SEPM, but it significantly increased LDH level in RVM (Table 1).
Table 1.
Viability and total LDH content in cell preparations from normoxic and chronically hypoxic rats after 1-h stabilization
| Group | Viability, %total cells | LDH, U/ml |
|---|---|---|
| Normoxia | ||
| Left ventricle | 51.7 ± 2.4 | 1.31 ± 0.33 |
| Septum | 50.6 ± 1.4 | 1.14 ± 0.29 |
| Right ventricle | 66.4 ± 1.9† | 1.27 ± 0.31 |
| Hypoxia | ||
| Left ventricle | 50.6 ± 3.0 | 1.32 ± 0.26 |
| Septum | 49.8 ± 3.2 | 1.10 ± 0.27 |
| Right ventricle | 62.1 ± 3.9† | 2.10 ± 0.44*† |
Values are means ± SE from 8 hearts in each group. LDH, lactate dehydrogenase.
P < 0.05 vs. corresponding normoxic group.
P < 0.05 vs. left ventricle and septum.
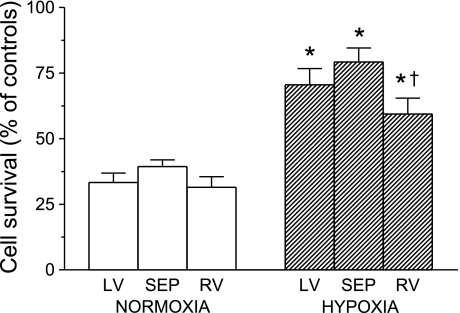
Exposure of cells to MI/R insult decreased their survival in all groups (Fig. 1). When expressed as a percentage of corresponding numbers in control experiments, only about 30–40% of rod-shaped myocytes survived in the normoxic group without significant differences among LVM, SEPM, and RVM. Chronic hypoxia markedly improved the relative survival by doubling the number of rod-shaped cells after MI/R in all three ventricular parts. However, the protective effect was significantly smaller in RVM than in SEPM (Fig. 1). MI/R did not affect the number of non-rod-shaped viable (nonblue) myocytes, except for the moderate increase in RVM from the normoxic group (data not shown).
Fig. 1.
Survival of cardiomyocytes after acute metabolic inhibition and reenergization (MI/R), expressed as a percentage of control cell survival in the absence of MI/R. Cells were isolated from the left ventricle (LV), septum (SEP), and right ventricle (RV) of rats adapted to chronic hypoxia and of normoxic animals. Values are means ± SE from 8 hearts in each group. *P < 0.05 vs. corresponding normoxic groups. †P < 0.05 vs. SEP.
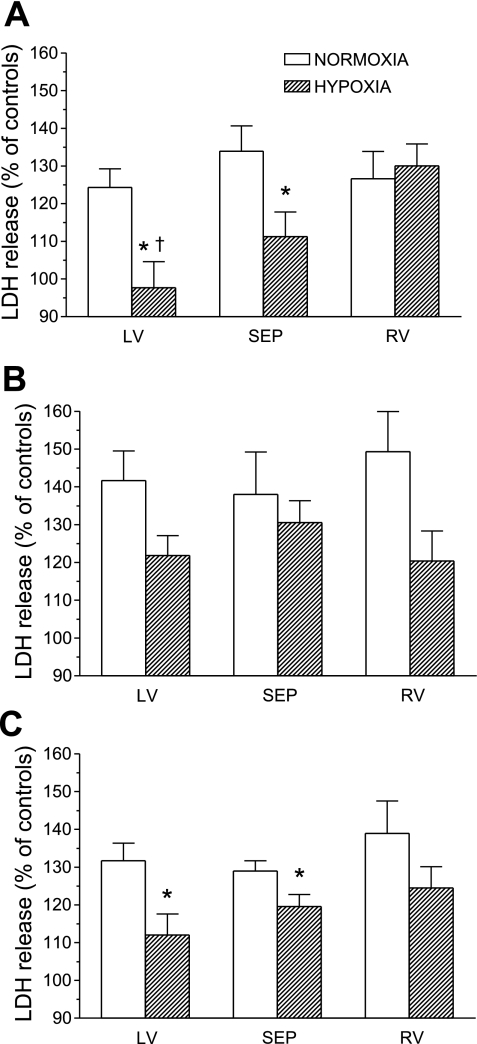
Figure 2, A–C, respectively, shows LDH release from cells during MI, during reenergization, and total release during MI/R, expressed as a percentage of appropriate control values. In LVM and RVM from normoxic rats, the LDH release during reenergization was higher than that caused by MI. The same is true for LVM and SEPM isolated from chronically hypoxic rats. Chronic hypoxia significantly reduced both MI-induced and total LDH release during MI/R from LVM and SEPM; the protective effect was more pronounced in LVM. LDH release from RVM and the release during the reenergization phase from myocytes of all three ventricular parts were not significantly decreased by chronic hypoxia (P =; 0.084 for LV).
Fig. 2.
Lactate dehydrogenase (LDH) release from cardiomyocytes during metabolic inhibition (A) and during reenergization (B), and total release during MI/R (C), expressed as a percentage of LDH release from control cells not exposed to MI/R. Cells were isolated from the LV, SEP, and RV of rats adapted to chronic hypoxia and of normoxic animals. Values are means ± SE from 8 hearts in each group. *P < 0.05 vs. corresponding normoxic groups. †P < 0.05 vs. RV.
These results demonstrate that cardiomyocytes (LVM and SEPM) isolated from rats adapted to chronic hypoxia retain the improved resistance against injury caused by MI/R.
Paxilline attenuates the protective effects of chronic hypoxia, while NS-1619 protects only cardiomyocytes from normoxic animals.
Starting viability of LVM in this set of experiments was higher than 50% and similar in normoxic and chronically hypoxic groups. Incubation of cells with paxilline and DMSO for 25 min did not affect their viability before MI, whereas NS-1619 decreased it by 10%. Total LDH levels in cell preparations were similar to those reported above and were affected by neither chronic hypoxia nor any drug treatments (data not shown).
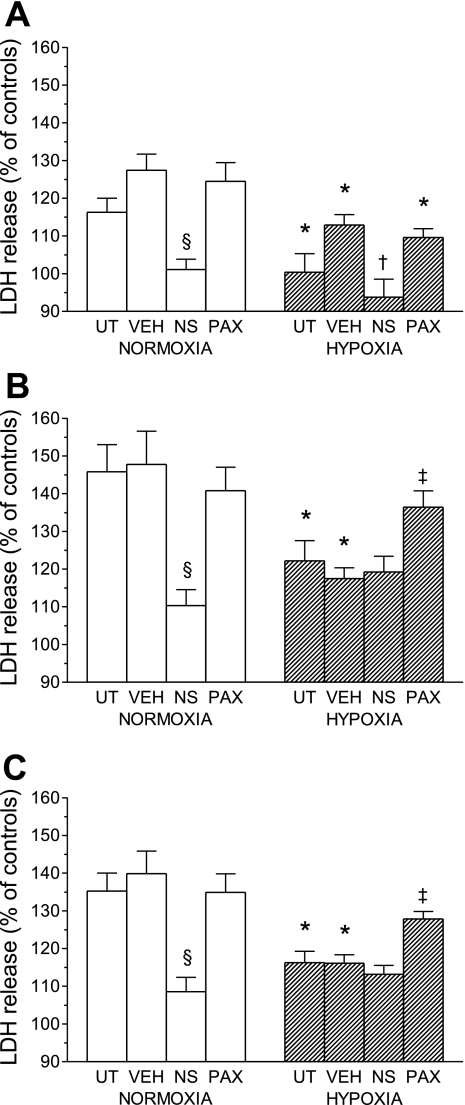
Treatment with DMSO had no effect on survival of rod-shaped myocytes after the MI/R insult in either normoxic or chronically hypoxic groups. NS-1619 significantly improved cell viability after MI/R in the normoxic group to a similar level observed in untreated or DMSO-treated cells of the chronically hypoxic group, but it had no additive effect in the chronically hypoxic group. On the other hand, paxilline attenuated the salutary effect of chronic hypoxia on rod-shaped cell survival after MI/R, but it did not influence viability in the normoxic group (Fig. 3).
Fig. 3.
Effects of NS-1619 (NS) and paxilline (PAX) on survival of cardiomyocytes after acute MI/R, expressed as a percentage of control cells in the absence of MI/R. Cells were isolated from the LV of rats adapted to chronic hypoxia and of normoxic animals. VEH, vehicle-treated cells; UT, untreated cells. Values are means ± SE from 8 hearts in each group. *P < 0.05 vs. corresponding normoxic groups. §P < 0.05 vs. UT, VEH, and PAX. $P < 0.05 vs. NS.
Figure 4, A–C, respectively, shows effects of drugs on LDH release from LVM during MI, during reenergization and total release during MI/R, expressed as a percentage of appropriate control values. In cells from both normoxic and chronically hypoxic rats and all treatment groups, the LDH release during the reenergization phase was higher than that induced by MI. DMSO treatment had no effect on LDH release. In the normoxic group, the total LDH release during MI/R was significantly reduced by NS-1619, which had no additive protective effect in the hypoxic group. Reciprocally, the improved resistance to injury in myocytes of the later group was significantly attenuated by paxilline, which did not influence LDH release in the normoxic group (Fig. 4C). Pattern of total LDH release during MI/R reflected that associated with the reenergization phase (Fig. 4B). In contrast, MI-induced LDH release was decreased by NS-1619 in both normoxic and hypoxic groups, and paxilline did not influence the salutary effect of chronic hypoxia (Fig. 4A).
Fig. 4.
Effects of NS and PAX on LDH release from cardiomyocytes during metabolic inhibition (A) and during reenergization (B), and total release during MI/R (C), expressed as a percentage of corresponding LDH release from control cells not exposed to MI/R. Cells were isolated from the LV of rats adapted to chronic hypoxia and of normoxic animals. Values are means ± SE from 8 hearts in each group. *P < 0.05 vs. corresponding normoxic groups. §P < 0.05 vs. UT, VEH, and PAX. †P < 0.05 vs. VEH and PAX. ‡P < 0.05 vs. VEH and NS.
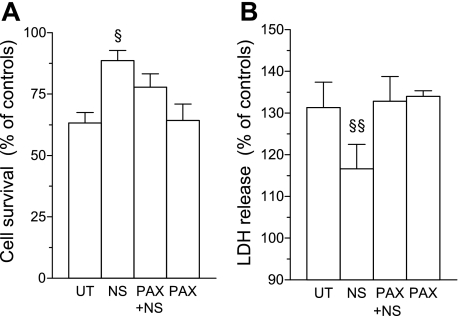
To verify that the protective effect of NS-1619 in cells from normoxic rats can be attributed to the activation of BKCa channels, the experiment was repeated with an additional group of cells that were treated with paxilline before the addition of NS-1619. As illustrated in Fig. 5, paxilline completely abolished the decrease in total LDH release from NS-1619-treated myocytes during MI/R, while its effect on viability did not reach statistical significance (P = 0.056).
Fig. 5.
Effect of PAX pretreatment on protection induced by NS in cardiomyocytes isolated from the LV of normoxic rats. Shown are cell survival (A) and total LDH release during MI/R (B), expressed as a percentage of control values in the absence of MI/R. PAX+NS, cells pretreated with PAX before adding NS. Values are means ± SE from 4 hearts. §P < 0.05 vs. UT and PAX. §§P < 0.05 vs. UT, PAX, and PAX+NS.
These results suggest that mitochondrial BKCa channels contribute to the improved resistance to injury of myocytes isolated from chronically hypoxic rats, and the involvement of these channels in the protective mechanism is confined predominantly to the reenergization phase.
Chronic hypoxia causes deglycosylation of the BKCa channel regulatory β1-subunit.
Western blot analysis detected two bands in cells from all three ventricular parts corresponding to molecular masses of ∼40 and ∼26 kDa that were blocked by a specific blocking peptide against BKCa channel regulatory β1-subunit. While in myocytes from normoxic rats the upper (∼40 kDa) band strongly predominated, chronic hypoxia significantly increased the relative abundance of the lower (∼26 kDa) band (Fig. 6, A and B). Consequently, the upper-to-lower band ratio decreased markedly in myocytes from the hypoxic group compared with normoxic controls (Fig. 6C). Based on previous reports (15, 37), we assumed that the band corresponding to the higher molecular mass could result from N-linked glycosylation of the protein subunit. This assumption was confirmed by enzymatic deglycosylation, which led to a complete disappearance of the upper band (Fig. 6C). It suggests that chronic hypoxia causes partial deglycosylation of the BKCa channel regulatory β1-subunit without affecting its total abundance.
Fig. 6.
Deglycosylation of the large-conductance Ca2+-activated K+ (BKCa) channel regulatory β1-subunit. A: representative Western blots of β1-subunit in cardiomyocytes isolated from the LV, septum (S), and RV show a dominant band at ∼40 kDa (band 1) in the normoxic group and increased abundance of an ∼26-kDa band (band 2) in the chronically hypoxic group. GAPDH was used as a loading control. Quantitative analysis revealed a shift from band 1 to band 2 induced by chronic hypoxia (B), resulting in a drop of the band 1-to-band 2 ratio (C). Values are means ± SE from 6 hearts in each group. *P < 0.05 vs. corresponding normoxic groups. D: the ∼40-kDa band was barely detectable after the enzymatic deglycosylation of samples (shown only for LV). H, chronic hypoxia; N, normoxia; N-DG, normoxia after deglycosylation.
DISCUSSION
The present work resulted in the following three major novel observations: 1) cardiomyocytes isolated from rats adapted to chronic hypoxia were more resistant to injury and cell death caused by acute MI/R; 2) these salutary effects were attenuated by the BKCa channel blocker paxilline, while the opener NS-1619 protected only cells isolated from control normoxic animals; and 3) chronic hypoxia led to partial deglycosylation of the BKCa channel regulatory β1-subunit without changing its total abundance.
Studies on the protective role of the mitochondrial BKCa channel using whole-animal models are complicated by its similar basic biophysical and pharmacological properties to that of the BKCa channel localized in plasma membrane of various cell types (31). To examine the potential involvement of mitochondrial BKCa channels in increased ischemic tolerance of chronically hypoxic rat hearts, we, therefore, used freshly isolated ventricular myocytes that most likely do not contain this type of channel in the sarcolemma (27). First, it was necessary to find out whether isolated myocytes are able to maintain the protected phenotype achieved by the in vivo hypoxic adaptation. Considering regional differences in ventricular myocyte characteristics (6) and distinct effects of chronic hypoxia on the left and right ventricles (25), LVM, SEPM, and RVM were examined separately. We demonstrated for the first time that the salutary effect of chronic hypoxia was retained in subsequently isolated cardiomyocytes, as evidenced by reduced LDH release and increased survival rate of cells exposed to MI/R, simulating acute “ischemia-reperfusion” injury. These data suggest that the cell isolation procedure does not negatively interfere with the robust myocardial protection induced by chronic hypoxia, and freshly isolated myocytes are, therefore, a suitable model to study its underlying mechanism. Similarly, it has been reported that ischemic preconditioning of the whole rat heart increased resistance of subsequently isolated ventricular myocytes to MI/R (28). Somewhat smaller improvement of cell viability and the absence of a significant effect on LDH release from RVM compared with LVM and SEPM in our study can be most likely attributed to an opposing influence of RVM hypertrophy, resulting from hypoxic pulmonary hypertension, because hypertrophy is known to increase susceptibility to myocardial injury caused by acute oxygen deprivation (13).
Opening of mitochondrial BKCa channels has been associated with improved ischemic tolerance in heart and other tissues, based mostly on pharmacological evidence. An increasing number of recent studies suggest the involvement of K+ flux through these channels in the short-lived protective mechanism of various forms of preconditioning (8, 9, 27, 32, 33). Our present results demonstrate for the first time that BKCa channels also play a role in the persistent protection of cardiac myocytes achieved by long-term adaptation of animals to hypoxia. The fact that the inhibitory effect of BKCa blocker paxilline on LDH release occurred only after the MI phase suggests that the channel opening prevented cell injury associated mainly with mitochondrial reenergization. In contrast, paxilline did not affect the reduction of infarct size and the improvement of postischemic contractile function recovery in isolated perfused hearts of chronically hypoxic infant rabbits (30), suggesting possible species- and/or age-dependent differences in the protective mechanism of chronic hypoxia.
It should be mentioned that better cell survival and reduced injury due to MI/R in the chronically hypoxic group were significantly attenuated but not completely abolished by paxilline. It means that other factors independent of BKCa opening may also play a role. This observation is consistent with the contribution of other mitochondrial K+ channels, such as KATP, to achieve a resistant cardiac phenotype by chronic hypoxia (21). Interestingly, it has been shown that protective effects of BKCa and KATP openers against ouabain-induced mitochondrial Ca2+ overload are independent of each other and involve different signaling pathways (29).
Pharmacological opening of BKCa channels with NS-1619 increased survival and limited MI/R-induced injury in cells from normoxic animals. However, no further protection was achieved in the presence of NS-1619 in myocytes from the chronically hypoxic group. It may suggest that hypoxic adaptation preceding the MI/R insult maintains BKCa channels or a related downstream protective pathway in a fully active or conditioned state. Unlike the plasma membrane BKCa channel, the mitochondrial counterpart increases its open probability during hypoxia (10). Although the mechanism is unknown, it may involve cAMP-dependent protein kinase (PKA), which has been shown to augment the channel activity (29) and mediate a BKCa-dependent myocardial protection induced by anesthetic preconditioning (27). Interestingly, cardioprotection induced by adaptation to repeated hypoxic exposures appears to involve signaling via β1-adrenoceptors and PKA, as indicated by blunting effects of respective pharmacological blockers metoprolol (20) and H89 (35).
However, as no agents strictly specific to the mitochondrial BKCa channel are currently available (14), the effects of the modulators used should be interpreted with caution. While no concerns have been raised with respect to the protection-blocking effect of paxilline (39), the opener NS-1619 seems to exhibit some BKCa-independent effects that may contribute to protection, such as the inhibition of L-type Ca2+ channels (26) or generation of reactive oxygen species (14). Moreover, it has been shown that NS-1619 does not promote matrix K+ influx solely through BKCa channels, but also through nonspecific ion transport mechanism (1). Nevertheless, the protective effect of NS-1619 on LDH release from normoxic myocytes was blunted by paxilline in our experiments, suggesting that it can be at least partly attributed to the opening of mitochondrial BKCa channels.
In general, the β-subunits exert important regulatory effects on BKCa channel activity by changing its calcium sensitivity and gating kinetics (24). The BKCa channel auxillary β1-subunit is abundant in cardiac myocytes (36) and exhibit mitochondrial localization (23, 32). Its gene expression is regulated by oxygen tension in a manner dependent on hypoxia-inducible factor-2α (4). However, we did not find any change in protein level of the β1-subunit in ventricular myocytes of chronically hypoxic rats compared with normoxic controls. Bautista et al. (4) recently demonstrated a marked drop in β1-subunit transcript levels in cultured neonatal cardiomyocytes exposed to severe hypoxia for 24–48 h, but this response was partly reversed after the prolongation of the hypoxic period to 72 h. It seems, therefore, that downregulation of β1-subunit expression is only a transient reaction to oxygen deprivation.
It has been shown that the β1-subunit plays an important role in cytoprotective effects mediated by the mitochondrial BKCa channel. For example, the activation of the channel and the improved ischemic tolerance of ventricular myocytes induced by 17β-estradiol resulted from its functional interaction with the β1-subunit (23). Furthermore, selective knockdown of mitochondrial BKCa channel β1-subunit with short interfering RNA blunted the delayed infarct size-limiting effect of sildenafil (32). In agreement with our present data, no de novo synthesis of the β1-subunit was detected after sildenafil treatment in the later study, suggesting that cardioprotection was dependent on activation of existing β1-subunits rather than its upregulation (32).
It has been suggested that chronic hypoxia can modulate BKCa channel function via posttranscriptional mechanisms (16). In the present study, Western blot analysis of cardiac myocytes identified two bands corresponding to the β1-subunit, and enzymatic removal of N-linked oligosaccharides resulted in a single band at a lower molecular mass. Quantitative analysis revealed that native β1-subunit occurred predominantly in a glycosylated form in cells from normoxic animals, while chronic hypoxia led to its marked deglycosylation, decreasing the ratio of glycosylated to nonglycosylated forms by almost 10-fold. Hagen and Sanders (15) demonstrated that glycosylation of the β1-subunit modifies biophysical properties of the BKCa channel in smooth muscle cells and inhibits its opening. In their experiments, enzymatic deglycosylation stimulated the channel activity by increasing its open probability and mean open time. However, it is unknown at present whether this mechanism may regulate the activity of BKCa channels localized in cardiomyocyte mitochondria and promote their involvement in cell protection.
In conclusion, our results demonstrate that ventricular myocytes isolated from chronically hypoxic rats retain the improved resistance against injury caused by MI/R. The blunting effects of paxilline and the absence of additive protection by NS-1619 suggest that chronic hypoxia leads to the activation of mitochondrial BKCa channels, which contribute to the protective mechanism. It remains to be determined whether the reduced glycosylation level of the BKCa channel regulatory β1-subunit, observed in myocytes of chronically hypoxic animals, plays a role in the activation of the channel and cell protection.
GRANTS
This work was supported by the Grant Agency of the Academy of Sciences of the Czech Republic (IAA500110804 to G. H. Borchert) and the Grant Agency of the Czech Republic (305/07/1008 to F. Kolář). C. Yang was supported by the National Institute of General Medical Sciences (GM081748).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We are grateful to Drs. C. Lingle, P. Kemp, and S. Brazier for helpful advices regarding the detection of the β-subunit, S. Brazier for providing us the positive control, and C. Lingle for useful comments to the manuscript. We thank J. Vasinova for excellent technical assistance.
REFERENCES
- 1. Aldakkak M, Stowe DF, Cheng Q, Kwok WM, Camara AKS. Mitochondrial matrix K+ flux independent of large-conductance Ca2+-activated K+ channel opening. Am J Physiol Cell Physiol 298: C530–C541, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aon MA, Cortassa S, Wei AC, Grunnet M, O'Rourke B. Energetic performance is improved by specific activation of K+ fluxes through KCa channels in heart mitochondria. Biochim Biophys Acta 1797: 71–80, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asemu G, Papousek F, Ostadal B, Kolar F. Adaptation to high altitude hypoxia protects the rat heart against ischemia-induced arrhythmias. Involvement of mitochondrial KATP channels. J Mol Cell Cardiol 31: 1821–1831, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Bautista L, Castro MJ, Lopez-Barneo J, Castellano A. Hypoxia inducible factor-2α stabilization and maxi-K+ channel β1-subunit gene repression by hypoxia in cardiac myocytes. Role in preconditioning. Circ Res 104: 1364–1372, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Bentzen BH, Osadchii O, Jespersen T, Hansen RS, Olesen SP, Grunnet M. Activation of big conductance Ca2+-activated K+ channels (BK) protects the heart against ischemia-reperfusion injury. Pflügers Arch 457: 979–988, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Borchert GH, Giggey M, Kolar F, Wong TM, Backx PH, Escriba PV. 2-Hydroxyoleic acid affects cardiomyocyte [Ca2+]i transient and contractility in a region-dependent manner. Am J Physiol Heart Circ Physiol 294: H1948–H1955, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Buhl SN, Jackson KY. Optimal conditions and comparison of lactate dehydrogenase catalysis of the lactate-to-pyruvate and pyruvate-to-lactate reactions in human serum at 25, 30, and 37°C. Clin Chem 24: 823–831, 1978 [PubMed] [Google Scholar]
- 8. Cao CM, Chen M, Wong TM. The KCa channel as a trigger for the cardioprotection induced by kappa-opioid receptor stimulation: its relationship with protein kinase C. Br J Pharmacol 145: 984–991, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cao CM, Xia Q, Gao Q, Chen M, Wong TM. Calcium-activated potassium channel triggers cardioprotection of ischemic preconditioning. J Pharmacol Exp Ther 312: 644–650, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Cheng Y, Gu XQ, Bednarczyk P, Wiedemann FR, Haddad GG, Siemen D. Hypoxia increases activity of the BK-channel in the inner mitochondrial membrane and reduces activity of the permeability transition pore. Cell Physiol Biochem 22: 127–136, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Eells JT, Henry MM, Gross GJ, Baker JE. Increased mitochondrial KATP channel activity during chronic myocardial hypoxia: is cardioprotection mediated by improved bioenergetics? Circ Res 87: 915–921, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Essop MF. Cardiac metabolic adaptations in response to chronic hypoxia. J Physiol 584: 715–726, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Friehs I, del Nido PJ. Increased susceptibility of hypertrophied hearts to ischemic injury. Ann Thorac Surg 75: S678–S684, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Gaspar T, Katakam P, Snipes JA, Kis B, Domoki F, Bari F, Busija DW. Delayed neuronal preconditioning by NS1619 is independent of calcium activated potassium channels. J Neurochem 105: 1115–1128, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hagen BM, Sanders KM. Deglycosylation of the β1-subunit of the BK channel changes biophysical properties. Am J Physiol Cell Physiol 291: C750–C756, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Hartness ME, Brazier SP, Peers C, Bateson AN, Ashford MLJ, Kemp PJ. Post-transcriptional control of human maxiK potassium channel activity and acute oxygen sensitivity by chronic hypoxia. J Biol Chem 278: 51422–51432, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Kang SH, Park WS, Kim N, Youm JB, Warda M, Ko JH, Ko EA, Han J. Mitochondrial Ca2+-activated K+ channels more efficiently reduce mitochondrial Ca2+ overload in rat ventricular myocytes. Am J Physiol Heart Circ Physiol 293: H307–H313, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Kolar F, Ostadal B. Molecular mechanisms of cardiac protection by adaptation to chronic hypoxia. Physiol Res 53, Suppl 1: S3–S13, 2004 [PubMed] [Google Scholar]
- 19. Kopecky M, Daum S. [Tissue adaptation to anoxia in rat myocardium] (in Czech). Cesk Fysiol 7: 518–521, 1958 [PubMed] [Google Scholar]
- 20. Mallet RT, Ryou MG, Williams AG, Howard L, Downey HF. β1-adrenergic receptor antagonism abrogates cardioprotective effects of intermittent hypoxia. Basic Res Cardiol 101: 436–446, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Neckar J, Szarszoi O, Koten L, Papousek F, Ostadal B, Grover GJ, Kolar F. Effects of mitochondrial KATP modulators on cardioprotection induced by chronic high altitude hypoxia in rats. Cardiovasc Res 55: 567–575, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Neckar J, Ostadal B, Kolar F. Myocardial infarct size-limiting effect of chronic hypoxia persists for five weeks of normoxic recovery. Physiol Res 53: 621–628, 2004 [PubMed] [Google Scholar]
- 23. Ohya S, Kuwata Y, Sakamoto K, Muraki K, Imazumi Y. Cardioprotective effects of estradiol include the activation of large-conductance Ca2+-activated K+ channels in cardiac mitochondria. Am J Physiol Heart Circ Physiol 289: H1635–H1642, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Orio P, Rojas P, Ferreira G, Latorre R. New disguises for an old channel: MaxiK channel β-subunits. News Physiol Sci 17: 156–161, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Ostadal B, Kolar F. Cardiac adaptation to chronic high-altitude hypoxia: Beneficial and adverse effects. Respir Physiol Neurobiol 158: 224–236, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Park WS, Kang SH, Son YK, Kim N, Ko JH, Kim HK, Ko EA, Kim CD, Han J. The mitochondrial Ca2+-activated K+ channel activator, NS 1619 inhibits L-type Ca2+ channels in rat ventricular myocytes. Biochem Biophys Res Commun 362: 31–36, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Redel A, Lange M, Jazbutyte V, Lotz C, Smul TM, Roewer N, Kehl F. Activation of mitochondrial large-conductance calcium-activated K+ channel via protein kinase A mediates desflurane-induced preconditioning. Anesth Analg 106: 384–391, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Rodrigo GC, Samani NJ. Ischemic preconditioning of the whole heart confers protection on subsequently isolated ventricular myocytes. Am J Physiol Heart Circ Physiol 294: H524–H531, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Sato T, Saito T, Saegusa N, Nakaya H. Mitochondrial Ca2+-activated K+ channels in cardiac myocytes. A mechanism of the cardioprotective effect and modulation by protein kinase A. Circulation 111: 198–203, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Shi Y, Jiang MT, Su J, Hutchins W, Konorev E, Baker JE. Mitochondrial big conductance KCa channel and cardioprotection in infant rabbit heart. J Cardiovasc Pharmacol 50: 497–502, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Skalska J, Piwonska M, Wyroba E, Surmacz L, Wieczorek R, Koszela-Piotrowska I, Zielinska J, Bednarczyk P, Dolowy K, Wilczynski GM, Szewczyk A, Kunz WS. A novel potassium channel in skeletal muscle mitochondria. Biochim Biophys Acta 1777: 651–659, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Wang X, Fisher PW, Xi L, Kukreja R. Essential role of mitochondrial Ca2+-activated and ATP-sensitive K+ channels in sildenafil-induced late cardioprotection. J Mol Cell Cardiol 44: 105–113, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Wang X, Yin C, Xi L, Kukreja RC. Opening of Ca2+-activated K+ channels triggers early and delayed preconditioning against I/R injury independent of NOS in mice. Am J Physiol Heart Circ Physiol 287: H2070–H2077, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Wu S, Li HY, Wong TM. Cardioprotection of preconditioning by metabolic inhibition in the rat ventricular myocyte. Involvement of κ-opioid receptor. Circ Res 84: 1388–1395, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Xie Y, Zhu Y, Zhu WZ, Chen L, Zhou ZN, Juan WJ, Yang HT. Role of dual-site phospholamban phosphorylation in intermittent hypoxia-induced cardioprotection against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 288: H2594–H2602, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, O'Rourke B. Cytoprotective role of Ca2+-activated K+ channels in the cardiac inner mitochondrial membrane. Science 298: 1029–1033, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Yang CT, Zeng XH, Xia XM, Lingle CJ. Interactions between β subunits of the KCNMB family and Slo3: β4 selectively modulates Slo3 expression and function. PLoS One 4: e6135, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhu HF, Dong JW, Zhu WZ, Ding HL, Zhou NZ. ATP-dependent potassium channels involved in the cardiac protection induced by intermittent hypoxia against ischemia/reperfusion injury. Life Sci 73: 1275–1287, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Zoratti M, De Marchi U, Gulbins E, Szabo I. Novel channels in the inner mitochondrial membrane. Biochim Biophys Acta 1787: 351–363, 2009 [DOI] [PubMed] [Google Scholar]