Abstract
We investigated the contribution of tetrodotoxin (TTX)-resistant sodium channels to the augmented exercise pressor reflex observed in decerebrated rats with femoral artery ligation. The pressor responses to static contraction, to tendon stretch, and to electrical stimulation of the tibial nerve were compared before and after blocking TTX-sensitive sodium channels on the L3-L6 dorsal roots of rats whose hindlimbs were freely perfused and rats whose femoral arteries were ligated 72 h before the start of the experiment. In the freely perfused group (n = 9), pressor (Δ22 ± 4 mmHg) and cardioaccelerator (Δ32 ± 6 beats/min) responses to contraction were attenuated by 1 μM TTX (Δ4 ± 1 mmHg, P < 0.05 and Δ17 ± 4 beats/min, P < 0.05, respectively). In the 72 h ligated group (n = 9), the augmented pressor response to contraction (32 ± 4 mmHg) was also attenuated by 1 μM TTX (Δ8 ± 2 mmHg, P < 0.05). The cardioaccelerator response to contraction was not significantly attenuated in these rats. In addition, TTX suppressed the pressor response to tendon stretch in both groups of rats. Electrical stimulation of the tibial nerve evoked similar pressor responses between the two groups (freely perfused: Δ74 ± 9 mmHg and 72 h ligated: Δ78 ± 5 mmHg). TTX attenuated the pressor response to the tibial nerve stimulation by about one-half in both groups. Application of the TTX-resistant sodium channel blocker A-803467 (1 μM) with TTX (1 μM) did not block the pressor response to tibial nerve stimulation to any greater extent than did application of TTX (1 μM) alone. Although the contribution of TTX-resistant sodium channels to the augmented exercise pressor reflex may be slightly increased in rats with chronic femoral artery ligation, TTX-resistant sodium channels on dorsal roots do not play a major role in the augmented exercise pressor reflex.
Keywords: static contraction, thin fiber muscle afferents, tetrodotoxin, sodium channel
exercise is well known to increase arterial pressure, heart rate (HR), and ventilation. Evidence in both animals and humans has been accumulating to demonstrate the importance of the exercise pressor reflex in evoking these effects (8, 16, 26, 32). The afferent arm of the exercise pressor reflex arc is comprised of thinly myelinated (group III) and unmyelinated (group IV) fibers, the endings of which respond, respectively, to mechanical and metabolic stimuli arising in the contracting muscles (13, 14). Conduction along the axons of these afferents is mediated by voltage-gated sodium channels (Nav1), some of which are blocked by tetrodotoxin (TTX) and some of which are not. TTX-sensitive (TTX-s) channels are found on myelinated axons as well as on unmyelinated ones. In contrast, TTX-resistant (TTX-r) channels are found on unmyelinated axons (22).
Voltage-gated sodium channels have been shown to be encoded by nine genes (4). Eight of the nine channels are expressed on dorsal root ganglion cells (22). Specifically, NaV1.1, NaV1.2, NaV1.3, NaV1.6, and NaV1.7 are sensitive to TTX, whereas NaV1.5, NaV1.8, and NaV1.9 are resistant to TTX. NaV1.4 is sensitive to TTX, but it is not present on dorsal root ganglion cells (22). Both TTX-s and TTX-r channels are found both on the cell bodies of dorsal root ganglion cells and along their axons (2, 3, 27). Particular attention has been paid to the NaV1.8 channel because it has been thought to be involved in the transmission of nociceptive information in rodents (12).
The roles played by TTX-s and TTX-r channels in eliciting the exercise pressor reflex are unknown. In the experiments to be described, we have focused on the role played by both TTX-s and TTX-r voltage-gated sodium channels in the dorsal roots, which comprise the conduction pathway of the afferent arm of the reflex. We examined the effect of blocking these channels on the exercise pressor reflex in decerebrated unanesthetized rats whose hindlimb muscles were freely perfused and in rats whose arterial supply to the hindlimb was occluded for 72 h before the start of the experiment. The latter preparation is believed to simulate blood flow to muscles in humans with peripheral artery disease (20, 34). Specifically, blood flow to the muscles whose arterial supply is ligated is normal at rest, whereas blood flow to these muscles during exercise is inadequate to meet demand (20, 35).
METHODS
All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University, Hershey Medical Center. Adult male rats (Sprague-Dawley, n = 60, weighing between 315 and 460 g) were used in this study. The rats were housed in a temperature-controlled room (24 ± 1°C) with a 12:12-h light-dark cycle. Rats were fed a standard diet and tap water ad libitum. Before an experiment (72 h), 46 of the 62 rats underwent surgery to induce unilateral femoral artery occlusion according to the procedure described elsewhere (20, 34). Briefly, rats were anesthetized with a mixture of 4% isoflurane and 100% oxygen; one femoral artery was isolated and then tightly ligated with 5–0 silk suture just distal to the inguinal ligament. With the use of radiolabeled microspheres, it has been shown that this femoral artery ligation procedure reduced blood flow reserve capacity to ∼10–20% of normal but allowed sufficient blood flow to meet resting requirements (20, 34). The rats were allowed to recover 72 h before the experiments were started. Femoral artery occlusion has been reported to have no effect on normal cage activity (29).
Surgical Preparation
On the day of the experiment, rats were anesthetized with a mixture of 4% isoflurane and 100% oxygen. The right jugular vein and common carotid artery were cannulated for the delivery of drugs and fluids and the measurement of arterial blood pressure, respectively. The carotid arterial catheter was connected to a pressure transducer (model P23 XL; Statham). HR was calculated beat to beat from the arterial pressure pulse (Gould Biotach). The trachea was cannulated, and the lungs were ventilated mechanically (Harvard Apparatus). Arterial blood gases and pH were measured by an automated blood-gas analyzer (model ABL-700; Radiometer). Pco2 and arterial pH were maintained within normal range by either adjusting ventilation or by intravenous administration of sodium bicarbonate (8.5%). A rectal temperature probe was inserted, and the core body temperature of the animal was maintained at 37–38°C by a water-perfused heating pad and a lamp.
The rat was placed in a Kopf stereotaxic frame. Dexamethasone (0.2 mg) was injected intravenously just before the decerebration procedure to minimize brain stem edema. The left common carotid artery was tied off, and a precollicular decerebration was performed. The plane of section was <1 mm anterior to the superior colliculi. All neural tissue rostral to the section was removed. To minimize cerebral hemorrhage, small pieces of oxidized regenerated cellulose (Ethicon; Johnson & Johnson) were placed on the internal skull surface, and the cranial cavity was packed with cotton. In our experiments, rats were decerebrated instead of anesthetized because the preponderance of the evidence indicates that anesthesia prevents the reflex in this species (23, 30, 33).
A laminectomy exposing the lower lumbar and sacral portions of the spinal cord (L1-L5) was performed. The rat was then secured in a customized spinal frame by clamps placed on rostral lumbar vertebrae and the pelvis. Using the skin on the back, we formed a pool that was filled with warm (37°C) mineral oil. The dura was cut and reflected, allowing visual identification of the spinal roots. The left L4 and L5 ventral roots were identified and cut close to their exits from the spinal cord. Around L3-L6 dorsal roots, a pool was formed for TTX application. A longitudinally halved plastic tube, which was 6 mm in length and 3 mm in transverse inner diameter, was put under the intact left side of L3-L6 dorsal roots. A sheet of laboratory film (Parafilm), 9 mm square, was placed underneath the tube. Next, both proximal and distal ends of the tube were sealed with silicone elastomer (Kwik-Sil; World Precision Instruments). The pool was first filled with 50 μl lactated Ringer solution. The segments L4 and L5 are the main input targets of afferent nerve fibers from the lower leg in the rat (17).
The calcaneal bone of a left hindlimb was severed, and the triceps surae muscles were isolated. Once the surgeries were completed, the anesthesia was withdrawn, and the lungs were ventilated with room air. A minimum recovery period of 90 min was employed after decerebration before beginning any experimental protocol.
Experimental Protocols
Static contraction and tendon stretch.
The cut peripheral ends of the L4 and L5 ventral roots were placed on shielded stimulating electrodes. Each calcaneal tendon was attached to a force transducer (model FT 10; Grass), which in turn was attached to a rack-and-pinion. The tendon was stretched so that baseline tension was set between 50 and 100 g. Static contraction was evoked by electrically stimulating (40 Hz, 0.1 ms, >2 times motor threshold) the peripheral ends of the L4 and L5 ventral roots. A muscle mechanoreceptor reflex (10) was evoked by stretching the triceps surae muscles by manually turning the rack-and-pinion that was attached to the calcaneal tendon. Baseline tension was set between 50 and 100 g. Both muscle contraction and tendon stretch lasted for 60 s. We attempted to match the magnitudes of the tension traces for static contraction and tendon stretch. The order of presentation of the two stimuli was varied randomly. After the end of contraction or tendon stretch (10 min), we blocked the TTX-s sodium channels by replacing the 50 μl of lactated Ringer solution in the pool with 50 μl of TTX (1 μM) in lactated Ringer solution. After application of TTX to the pool (20 min), static contraction and tendon stretch were repeated. Next, the dorsal roots were repeatedly rinsed with Ringer solution every 10 min. In eight experiments, static contraction and tendon stretch were performed 90 min after removal of TTX.
Tibial nerve stimulation.
Thirty two rats were artificially ventilated with room air and were paralyzed with pancuronium bromide (initial dose: 1 mg/kg iv followed by supplementary doses of 0.2 mg/kg every 30 min). The left tibial nerve was exposed through a midline linear incision in the posterior thigh and was then dissected free and placed on a stimulating electrode. The intact left tibial nerve was electrically stimulated (20 Hz; 0.75 ms) at supramaximal current intensities so that maximal pressor response could be obtained. After the control stimulation, TTX-s sodium channels were blocked by applying 50 μl of the TTX (1 μM) solution in the pool in 14 of the 32 rats. After 20 min of the application of TTX, electrical stimulation of tibial nerve was repeated. Next, both TTX-s and TTX-r channels were blocked by applying 50 μl of lidocaine (2%) into the pool. The tibial nerve was then stimulated after 20 min of the incubation time. In 7 of 32 experiments, 1 or 100 μM of A-803467, a potent and selective NaV1.8 antagonist (12), and 1 μM of TTX solution were coapplied to the pool before testing lidocaine. In another 5 of 32 experiments, 3 μM of TTX was also tested before lidocaine. In the remaining six rats, 1, 10, and 100 μM of A-803467 solution was applied to the pool before testing lidocaine. The volume of each of these solutions was 50 μl.
Compound action potentials.
Compound action potentials evoked by stimulating the tibial nerve were recorded from the left L5 dorsal root, which was cut near the spinal cord and was carefully separated into two to three rootlets (7, 15). One rootlet was placed on one foot of a bipolar hook electrode. The other foot was grounded to the rat with a thin string soaked in saline. The left sciatic nerve was exposed. Skin flaps were raised to enclose a pool of mineral oil that covered the exposed regions of nerve. The tibial nerve was stimulated through a pair of shielded hook electrodes at 0.5 Hz (square-wave pulse of 1 ms duration and supramaximal voltage). The evoked compound action potential was passed through a high-impedance probe (model HIP 511; Grass Instruments), amplified, and filtered (0.3–1 kHz; model P 511; Grass Instruments). The compound action potentials were displayed on a computer monitor (Spike 2; Cambridge Electronics Design, Cambridge, UK) and on a storage oscilloscope (model HP 54603B). The compound potentials from 30 stimulus presentations were summed, and the areas under Aδ (i.e., group III) and the C (i.e., group IV) waves were measured. Next, TTX was applied to a pool at 0.1 and 1 μM concentrations in 5 of 10 rats, and A-803467 was applied at 1, 10, and 100 μM in the remaining 5 rats. The compound action potentials evoked by electrical stimulation of the tibial nerve were then reassessed. Last, the effect of 2% of lidocaine applied to the pool was assessed in the same manner as that described for TTX and A-803467. Thirty minutes were allowed to elapse between each measurement. Before stimulating the tibial nerve, the rats were paralyzed with pancuronium bromide (initial dose: 1 mg/kg iv followed by supplementary doses of 0.2 mg/kg every 30 min).
Data Analysis
Arterial blood pressure, HR, muscle tension, and the electrocardiogram were recorded with a Spike 2 data acquisition system (CED; Cambridge) and stored on a computer hard drive (Dell). Mean arterial pressure (MAP) is expressed in millimeters mercury and HR in beats per minute. The initial 60-s values were used to compare the differences between baseline and the response to each maneuver. In addition, the time course of the MAP and HR responses to static contraction, tendon stretch, and electrical stimulation of the tibial nerve were analyzed at 2 s following the onset of each maneuver and then at each 5-s time point until the maneuver ended. The tension-time index was calculated by integrating the area between the tension trace and the baseline level (Spike 2) and is expressed in kilograms times seconds (kg·s).
All values are expressed as means ± SE. Statistical comparisons were performed with either a one-way repeated-measures ANOVA or two-way repeated-measures ANOVA. Next, post hoc tests were performed with the Tukey test between individual means. Bonferroni post hoc tests were used to determine significant differences between time course means. The criterion for statistical significance was set at P < 0.05.
RESULTS
Contraction
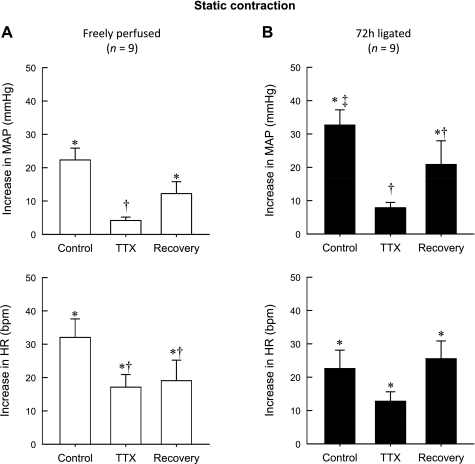
In control rats (n = 9) whose hindlimbs were freely perfused, TTX (1 μM) markedly attenuated the peak pressor response to static contraction (22 ± 4 mmHg before TTX vs. 4 ± 1 mmHg during TTX, P < 0.001; Fig. 1A). After TTX was removed from the pool (90 min), the peak pressor response to contraction partially recovered (4 ± 1 mmHg during TTX vs. 12 ± 4 mmHg during recovery; P < 0.05; Fig. 1A). Likewise, TTX attenuated the peak cardioacclerator response to contraction (32 ± 6 beats/min before TTX vs. 17 ± 4 beats/min during TTX, P < 0.001). After TTX was removed from the pool (90 min), the peak cardioaccelerator response did not recover (19 ± 6 beats/min during recovery vs. 17 ± 4 beats/min during TTX; P > 0.05; Fig. 1A and Tables 1 and 2).
Fig. 1.
Peak pressor and cardioaccelerator responses to static contraction in 9 rats whose hindlimbs were freely perfused (A) and in 9 other rats whose femoral arteries were ligated 72 h before the start of the experiment (B). Note that control represents the responses to contraction before tetrodotoxin (TTX, 1 μM) was applied to the pool surrounding the dorsal roots. Likewise, TTX represents the responses to contraction while TTX (1 μM) was applied to the pool surrounding the dorsal roots, and recovery represents the responses after TTX was removed from the dorsal roots. *The peak response was significantly greater than the corresponding baseline (P < 0.05). †The peak response was significantly less than its corresponding control value (P < 0.05). ‡The peak response was significantly greater than the peak response of the control condition in rats whose hindlimbs were freely perfused. MAP, mean arterial pressure; HR, heart rate.
Table 1.
Baseline MAP and HR
| n | Condition | MAP, mmHg | HR, beats/min | |
|---|---|---|---|---|
| Freely perfused | ||||
| Static contraction | 9 | Control | 81 ± 6 | 395 ± 9 |
| TTX | 84 ± 7 | 385 ± 11 | ||
| Recovery | 86 ± 6 | 403 ± 14 | ||
| Tendon stretch | 9 | Control | 74 ± 5 | 408 ± 14 |
| TTX | 85 ± 7 | 386 ± 11 | ||
| Recovery | 89 ± 6 | 410 ± 14 | ||
| Tibial nerve stimulation | 7 | Control | 81 ± 8 | 451 ± 8 |
| TTX | 87 ± 10 | 439 ± 12 | ||
| Recovery | 76 ± 9 | 441 ± 13 | ||
| Ligated (72 h) | ||||
| Static contraction | 9 | Control | 97 ± 6 | 408 ± 21 |
| TTX | 91 ± 6 | 403 ± 17 | ||
| Recovery | 105 ± 8 | 416 ± 10 | ||
| Tendon stretch | 9 | Control | 92 ± 7 | 421 ± 21 |
| TTX | 95 ± 7 | 396 ± 18 | ||
| Recovery | 100 ± 5 | 422 ± 6 | ||
| Tibial nerve stimulation | 7 | Control | 102 ± 6 | 488 ± 16 |
| TTX | 97 ± 4 | 478 ± 12 | ||
| Recovery | 93 ± 4 | 480 ± 9 |
Values are means ± SE; n, no. of rats. MAP, mean arterial pressure; HR, heart rate; TTX, tetrodotoxin.
Table 2.
TTI during static contraction and tendon stretch
| n | Condition | TTI, kg · s | |
|---|---|---|---|
| Freely perfused | 9 | ||
| Contraction | Control | 33 ± 3 | |
| TTX | 31 ± 1 | ||
| Recovery | 32 ± 2 | ||
| Stretch | Control | 34 ± 1 | |
| TTX | 35 ± 1 | ||
| Recovery | 34 ± 2 | ||
| Ligated (72 h) | 9 | ||
| Contraction | Control | 26 ± 3 | |
| TTX | 26 ± 3 | ||
| Recovery | 32 ± 2 | ||
| Stretch | Control | 32 ± 1 | |
| TTX | 34 ± 1 | ||
| Recovery | 33 ± 2 |
Values are means ± SE; n, no. of rats. TTI, tension-time index.
As shown previously (31), the peak pressor response to static contraction in rats whose femoral artery was ligated 72 h before the start of the experiment (n = 9) was significantly greater than that in rats whose hindlimbs were freely perfused (P < 0.05; Fig. 1B). In the rats whose femoral artery was ligated, TTX (1 μM) attenuated the peak pressor response to contraction (33 ± 5 mmHg before TTX vs. 8 ± 2 mmHg during TTX; P = 0.004). After TTX was removed from the pool (90 min), the response to contraction partially recovered (8 ± 2 mmHg during TTX vs. 21 ± 7 mmHg after TTX; P < 0.05). The peak cardioaccelerator response to contraction appeared to be reduced by TTX, but the effect was not significant (23 ± 5 beats/min before TTX, 15 ± 3 beats/min during TTX; P > 0.05; Fig. 1B).
In five rats whose femoral artery was ligated, we also examined the effect of 100 nM TTX on the exercise pressor reflex. Three of these five were also tested in the 1 μM TTX experiments described above. We found that peak pressor response to contraction was significantly reduced by 100 nM TTX (from 26 ± 8 to 6 ± 1 mmHg; P = 0.016). The peak cardioaccelerator response to contraction appeared to be reduced by TTX, but the effect was not significant (26 ± 10 beats/min before TTX and 9 ± 3 beats/min during TTX; P > 0.05).
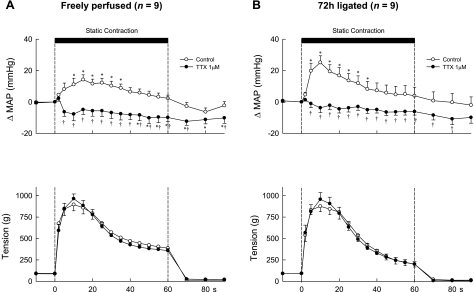
Next, we examined the effect of TTX on the topography of the pressor response to static contraction. We did so by plotting the change in MAP from baseline at 2 s after the start of contraction, at 5 s after the start of contraction, and at every 5-s interval thereafter for 90 s. Before TTX (1 μM) was added to the pool, static contraction, regardless of whether the femoral artery was occluded or not, rapidly increased MAP, an effect that reached peak levels between 10 and 15 s from the onset of tension development (Fig. 2). Subsequently, the increases in MAP gradually decreased from peak levels even though the hindlimb muscles continued to contract. Static contraction also rapidly increased HR. The increased HR remained elevated throughout the contraction period (data not shown).
Fig. 2.
Temporal plots of the pressor responses and the tension development to static contraction in 9 rats whose hindlimbs were freely perfused (A) and in 9 other rats whose femoral arteries were ligated 72 h before the start of the experiment (B). Open circles, data points during the control period; filled circles, data points while TTX (1 μM) was applied to the pool surrounding the dorsal roots. Note that the first data point after the start of contraction (vertical broken line) was taken 2 s after its start, and every point afterward was taken at 5-s intervals from the start of contraction. *A data point for the change in mean arterial pressure (ΔMAP) was greater (P < 0.05) than its corresponding baseline value. †A data point for the change in mean arterial pressure was less (P < 0.05) than its corresponding control value.
In rats whose hindlimbs were freely perfused, addition of TTX (1 μM) to the pool surrounding the dorsal roots abolished the pressor response to static contraction. Moreover, arterial pressure decreased below baseline levels, an effect that was significant during the last 15 s of the contraction period (Fig. 2). In the rats whose femoral arteries were ligated, addition of TTX (1 μM) to the pool also abolished the pressor response to contraction. In contrast to the rats whose hindlimbs were freely perfused, arterial pressure during contraction did not decrease below its baseline level, an effect presumably caused by ligation of the femoral artery (Fig. 2).
Tendon Stretch
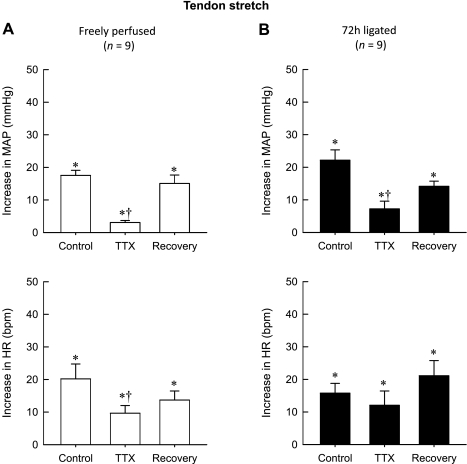
In control rats (n = 9) whose hindlimbs were freely perfused, TTX (1 μM) markedly attenuated the peak pressor response to tendon stretch (18 ± 2 mmHg before TTX vs. 3 ± 1 mmHg during TTX, P < 0.001; Fig. 3A). After TTX was removed from the pool (90 min), the peak pressor response to stretch partially recovered (3 ± 1 mmHg during TTX vs. 15 ± 3 during recovery; P < 0.05; Fig. 3A). Likewise, TTX attenuated the peak cardioaccelerator response to stretch (20 ± 5 beats/min before TTX vs. 10 ± 2 beats/min during TTX, P = 0.014). After TTX was removed from the pool (90 min), the peak cardioaccelerator response did not recover (14 ± 3 beats/min during recovery vs. 10 ± 2 beats/min during TTX; P > 0.05; Fig. 3A and Tables 1 and 2).
Fig. 3.
Peak pressor and cardioaccelerator responses to tendon stretch in 9 rats whose hindlimbs were freely perfused (A) and in 9 other rats whose femoral arteries were ligated 72 h before the start of the experiment (B). Note that control represents the responses to stretch before TTX (1 μM) was applied to the pool surrounding the dorsal roots. Likewise, TTX represents the responses to stretch while TTX (1 μM) was applied to the pool surrounding the dorsal roots, and recovery represents the responses after TTX was removed from the dorsal roots. *The peak response was significantly greater than its corresponding baseline (P < 0.05). †The peak response was significantly less (P < 0.05) than its corresponding control value.
The peak pressor response to tendon stretch in rats whose femoral artery was ligated 72 h before the start of the experiment (n = 9) was not significantly greater than that in rats whose hindlimbs were freely perfused (P > 0.05; Fig. 3). In the rats whose femoral artery was ligated, TTX (1 μM) attenuated the peak pressor response to stretch (22 ± 3 mmHg before TTX vs. 7 ± 2 mmHg during TTX; P < 0.001). After TTX was removed from the pool (90 min), the response to stretch partially recovered (7 ± 2 mmHg during TTX vs. 14 ± 2 mmHg after TTX; P < 0.05). TTX had no effect on the peak cardioaccelerator response to tendon stretch, with the increase in HR averaging 23 ± 5 beats/min before TTX, 15 ± 3 beats/min during TTX, and 29 ± 4 beats/min during recovery (P > 0.05; Fig. 3B).
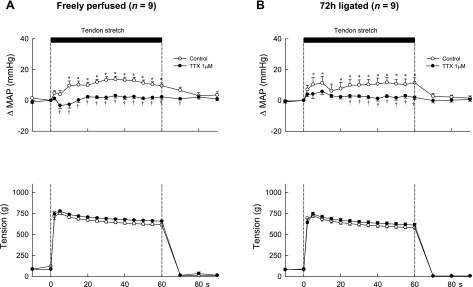
Examination of the topography of the pressor response to tendon stretch before and during application of TTX (1 μM) to the pool was performed in the same manner as that done for contraction. TTX almost abolished the pressor response to stretch both in rats whose hindlimbs were freely perfused and in rats whose femoral arteries were ligated (Fig. 4, A and B). Moreover, tendon stretch did not significantly decrease or increase arterial pressure below the baseline levels when TTX was added to the pool in either group.
Fig. 4.
Temporal plots of the pressor responses and the tension development to tendon stretch in 9 rats whose hindlimbs were freely perfused (A) and in 9 other rats whose femoral arteries were ligated 72 h before the start of the experiment (B). Open circles, data points during the control period; filled circles, data points while TTX (1 μM) was applied to the pool surrounding the dorsal roots. Note that the first data point after the start of stretch (vertical broken line) was taken 2 s after its start, and every point afterward was taken at 5-s intervals from the start of stretch. *A data point for the change in mean arterial pressure was greater (P < 0.05) than its corresponding baseline value. †A data point for the change in mean arterial pressure was less (P < 0.05) than its corresponding control value.
Tibial Nerve Stimulation
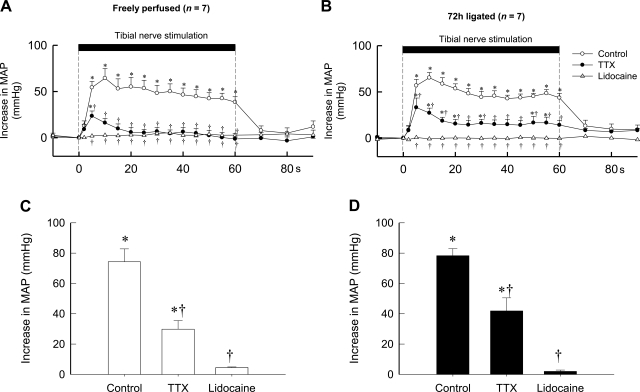
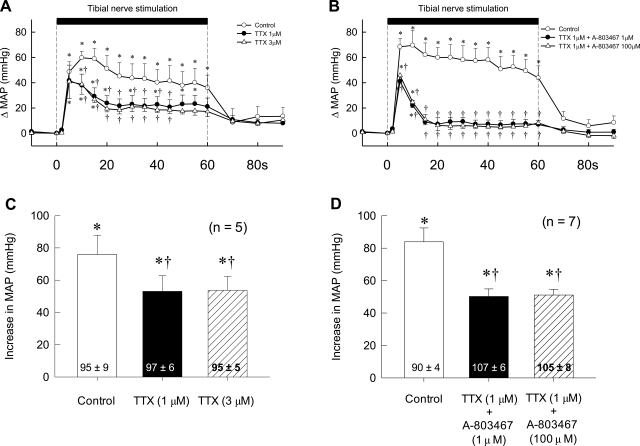
The large peak pressor response evoked by electrical stimulation of the tibial nerve was not different between rats whose hindlimbs were freely perfused and rats whose femoral arteries were ligated for 72 h. The peak response was attenuated by about one-half by TTX (1 μM) and was almost abolished by lidocaine in both groups of rats (Fig. 5). In five rats whose femoral arteries were ligated for 72 h, 3 μM of TTX did not attenuate the pressor response to tibial nerve stimulation any more than did 1 μM of TTX (Fig. 6). Likewise, the cardioaccelerator responses to tibial nerve stimulation and their attenuation by TTX were similar between the two groups. The topographies of the pressor response to tibial nerve stimulation before, after TTX, and after lidocaine were similar between the two groups (Fig. 5). In seven rats whose femoral arteries were ligated, the combined solution of 1 μM TTX and either 1 or 100 μM A-803467, a selective antagonist to NaV1.8 channels, had no effect on the pressor response to tibial nerve stimulation to any greater extent than did 1 μM TTX alone (P > 0.05; Fig. 6).
Fig. 5.
Effects of TTX (1 μM) and lidocaine (2%) applied to the pool surrounding the dorsal roots on the pressor response to electrical stimulation of the tibial nerve at current intensities that recruited group IV afferents. A: temporal plot of the effects of these agents in 7 rats whose hindlimbs were freely perfused. B: temporal plot of the effects of these agents in 7 rats whose femoral arteries were ligated 72 h before the start of the experiment. Open circles, control response; filled circles, response while TTX was applied to the pool; open triangles, response while lidocaine was applied to the pool. Note that the first data point after the start of stimulation (vertical broken line) was taken 2 s after its start, and every point afterward was taken at 5-s intervals from the start of stretch. C: peak responses (open bars) to electrical stimulation of the tibial nerve in 7 rats whose hindlimbs were freely perfused. D: peak pressor responses (filled bars) to electrical stimulation of the tibial nerve in 7 other rats whose femoral arteries were ligated 72 h before the start of the experiment. *The change in mean arterial pressure was greater (P < 0.05) than its corresponding baseline value. †The change in mean arterial pressure was less (P < 0.05) than its corresponding control value.
Fig. 6.
Effects of TTX and A-803467 applied to the pool surrounding the dorsal roots on the pressor response to electrical stimulation of the tibial nerve at current intensities that recruited group IV afferents. A: temporal plot of the effects of 1 μM TTX (filled circles) and 3 μM TTX (open triangles) in 5 rats whose femoral arteries were ligated 72 h before the start of the experiment. B: temporal plot of the effects of TTX (1 μM) plus A-803467 (1 μM) (filled circles) as well as the effects of TTX (1 μM) plus A-803467 (100 μM) (open triangles) in 7 other rats on the pressor responses to stimulation of the tibial nerve. Open circles, control responses in both A and B. Note that, in both A and B, the first data point after the start of stimulation (vertical broken line) was taken 2 s after its start, and every point afterward was taken at 5-s intervals from the start of stimulation. C: peak responses to electrical stimulation of the tibial nerve in the 5 rats shown in A. D: peak responses to electrical stimulation of the tibial nerve in the 7 rats shown in B. Values inside bars are baseline values. *The change in mean arterial pressure was greater (P < 0.05) than its corresponding baseline value. †The change in mean arterial pressure was less (P < 0.05) than its corresponding control value.
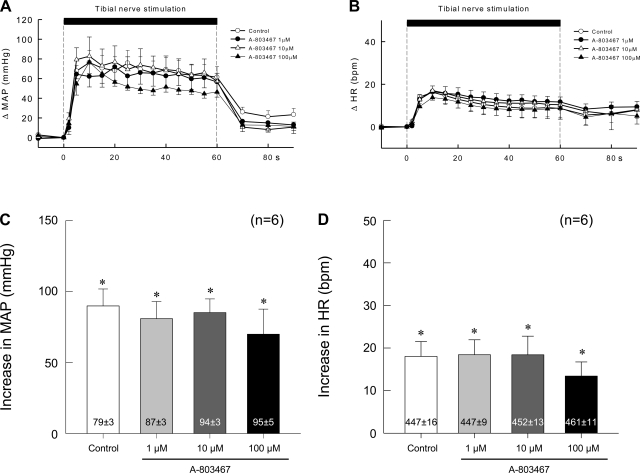
The peak pressor and cardioaccelerator responses to tibial nerve stimulation were not changed significantly (P > 0.05) by addition of 1, 10, or 100 μM of the selective NaV1.8 antagonist A-803467 to the pool surrounding the dorsal roots of six rats in which the femoral artery was ligated (Fig. 7). These experiments were performed in the absence of TTX. Moreover, none of three concentrations of the NaV1.8 antagonist had any significant effect on the topography of the pressor and cardioaccelerator responses to tibial nerve stimulation (Fig. 7).
Fig. 7.
Effects of 3 concentrations of A-803467 (1, 10, and 100 μM) applied to the pool surrounding the dorsal roots on the pressor responses to electrical stimulation of the tibial nerve at current intensities that recruited group IV afferents. Data are from 6 rats whose femoral arteries were ligated 72 h before the start of the experiment. A and B: temporal plots of the data. C and D: peak pressor and cardioaccelerator responses to stimulation. Values inside bars are baseline values. *A significant increase (P < 0.05) over the corresponding baseline value that is given in the vertical bar.
Compound Action Potentials
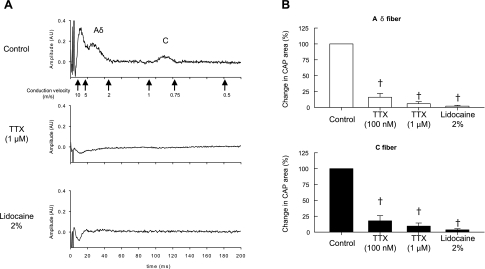
The effect of TTX on the compound action potential evoked by supramaximal stimulation of the tibial nerve was determined in five rats whose femoral arteries were ligated for 72 h (Fig. 8). TTX at a concentration of 100 nM blocked most of the compound action potentials arising from stimulation of group III and IV afferents. In addition, TTX at a concentration of 1 μM abolished the compound action potential evoked by stimulation of group III afferents and reduced the compound action potential evoked by stimulation of group IV afferents by ∼90% (Fig, 8). Application of 2% lidocaine to the spinal pool completely blocked the compound action potentials evoked by stimulation of group III and IV afferents. TTX at both concentrations as well as lidocaine also blocked the compound action potential evoked by stimulation of group I and II afferents.
Fig. 8.
Effects of TTX (100 nM and 1 μM) and lidocaine (2%) applied to the pool surrounding the dorsal roots on the compound action potential evoked by single-pulse electrical stimulation of the tibial nerve in 5 rats whose femoral artery was ligated 72 h before the start of the experiment. A: recording of the compound action potential recorded from a filament of the L4 dorsal root. Note that both TTX (1 μM) and lidocaine abolished the compound action potential arising from the stimulation of group III and IV afferents in this particular instance. B: summary data from 5 rats in which the effects of TTX and lidocaine on the compound action potential (CAP) are expressed as a percentage of the control area. †A significant decrease (P < 0.05) in area of the CAP from control values.
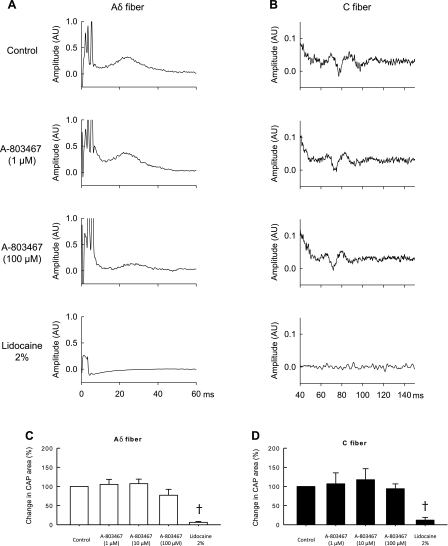
The effect of the selective Nav1.8 blocker A-803467 on the compound action potential evoked by supramaximal stimulation of the tibial nerve was determined in five rats whose femoral arteries were ligated (Fig. 9). These five rats were not used in the above experiments in which TTX was used to block compound action potentials. Contrary to our expectations, A-803467 at concentrations of 1 and 10 μM had no effect on the compound action potentials evoked by the stimulation of either group III or IV afferents. In two of the five rats tested, 100 μM A-803467 slightly attenuated the Aδ wave of the compound action potential evoked by group III stimulation (Fig. 9C), but in none of the five tested did it have any effect on the C wave of the compound action potential evoked by stimulation of group IV afferents. In addition, none of the three concentrations of A-803467 had an effect on the compound action potential evoked by stimulation of group I and II afferents.
Fig. 9.
Effects of A-803467 (1, 10, and 100 μM) applied to the pool surrounding the dorsal roots on the compound action potential evoked by single-pulse electrical stimulation of the tibial nerve in 5 rats whose femoral artery was ligated 72 h before the start of the experiment. A and B: individual compound action potential recorded from a filament of the L4 dorsal root. Note that the 1 μM concentration of A-803467 had no effect on the compound action potential evoked by stimulation of group III and IV afferents; in this example, the 100 μM concentration of A-803467 slightly decreased the group III component of the compound action potential but had no effect on the group IV component. C and D: summary data from 6 rats in which the effects of A-803467 (1, 10, and 100 μM) and lidocaine (2%) on the compound action potential evoked by stimulation of the tibial nerve are expressed as a percentage of the control area. †A significant decrease (P < 0.05) in area of the compound action potential from control values.
DISCUSSION
We found that application of TTX (1 μM) to the L3 through L6 dorsal roots greatly reduced the peak pressor responses to static contraction of the hindlimb muscles both in rats whose hindlimbs were freely perfused and in rats whose femoral artery was ligated. We also found that TTX abolished these pressor responses when MAP was plotted and averaged over the entire contraction period. We interpret these findings to indicate that TTX-s, but not TTX-r, channels in the axons of group IV afferents play an essential role in evoking the exercise pressor reflex. The presence of TTX-r channels on the axons of the group IV afferents was revealed when we stimulated electrically the tibial nerve in paralyzed rats. This stimulation evoked a pressor response, which though attenuated by TTX, was still substantial.
The concentration of TTX (1 μM) used in our experiments was shown by Steffens et al. (25) to abolish the cord dorsum potential evoked by supramaximal stimulation of myelinated afferent fibers. In contrast, this concentration of TTX only attenuated the cord dorsum potential evoked by stimulation of unmyelinated afferent fibers (25). Likewise, Steffens et al. (25) showed that TTX (1 μM) abolished the impulse activity arising from myelinated afferent fibers in the lumbar dorsal roots of rats but only attenuated the impulse activity arising from their unmyelinated counterparts. These findings indicated that application of TTX (1 μM) to the lumbar dorsal roots of rats blocked TTX-s channels but did not block TTX-r channels. These findings further indicated that TTX-r channels on unmyelinated afferents in the lumbar dorsal roots of rats were capable of synaptically activating dorsal horn cells in the cord (25).
In our experiments, application of TTX (1 μM) to the pool surrounding the dorsal roots abolished the group III wave of the compound action potential arising from electrical stimulation of the tibial nerve and attenuated the group IV wave of this compound action potential by ∼90%. At first glance, one might interpret our findings that this concentration blocked both TTX-s as well as most TTX-r channels. This interpretation is most probably incorrect because the latter part of the cord dorsum potential evoked by electrical stimulation of C-fibers in the sural nerve remained after application of TTX (1 μM) to the lumbar dorsal roots, whereas the Aδ component was abolished (25). Some of the large TTX-induced reduction of the group IV wave of the compound action potential in our experiments was probably because of its temporal dispersion, which becomes more pronounced after application of this toxin (19, 25).
In our experiments, TTX (1 μM) greatly attenuated the pressor reflex response to tendon stretch, regardless of whether the hindlimb was freely perfused or not (24). This attenuation was caused by the blockade of TTX-s channels on thin fiber afferents supplying the hindlimb muscles. In rats, the effect of tendon stretch on the discharge of group III and IV muscle afferents has not been described. In cats and dogs, however, tendon stretch stimulated primarily group III afferents (13, 18), the axons of which possess TTX-s channels but are not thought to possess any TTX-r channels (22). The pressor response to stretch that remained after TTX most likely was mediated by TTX-r channels, which are thought to exist only on unmyelinated (i.e., group IV) afferents. To the extent that this is the case, then the muscle mechanoreceptor reflex in rats is evoked by a different population of afferents than is this reflex in cats and dogs. This might not be too surprising because in the rat the boundary defining group III afferents is shifted downward from that in cats or dogs. Specifically, group III afferents in rats conduct impulses within the range of 1.5–10 m/s, whereas these afferents in cats and dogs conduct impulses within the range of 2.5–30 m/s (13, 19). Consequently, some of the thin fiber afferents responsive to stretch in rats might be unmyelinated or be thinly myelinated, which in turn might increase their probability of possessing TTX-r channels.
An important limitation to any interpretation of our findings concerns the location of where we administered TTX. Voltage-gated sodium channels that are resistant to TTX are found on the cell body (5), on the peripheral nerve (21), on the sensory terminal (3), as well as on the dorsal roots (25), the site where we administered TTX. Consequently, any conclusion drawn from our findings must be limited to the role played by TTX-r voltage-gated sodium channels in the dorsal roots, and great caution must be extended to any speculation concerning the role played by these channels on other parts of the group IV neuron.
We found that ligating the femoral artery 72 h before the start of our experiments increased the magnitude of the exercise pressor reflex but had only minimal or no effect either on the pressor response to tendon stretch (24) or on the pressor response to electrical stimulation of the tibial nerve at current intensities that recruited unmyelinated afferent fibers. Our interest in the effect of femoral ligation on the pressor responses to these maneuvers was prompted by previous studies showing that tissue damage or the administration of inflammatory mediators increased the number of TTX-r voltage-gated sodium channels as well as the currents derived from them (6, 28). Perhaps we should not be too surprised at our finding that TTX-r sodium channels did not appear to play much of a role in the exaggerated response to contraction because femoral artery ligation has been shown to have no effect on resting blood flow to the hindlimb in rats at rest (20, 34). Consequently, the rats used in our experiments had little or no prior “ischemic damage” done to their hindlimbs, and therefore little stimulus to generate TTX-r channels.
In our experiments, we attempted to block TTX-r channels with A-803467, a substance that has been reported to be an effective and selective antagonist to NaV1.8 channels (12). We found little evidence that this antagonist blocked the group IV wave of the compound action potential or that it attenuated the pressor response to electrical stimulation of the tibial nerve. We can offer three explanations for our findings. The first is that A-803467 is not an effective antagonist to NaV1.8 channels, a possibility that seems unlikely based on the evidence presented previously (12). The second is that other TTX-r channels, such as NaV1.9 channels (1, 11), compensated for the loss of input from NaV1.8 channels. The third, and the most likely, is that TTX-r channels play little or no role in evoking the exercise pressor reflex in freely perfused limbs and that the number of these channels was not increased by femoral artery ligation.
We could find no evidence that TTX-r channels, namely NaV1.8 and NaV1.9 (9), in the dorsal root axons of group IV muscle afferents played an important role in the generation of the exercise pressor reflex while the hindlimb was freely perfused or while its femoral artery was ligated for 72 h before the start of the experiment. In contrast, we found that TTX-r channels played a role in the generation of the pressor response to electrical stimulation of the tibial nerve at current intensities that recruited group IV afferents. This latter finding was unlikely to be attributable to an incomplete blockade of TTX-s channels because the pressor response to electrical stimulation was no different when the concentration of TTX was increased from 1 to 3 μM.
GRANTS
This work was supported by National Institutes of Health Grants RO1 AR-059397 and PO 1 HL-096570 as well as by a grant from the Pennsylvania Department of Health using Tobacco CURE Funds.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Sarah Simmonds for technical assistance.
REFERENCES
- 1. Baker MD, Chandra SY, Ding Y, Waxman SG, Wood JN. GTP-induced tetrodotoxin-resistant Na+ current regulates excitability in mouse and rat small diameter sensory neurones. J Physiol 548: 373–382, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Black JA, Renganathan M, Waxman SG. Sodium channel Na(v)1.6 is expressed along nonmyelinated axons and it contributes to conduction. Brain Res Mol Brain Res 105: 19–28, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Brock JA, McLachlan EM, Belmonte C. Tetrodotoxin-resistant impulses in single nociceptor nerve terminals in guinea-pig cornea. J Physiol 512: 211–217, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev 57: 397–409, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Elliot AA, Elliot JR. Characterization of TTX-sensitive and TTX-resistant sodium currents in small cells from adult rat dorsal root ganglia. J Physiol 463: 39–56, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. England S, Bevan S, Docherty RJ. PGE2 modulates the tetrodotoxin-resistant sodium current in neonatal rat dorsal root ganglion neurones via the cyclic AMP-protein kinase A cascade. J Physiol 495: 429–440, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Erlanger J, Gasser HS. Electrical Signs of Nervous Activity. Philadelphia, PA: Univ of Pennsylvania Press, 1937 [Google Scholar]
- 8. Fernandes A, Galbo H, Kjaer M, Mitchell JH, Secher NH, Thomas SN. Cardiovascular and ventilatory responses to dynamic exercise during epidural anesthesia in man. J Physiol 420: 281–293, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fjell J, Hjelmstrom P, Hormuzdiar W, Milenkovic M, Aglieco F, Tyrrell L, Dib-Hajj S, Waxman SG, Black JA. Localization of the tetrodotoxin-resistant sodium channel NaN in nociceptors. Neuroreport 11: 199–202, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Hayes SG, Kindig AE, Kaufman MP. Comparison between the effect of static contraction and tendon stretch on the discharge of group III and IV muscle afferents. J Appl Physiol 99: 1891–1896, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Herzog RI, Cummins TR, Waxman SG. Persistent TTX-resistant Na+ current affects resting potential and response to depolarization in simulated spinal sensory neurons. J Neurophysiol 86: 1351–1364, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Jarvis MF, Honore P, Shieh CC, Chapman M, Joshi S, Zhang XF, Kort M, Carroll W, Marron B, Atkinson R, Thomas J, Liu D, Krambis M, Liu Y, McGaraughty S, Chu K, Roeloffs R, Zhong C, Mikusa JP, Hernandez G, Gauvin D, Wade C, Zhu C, Pai M, Scanio M, Shi L, Drizin I, Gregg R, Matulenko M, Hakeem A, Gross M, Johnson M, Marsh K, Wagoner PK, Sullivan JP, Faltynek CR, Krafte DS. A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc Natl Acad Sci USA 104: 8520–8525, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983 [DOI] [PubMed] [Google Scholar]
- 14. Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol 57: 644–650, 1984 [DOI] [PubMed] [Google Scholar]
- 15. Matthews PBC. Mammalian Muscle Receptors and their Central Actions. London, UK: Arnold, 1972 [Google Scholar]
- 16. Mitchell JH, Reeves DR, Rogers HB, Secher NH. Epidural anesthesia and cardiovascular responses to static exercise in man. J Physiol 417: 13–24, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Molander C, Grant G. Spinal cord projections from hindlimb muscle nerves in the rat studied by transganglionic transport of horseradish peroxidase, wheat germ agglutinin conjugated horseradish peroxidase, or horseradish peroxidase with dimethylsulfoxide. J Comp Neurol 260: 246–255, 1987 [DOI] [PubMed] [Google Scholar]
- 18. Paintal AS. Functional analysis of group III afferent fibres of mammalian muscles. J Physiol 152: 250–270, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pinto V, Derkach VA, Safronov BV. Role of TTX-sensitive and TTX-resistant sodium channels in Adelta- and C-fiber conduction and synaptic transmission. J Neurophysiol 99: 617–628, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Prior BM, Lloyd PG, Ren J, Li H, Yang HT, Laughlin MH, Terjung RL. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol 287: H2434–H2447, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Quasthoff S, Grosskreutz J, Schroder JM, Schneider U, Grafe P. Calcium potentials and tetrodotoxin-resistant sodium potentials in unmyelinated C fibres of biopsied human sural nerve. Neuroscience 69: 955–965, 1995 [DOI] [PubMed] [Google Scholar]
- 22. Rush AM, Cummins TR, Waxman SG. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J Physiol 579: 1–14, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smith SA, Mitchell GS, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537: 961–970, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stebbins CL, Brown B, Levin D, Longhurst JC. Reflex effect of skeletal muscle mechanoreceptor stimulation on the cardiovascular system. J Appl Physiol 65: 1539–1547, 1988 [DOI] [PubMed] [Google Scholar]
- 25. Steffens H, Hoheisel U, Eek B, Mense S. Tetrodotoxin-resistant conductivity and spinal effects of cutaneous C-fibre afferents in the rat. Neurosci Res 39: 413–419, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Strange S, Secher NH, Pawelczyk JA, Karpakka J, Christensen NJ, Mitchell JH, Saltin B. Neural control of cardiovascular responses and of ventilation during dynamic exercise in man. J Physiol 470: 693–704, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Strassman AM, Raymond SA. Electrophysiological evidence for tetrodotoxin-resistant sodium channels in slowly conducting dural sensory fibers. J Neurophysiol 81: 413–424, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Tanaka M, Cummins TR, Ishikawa K, Dib-Hajj SD, Black JA, Waxman SG. SNS Na+ channel expression increases in dorsal root ganglion neurons in the carrageenan inflammatory pain model. Neuroreport 9: 967–972, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Taylor JC, Li Z, Yang HT, Laughlin MH, Terjung RL. Alpha-adrenergic inhibition increases collateral circuit conductance in rats following acute occlusion of the femoral artery. J Physiol 586: 1649–1667, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Toney GM, Mifflin SW. Mediators of contraction-evoked skeletal muscle depressor response in anesthetized rats. J Appl Physiol 81: 578–585, 1996 [DOI] [PubMed] [Google Scholar]
- 31. Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol 299: H106–H113, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Victor RG, Pryor SL, Secher NH, Mitchell JH. Effects of partial neuromuscular blockade on sympathetic nerve responses to static exercise in humans. Circ Res 65: 468–476, 1989 [DOI] [PubMed] [Google Scholar]
- 33. Vissing J, Wilson LB, Mitchell JH, Victor RG. Static muscle contraction reflexly increases adrenal sympathetic nerve activity in rats. Am J Physiol Regul Integr Comp Physiol 261: R1307–R1312, 1991 [DOI] [PubMed] [Google Scholar]
- 34. Yang HT, Feng Y, Allen LA, Protter A, Terjung RL. Efficacy and specificity of bFGF increased collateral flow in experimental peripheral arterial insufficiency. Am J Physiol Heart Circ Physiol 278: H1966–H1973, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Yang HT, Terjung RL. Angiotensin-converting enzyme inhibition increases collateral-dependent muscle blood flow. J Appl Physiol 75: 452–457, 1993 [DOI] [PubMed] [Google Scholar]