Abstract
Many studies have implicated both angiotensin II (ANG II) and aldosterone (Aldo) in the pathogenesis of hypertension, the progression of renal injury, and cardiac remodeling after myocardial infarction. In several cases, ANG II and Aldo have been shown to have synergistic interactions in the periphery. In the present studies, we tested the hypothesis that ANG II and Aldo interact centrally in Aldo- and ANG II-induced hypertension in male rats. In rats with blood pressure (BP) and heart rate (HR) measured by DSI telemetry, intracerebroventricular (icv) infusions of the mineralocorticoid receptor (MR) antagonists spironolactone and RU28318 or the angiotensin type 1 receptor (AT1R) antagonist irbesartan significantly inhibited Aldo-induced hypertension. In ANG II-induced hypertension, icv infusion of RU28318 significantly reduced the increase in BP. Moreover, icv infusions of the reactive oxygen species (ROS) scavenger tempol or the NADPH oxidase inhibitor apocynin attenuated Aldo-induced hypertension. To confirm these effects of pharmacological antagonists, icv injections of either recombinant adeno-associated virus carrying siRNA silencers of AT1aR (AT1aR-siRNA) or MR (MR-siRNA) significantly attenuated the development of Aldo-induced hypertension. The immunohistochemical and Western blot analyses of AT1aR-siRNA- or MR-siRNA-injected rats showed a marked reduction in the expression of AT1R or MR in the paraventricular nucleus compared with scrambled siRNA rats. When animals from all studies underwent ganglionic blockade with hexamethonium, there was a smaller reduction in the fall of BP in animals receiving icv AT1R or MR antagonists. These results suggest that ANG II and Aldo interact in the brain in a mutually cooperative manner such that the functional integrity of both brain AT1R and MR are necessary for hypertension to be induced by either systemic ANG II or Aldo. The pressor effects produced by systemic ANG II or Aldo involve increased central ROS and sympathetic outflow.
Keywords: blood pressure, reactive oxygen species, sympathetic nervous system
it is well established that the renin-angiotensin-aldosterone (Aldo) system (RAAS) is a major regulator of sodium and potassium balance, blood volume, and blood pressure (BP) (38). Angiotensin II (ANG II) has been considered to be the principal effector of the RAAS. The major cardiovascular actions of ANG II are mediated via the angiotensin type 1 receptor (AT1R), and AT1R blockers have been shown to have therapeutic benefits in the treatment of hypertension and heart failure (6, 55). In recent years, Aldo has also become recognized as an important component and mediator of the effects of the RAAS, and this steroid plays an important role in the pathophysiology of cardiovascular disease. The additional benefit of the use of mineralocorticoid receptor (MR) antagonists in conjunction with ANG II therapies highlights the clinical relevance of an ANG II/Aldo-interdependent humoral network (47). Therefore, a better understanding of the variety and sites of such interactions will lead to insights providing improved treatment of patients.
Besides the well-known effect of ANG II in stimulating Aldo production from the adrenal cortex, a reciprocal interaction has been reported between hormones in extra-adrenal tissues (46, 52, 61). Aldo increases ANG II binding, upregulates the expression of AT1R and angiotensin-converting enzyme (ACE), and potentiates ANG II-stimulated intracellular signaling and proliferation in peripheral cardiovascular tissues (7, 15–17, 34, 35, 46, 53, 57). In cultured rat neonatal cardiomyocytes, an ANG II receptor antagonist has been shown to inhibit Aldo-induced IL-18 expression (7). Moreover, Lemarié et al. (30) demonstrated that vascular smooth muscle cell (VSMC) activation of signaling pathways such as ERK1/2, JNK, and NF-κB in response to Aldo requires a functional AT1aR. These results suggest that Aldo induces vascular damage, endothelial dysfunction, and myocardial fibrosis, which depends in part on activation of an ANG II/AT1R-mediated pathway. Conversely, ANG II directly stimulates nuclear localization of MR, and MR antagonism inhibits ANG II-mediated, MR-dependent gene expression in human coronary artery smooth muscle cells. ANG II-mediated MR activation is also inhibited by an AT1R antagonist, demonstrating a link between AT1R activation and MR transcriptional activation (24). Accordingly, a study by Min et al. (35) demonstrated that Aldo and ANG II can synergistically promote rat VSMC mitogenesis via the interaction of the AT1R with MR. Taken together, these studies from peripheral cardiovascular tissues strongly suggest that ANG II and Aldo may modulate signaling pathways and cardiovascular function either synergistically or via a cross-talk of their receptors or their respective messenger systems. Recently, it has been shown that Aldo significantly increased the number and expression of AT1Rs and ACEs in the brain (63, 64). Mineralocorticoid pretreatment can enhance ANG II-induced neuronal excitation in the preoptic/medial septum region (36). However, there have been few studies investigating the central interactions between ANG II and Aldo.
Numerous in vitro and in vivo studies have demonstrated a direct role of Aldo in the development of cardiovascular disease via oxidative stress (11, 36, 43, 48). Long-term administration of an NADPH oxidase inhibitor, apocynin, in the drinking water to deoxycorticosterone acetate (DOCA)-salt rats significantly decreased systolic BP and superoxide production in aortic rings compared with that of rats treated with DOCA-salt alone (1). In cultured rat aortic endothelial cells, Aldo induced a time- and dose-dependent increase in superoxide generation, activated NADPH oxidase, and Rac1 (small GTP-binding protein). An MR antagonist, apocynin, or adenoviral gene transfer of dominant-negative Rac1 abolished Aldo-induced superoxide generation (23). DOCA and Aldo have also been shown to increase mRNA levels of NADPH oxidase components (1, 17). These findings indicate that mineralocorticoid excess increases superoxide production through an activated NADPH oxidase pathway, thereby contributing to vascular injury and hypertension. The present experiments extended these studies to determine whether similar functional pathways occur in the central nervous system (CNS) and if they are responsible for elevated BP during mineralocorticoid excess.
Recently, adenoviral-mediated delivery of small interfering RNA (siRNA) to selectively silence AT1aR or MR has been demonstrated to be a powerful tool to investigate their role in cardiovascular pathophysiology (31, 54, 58). For example, Sun et al. (54) reported that siRNA inhibition of MRs prevents the development of cold-induced hypertension. Selective knockdown of AT1aR by siRNA inhibited ANG II endocytosis and Na+/H+ exchanger isoform 3 (NHE-3) expression in immortalized rabbit proximal tubule cells (22). In the present studies, to confirm the effects we observed with pharmacological antagonists, we used intracerebroventricular (icv) injections of AT1aR-siRNA and MR-siRNA to induce stable and localized knockdown of AT1aR and MR, respectively, and then examined the effects of silencing these central receptors on Aldo-induced hypertension.
Taken together, the present studies tested the hypotheses that ANG II and Aldo interact centrally in Aldo- and ANG II-induced hypertension and that the hypertensive effects of ANG II and Aldo involve increased central reactive oxygen species (ROS) and sympathetic outflow.
METHODS
Animals
Sprague-Dawley rats (115 males, 10–12 wk old) were used and obtained from Harlan Sprague Dawley (Indianapolis, IN). They were housed in temperature- and light-controlled animal quarters and were provided with rat chow (7013 NIH-31 modified rat diet, 0.25% NaCl) ad libitum. In Aldo-induced hypertension studies, the rats were divided into the following groups: 1) central vehicle infusion (n = 8), 2) central infusion of MR antagonist RU28318 (n = 6), 3) central infusion of MR antagonist spironolactone (n = 6), 4) central AT1R antagonist irbesartan infusion (n = 6), 5) central ROS scavenger tempol (n = 6), 6) central NADPH oxidase inhibitor apocynin infusion (n = 6), 7) icv scrambled (SCM)-siRNA injection (n = 7), 8) icv MR-siRNA injection (n = 6), and 9) icv AT1aR-siRNA injection (n = 7). To induce hypertension with Aldo, the rats were infused subcutaneously with Aldo combined with 1% NaCl as the sole drinking fluid. One-percent NaCl intakes were measured daily. Control experiments were also conducted by giving animals 1% NaCl to drink or central RU28318, spironolactone, irbesartan, tempol, apocynin, SCM-siRNA, MR-siRNA, or AT1aR-siRNA (n = 4–6/group) without subcutaneous Aldo infusions. In ANG II-induced hypertension studies, rats were divided into 2 groups: 1) central vehicle infusion (n = 6) and 2) central infusion of MR antagonist RU28318 (n = 6). The rats were infused with systemic ANG II during these central treatments.
All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Iowa Animal Care and Use Committee.
Surgical Procedures
Telemetry probe implantation.
Implantable rat BP transmitters (TA11PA-C40; Data Sciences International, St. Paul, MN) were used to directly measure arterial blood pressure in individual animals. The rats were anesthetized with a ketamine-xylazine mixture (100 and 10 mg/kg). The femoral artery of the rat was accessed with a ventral incision. The right femoral artery was isolated, and the catheter of a telemetry probe was inserted into the vessel. Through the same ventral incision a pocket along the right flank was formed. The body of the transmitter was slipped into the pocket and secured with tissue adhesive, and the ventral incision was then closed with suture.
Chronic icv cannula and osmotic pump implantation.
After baseline BP and heart rate (HR) recordings were obtained, rats were again anesthetized with a ketamine-xylazine mixture. An icv cannula with an osmotic pump (model 2004, 28 days; Alzet, Cupertino, CA) was implanted into the right lateral ventricle (the coordinates were 0.9 mm caudal, 1.5 mm lateral to bregma, and 4.5 mm below the skull surface) for chronic infusions of spironolactone (250 ng/h; Sigma, St. Louis, MO), RU28318 (1.1 μg/h; Tocris Bioscience, Ellisville, MO), irbesartan (125 μg/day; Sigma), tempol (200 nm·kg−1·min−1; Sigma), and apocynin (4 μg·kg−1·min−1, Sigma). In separate groups of rats, adeno-associated virus (AAV)-SCM-siRNA, AAV-MR-siRNA, or AAV-AT1aR-siRNA (2 μl of 1.3 × 1012 genomic particles/ml; GeneDetect, Bradenton, FL) were injected into the lateral ventricle. At the same time, osmotic pumps (model 2004; Alzet) containing Aldo (1.8 μg·kg−1·h−1, Sigma) were implanted subcutaneously in the back, and tap water was changed to 1% NaCl. In separate groups of rats receiving central infusions of vehicle or RU28318 (1.1 μg/h), ANG II (100 ng·kg−1·min−1; Sigma) was infused systemically by osmotic pumps. The dosages of all drugs used above were chosen on the basis of published in vivo studies (12, 14, 18, 26, 28, 33, 56). At the end of each experiment, animals were euthanized and perfused transcardially with saline followed by fixative. The locations of the lateral ventricle cannula implantations were verified histologically.
Western blotting analysis.
The protein sample was mixed with an equal volume of SDS-PAGE buffer and loaded on the 10% SDS-PAGE gel for electrophoresis and then transferred to a PVDF membrane (Immun-Blot; Bio-Rad Laboratories). The membrane was probed with rabbit polyclonal antibody for anti-MR (1:500, SC-11412; Santa Cruz Biotechnology, Santa Cruz, CA), anti-AT1R (1:500, SC-1173; Santa Cruz Biotechnology), or anti-β-actin (1:2,500, no. 4967; Cell Signaling Technology). This was followed by horseradish peroxidase-labeled anti-rabbit secondary antibody (Santa Cruz Biotechnology) and then treatment with enhanced chemiluminescence reagent (Supersignal Substrate Western Blotting; Pierce Chemical). Band intensities were quantified with NIH Image J software and normalized to β-actin.
Fluorescent immunohistochemistry.
Immunohistochemical (IHC) studies were performed to confirm MR or AT1R knockdown in the paraventricular nucleus (PVN). Brain sections were incubated with a rabbit polyclonal anti-AT1R antibody (1:500; Santa Cruz Biotechnology) or a mouse monoclonal anti-MR antibody (generated in the laboratory of C. E. Gomez-Sanchez) in 5% donkey normal serum with 0.2% Triton X-100 for 72 h at 4°C. After being washed thoroughly with PBS, sections were incubated with Rhodamine Red-X-conjugated AffiniPure donkey anti-rabbit or anti-mouse IgG (1:100; Jackson ImmunoResearch Laboratories, West Grove, PA) in PBS for 24 h at 4°C. Fluorescence was then identified using confocal microscopy.
Experimental Protocol
Measurement of BP and HR.
All rats were allowed 7 days to recover from transmitter implantation surgery before any measurements were made. Thereafter, BP and HR were telemetrically recorded and stored with the Dataquest ART data acquisition system (Data Sciences International).
In the control experiments, we tested whether 1% NaCl alone or central blockade of AT1R, MR, or ROS production had effects on basal mean arterial pressure (MAP) and HR. Animals were given 1% NaCl to drink or central spironolactone, RU28318, irbesartan, tempol, apocynin, AAV-SCM-siRNA, MR-siRNA, or AT1aR-siRNA for 21 or 28 days without Aldo treatments.
In Aldo-induced hypertension studies, we tested the BP and HR responses to central blockade of AT1R, MR, or ROS production during Aldo infusion. Intracerebroventricular cannulas with osmotic pumps were implanted into the right lateral ventricle for chronic infusions of vehicle, spironolactone, RU28318, irbesartan, tempol, or apocynin for 28 days. At the same time, rats were infused subcutaneously with Aldo combined with 1% NaCl as the sole drinking fluid. To confirm the effects of the pharmacological antagonists, 2 μl of AAV-SCM-siRNA, AAV-MR-siRNA, or AAV-AT1aR-siRNA was injected into the lateral ventricle, whereas Aldo was infused for 21 days subcutaneously, and 1% NaCl replaced water as the drinking fluid.
In ANG II-induced hypertension studies, we tested the effects of central blockade of MR on ANG II-induced hypertension. Intracerebroventicular cannulas with osmotic pumps were implanted into the right lateral ventricle for chronic infusions of vehicle or RU28318 for 22 days. On day 7, osmotic pumps filled with ANG II were implanted subcutaneously.
Evaluation of BP responses to autonomic blockade.
BP was also measured in the presence of the ganglionic blocker hexamethonium (30 mg/kg ip). Ganglionic blockade was repeated two times in each animal, during baseline and after 28 days of Aldo infusion. On the day of ganglionic blockade experiments, BP was recorded for 20 min before and after hexamethonium injection. After hexamethonium injection, the largest decrease in BP occurred within 5 min. This nadir was recorded as the maximum fall in BP.
MR or AT1R protein expression measurements.
Following 21 days of icv injection of AAV-SCM-siRNA, AAV-MR-siRNA, or AAV-AT1aR-siRNA, rats were deeply anesthetized with isoflurane. The brains were removed and stored at −80°C until assay. Brains were cut into consecutive 100-μm sections in a cryostat at −20°C, and micropunches were made to collect the PVN. Total cellular protein was isolated from tissue punches of the PVN and analyzed for protein expression of MR or AT1R by Western blotting.
Data Analysis
MAP and HR were collected for 5 baseline days and then for 28 consecutive days during spironolactone, RU28318, irbesartan, tempol, apocynin, MR-siRNA, AT1aR-siRNA, and Aldo or ANG II pump implantation. MAP and HR are presented as mean daily values averaged from daytime and nighttime measurements. Difference scores for MAP and for HR were calculated for each animal based on the mean of the 5-day baseline values subtracted from the mean of the final 7 days of treatment. One-way ANOVAs for the experimental groups were then conducted on the means of calculated difference scores. After a significant ANOVA was established, follow-up t-tests were conducted to test for differences between pairs of mean change scores. To test differences in the mean of 5 days baseline vs. the mean of the final 7 days of treatment, t-tests were performed.
For analysis of 1% NaCl intakes, mean intakes for each experimental group were averaged from daily measurements during 21 or 28 days of treatment. A one-way ANOVA was conducted on these mean values.
Student t-tests were conducted to test for differences in MR or AT1R protein expression in MR-siRNA- or AT1aR-siRNA-treated animals vs. SCM-siRNA-treated animals.
All data are expressed as means ± SE. Statistical significance was set at P < 0.05.
RESULTS
Effects of Central Blockade of MR or AT1R on Aldo/NaCl-Induced Hypertension
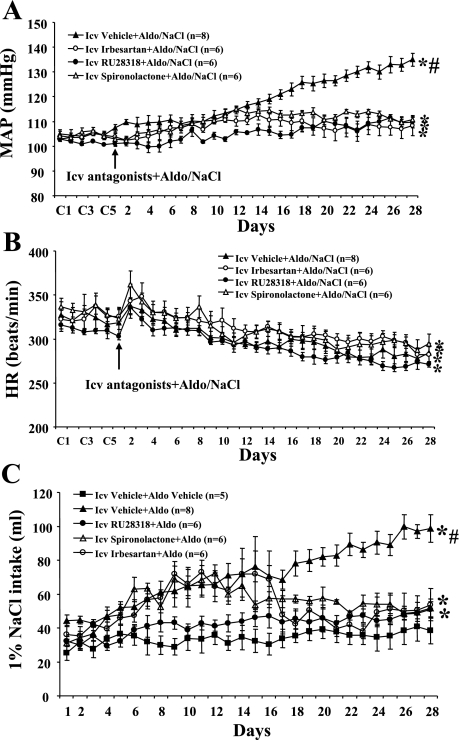
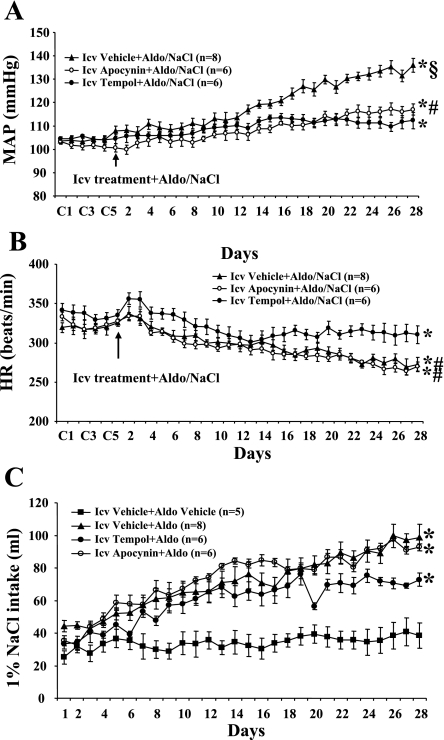
Baseline MAP was unaltered (P > 0.05) when 1% NaCl was given alone (Δ3.1 ± 2.5 mmHg; n = 5) and after central infusion of spironolactone (Δ1.7 ± 3.8 mmHg; n = 4), RU28318 (Δ3.5 ± 2.6 mmHg; n = 4), or irbesartan (Δ2.5 ± 2.9 mmHg; n = 4) when the control (vehicle) for Aldo was given for 28 days. Central infusion of either spironolactone or RU28318 (Fig. 1A) for 28 days significantly attenuated the increase in MAP induced by Aldo/NaCl (Δ7.4 ± 1.5 and Δ7.6 ± 2.1, respectively; n = 6, P < 0.05) compared with that seen in rats with central vehicle plus systemic Aldo (Δ26.9 ± 1.5 mmHg; n = 8). Likewise, icv infusion of irbesartan (Fig. 1A) also inhibited the increase in MAP induced by Aldo/NaCl (Δ7.5 ± 2.0 mmHg; n = 6, P < 0.05). Systemic Aldo infusion produced significant, comparable decreases in HR in all groups (central vehicle −42.4 ± 6.6, central irbesartan −37.3 ± 3.5, central RU28318 −38.2 ± 2.7, central spironolactone −39.1 ± 9.0 beats/min; Fig. 1B).
Fig. 1.
The effect of chronic intracerebroventricular (icv) infusions of the mineralocorticoid receptor (MR) antagonist angiotensin type 1 receptor (AT1R) antagonist on aldosterone (Aldo)-induced hypertension in male rats. Daily mean arterial pressures (MAP; A) and heart rates (HR; B) before and during systemic infusion of Aldo and 1% NaCl access in icv vehicle-, spironolactone-, RU28318-, or irbesartan-treated rats. C: daily 1% NaCl intake during Aldo infusions in male rats treated with central AT1R or MR antagonist. C1, C3, and C5 denote control days, followed by 28 days of systemic Aldo infusion. *P < 0.05 compared with baseline MAP and HR or 1% NaCl intake without treatment; #P < 0.05 compared with antagonist-treated rats.
Average daily 1% NaCl intake was 34.8 ± 6.8 ml when the control (vehicle) for Aldo treatment was given for 28 days (Fig. 1C). Systemic infusion of Aldo significantly increased 1% NaCl intake in rats with central vehicle treatment (71.5 ± 6.0 ml/day, P < 0.05; Fig. 1C). Central infusions of AT1R or MR antagonists significantly attenuated the increases in 1% NaCl intake induced by systemic Aldo (RU28318 44.2 ± 3.9, spironolactone 55.2 ± 2.9, and irbesartan 55.1 ± 6.9 ml/day, P < 0.05; Fig. 1C).
Effects of icv Injection of AAV-AT1aR-siRNA or AAV-MR-siRNA on Aldo-Induced Hypertension
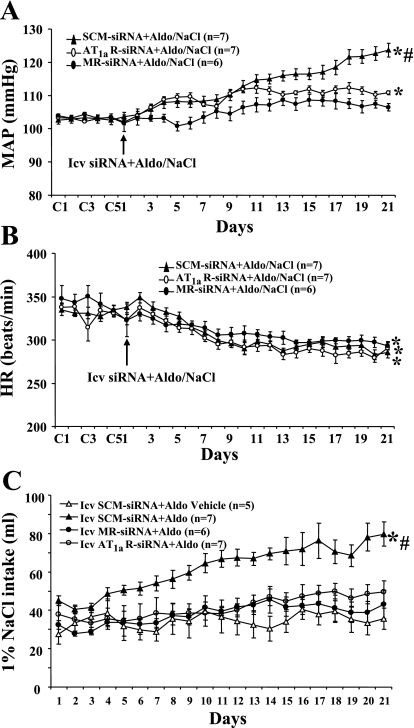
With subcutaneous infusions of vehicle rather than Aldo for 21 days, there were no changes (P > 0.05) in MAP or HR after icv injection of SCM-siRNA (Δ2.5 ± 3.1 mmHg, Δ3.9 ± 8.4 beats/min; n = 6), AT1aR-siRNA (Δ 1.5 ± 2.4 mmHg, Δ2.4 ± 7.5 beats/min; n = 6), or MR-siRNA (Δ 1.1 ± 2.8 mmHg, Δ4.4 ± 8.6 beats/min; n = 6). Twenty-one days of Aldo infusion resulted in a 17.3 ± 2.1-mmHg (n = 7, P <0.05) increase in MAP in rats treated with central AAV-SCM-siRNA. Intracerebroventricular injections of AAV-MR-siRNA or of AAV-AT1aR-siRNA both significantly attenuated (Fig. 2A) the increase in MAP induced by Aldo/NaCl (MR-siRNA Δ4.1 ± 1.7, n = 6; AT1aR-siRNA Δ8.3 ± 0.8, n = 7, P <0.05).
Fig. 2.
The effect of icv injection of adeno-associated virus (AAV)-scrambled (SCM)-siRNA, AAV-AT1aR-siRNA, or AAV-MR-siRNA on Aldo/NaCl-induced hypertension in male rats. Daily MAP (A) and HR (B) before and during systemic infusion of Aldo in icv SCM-siRNA-, AT1aR-siRNA-, or MR-siRNA-injected male rats. C: daily 1% NaCl intake during Aldo infusions in male rats treated with siRNA for AT1aR or MR. Control days followed by 21 days of systemic Aldo infusion. *P <0.05 compared with baseline MAP and HR or 1% NaCl intake without treatment; #P < 0.05 compared with rats given central AT1aR-siRNA or MR-siRNA.
The chronic Aldo infusion produced a significant decrease in HR in all groups (P < 0.05; Fig. 2B). The fall in HR during Aldo/NaCl treatment was similar in all groups (SCM-siRNA −42.2 ± 6.2, AT1aR-siRNA −46.4 ± 5.2, MR-siRNA −45.7 ± 4.8 beats/min, P > 0.05).
Systemic infusion of Aldo significantly increased 1% NaCl intake in rats with icv injection of SCM-siRNA (61.8 ± 4.7 ml/day, P < 0.05; Fig. 2C). Intracerebroventricular injection of either MR-siRNA or AT1aR-siRNA significantly reduced Aldo-induced increases in 1% NaCl intake (37.9 ± 3.9 and 43.5 ± 4.3 ml/day, respectively, P < 0.05; Fig. 2C).
AAV Delivery of siRNA Effectively Silences AT1aR
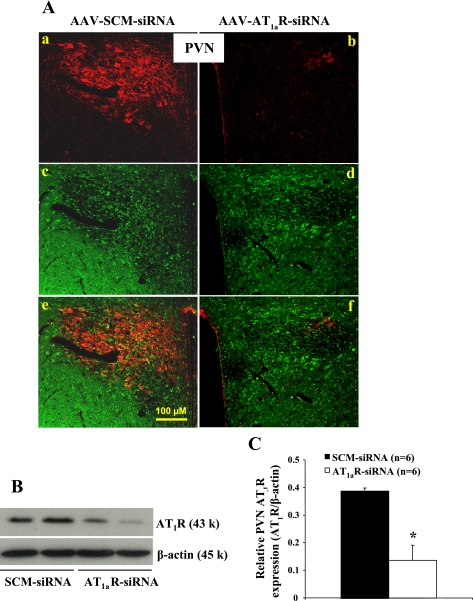
The effect of viral delivery of siRNA to knockdown AT1aR was verified by IHC and Western blot analyses. AAV-siRNA constructs contained a green fluorescent protein (GFP) construct that served as a marker indicating the site of delivery and cells affected. Three weeks after icv injections of AAV-AT1aR-siRNA, highly robust GFP expression presented throughout the forebrain, including the PVN (Fig. 3A), the subfornical organ (data not shown), and the organum vasculosum of the lamina terminalis (data not shown). Immunohistochemistry showed the colocalization of AT1R and the marker of siRNA GFP and a remarkable reduction in the expression of AT1R in the PVN (Fig. 3A). To confirm effective silencing of AT1R in the PVN with these viral constructs, we performed a Western blot analysis on the micropunches taken from the PVN. As shown in Fig. 3, B and C, AT1R protein level was significantly diminished in rats treated with AAV-AT1aR-siRNA by ∼65% when compared with rats with AAV-SCM-siRNA.
Fig. 3.
Immunohistochemical and Western blot analyses of AT1R protein expression in the paraventricular nucleus (PVN) from rats receiving icv injections of SCM-siRNA or AT1aR-siRNA. A: adenovirus-mediated delivery of RNA interference effectively silences AT1R (red in the cytoplasm) expression in the PVN. Green in the cytoplasm represents green fluorescent protein (GFP), a marker for virus transfection. Images in a–d are merged in e and f. B: representative Western blots of AT1R and β-actin. C: the results of Western blot analysis represent the change in AT1R protein expression, which was normalized with β-actin in the PVN. *P < 0.05 compared with SCM-siRNA treatment.
AAV Delivery of siRNA Effectively Silences MR
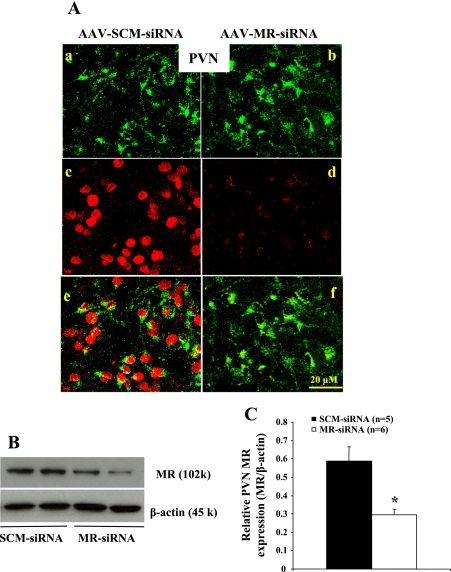
Figure 4 presents a set of representative IMC and Western blots showing GFP, MR protein expression, and β-actin in the PVN 3 wk after icv injection of siRNA. IMC analysis of the PVN showed that AAV-MR-siRNA knocked down MR in this structure (Fig. 4A). The Western blot analysis indicated that MR protein was decreased by ∼50% (Fig. 4, B and C).
Fig. 4.
Immunohistochemical and Western blot analyses of MR protein expression in the PVN from rats receiving icv injections of SCM-siRNA or MR-siRNA. A: adenovirus-mediated delivery of RNA interference effectively silences MR (red in the nucleus) expression in the PVN. Green in the cytoplasm represents GFP, a marker for virus transfection. Images in a–d are merged in e and f. B: representative Western blots of MR and β-actin. C: the results of Western blot analysis represent the change in MR protein expression, which was normalized with β-actin in the PVN. *P < 0.05 compared with SCM-siRNA treatment.
Effects of Central ROS Blockade on Aldo/NaCl-Induced Hypertension
Intracerebroventricular infusion of apocynin alone or tempol alone for 28 days did not affect baseline MAP (Δ2.4 ± 1.4 mmHg, n = 5, and Δ1.1 ± 3.4 mmHg, n = 5, respectively) and HR (Δ4.2 ± 9.1 and Δ5.4 ± 6.3 beats/min, respectively). Twenty-eight days of Aldo infusion resulted in a 29.1 ± 2.3-mmHg (n = 8, P <0.05) increase in MAP in rats treated with central vehicle. Central infusion of either apocynin or tempol (Fig. 5A) attenuated the Aldo/NaCl-induced increase in MAP (n = 6, P <0.05). As shown in Fig. 5B, Aldo infusion produced a significant decrease in HR in all groups (P <0.05). However, compared with the effects of apocynin, tempol induced a greater decrease in MAP (Δ6.8 ± 1.5 vs. Δ14.4 ± 1.9 mmHg, P < 0.05) and a smaller decrease in HR (−35.6 ± 4.0 vs. −47.0 ± 5.9 beats/min, P < 0.05).
Fig. 5.
The effect of chronic icv infusions of an NADPH oxidase or reactive oxygen species (ROS) scavenger on Aldo/NaCl-induced hypertension in male rats. Daily MAP (A) and HR (B) before and during systemic infusions of Aldo and access to 1% NaCl in icv vehicle-, apocynin-, or tempol-treated rats. C: daily 1% NaCl intake during Aldo infusions in male rats treated with central NADPH oxidase inhibitor or ROS scavenger. Control days followed by 28 days of central apocynin or tempol and systemic Aldo infusion. *P < 0.05 compared with baseline MAP and HR or 1% NaCl intake without treatment; #P < 0.05 compared with rats given tempol; §P < 0.05 compared with rats given apocynin or tempol.
Systemic infusion of Aldo significantly increased 1% NaCl intake in rats with central vehicle treatment (71.5 ± 6.0 ml/day, P < 0.05; Fig. 5C). Central infusion of the ROS scavenger or NADPH oxidase inhibitor had no effect on Aldo-induced 1% NaCl intake (tempol 59.8 ± 4.5, apocynin 73.5 ± 1.2 ml/day, P > 0.05; Fig. 5C).
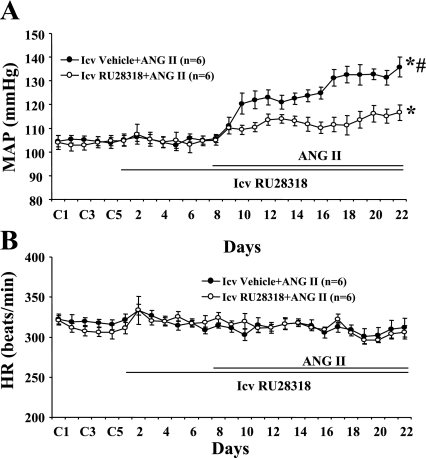
Effects of Central Infusion of RU28318 on ANG II-Induced Hypertension
Baseline MAP in rats was unaltered during central infusion of RU28318 (Fig. 6A). Central RU28318 (n = 6; Fig. 6A) significantly inhibited the increase in MAP induced by ANG II (Δ9.8 ± 4.9 mmHg, P < 0.05) compared with that seen in rats with central vehicle plus systemic ANG II (Δ28.0 ± 5.3 mmHg, n = 6). ANG II infusion did not significantly change HR in any group (Fig. 6B).
Fig. 6.
The effect of chronic icv infusions of a MR antagonist on angiotensin II (ANG II)-induced hypertension in male rats. Daily MAP (A) and HR (B) before and during systemic infusions of ANG II in icv vehicle- or RU28318-treated rats. Control days followed by 22 days of central RU28318 and 14 days of systemic ANG II infusion. *P < 0.05 compared with baseline; #P < 0.05 compared with rats given RU28318.
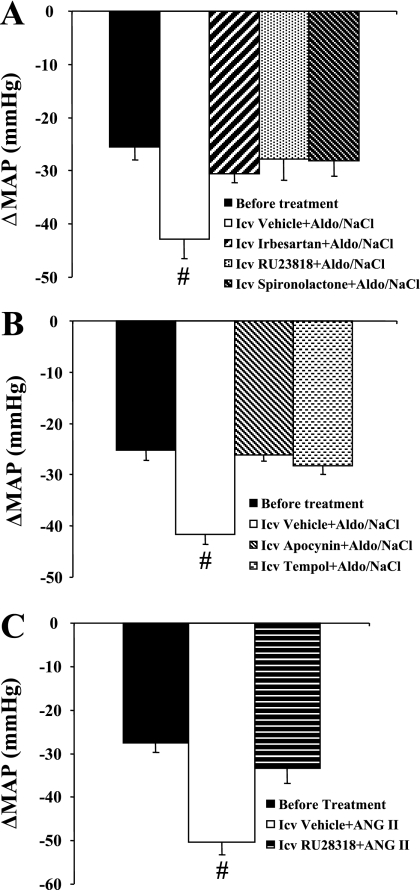
Effects of Autonomic Blockade on BP
Figure 7 shows the decreases in BP with acute ganglionic blockade in all groups. The average reduction in the BP response to hexamethonium injection before central treatment and systemic infusion of Aldo or ANG II was −26.1 ± 2.3 mmHg, and there was no difference between groups (Fig. 7). In Aldo-induced hypertension, following 28 days of Aldo infusion, acute hexamethonium injection resulted in a greater reduction in BP in rats with central vehicle (−42.3 ± 2.8 mmHg, P < 0.05; Fig. 7, A and B) compared with central irbesartan- (−30.6 ± 1.6; Fig. 7A), RU28318- (−27.8 ± 3.8 mmHg; Fig. 7A), spironolactone- (−28.1 ± 2.9 mmHg; Fig. 7A), apocynin- (−26.2 ± 1.1 mmHg; Fig. 7B), or tempol-treated (−28.2 ± 1.7 mmHg; Fig. 7B) rats. In ANG II-induced hypertension, the BP reduction was greater in response to hexamethonium injection in central vehicle-treated rats (−50.2 ± 3.0 mmHg; Fig. 7C) than that seen in central RU28318-treated rats (−33.4 ± 3.4 mmHg, P < 0.05) after 14 days of ANG II infusion.
Fig. 7.
MAP in response to ganglionic blockade with hexamethonium before Aldo or ANG II infusions were begun and on the last day of Aldo or ANG II infusion in all groups. #P < 0.05 compared with rats receiving central treatment with AT1R or MR antagonist (A), apocynin or tempol (B), and RU28318 (C).
DISCUSSION
The main findings of these studies are that 1) central blockade of either MRs or the generation of ROS significantly inhibits Aldo/NaCl-induced hypertension, 2) central antagonism of AT1R attenuates Aldo/NaCl-induced hypertension and central antagonism of MR attenuates ANG II-induced hypertension, 3) central knockdown of either AT1aR or MR by AAV-siRNA also attenuates Aldo/NaCl-induced hypertension, and 4) the attenuated BP effect on Aldo/NaCl- or ANG II-induced hypertension in rats produced by central blockade of AT1R, MR, or ROS production is associated with decreased sympathetic outflow. Taken together, these results suggest that ANG II and Aldo interact in the brain in a mutually cooperative manner such that disruption of the functional integrity of either AT1R or MR will block the development of hypertension produced by either systemic treatment with ANG II or Aldo. Furthermore, the pressor effects of ANG II and Aldo involve increased central ROS and sympathetic outflow.
Besides the systemic or circulating RAAS, many tissues, including the vasculature, heart, and brain, have the potential for local ANG II or Aldo production through the tissue-specific local RAAS (19, 22). Recently, many interactions between ANG II and Aldo in the cardiovascular system have been highlighted. Aldo increased ANG II binding (16, 57), upregulated the expression of AT1R and ACE in rat endothelial cells (53), neonatal rat cardiomyocytes (15), and aortic tissues (17), and potentiated ANG II-stimulated intracellular signaling and proliferation in rat VSMCs (7, 34, 35). Studies from Yu et al. (63) and Zhang et al. (64) have demonstrated that icv infusion of Aldo increases expression of AT1R and ACE mRNA in hypothalamic tissue. Similarly, Aldo was elevated in heart failure in the brain in proportion to the increase in the circulation. This increase resulted in MR-mediated stimulation of hypothalamic ACE and AT1R mRNA and protein. Moreover, release of angiotensin peptides in the hypothalamus was greater in DOCA-salt-treated rats than in control rats (27). These results suggest that Aldo actions in the brain may influence local production of ANG II and switch the actions of ANG II toward the progression of cardiovascular disorders.
DOCA/Aldo-salt hypertension is associated with markedly depressed plasma renin activity and reduced circulating ANG II (2). One study has shown that treatment of DOCA-salt rats with the ANG II receptor inhibitor losartan in the drinking water did not correct hypertension or endothelium-dependent vessel relaxation (49). This observation would argue against the importance of a role for local RAAS in the systemic vasculature in this form of experimental hypertension. However, another recent study demonstrated that increased systolic BP and vascular inflammatory changes were attenuated by peripheral treatment with either an MR antagonist or an AT1R antagonist. This might suggest that both the ANG II-dependent pathway resulting from vascular ACE upregulation and the ANG II-independent pathway are involved in the mechanisms underlying the development of hypertension, vascular inflammation, and oxidative stress induced by Aldo (17). It has been shown that activation of central AT1R and MR is essential for the pressor effects caused by systemic ANG II and Aldo excess, respectively (13). In the present study, we found that systemic Aldo-induced increases in BP were attenuated not only by central MR antagonists but also by a central AT1R antagonist. The fact that a central AT1R antagonist exerts an antihypertensive effect indicates that enhanced CNS renin-angiotensin activity is involved in the maintenance of hypertension in Aldo/NaCl hypertensive rats. This result is consistent with recent studies showing that VSMC activation of signaling pathways in response to Aldo requires functional AT1R (30) and that blockade of angiotensin formation or AT1R in the hypothalamus lowers BP in DOCA-salt hypertensive rats (20, 27).
It has become evident that ANG II stimulates both systemic and local Aldo production. Aldo may influence the signaling of trafficking of the AT1R (29), and some of the cellular effects of ANG II occur through Aldo-dependent pathways (41, 59). For example, it has been shown that the ANG II-induced biventricular damage is mediated by a mechanism partially dependent on caveolin-1 and signaling via MR/Aldo (41). Aldo blockade restored or prevented vascular and cardiac inflammation and remodeling caused by the long-term renin-angiotensin enhancement or ANG II infusion (44, 46, 59) and by ANG II-mediated oxidative stress (9, 59). Jaffe and Mendelsohn (24) have reported that ANG II directly activates the MR independent of Aldo production by VSMC, which provides further support for the evolving role of MR as a signal effector for ANG II. However, the available evidence as to whether MR action is sustained with chronic ANG II infusion, and whether this plays a role in mediating ANG II hypertension, is not completely consistent. In transgenic mice overproducing ANG II, the beneficial effects of peripheral Aldo blockade by spironolactone on cardiac and vascular remodeling were independent of BP (46, 50). In mice, the BP increase produced by systemic infusion of ANG II was not significantly different from animals treated with systemic spironolactone; however, there was a tendency for lower BP during the 1st week of treatment (52). In transgenic rats overexpressing human renin and angiotensinogen genes, oral administration of FAD286, an Aldo synthase inhibitor, ameliorated ANG II-induced organ damage and hypertension (8). Recently, a study from Huang et al. (18) showed that central infusion of FAD286 or MR antagonists (spironolactone and eplerenone) attenuated systemic ANG II-induced hypertension. These discrepancies may be attributed to differences in species, BP measurement (tail cuff vs. telemetry), or the method of antagonist administration (peripheral vs. central). In the present study, another selective MR antagonist, RU28318, was used to attenuate ANG II-induced hypertension. This observation supports the conclusion that a central MR-dependent mechanism promotes ANG II-induced cardiovascular disease.
A key feature of the present study involved the use of AAV delivery of siRNA for selective silencing of brain AT1aR or MR. Although this approach has only recently been developed, it has already proven to be a powerful strategy for unraveling molecular mechanisms in the CNS (4, 39). Several reports have shown that AT1aR and AT1bR subtypes each exhibit unique regulatory functions (3, 21, 32). AT1aR and AT1bR share 95% homology in mRNA and amino acid sequences and have similar ANG II binding characteristics (5). It is difficult to differentiate between the subtypes because there are no specific agonists or antagonists for AT1aR or AT1bR. In addition, recent studies have questioned the specificity of currently available pharmacological antagonists of MR (51). Therefore, we and others have used AAV delivery of siRNA to selectively silence AT1aR or MR in the CNS. In agreement with previous studies (4), icv injections of siRNA induced significant but incomplete silencing of MR or AT1aR within brain tissue by 50 to 65%. Nevertheless, these central receptor knockdowns resulted in significantly attenuating Aldo-induced hypertension, which confirms results produced by pharmacological antagonists on Aldo-induced increases in BP. In future studies, this powerful tool can be used to chronically knock down AT1aR or MR in specific nuclei such as the PVN or the subfornical organ to determine which brain areas are responsible for the interaction between ANG II and Aldo.
We and others have reported previously that central scavenging of ROS by tempol or central blockade of NADPH oxidase attenuated the ANG II-induced increase in BP (39, 62) and that this attenuated effect of ANG II on the BP response involves a decrease in sympathetic outflow (62). Aldo has also recently been shown to increase superoxide production via MR- and AT1R-mediated activation of NADPH oxidase and induce cardiovascular injury (1, 17, 23, 49, 64). This is further supported by a recent study showing that Aldo-induced oxidative stress was blocked not only by a MR antagonist but also by an antioxidant and an AT1R antagonist (17). Furthermore, increasing the central level of Aldo by direct icv infusion or by heart failure resulted in enhanced NADPH oxidase activity and ROS production in the PVN through activation of brain renin-angiotensin activity, thereby leading to increased neuronal activity and sympathetic drive (63, 64). In agreement with these previous results, the present study demonstrated that central infusion of tempol or apocynin significantly inhibits Aldo/NaCl-induced hypertension and that the reduction of BP in response to ganglionic blockade was less in rats with central blockade of AT1R, MR, or ROS production compared with that in rats with central vehicle during systemic Aldo or ANG II infusion. These observations in combination with the previously described results suggest that enhanced oxidative stress resulting from increased ROS production by NADPH oxidase is a common pathway mediating the actions of both ANG II and Aldo. The consequence of this increase in ROS is a central activation of sympathetic drive to the vasculature, heart, and kidney, which at least partially contributes to the development of ANG II- and Aldo-induced hypertension.
Aldo and ANG II are implicated in the genesis of salt appetite (10). Central blockade of either MR or AT1R or formation of endogenous ANG II reduced salt appetite caused by sodium depletion (45, 60). However, central blockade of formation of ANG II failed to reduce salt appetite evoked by pharmacological doses of DOCA (10). Similarly, icv administration of a MR antagonist markedly attenuated hypertension in the DOCA/NaCl model but had no effect on saline intake (25). In contrast to these results, we found that central blockade of MR or AT1R either by a pharmacological antagonist or by use of an AAV-siRNA decreased both the increases in BP and 1% NaCl intake produced by the systemic Aldo infusion. An explanation for these functional disassociations is not readily apparent. One CNS-mediated mechanism to facilitate the development of DOCA/NaCl or Aldo/NaCl hypertension would be by enhancing the intake of saline. Early studies showed that the destruction of central catecholaminergic neurons prevented the DOCA/saline-induced hypertension and inhibited saline intake, but the failure of the BP to rise was not secondary to a reduction in saline intake, since intact rats subjected to restricted saline intake to match that of experimental animals still became hypertensive (42). Peysner et al. (40) demonstrated that icv Aldo produced sustained dose-responsive increases in BP, which appear to be independent of alterations in sodium ingestion. As suggested by Osborn et al. (37), such results indicate that the pathways mediating the BP responses to Aldo/NaCl treatment may be separate from those controlling thirst and salt appetite. Therefore, the lower saline intake induced by central blockade of MR or AT1R in the present study in all likelihood cannot account entirely for the reduction in BP, because we also found that central blockade of ROS production induced by systemic Aldo infusion changed the BP responses to Aldo without attenuating saline intake.
In summary, the present results indicate that brain AT1Rs are involved in mediating the hypertensive response produced by systemic Aldo administration and that brain MRs are implicated in the expression of high BP induced by systemic ANG II. Activation of central RAAS may exert enhanced cardiovascular damage and elevated BP through a synergistic and cooperative interaction of Aldo and ANG II.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-62261, HL-59676, HL-14388, and HL-98207, National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-66086, and National Institute of Mental Health Grant MH-80241.
REFERENCES
- 1. Beswick RA, Dorrance AM, Leite R, Webb RC. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension 38: 1107–1111, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Beswick RA, Zhang H, Marable D, Catravas JD, Hill WD, Webb RC. Long-term antioxidant administration attenuates mineralocorticoid hypertension and renal inflammatory response. Hypertension 37: 781–786, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Burson JM, Aguilera G, Gross KW, Sigmund CD. Differential expression of angiotensin receptor 1A and 1B in mouse. Am J Physiol Endocrinol Metab 267: E260–E267, 1994 [DOI] [PubMed] [Google Scholar]
- 4. Chen Y, Chen H, Hoffmann A, Cool DR, Diz DI, Chappell MC, Chen AF, Morris M. Adenovirus-mediated small-interference RNA for in vivo silencing of angiotensin AT1a receptors in mouse brain. Hypertension 47: 230–237, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Chiu AT, Dunscomb J, Kosierowski J, Burton CR, Santomenna LD, Corjay MH, Benfield P. The ligand binding signatures of the rat AT1a, AT1b and the human AT1 receptors are essentially identical. Biochem Biophys Res Commun 197: 440–449, 1993 [DOI] [PubMed] [Google Scholar]
- 6. de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 52: 415–472, 2000 [PubMed] [Google Scholar]
- 7. Doi T, Sakoda T, Akagami T, Naka T, Mori Y, Tsujino T, Masuyama T, Ohyanagi M. Aldosterone induces interleukin-18 through endothelin-1, angiotensin II, Rho/Rho-kinase, and PPARs in cardiomyocytes. Am J Physiol Heart Circ Physiol 295: H1279–H1287, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Fiebeler A, Nussberger J, Shagdarsuren E, Rong S, Hilfenhaus G, Al-Saadi N, Dechend R, Wellner M, Meiners S, Maser-Gluth C, Jeng AY, Webb RL, Luft FC, Muller DN. Aldosterone synthase inhibitor ameliorates angiotensin II-induced organ damage. Circulation 111: 3087–3094, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Fiebeler A, Schmidt F, Muller DN, Park JK, Dechend R, Bieringer M, Shagdarsuren E, Breu V, Haller H, Luft FC. Mineralocorticoid receptor affects AP-1 and nuclear factor-kappab activation in angiotensin II-induced cardiac injury. Hypertension 37: 787–793, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Fluharty SJ, Epstein AN. Sodium appetite elicited by intracerebroventricular infusion of angiotensin II in the rat: II. Synergistic interaction with systemic mineralocorticoids. Behav Neurosci 97: 746–758, 1983 [DOI] [PubMed] [Google Scholar]
- 11. Funder JW. Aldosterone, mineralocorticoid receptors and vascular inflammation. Mol Cell Endocrinol 217: 263–269, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Geerling JC, Engeland WC, Kawata M, Loewy AD. Aldosterone target neurons in the nucleus tractus solitarius drive sodium appetite. J Neurosci 26: 411–417, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gómez-Sánchez EP. Central hypertensive effects of aldosterone. Front Neuroendocrinol 18: 440–462, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Gómez-Sánchez EP, Fort CM, Gómez-Sánchez CE. Intracerebroventricular infusion of RU28318 blocks aldosterone-salt hypertension. Am J Physiol Endocrinol Metab 258: E482–E484, 1990 [DOI] [PubMed] [Google Scholar]
- 15. Harada E, Yoshimura M, Yasue H, Nakagawa O, Nakagawa M, Harada M, Mizuno Y, Nakayama M, Shimasaki Y, Ito T, Nakamura S, Kuwahara K, Saito Y, Nakao K, Ogawa H. Aldosterone induces angiotensin-converting-enzyme gene expression in cultured neonatal rat cardiocytes. Circulation 104: 137–139, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Hatakeyama H, Miyamori I, Fujita T, Takeda Y, Takeda R, Yamamoto H. Vascular aldosterone: biosynthesis and a link to angiotensin II-induced hypertrophy of vascular smooth muscle cells. J Biol Chem 269: 24316–24320, 1994 [PubMed] [Google Scholar]
- 17. Hirono Y, Yoshimoto T, Suzuki N, Sugiyama T, Sakurada M, Takai S, Kobayashi N, Shichiri M, Hirata Y. Angiotensin II receptor type 1-mediated vascular oxidative stress and proinflammatory gene expression in aldosterone-induced hypertension: the possible role of local renin-angiotensin system. Endocrinology 148: 1688–1696, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Huang BS, Ahmadi S, Ahmad M, White RA, Leenen FH. Central neuronal activation and pressor responses induced by circulating ANG II: role of the brain aldosterone-“ouabain” pathway. Am J Physiol Heart Circ Physiol 299: H422–H430, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Huang BS, White RA, Jeng AY, Leenen FH. Role of central nervous system aldosterone synthase and mineralocorticoid receptors in salt-induced hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 296: R994–R1000, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Itaya Y, Suzuki H, Matsukawa S, Kondo K, Saruta T. Central renin-angiotensin system and the pathogenesis of DOCA-salt hypertension in rats. Am J Physiol Heart Circ Physiol 251: H261–H268, 1986 [DOI] [PubMed] [Google Scholar]
- 21. Iwai N, Inagami T, Ohmichi N, Nakamura Y, Saeki Y, Kinoshita M. Differential regulation of rat AT1a and AT1b receptor mRNA. Biochem Biophys Res Commun 188: 298–303, 1992 [DOI] [PubMed] [Google Scholar]
- 22. Iwanami J, Mogi M, Iwai M, Horiuchi M. Inhibition of the renin-angiotensin system and target organ protection. Hypertens Res 32: 229–237, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Iwashima F, Yoshimoto T, Minami I, Sakurada M, Hirono Y, Hirata Y. Aldosterone induces superoxide generation via Rac1 activation in endothelial cells. Endocrinology 149: 1009–1014, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Jaffe IZ, Mendelsohn ME. Angiotensin II and aldosterone regulate gene transcription via functional mineralocorticoid receptors in human coronary artery smooth muscle cells. Circ Res 96: 643–650, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Janiak PC, Lewis SJ, Brody MJ. Role of central mineralocorticoid binding sites in development of hypertension. Am J Physiol Regul Integr Comp Physiol 259: R1025–R1034, 1990 [DOI] [PubMed] [Google Scholar]
- 26. Kagiyama S, Tsuchihashi T, Abe I, Matsumura K, Fujishima M. Central infusion of l-arginine or superoxide dismutase does not alter arterial pressure in SHR. Hypertens Res 23: 339–343, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Kubo T, Yamaguchi H, Tsujimura M, Hagiwara Y, Fukumori R. Blockade of angiotensin receptors in the anterior hypothalamic preoptic area lowers blood pressure in DOCA-salt hypertensive rats. Hypertens Res 23: 109–118, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Leenen FH, Yuan B. Prevention of hypertension by irbesartan in Dahl S rats relates to central angiotensin II type 1 receptor blockade. Hypertension 37: 981–984, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Lemarié CA, Paradis P, Schiffrin EL. New insights on signaling cascades induced by cross-talk between angiotensin II and aldosterone. J Mol Med 86: 673–678, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Lemarié CA, Simeone SM, Nikonova A, Ebrahimian T, Deschênes ME, Coffman TM, Paradis P, Schiffrin EL. Aldosterone-induced activation of signaling pathways requires activity of angiotensin type 1a receptors. Circ Res 105: 852–859, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Li XC, Zhuo JL. Selective knockdown of AT1 receptors by RNA interference inhibits Val5-ANG II endocytosis and NHE-3 expression in immortalized rabbit proximal tubule cells. Am J Physiol Cell Physiol 293: C367–C378, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Llorens-Cortes C, Greenberg B, Huang H, Corvol P. Tissular expression and regulation of type 1 angiotensin II receptor subtypes by quantitative reverse transcriptase-polymerase chain reaction analysis. Hypertension 24: 538–548, 1994 [DOI] [PubMed] [Google Scholar]
- 33. Mann JF, Johnson AK, Ganten D. Plasma angiotensin II: dipsogenic levels and angiotensin-generating capacity of renin. Am J Physiol Regul Integr Comp Physiol 238: R372–R377, 1980 [DOI] [PubMed] [Google Scholar]
- 34. Mazak I, Fiebeler A, Muller DN, Park JK, Shagdarsuren E, Lindschau C, Dechend R, Viedt C, Pilz B, Haller H, Luft FC. Aldosterone potentiates angiotensin II-induced signaling in vascular smooth muscle cells. Circulation 109: 2792–2800, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Min LJ, Mogi M, Li JM, Iwanami J, Iwai M, Horiuchi M. Aldosterone and angiotensin II synergistically induce mitogenic response in vascular smooth muscle cells. Circ Res 97: 434–442, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Omouessi ST, Falconetti C, Chapleur M, Fernette B, Thornton SN. Mineralocorticoid pretreatment enhances angiotensin II-induced neuronal excitation but not salt drinking in male Fischer rats. J Neuroendocrinol 19: 109–115, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Osborn JW, Jacob F, Hendel M, Collister JP, Clark L, Guzman PA. Effect of subfornical organ lesion on the development of mineralocorticoid-salt hypertension. Brain Res 1109: 74–82, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Perazella MA, Setaro JF. Renin-angiotensin-aldosterone system: fundamental aspects and clinical implications in renal and cardiovascular disorders. J Nucl Cardiol 10: 184–196, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Peterson JR, Burmeister MA, Tian X, Zhou Y, Guruju MR, Stupinski JA, Sharma RV, Davisson RL. Genetic silencing of Nox2 and Nox4 reveals differential roles of these NADPH oxidase homologues in the vasopressor and dipsogenic effects of brain angiotensin II. Hypertension 54: 1106–1114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peysner K, Henry CA, Malvin RL. Central infusion of aldosterone increases blood pressure by mechanisms independent of Na retention. Clin Exp Hypertens A 12: 399–414, 1990 [DOI] [PubMed] [Google Scholar]
- 41. Pojoga LH, Romero JR, Yao TM, Loutraris P, Ricchiuti V, Coutinho P, Guo C, Lapointe N, Stone JR, Adler GK, Williams GH. Caveolin-1 ablation reduces the adverse cardiovascular effects of N-omega-nitro-l-arginine methyl ester and angiotensin II. Endocrinology 151: 1236–1246, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reid JL, Zivin JA, Kopin IJ. Central and peripheral adrenergic mechanisms in the development of deoxycorticosterone-saline hypertension in rats. Circ Res 37: 569–579, 1975 [DOI] [PubMed] [Google Scholar]
- 43. Rocha R, Funder JW. The pathophysiology of aldosterone in the cardiovascular system. Ann NY Acad Sci 970: 89–100, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Rocha R, Martin-Berger CL, Yang P. Selective aldosterone blockade prevents angiotensin II/salt-induced vascular inflammation in the rat heart. Endocrinology 143: 4828–4836, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Sakai RR, Nicolaïdis S, Epstein AN. Salt appetite is suppressed by interference with angiotensin II and aldosterone. Am J Physiol Regul Integr Comp Physiol 251: R762–R768, 1986 [DOI] [PubMed] [Google Scholar]
- 46. Sakurabayashi-Kitade S, Aoka Y, Nagashima H, Kasanuki H, Hagiwara N, Kawana M. Aldosterone blockade by spironolactone improves the hypertensive vascular hypertrophy and remodeling in angiotensin II overproducing transgenic mice. Atherosclerosis 206: 54–60, 2009 [DOI] [PubMed] [Google Scholar]
- 47. Sato A, Saruta T, Funder JW. Combination therapy with aldosterone blockade and renin-angiotensin inhibitors confers organ protection. Hypertens Res 29: 211–216, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Schiffrin EL. Effects of aldosterone on the vasculature. Hypertension 47: 312–318, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Somers MJ, Mavromatis K, Galis ZS, Harrison DG. Vascular superoxide production and vasomotor function in hypertension induced by deoxycorticosterone acetate-salt. Circulation 101: 1722–1728, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Stas S, Whaley-Connell A, Habibi J, Appesh L, Hayden MR, Karuparthi PR, Qazi M, Morris EM, Cooper SA, Link CD, Stump C, Hay M, Ferrario C, Sowers JR. Mineralocorticoid receptor blockade attenuates chronic overexpression of the renin-angiotensin-aldosterone system stimulation of reduced nicotinamide adenine dinucleotide phosphate oxidase and cardiac remodeling. Endocrinology 148: 3773–3780, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Struthers A, Krum H, Williams GH. A comparison of the aldosterone-blocking agents eplerenone and spironolactone. Clin Cardiol 31: 153–158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sturgis LC, Cannon JG, Schreihofer DA, Brands MW. The role of aldosterone in mediating the dependence of angiotensin hypertension on IL-6. Am J Physiol Regul Integr Comp Physiol 297: R1742–R1748, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sugiyama T, Yoshimoto T, Tsuchiya K, Gochou N, Hirono Y, Tateno T, Fukai N, Shichiri M, Hirata Y. Aldosterone induces angiotensin converting enzyme gene expression via a JAK2-dependent pathway in rat endothelial cells. Endocrinology 146: 3900–3906, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Sun Z, Bello-Roufai M, Wang X. RNAi inhibition of mineralocorticoid receptors prevents the development of cold-induced hypertension. Am J Physiol Heart Circ Physiol 294: H1880–H1887, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Timmermans PB. Angiotensin II receptor antagonists: an emerging new class of cardiovascular therapeutics. Hypertens Res 22: 147–153, 1999 [DOI] [PubMed] [Google Scholar]
- 56. Taylor NE, Glocka P, Liang M, Cowley AW., Jr NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension 47: 692–698, 2006 [DOI] [PubMed] [Google Scholar]
- 57. Ullian ME, Schelling JR, Linas SL. Aldosterone enhances angiotensin II receptor binding and inositol phosphate responses. Hypertension 20: 67–73, 1992 [DOI] [PubMed] [Google Scholar]
- 58. Vázquez J, Correa de Adjounian MF, Sumners C, González A, Diez-Freire C, Raizada MK. Selective silencing of angiotensin receptor subtype 1a (AT1aR) by RNA interference. Hypertension 45: 115–119, 2005 [DOI] [PubMed] [Google Scholar]
- 59. Virdis A, Neves MF, Amiri F, Viel E, Touyz RM, Schiffrin EL. Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension 40: 504–510, 2002 [DOI] [PubMed] [Google Scholar]
- 60. Weiss ML, Moe KE, Epstein AN. Interference with central actions of angiotensin II suppresses sodium appetite. Am J Physiol Regul Integr Comp Physiol 250: R250–R259, 1986 [DOI] [PubMed] [Google Scholar]
- 61. Xiao F, Puddefoot JR, Barker S, Vinson GP. Mechanism for aldosterone potentiation of angiotensin II-stimulated rat arterial smooth muscle cell proliferation. Hypertension 44: 340–345, 2004 [DOI] [PubMed] [Google Scholar]
- 62. Xue B, Zhao Y, Johnson AK, Hay M. Central estrogen inhibition of angiotensin II-induced hypertension in male mice and the role of reactive oxygen species. Am J Physiol Heart Circ Physiol 295: H1025–H1032, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yu Y, Wei SG, Zhang ZH, Gomez-Sanchez E, Weiss RM, Felder RB. Does aldosterone upregulate the brain renin-angiotensin system in rats with heart failure? Hypertension 51: 727–733 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang ZH, Yu Y, Kang YM, Wei SG, Felder RB. Aldosterone acts centrally to increase brain renin-angiotensin system activity and oxidative stress in normal rats. Am J Physiol Heart Circ Physiol 294: H1067–H1074, 2008 [DOI] [PubMed] [Google Scholar]