Abstract
The differentiation of valvular interstitial cells (VICs) to a myofibroblastic or osteoblast-like phenotype is commonly found in calcific valvular stenosis, although the molecular-level mechanisms of this process remain poorly understood. Due to the role of the Rho pathway in various vascular diseases and in the expression of a myofibroblast phenotype, the present study was inspired by the hypothesis that Rho activation is involved in regulating cellular processes related to valve calcification. It was found that increased RhoA and Rho kinase (ROCK) activity was associated with increased nodule formation in VIC cultures in vitro, and intentional induction of RhoA activity led to a further increase in nodules and expression of α-smooth muscle actin. VICs treated with ROCK inhibitors were also examined for nodule formation, proliferation, apoptosis, and expression of myofibroblastic or osteoblastic markers. ROCK inhibition dramatically reduced myofibroblast-regulated nodule formation in VIC cultures, as evidenced by a decrease in nodule number, total nodule area, α-smooth muscle actin-positive stress fibers, apoptosis, and gene expression of myofibroblast-related phenotypic markers. Meanwhile, ROCK inhibition was less effective at reducing nodule formation associated with osteogenic activity. In fact, ROCK inhibition increased the expression of alkaline phosphatase and effected only a modest decrease in nodule number when applied to VIC cultures with higher osteogenic activity. Thus, the Rho pathway possesses a complex role in regulating the VIC phenotype and nodule formation, and it is hoped that further elucidation of these molecular-level events will lead to an improved understanding of valvular disease and identification of potential treatments.
Keywords: heart valve, signaling pathways, extracellular matrix, nodule formation, myofibroblast
although calcific aortic valve disease (CAVD) is the third most common cardiovascular disorder in the United States (45), it remains relatively poorly understood at the molecular level. Valvular interstitial cells (VICs) are the most prominent cell type in the native valve leaflet and are believed to play a major role in CAVD (7, 44, 53, 58). Many factors are thought to influence valve calcification, including various cytokines (21, 28, 61), mechanical stimuli (38, 39, 65), and extracellular matrix (ECM) composition (16, 33, 47). Although the majority of VICs within healthy adult valves are quiescent, fibroblast-like cells, the overall VIC population is both heterogeneous and plastic, capable of differentiation toward multiple different mesenchymal lineages (4, 28). The calcification of valves has been associated with the transdifferentiation of quiescent VICs to a contractile, myofibroblastic phenotype [termed activated VICs (aVICs)] or an osteoblast-like phenotype [termed osteoblast-like VICs (obVICs)] (28).
Although myofibroblasts are present in the resident VIC population in aortic valves, they represent <1% of the total VIC population in healthy valves (39). In contrast, calcified valves and nodule-forming VIC cultures tend to be rich in VICs that have differentiated to a myofibroblastic phenotype and stain positively for α-smooth muscle actin (α-SMA) (41, 61). These α-SMA-positive valvular myofibroblasts are believed to participate in valve calcification via dystrophic mechanisms. Dystrophic calcification is found in ∼83% of calcified valves (32) and is accompanied by both a significant increase in apoptosis and the increased presence and prolonged activation of α-SMA-positive cells (28). Meanwhile, ossification is associated with deposition of bone minerals by obVICs, mirroring the process of bone tissue formation (44). Ossification generally does not involve apoptotic mechanisms and is found in a minority (∼13%) of calcified valves (32).
Rho family GTPases (which includes Rho, Rac, and Cdc42) are regulatory molecules that, in addition to other functions, provide a link between cell surface receptors and actin cytoskeleton organization (15). RhoA has been directly implicated in regulating the formation of actin stress fibers, as evidenced by the induction of stress fibers and focal adhesions in fibroblasts upon microinjection of an activated mutant form of RhoA (46). As discussed in the following sections, there are numerous indicators that point toward the Rho/Rho kinase (ROCK) signaling pathway as a potential regulator of heart valve calcification. Namely, the Rho/ROCK pathway is involved in 1) inducing contractility and stress fiber formation, which are features found in diseased valves (6, 7, 41, 42, 46, 48, 61); 2) effecting the actions of transforming growth factor (TGF)-β1, which is a known potent inducer of VIC calcification (1, 21, 47, 61); and 3) positively regulating mineralization in other cell populations, such as smooth muscle cells (SMCs) (25, 51) and mesenchymal stem cells (30).
In the cardiovascular system, Rho plays important roles in regulating proper smooth muscle and endothelial cell function (34, 48, 66). ROCK may be present in two isoforms, ROCK1 and ROCK2, with the latter being highly expressed in the heart (54). ROCK is the downstream effector molecule of RhoA (49), and it also mediates a wide range of cell behaviors, such as morphology (50), migration (46), proliferation (10), and apoptosis (37). However, there is growing evidence that Rho and ROCK contribute to the initiation and progression of various cardiovascular diseases (27, 48). In the context of vascular pathologies, ROCK is involved in vascular inflammation and remodeling (24), restenosis (52), hypertension, atherosclerosis (29), and calcification (51). For these reasons, Rho/ROCK also represents a powerful therapeutic target (19, 45, 54). Clinical administration of a ROCK inhibitor (fasudil) has been successful in treating several of the aforementioned cardiovascular diseases (27, 54). Furthermore, 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, commonly termed “statins,” are thought to exert many of their pleiotropic effects via inhibition of the Rho pathway (12, 25, 27). Statins can inhibit the isoprenylation of small GTPases such as Rho (12), and the anticalcific effects of statins on vascular SMCs are believed to be related to inhibition of both RhoA and ROCK (25). A recent publication from our own group (33) has also linked statin-induced decreases in the calcific activity of VICs to inhibition of ROCK.
There is currently no Federal Drug Administration-approved treatment available to halt the progression of valvular calcification. This lack of therapeutics is due, in part, to our incomplete understanding of the etiology of valvular disease and its underlying mechanisms. We propose that the RhoA/ROCK pathway is involved in mediating the processes that can eventually lead to valvular calcification and thus studied the role of RhoA/ROCK signaling in regulating the phenotype and formation of nodules in in vitro cultures of VICs. The goal of such an investigation was to gain a better understanding of the factors that regulate CAVD with the hopes of identifying potential therapeutic targets for preventing its occurrence or stopping its progression.
MATERIALS AND METHODS
All chemicals and cell culture solutions were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
VIC isolation and culture.
VICs were isolated from porcine aortic valve leaflets (Hormel, Austin, MN) by collagenase digestion as previously described (22) and cultured in growth medium (15% FBS, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin in medium 199) at 37°C and 5% CO2 for two to four passages. Unless otherwise specified, VICs used in all experiments were seeded at a density of 50,000 cells/cm2 onto 24- or 96-well plates. During the execution of experiments, VICs were cultured in low-serum medium (1% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine in medium 199), and the medium was changed each day until day 5.
Substrate preparation.
To produce culture environments that differ in their ability to support VIC nodule formation, culture surfaces were modified as described in Ref. 47. To produce environments that do not support nodules, tissue culture polystyrene (TCPS) plates (24 or 96 wells) were coated with type I collagen (Coll; Inamed Biomaterials, Fremont, CA, 2 μg/cm2) or fibronectin (FN; 5 μg/cm2). For nodule-forming environments, TCPS wells were coated with fibrin (FB; 1.5 μg/cm2) as described previously (14) or left untreated. The amounts of adsorbed proteins were measured on separate plates using a MicroBCA Protein Assay (Pierce, Rockford, IL) according to the manufacturer's instructions to verify adsorption.
RhoA and ROCK activity assays.
ROCK activity was assayed using an ELISA-based Cyclex ROCK assay kit (Cyclex, Nagano, Japan). After 1, 3, or 5 days of culture in nodule-forming or negative control environments, VIC samples were lysed at 4°C in 200 μl TRI reagent (Molecular Research Center, Cincinnati, OH) supplemented with 1× protease inhibitor cocktail (BD Biosciences, San Jose, CA) and processed to obtain RNA, DNA, and protein fractions. After the RNA fraction of the TRI lysate was removed as described below, the remaining solution, which contained DNA and proteins, was mixed with 0.2 ml ethanol/600 μl TRI reagent, stored at room temperature for 5 min, and then centrifuged at 2,000 g for 5 min. After centrifugation, DNA was precipitated, and 300 μl of the aqueous phase were further processed for protein isolation by mixing with 900 μl acetone; this mixture was then centrifuged at 12,000 g for 10 min. Proteins were precipitated at the bottom of the tube and washed with 0.5 ml wash solution (0.3 M guanidine hydrochloride in 95% ethanol and 2.5% glycerol). After being centrifuged at 8,000 g for 5 min, the supernatant was removed, and the protein pellets were further washed two times with 1 ml of the washing solution. Protein pellets were purified with a final wash of 1 ml ethanol containing 2.5% glycerol with gentle rotation for 10 min. Proteins were then air dried, dissolved in 300 μl of 1% SDS, and stored at 4°C for use in the ROCK activity assay.
Using these purified protein samples, the ROCK activity assay was then executed according to the manufacturer's instructions. The principle of this assay involves incubating samples in plates precoated with a substrate corresponding to the COOH-terminus of the myosin-binding subunit of myosin phosphatase (MBS), which contains a threonine residue (Thr696) that can be phosphorylated by ROCK (both ROCK1 and ROCK2). This is followed by the addition of the detection antibody, which is the horseradish peroxidase (HRP) conjugate of AF20, an antibody that specifically detects only the phosphorylated form of Thr696 on MBS. Chromogenic development was performed using tetramethylbenzidine, with the final sample absorbance read at 450 nm (Synergy HT plate reader, Bio-Tek Instruments, Winooski, VT).
RhoA activity was assayed using an ELISA-based G-LISA RhoA Activation Assay (Cytoskeleton, Denver, CO). This quantitative assay detects the active GTP-bound form of RhoA but not the inactive GDP-bound form. Cells were lysed with kit-provided buffer, and a portion of the lysate from each condition was analyzed for total protein concentration for normalization of sample loading. Samples were then loaded in the assay plate, and the manufacturer's instructions were followed to detect RhoA. Absorbance results of the final chromogenic reaction were read at 490 nm.
Rho pathway stimulation and inhibition.
In Rho stimulation experiments, VICs on the aforementioned coatings (Coll, FN, FB, and uncoated TCPS) were treated with oleoyl-l-α-lysophosphatidic acid sodium salt (LPA; 20 μM, Cayman Chemical, Ann Arbor, MI). LPA is a natively occurring phospholipid that activates the small GTPases Ras, Rac, and RhoA (55). Thus, to isolate the contribution of Rho in LPA-induced effects, cells were also treated with a combination of LPA and the ROCK inhibitor (S)-(+)-2-methyl-1-[(4-methyl-5-isoquinolinyl)sulfonyl]homopiperazine (H1152; 5 μM, Calbiochem, San Diego, CA) (18). All treatments were administered starting 24 h after cell seeding and were reapplied every other day until day 5, at which point nodule formation was assessed as described below.
In Rho inhibition experiments, VICs on ECM-coated substrates were treated with H1152, a specific ROCK inhibitor (18). Twenty-four hours after cell seeding, medium in all plates was changed and supplemented with H1152 (5 μM) or left untreated. This inhibitor was applied every other day until day 5, at which point nodules, apoptosis, and phenotype were assessed as described below. These experiments were repeated using an alternative ROCK inhibitor, (R)-(+)-trans-4-(1-aminoethyl)-N-(4-pyridyl)cyclohexanecarboxamide dihydrochloride monohydrate (Y27632; 5 μM, Calbiochem), to confirm the ROCK specificity of these inhibition experiments. Both H1152 and Y27632 are cell permeable, highly specific, and potent (Ki: 1.6 and 140 nM, respectively) compounds that inhibit ROCK by competing with ATP for its binding to the kinase (18). H1152 exhibits weaker affinity for other serine/threonine kinases (Ki: 630 nM for PKA, 9.27 mM for PKC, and 10.1 mM for myosin light chain kinase) and is more potent and selective than Y27632. The ability of LPA to stimulate ROCK activity and the ability of both H1152 and Y27632 to inhibit ROCK activity was confirmed by performing a ROCK activity assay on samples treated with LPA, H1152, or Y27632 as described above.
Quantification of nodule number and size.
After 5 days of culture in the presence of absence of LPA or a ROCK inhibitor, VIC cultures were stained with alizarin red S, which stains mineralized deposits red, to facilitate the quantification of nodules. Cultures were fixed with 10% neutral buffered formalin, stored at 4°C overnight, and stained with a 2% solution of alizarin red S in PBS. Positively stained nodules were manually counted under a microscope (Olympus IX51 with a Hamamatsu 285 digital camera and Simple PCI digital imaging software, Compix, Imaging Systems, Cranberry Township, PA). Nodule size was measured using ImageJ software (NIH; http://rsb.info.nih.gov/ij/), and photomicrographs were captured under ×40 and ×100 magnifications.
α-SMA detection.
Standard immunocytochemical methods were used to qualitatively examine α-SMA expression. Briefly, samples were fixed in 3.7% formaldehyde, blocked overnight in 5% BSA in PBS, permeabilized with 0.1% Triton X-100, and then incubated with an antibody to α-SMA (monoclonal, mouse, clone 1A4, 5 μg/ml) for 1.5 h, goat anti-mouse Alexa Fluor 488 (Invitrogen, 1:1,000 dilution) for 1 h, and photographed at ×100 and ×400.
Similar procedures were followed to obtain quantitative data for the amount of α-SMA in VIC cultures. On day 3 of culture, VICs were fixed, permeabilized, blocked as described above, and then incubated with anti-α-SMA primary antibody for 2.5 h. Samples were then washed several times with PBS and incubated with HRP-conjugated goat anti-mouse antibody (Pierce) diluted 1:5,000 in 1% BSA in PBS and applied to all plates for 1 h, followed by a 30-min incubation with 1-Step Turbo TMB-ELISA (Thermo Fisher Scientific, Waltham, MA). Development of the colorimetric reaction was stopped with 1 N H2SO4, and absorbance was read at 450 nm. After α-SMA detection, all plates were washed several times with PBS, counterstained with 4′,6-diamidino-2-phenylindole (DAPI; 1 μg/ml), and measured for fluorescence (excitation: 340 nm and emission: 440 nm) to normalize absorbance results to cell number.
Quantification of cell proliferation.
At time points of 3 and 5 days, proliferation was measured via the Click-iT EdU quantitative proliferation assay (Invitrogen) according to the manufacturer's instructions. Briefly, VICs were incubated for 10 h with 10 μM 5-ethynyl-2′-deoxyuridine (EdU), a nucleoside analog of thymidine incorporated during cell division, followed by fixation in 3.7% formaldehyde and permeabilization in 0.5% Triton X-100. The detection chemistry is based on a click reaction between the EdU alkyne group and the azide in Alexa Fluor 488, resulting in fluorescent green staining of proliferating cells. Cell nuclei were counterstained with DAPI (1 μg/ml), and cellular proliferation was calculated as the number of proliferating cells divided by the total number of cells in 4 photomicrographs/well (4 wells/condition).
Apoptosis assay.
Apoptosis was measured using an ELISA-based HT TiterTACS Assay kit (Trevigen, Gaithersburg, MD), which detects DNA fragmentation. At days 1 and 5, cells were fixed in 3.7% buffered formaldehyde solution for 7 min, washed with PBS, and postfixed in 100% methanol for 20 min. Following the manufacturer's instructions, cells were permeabilized with proteinase K, quenched with 2.5% H2O2 in methanol, and then incubated with the labeling reaction mix (TdT, biotin-dNTP, and unlabeled dNTP) to label breaks in DNA. Streptavidin-HRP and then TACS-Sapphire were added to the wells to detect apoptotic cells; the reaction was stopped with 2 N HCl, and absorbance was read at 450 nm.
RNA isolation.
As noted earlier, VIC samples were lysed in TRI reagent; RNA was then isolated from this lysate according to the manufacturer's instructions. The homogenate was stored at room temperature for 5 min to complete the dissociation of nucleoprotein complexes, at which point 0.15 ml chloroform/600 μl TRI reagent was added to the homogenate, followed by centrifugation at 13,000 g for 15 min. After centrifugation, RNA was precipitated from the upper aqueous phase by adding 0.3 ml isopropanol/600 μl TRI reagent to the tubes and then centrifuging at 13,000 g for 8 min. After this centrifugation step, the RNA pellet was washed with 75% ethanol and centrifuged at 8,000 g for 5 min. The RNA pellet was air dried and dissolved in 75 μl H2O at 60°C for 15 min. RNA samples were stored at −20°C until subsequent use.
Quantitative real-time PCR analysis.
Custom primers for various markers of myofibroblastic and osteogenic activity were obtained from Invitrogen and are shown in Table 1. For cDNA construction, 250 ng of original RNA isolated from samples were reverse transcribed using iScript (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions.
Table 1.
Sequences for real-time PCR
| Primers | |
|---|---|
| Alkaline phosphatase | |
| Forward | 5′-ATGAGCTCAACCGGAACAA-3′ |
| Reverse | 5′-GTGCCCATGGTCAATCCT-3′ |
| Core-binding factor-α1 | |
| Forward | 5′-GAGGAACCGTTTCAGCTTACTG-3′ |
| Reverse | 5′-CGTTAACCAATGGCACGAG-3′ |
| Heat shock protein 47 | |
| Forward | 5′-GCTGCTCGTCAACGCCATGT-3′ |
| Reverse | 5′- CCATCCAGGTCTTCAGCTGC-3′ |
| Matrix metalloproteinase-1 | |
| Forward | 5′-AAGCAGACATAATGTATCCTTTGTCA-3′ |
| Reverse | 5′-TGAGCATCCCCTCCAATACCT-3′ |
| Matrix metalloproteinase-13 | |
| Forward | 5′-ACAAGGGATCCAGTCTCTCTATGGT-3′ |
| Reverse | 5′-TCCAGGGATAATGAAGGATCACA-3′ |
| Osteocalcin | |
| Forward | 5′-TCAACCCCGACTGCGACGAG-3′ |
| Reverse | 5′-TTGGAGCAGCTGGGATGATGG-3′ |
| Transforming growth factor-β1 | |
| Forward | 5′-CGAGCCAGAGGCGGACTAC-3′ |
| Reverse | 5′-TTGGTTGCCGCTTTCCA-3′ |
| β-Actin | |
| Forward | 5′-ATGGTGGGTATGGGTCAGAA-3′ |
| Reverse | 5′-CGGAGCTCGTTGTAGAAGGT-3′ |
Samples were processed for real-time PCR by combining 0.5 μl of the cDNA construction, 5 μM primers, and SYBR green SuperMix (Bio-Rad) in a 15-μl reaction as specified in the manufacturer's protocol. A standard thermocycling protocol was used: 40 cycles of denaturing at 95°C for 15 s followed by annealing at 60°C for 1 min; this was followed by a melting curve analysis for 80 cycles of 55°C + 0.5°C/cycle, 10 s/cycle, to further confirm the purity of the final PCR products, with each condition performed in triplicate (iCycler iQ Real-Time PCR Instrument, Bio-Rad). Data analysis was performed using a standard comparative threshold cycle (Ct) (or ΔΔCt) method. The Ct values of all samples were first normalized to β-actin as an internal control, and the ΔCt values for experimental samples were then further normalized to the negative control (VICs on Coll, which represented a condition that did not support nodule formation).
Statistics.
All experiments were performed a minimum of three separate times, with n ≥ 3. Data were compared using ANOVA with Tukey's honestly significant difference post hoc test. P values of ≤0.05 were considered statistically significant. Data are presented as means ± SD.
RESULTS
Activation of intracellular signaling pathways.
To better understand the factors that cause VICs to form nodules, it is valuable to compare a condition that does not permit nodule formation against a condition that is associated with extensive nodule formation. Previous work (13, 14, 33, 47) demonstrated that this goal can be achieved by culturing VICs on substrates coated with different ECM-derived molecules. Spontaneously nodule-forming cultures of VICs can be formed by culturing the cells on either FB or unmodified TCPS, whereas VICs on Coll or FN are relatively resistant to nodule formation. Capitalizing on these differences, these four substrates were used to generate VIC cultures that exhibited differential nodule-forming capacities to be studied with respect to RhoA/ROCK characterization. It should be noted that nodule formation and related cellular events in this study were examined in the absence of osteogenic differentiation medium.
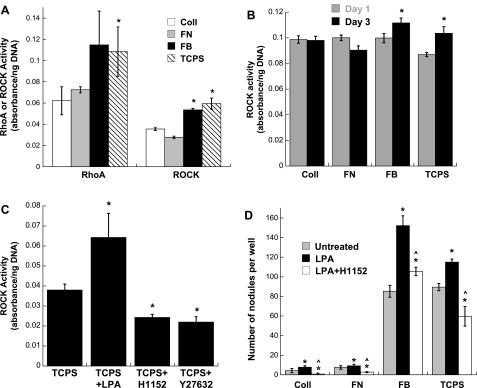
The active forms of RhoA (RhoA-GTP) and ROCK were quantified in both quiescent and nodule-forming VIC cultures. Compared with VIC cultures that did not allow nodule formation, the activation of both RhoA and ROCK in nodule-forming cultures was significantly increased at the day 5 time point (Fig. 1A). ROCK activity was also quantified at time points that preceded nodule formation, namely, days 1 and 3 of VIC culture on the different substrates. No significant differences in ROCK activity across these culture conditions were observed on day 1, but ROCK activity did display statistically significant trends as early as day 3 (Fig. 1B). Specifically, ROCK activity in VICs cultured in nodule-forming environments (TCPS and FB) was significantly higher than that found in the VIC cultures that did not support nodules (Coll and FN).
Fig. 1.
A: activity of RhoA (as RhoA-GTP) and Rho kinase (ROCK) in porcine valvular interstitial cells (VICs) on day 5 of culture in different environments. Fibrin (FB) and tissue culture polystyrene (TCPS) represent environments that support nodule formation, whereas fibronectin (FN) and collagen (Coll) represent environments that do not. *P < 0.05 compared with Coll. B: ROCK activity in VIC cultures at two earlier time points, days 1 and 3. n = 4 samples/condition. *P < 0.05 compared with both Coll and FN on day 3. C: ROCK activity in VIC cultures treated with the RhoA stimulant lysophosphatidic acid (LPA) or the ROCK inhibitors H1152 and Y27632 (day 5). *P < 0.01 compared with the untreated TCPS condition. D: impact of direct Rho activation (via administration of LPA) on the formation of nodules in VIC cultures. LPA was also administered in the presence of a ROCK inhibitor (H1152) to further understand the contribution of Rho to nodule formation. n = 4 samples/condition. *P < 0.04 compared with the untreated condition; ^P < 0.05 compared with the LPA-treated condition.
A validation experiment confirmed that administration of the Rho activation agent LPA significantly elevated ROCK activity, whereas administration of the ROCK inhibitors H1152 or Y27632 successfully decreased ROCK activity in VIC cultures (Fig. 1C). The elevation of ROCK activity induced by LPA was also accompanied by a corresponding increase in nodule formation in VIC cultures (Fig. 1D). Stimulation with LPA increased nodule formation on all substrate surfaces, including those that do not normally permit nodule formation (e.g., Coll and FN). Because LPA can stimulate Rac and Ras GTPases in addition to Rho, the contribution of Rho activation in these experiments was explored by concomitant administration of LPA with the ROCK inhibitor H1152. Through this treatment, we were able to discern whether the increase in nodule formation obtained via the application of LPA was due to its activation of Rho or of the alternative pathways (Rac and Ras) that it affects. When administered in combination with a Rho pathway inhibitor (H1152), LPA was no longer able to produce an increase in VIC nodule formation (Fig. 1D), indicating that the increased Rho activity was at least partly responsible for the increase in nodule formation induced by LPA.
Inhibition of the Rho pathway affects VIC nodule formation.
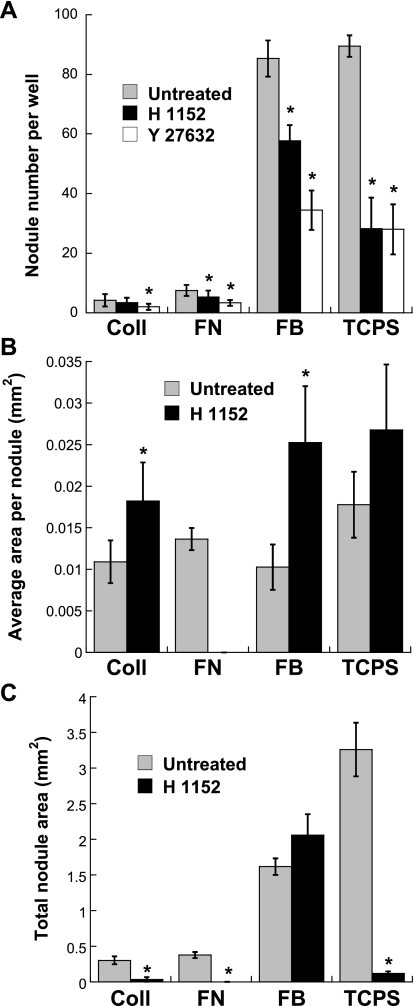
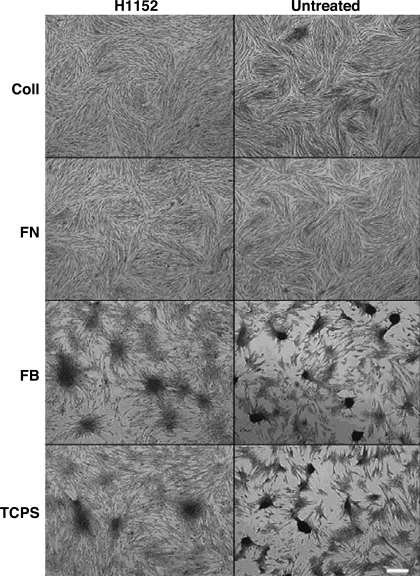
Blocking the Rho pathway via treatment with H1152 led to a significant decrease in the number of nodules formed in all conditions (Fig. 2A). The Rho specificity of these results was confirmed via administration of a different ROCK inhibitor, Y27632, which also significantly reduced nodule formation in all conditions (Fig. 2A). However, while treatment with H1152 was shown to significantly reduce nodule number in all conditions, this decrease in nodule number was consistently accompanied by significant increases in average nodule size (Fig. 2B) for all conditions and the total calcified area (Fig. 2C) in the case of FB. Also, although ROCK inhibition reduced the total nodule area in several conditions, H1152 treatment was much less effective when applied to VICs on FB. These observations were qualitatively confirmed by representative photomicrographs of the VIC cultures (Fig. 3). The occurrence of dramatically larger nodules in the H1152-treated conditions is clearly shown in these photomicrographs.
Fig. 2.
Characterization of day 5 nodule formation in VIC cultures after inhibition of ROCK via H1152 and Y27632. A: total nodule number. B: average nodule size. C: total nodule area/well. NA, not applicable, as the presence of zero nodules means that individual nodule sizes could not be measured. *P < 0.0001 compared with the corresponding untreated extracellular matrix (ECM) condition.
Fig. 3.
Photomicrographs showing day 5 nodule formation by VICs cultured on FN, FB, Coll, or TCPS in the presence or absence of H1152. Magnification: ×400. Scale bar = 100 μm.
Relationship between α-SMA and the extent of nodule formation.
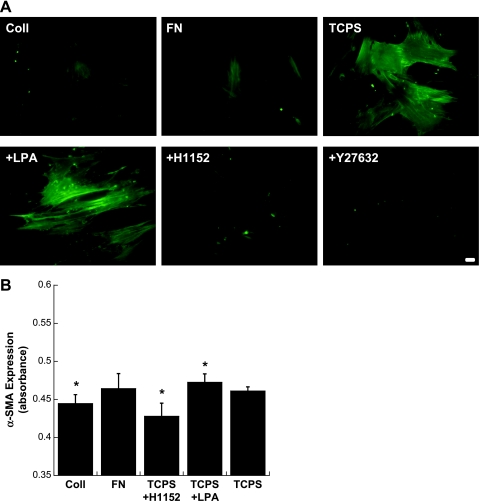
Staining for α-SMA, a cytoskeletal protein associated with contractility and activation of the Rho pathway (15), revealed a similar trend to that found for nodule formation. Specifically, VICs cultured on Coll or FN or treated with ROCK inhibitors while cultured on TCPS displayed relatively weak and diffuse α-SMA staining, as shown in Fig. 4A. In contrast, α-SMA-positive stress fibers were evident in VICs cultured on TCPS, and LPA (Rho activation) treatment of these cells further enhanced the intensity of α-SMA expression. Consistent with previous reports (2, 47), cells located in nodules stained positively for α-SMA (Supplemental Material, Supplemental Fig. 1),1 although the three-dimensional nature of the nodule and the lower magnification needed to image the entire nodule do not allow individual stress fibers to be distinguished. Quantitative analysis of α-SMA further confirmed the presence of increased levels of α-SMA in VIC cultures that contained a greater number of nodules (Fig. 4B). Specifically, the addition of LPA significantly increased α-SMA levels, whereas culture on Coll or treatment with H1152 significantly reduced α-SMA levels compared with untreated TCPS.
Fig. 4.
A: fluorescent photomicrographs of VIC cultures stained for α-smooth muscle actin (α-SMA). Bottom photomicrographs are VICs on TCPS treated with the indicated Rho activator or inhibitor. Magnification: ×400. Scale bar = 10 μm. B: quantitative measurement of α-SMA expression via a modified ELISA approach. n = 6 samples/condition. *P < 0.05 compared with TCPS.
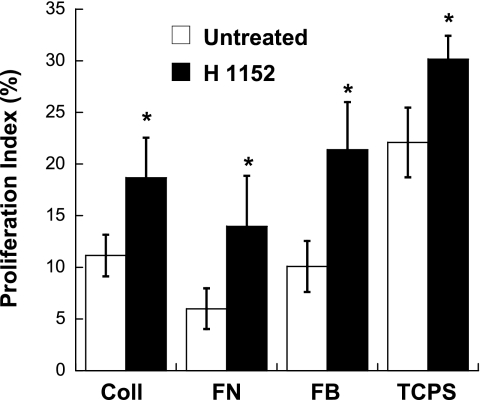
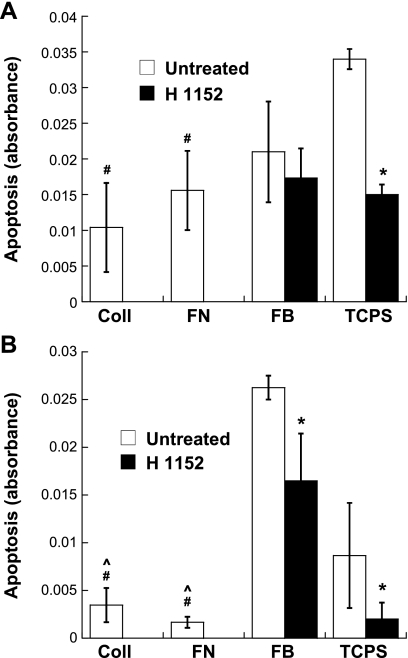
Impact of ROCK inhibition on VIC proliferation and apoptosis.
The Rho pathway can affect many cell processes, including proliferation and apoptosis, which are often involved in the progression of calcific valvular disease (3, 43). Thus, proliferation and apoptosis of VICs were examined in the presence or absence of the ROCK inhibitor H1152. As shown in Fig. 5, administration of H1152 significantly increased VIC proliferation in all conditions. The photomicrographs shown in Supplemental Fig. 2 also support this finding. Quantitative measurement of apoptosis in VIC cultures revealed dramatic differences across the various culture conditions. On day 1, before the formation of any nodules in any condition, trends in nodule formation had already emerged when the culture substrates that supported nodule formation (TCPS and FB) were compared with the substrates that did not (Coll and FN) or when H1152 administration compared with no treatment (Fig. 6A). Specifically, in the absence of H1152, the least amount of apoptosis was found on Coll and FN, whereas TCPS was associated with the highest apoptosis level. Also on day 1, the addition of H1152 had already significantly decreased apoptosis of VICs on all surfaces except FB. At the day 5 time point, these basic trends had strengthened even further (Fig. 6B). Notably, apoptosis of VICs in the Coll and FN environments was significantly lower than in both of the nodule-forming environments. Moreover, by day 5, H1152 treatment was associated with significantly decreased apoptosis levels for all conditions, including FB.
Fig. 5.
Proliferation of VICs cultured on Coll, FN, FB, or TCPS with or without H1152 treatment for 3 days. Data are presented as percent proliferation, which was calculated as the number of proliferating cells divided by the total number of cells in each field of view. n = 4 samples/condition. *P < 0.04 compared with the untreated condition.
Fig. 6.
Apoptosis of VICs cultured on all four substrates in the presence or absence of H1152 after 1 day (A) and 5 days (B) of culture. n = 3 samples/condition. *P < 0.05 compared with the untreated condition; #P < 0.05 compared with untreated TCPS; ^P < 0.05 compared with untreated FB.
Impact of ROCK inhibition on the VIC phenotype.
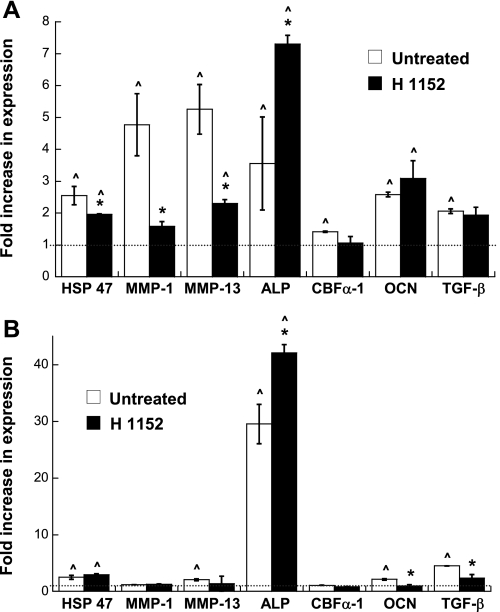
The expression of various markers of aVICs and obVICs (or dystrophic and ossific calcification, respectively) was quantified via real-time PCR to examine whether the nodule formation trends induced by H1152 treatment were due to stimulation of a myofibroblastic or osteoblastic cell phenotype. To meet this objective, these analyses focused on the conditions in which nodule formation was consistently achieved, namely, FB and TCPS. Previous publications describing VIC nodule formation and calcification have shown that calcifying VICs express markers related to their myofibroblastic phenotype [i.e., matrix metalloproteinase (MMP)-1, MMP-13, and heat shock protein (HSP)47] and/or markers related to osteogenic differentiation [i.e., alkaline phosphatase (ALP), osteocalcin (OCN), and core-binding factor-α1 (CBF-α1)] (14, 28, 31, 41, 47, 56, 58). Although TGF-β1 expression is more frequently associated with a myofibroblastic VIC phenotype, it can also participate in the osteogenic differentiation of vascular cell types (61, 62, 64).
The results shown in Fig. 7A demonstrate that the trends in nodule formation by VICs on TCPS were generally matched by the changes in the expression of myofibroblastic and osteoblastic markers. Specifically, compared with a noncalcifying negative control, untreated VICs cultured on TCPS demonstrated elevated mRNA levels for all markers analyzed: HSP47, MMP-1, MMP-13, ALP, CBF-α1, OCN, and TGF-β1. In a trend that mimicked the H1152-induced reduction of nodules in these cultures, the addition of H1152 to VICs on TCPS decreased the expression of several of the disease markers (HSP47, MMP-1, and MMP-13) relative to the untreated TCPS condition. Meanwhile, the results shown in Fig. 7B demonstrate that untreated VICs on FB also exhibited elevated expression levels of most markers analyzed (HSP47, MMP-13, ALP, OCN, and TGF-β1) relative to the negative control. In keeping with the minimal reduction in nodule formation obtained for H1152-treated VICs on FB, treatment with H1152 did not drastically alter expression of most of these markers, with only TGF-β1 expression being significantly lowered by the addition of H1152. Interestingly, in both TCPS and FB environments, the addition of H1152 resulted in a dramatic increase in ALP expression.
Fig. 7.
Phenotypic characterization (via real-time PCR) of VICs cultured on the procalcific surfaces, namely, TCPS (A) and FB (B), for 5 days. Heat shock protein 47 (HSP47), matrix metalloproteinase (MMP)-1, and MMP-13 are related to myofibroblast activity, whereas alkaline phosphatase (ALP), core-binding factor-α1 (CBF-α1), and osteocalcin (OCN) are indicative of osteoblast-like activity. Transforming growth factor (TGF)-β1 expression can be associated with either the myofibroblast or osteoblast phenotype. The dotted line represents the level of gene expression in the negative control condition, which did not permit nodule formation. n = 3 samples/condition. *P < 0.05 compared with the untreated condition; ^P < 0.05 compared with the negative control.
DISCUSSION
Much of what is currently known about valvular disease comes from the end-point analysis of explanted diseased valves (3, 31, 44, 57, 63). Unfortunately, such analyses can provide only limited information about the stimuli and underlying processes that contribute to the initiation and progression of valve calcification. Recent studies (32, 35) have implicated the Runx2/CBF-α1 and canonical Wnt/Lrp5/β-catenin pathways, which are involved in osteogenesis, as positive regulators of valve calcification. However, it is unlikely that valve calcification can be fully explained by the regulation of solely these two pathways. Because of its involvement in contractile mechanisms and various types of vascular disease, the Rho pathway emerged as a likely candidate for participation in valve calcification. Thus, in this work, we took a step toward characterizing the role of RhoA and ROCK in regulating the VIC phenotype and the in vitro formation of nodules by these cells. Although not equivalent to the direct measurement of calcification, the processes of VIC differentiation, proliferation, apoptosis, and nodule formation can serve as important indicators of the development of VIC dysfunction.
Our results indicate that aVICs share several similarities with other myofibroblastic cell types, where it has been demonstrated that the activation of Rho and ROCK activity accompanies the differentiation of fibroblasts into myofibroblasts, expression of α-SMA, assembly of a robust stress fiber network, and subsequent generation of continuous isometric tension on the cell's extracellular environment (17, 59, 67). This Rho-induced sustained contraction of myofibroblasts has been found to result in the deformation of the ECM structure (59), a phenomenon that has also been documented in VIC populations, acting as a critical step preceding nodule formation (61). In our VIC cultures, both RhoA and ROCK activity as well as α-SMA-positive stress fibers were found to be higher in cultures that formed nodules (FB and TCPS) than in those that were less permissive of nodule formation (Coll and FN), suggesting that Rho upregulation may be related to nodule formation. These findings were confirmed via experiments that measured the impact of intentional Rho activation or inhibition on nodule formation. Namely, administration of an agent that induced Rho activation (LPA) further increased nodule formation in all conditions, whereas administration of a ROCK inhibitor (H1152 or Y27632) dramatically decreased nodules in these cultures. Because LPA can activate other small GTPases, such as Ras and Rac, it was also administered concomitantly with H1152 to elucidate the contribution of Rho in these results. Nodule formation in cultures that received both LPA and H1152 was lowered back to levels of the unstimulated controls, demonstrating that the increase in nodule formation achieved via administration of LPA was likely due to its ability to elevate Rho activity.
With respect to ROCK inhibition, it was found that the addition of H1152 significantly decreased the number of nodules across all culture conditions. However, the magnitude of that decrease on FB was far smaller than that seen for the TCPS condition. Moreover, the average area of nodules in the FB condition increased by 2.5-fold upon blocking ROCK, resulting in a net increase in total nodule area relative to the untreated control. It is also worth noting that untreated VICs on FB exhibited almost a 30-fold increase in ALP expression levels compared with the negative control, whereas the ALP increase on TCPS was much more modest (∼3.5-fold). As noted earlier, the heterogeneous VIC population can differentiate to give rise to multiple phenotypes of cells, including aVICs (myofibroblasts) and obVICs (osteoblast-like cells), that can participate in dystrophic and ossific calcification processes, respectively. Thus, our findings come together to provide further evidence for a hypothesis proposed in our previous work (47), namely, that VIC nodule formation on FB involves more osteogenic activity than the nodule formation events that occur on TCPS. Moreover, with respect to the VIC phenotype, our results indicate that ROCK inhibition was most effective at lowering markers related to myofibroblastic activity (HSP47 and MMPs). Because Rho is an effector for a myofibroblastic phenotype in VICs, it is reasonable to expect that blocking this contractile-related pathway would have the greatest success in inhibiting the dystrophic, but not ossific, mechanisms that lead to nodule formation. Application of this reasoning to these experiments would explain why H1152 drastically decreased nodule formation in the TCPS environment but not in the FB condition, where the latter may be forming nodules via both dystrophic and osteogenic mechanisms.
As noted in the presentation of phenotype results, the administration of H1152 also resulted in a large upregulation of ALP expression by VICs on both TCPS and FB. This increase in ALP upon ROCK inhibition is, in fact, consistent with recent findings describing the mineralization of mesenchymal cells and vascular SMCs after treatment with a ROCK inhibitor (5, 23). Specifically, the application of fasudil or Y27632 to stromal stem cell lines or SMCs, respectively, resulted in a significant elevation of ALP (5, 23) and mineralization through ossific mechanisms (23). In our work, the concomitant decrease in apoptotic cells with an increase in osteoblastic activity may indicate that many of the nodules formed on cultures treated with H1152 were via osteogenic more so than dystrophic mechanisms. Although the factors that regulate nodule size are not known, the alteration in the type of calcification that we propose is occurring in these conditions may provide an explanation for the significant differences in nodule morphology and size observed upon H1152 treatment. Together with the findings that H1152 was most effective at lowering expression of only the myofibroblastic genes, these observations further support the emerging indication that blockade of ROCK can successfully inhibit nodule formation that occurs via dystrophic mechanisms but not that which occurs via osteogenic activity.
While it is clear that ROCK inhibition via H1152 exerted some nodule inhibition effects by virtue of its ability to regulate the VIC phenotype and attenuate the expression of myofibroblastic markers, this compound also impacted other VIC functions that may participate in processes that are important in valve calcification. Most notably, H1152 treatment significantly inhibited apoptosis in VIC cultures. Although not all cell types respond to ROCK inhibition with a decrease in apoptosis (36), in most cell types the actin-myosin II contractile forces generated via ROCK activity are necessary for the membrane blebbing that is an essential part of apoptosis (8, 9). In the present study, the presence of differences in apoptosis before the formation of any nodules suggests that the decreased apoptotic activity contributed to decreased nodule formation rather than being a resulting consequence of simply having less nodules in the sample. Thus, it is possible that H1152 also acts to inhibit nodule formation via the inhibition of apoptosis. This finding is consistent with the previous results (40) that demonstrated that increased apoptosis precedes calcification in SMC cultures and that these apoptotic bodies may act as nucleating structures for calcium crystal formation. Recent work by our group (14) also supports these data, as we found that administration of a direct apoptosis inhibitor can significantly decrease apoptosis in a dose-dependent manner. Thus, in the present study, the rather modest decrease in apoptosis of H1152-treated VICs on FB may be yet another factor contributing to the lack of inhibition of nodule formation found for this condition. Cell proliferation is also considered a hallmark feature of aortic valve disease (43), but a proliferation assay demonstrated that the decrease in nodule number caused by H1152 was not due to a decrease in VIC proliferation. In fact, in contrast to findings for SMCs (52), inhibition of Rho kinase activity in VIC cultures significantly increased cell proliferation. It is possible that this increase in proliferation contributed to the formation of larger nodules in H1152-treated cultures, although further experiments are needed to confirm this hypothesis.
The nodule-forming conditions created for this work were achieved via adsorption of different protein coatings to the culture substrates, so it is likely that integrin-initiated events underlie many of the observations made herein. Diseased valves present with altered ECM composition and arrangement (16, 31), so the use of ECM conditions to control VIC function has physiological relevance. However, a cursory examination of potential integrin involvement in these results revealed that a distinct correlation between the activation of specific integrins and calcification trends is unlikely (13). For instance, VICs bind to both FN and FB primarily through the α5β1-receptor (11, 26), yet the nodule formation and Rho inhibition results for VICs on FN and FB were strikingly different from each other. Regardless of specific integrin roles, however, the results in this work still clearly demonstrate that VIC nodule formation is strongly impacted by Rho pathway activity.
Although the findings herein provide evidence for a relationship between Rho pathway activity and VIC behaviors associated with valve calcification, there exist many limitations with respect to applying this work to understanding CAVD. As noted earlier, these experiments focused on the role of Rho signaling in VIC functions that may be indicative of calcification but are not directly equivalent to calcification. Moreover, although the formation of alizarin red S-positive nodules in VIC cultures is often characterized during in vitro investigations of factors involved in valve calcification (1, 2, 20, 47), it is not known whether the formation of these nodules in two-dimensional culture environments is indeed directly relevant to the complex formation of calcific nodules that occurs in native diseased valves. Finally, although the focus of this investigation was the Rho/ROCK pathway, there exist many other potential avenues by which the specific culture environments examined herein may be stimulating VIC differentiation and nodule formation. From our own work on the subject, it is known that MAPK/ERK signaling is also significantly elevated in these nodule-forming environments (14), wheras other factors, such as the sequestration of growth factors by the ECM or the differential production of cytokines that mediate VIC differentiation, are also likely to play a role in regulating VIC dysfunction.
In conclusion, the findings presented herein indicate that the Rho/ROCK signaling pathway plays a role in regulating the cellular events that are often associated with VIC calcification, including the differentiation of VICs into a myofibroblastic or osteoblastic phenotype. Blocking this signaling results in decreased nodule formation in VIC cultures, most likely via the inhibition of apoptosis and inhibition of VIC activation to a contractile, myofibroblast phenotype. Meanwhile, blocking the Rho pathway does not appear to inhibit calcification related to osteogenic mechanisms. Of course, the Rho pathway does not act in isolation. Many other pathways intersect with Rho, and other kinases work in parallel with ROCK to regulate contraction (60). However, despite this simplified view of valve calcification, it is hoped that these results will help us to better understand the etiology of valvular disease and contribute to the development of potential treatments.
GRANTS
This work was supported by provided by National Science Foundation CAREER Award CBET-0547374 and National Heart, Lung, and Blood Institute Grant 1-R01-HL 093281 (to K. S. Masters) and by a Herb Fellowship (to X. Gu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Footnotes
Supplemental Material for this article is available at the American Journal of Physiology-Heart and Circulatory Physiology website.
REFERENCES
- 1. Benton JA, Kern HB, Anseth KS. Substrate properties influence calcification in valvular interstitial cell culture. J Heart Valve Dis 17: 689–699, 2008 [PMC free article] [PubMed] [Google Scholar]
- 2. Benton JA, Kern HB, Leinwand LA, Mariner PD, Anseth KS. Statins block calcific nodule formation of valvular interstitial cells by inhibiting α-smooth muscle actin expression. Arterioscler Thromb Vasc Biol 29: 1950–1957, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caira FC, Stock SR, Gleason TG, McGee EC, Huang J, Bonow RO, Spelsberg TC, McCarthy PM, Rahimtoola SH, Rajamannan NM. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol 47: 1707–1712, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen JH, Yip CY, Sone ED, Simmons CA. Identification and characterization of aortic valve mesenchymal progenitor cells with robust osteogenic calcification potential. Am J Pathol 174: 1109–1119, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen NX, Chen X, O'Neill KD, Atkinson SJ, Moe SM. RhoA/Rho kinase (ROCK) alters fetuin-A uptake and regulates the calcification in bovine vascular smooth muscle cells (BVSMC). Am J Physiol Renal Physiol 299: F674–F680, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen S, Crawford M, Day RM, Briones VR, Leader JE, Jose PA, Lechleider RJ. RhoA modulates Smad signaling during transforming growth factor-beta-induced smooth muscle differentiation. J Biol Chem 281: 1765–1770, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chester AH, Taylor PM. Molecular and functional characteristics of heart-valve interstitial cells. Philos Trans R Soc Lond B Biol Sci 362: 1437–1443, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coleman ML, Olson MF. Rho GTPase signalling pathways in the morphological changes associated with apoptosis. Cell Death Differ 9: 493–504, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Croft DR, Coleman ML, Li S, Robertson D, Sullivan T, Stewart CL, Olson MF. Actin-myosin-based contraction is responsible for apoptotic nuclear disintegration. J Cell Biol 168: 245–255, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Croft DR, Olson MF. The Rho GTPase effector ROCK regulates cyclin A, cyclin D1, and p27Kip1 levels by distinct mechanisms. Mol Cell Biol 26: 4612–4627, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fayet C, Bendeck MP, Gotlieb AI. Cardiac valve interstitial cells secrete fibronectin and form fibrillar adhesions in response to injury. Cardiovasc Pathol 16: 203–211, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature 343: 425–430, 1990 [DOI] [PubMed] [Google Scholar]
- 13. Gu X, Masters KS. Regulation of valvular interstitial cell calcification by adhesive peptide sequences. J Biomed Mater Res A 93: 1620–1630, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gu X, Masters KS. Role of the MAPK/ERK pathway in valvular interstitial cell calcification. Am J Physiol Heart Circ Physiol 296: H1748–H1757, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hall A. Rho GTPases and the actin cytoskeleton. Science 279: 509–514, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Hinton RB, Jr, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, Yutzey KE. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res 98: 1431–1438, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Hinz B, Gabbiani G. Mechanisms of force generation and transmission by myofibroblasts. Curr Opin Biotechnol 14: 538–546, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Ikenoya M, Hidaka H, Hosoya T, Suzuki M, Yamamoto N, Sasaki Y. Inhibition of Rho-kinase-induced myristoylated alanine-rich C kinase substrate (MARCKS) phosphorylation in human neuronal cells by H-1152, a novel and specific Rho-kinase inhibitor. J Neurochem 81: 9–16, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, Narumiya S. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett 404: 118–124, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Jian B, Narula N, Li QY, Mohler ER, 3rd, Levy RJ. Progression of aortic valve stenosis: TGF-β1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann Thorac Surg 75: 456–465, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Jian B, Xu J, Connolly J, Savani RC, Narula N, Liang B, Levy RJ. Serotonin mechanisms in heart valve disease I: serotonin-induced up-regulation of transforming growth factor-β1 via G-protein signal transduction in aortic valve interstitial cells. Am J Pathol 161: 2111–2121, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johnson CM, Hanson MN, Helgeson SC. Porcine cardiac valvular subendothelial cells in culture: cell isolation and growth characteristics. J Mol Cell Cardiol 19: 1185–1193, 1987 [DOI] [PubMed] [Google Scholar]
- 23. Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Sugimoto T. Fasudil hydrochloride induces osteoblastic differentiation of stromal cell lines, C3H10T1/2 and ST2, via bone morphogenetic protein-2 expression. Endocr J 57: 415–421, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Kataoka C, Egashira K, Inoue S, Takemoto M, Ni W, Koyanagi M, Kitamoto S, Usui M, Kaibuchi K, Shimokawa H, Takeshita A. Important role of Rho-kinase in the pathogenesis of cardiovascular inflammation and remodeling induced by long-term blockade of nitric oxide synthesis in rats. Hypertension 39: 245–250, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Kizu A, Shioi A, Jono S, Koyama H, Okuno Y, Nishizawa Y. Statins inhibit in vitro calcification of human vascular smooth muscle cells induced by inflammatory mediators. J Cell Biochem 93: 1011–1019, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Latif N, Sarathchandra P, Taylor PM, Antoniw J, Yacoub MH. Molecules mediating cell-ECM and cell-cell communication in human heart valves. Cell Biochem Biophys 43: 275–287, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Liao JK, Seto M, Noma K. Rho kinase (ROCK) inhibitors. J Cardiovasc Pharmacol 50: 17–24, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu AC, Joag VR, Gotlieb AI. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol 171: 1407–1418, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mallat Z, Gojova A, Sauzeau V, Brun V, Silvestre JS, Esposito B, Merval R, Groux H, Loirand G, Tedgui A. Rho-associated protein kinase contributes to early atherosclerotic lesion formation in mice. Circ Res 93: 884–888, 2003 [DOI] [PubMed] [Google Scholar]
- 30. McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 6: 483–495, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Mohler ER., 3rd Mechanisms of aortic valve calcification. Am J Cardiol 94: 1396–1402, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Mohler ER, 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation 103: 1522–1528, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Monzack EL, Gu X, Masters KS. Efficacy of simvastatin treatment of valvular interstitial cells varies with the extracellular environment. Arterioscler Thromb Vasc Biol 29: 246–253, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nishimura J, Sakihara C, Zhou Y, Kanaide H. Expression of rho A and rho kinase mRNAs in porcine vascular smooth muscle. Biochem Biophys Res Commun 227: 750–754, 1996 [DOI] [PubMed] [Google Scholar]
- 35. O'Brien KD. Pathogenesis of calcific aortic valve disease: a disease process comes of age (and a good deal more). Arterioscler Thromb Vasc Biol 26: 1721–1728, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Olson MF. Applications for ROCK kinase inhibition. Curr Opin Cell Biol 20: 242–248, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Orlando KA, Pittman RN. Rho kinase regulates phagocytosis, surface expression of GlcNAc, and Golgi fragmentation of apoptotic PC12 cells. Exp Cell Res 312: 3298–3311, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Otto CM. Calcification of bicuspid aortic valves. Heart 88: 321–322, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pho M, Lee W, Watt DR, Laschinger C, Simmons CA, McCulloch CA. Cofilin is a marker of myofibroblast differentiation in cells from porcine aortic cardiac valves. Am J Physiol Heart Circ Physiol 294: H1767–H1778, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM, Weissberg PL. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ Res 87: 1055–1062, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Rabkin-Aikawa E, Farber M, Aikawa M, Schoen FJ. Dynamic and reversible changes of interstitial cell phenotype during remodeling of cardiac valves. J Heart Valve Dis 13: 841–847, 2004 [PubMed] [Google Scholar]
- 42. Rabkin-Aikawa E, Mayer JE, Jr, Schoen FJ. Heart valve regeneration. Adv Biochem Eng Biotechnol 94: 141–179, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Rajamannan NM. Calcific aortic stenosis: lessons learned from experimental and clinical studies. Arterioscler Thromb Vasc Biol 29: 162–168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg T. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation 107: 2181–2184, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ren J, Fang CX. Small guanine nucleotide-binding protein Rho and myocardial function. Acta Pharmacol Sin 26: 279–285, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Ridley AJ. Rho GTPases and cell migration. J Cell Sci 114: 2713–2722, 2001 [DOI] [PubMed] [Google Scholar]
- 47. Rodriguez KJ, Masters KS. Regulation of valvular interstitial cell calcification by components of the extracellular matrix. J Biomed Mater Res A 90: 1043–1053, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rolfe BE, Worth NF, World CJ, Campbell JH, Campbell GR. Rho and vascular disease. Atherosclerosis 183: 1–16, 2005 [DOI] [PubMed] [Google Scholar]
- 49. Sahai E, Ishizaki T, Narumiya S, Treisman R. Transformation mediated by RhoA requires activity of ROCK kinases. Curr Biol 9: 136–145, 1999 [DOI] [PubMed] [Google Scholar]
- 50. Sahai E, Olson MF, Marshall CJ. Cross-talk between Ras and Rho signalling pathways in transformation favours proliferation and increased motility. EMBO J 20: 755–766, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Saito E, Wachi H, Sato F, Seyama Y. 7-Ketocholesterol, a major oxysterol, promotes Pi-induced vascular calcification in cultured smooth muscle cells. J Atheroscler Thromb 15: 130–137, 2008 [DOI] [PubMed] [Google Scholar]
- 52. Sawada N, Itoh H, Ueyama K, Yamashita J, Doi K, Chun TH, Inoue M, Masatsugu K, Saito T, Fukunaga Y, Sakaguchi S, Arai H, Ohno N, Komeda M, Nakao K. Inhibition of rho-associated kinase results in suppression of neointimal formation of balloon-injured arteries. Circulation 101: 2030–2033, 2000 [DOI] [PubMed] [Google Scholar]
- 53. Schoen FJ. Evolving concepts of cardiac valve dynamics: the continuum of development, functional structure, pathobiology, and tissue engineering. Circulation 118: 1864–1880, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Shimokawa H, Takeshita A. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol 25: 1767–1775, 2005 [DOI] [PubMed] [Google Scholar]
- 55. Stahle M, Veit C, Bachfischer U, Schierling K, Skripczynski B, Hall A, Gierschik P, Giehl K. Mechanisms in LPA-induced tumor cell migration: critical role of phosphorylated ERK. J Cell Sci 116: 3835–3846, 2003 [DOI] [PubMed] [Google Scholar]
- 56. Stephens EH, Grande-Allen KJ. Age-related changes in collagen synthesis and turnover in porcine heart valves. J Heart Valve Dis 16: 672–682, 2007 [PubMed] [Google Scholar]
- 57. Suvorova EI, Buffat PA. Pathological mineralization of cardiac valves: causes and mechanism. J Long Term Eff Med Implants 15: 355–368, 2005 [DOI] [PubMed] [Google Scholar]
- 58. Taylor PM, Batten P, Brand NJ, Thomas PS, Yacoub MH. The cardiac valve interstitial cell. Int J Biochem Cell Biol 35: 113–118, 2003 [DOI] [PubMed] [Google Scholar]
- 59. Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3: 349–363, 2002 [DOI] [PubMed] [Google Scholar]
- 60. Van Eyk JE, Arrell DK, Foster DB, Strauss JD, Heinonen TY, Furmaniak-Kazmierczak E, Cote GP, Mak AS. Different molecular mechanisms for Rho family GTPase-dependent, Ca2+-independent contraction of smooth muscle. J Biol Chem 273: 23433–23439, 1998 [DOI] [PubMed] [Google Scholar]
- 61. Walker GA, Masters KS, Shah DN, Anseth KS, Leinwand LA. Valvular myofibroblast activation by transforming growth factor-β: implications for pathological extracellular matrix remodeling in heart valve disease. Circ Res 95: 253–260, 2004 [DOI] [PubMed] [Google Scholar]
- 62. Wang N, Wang X, Xing C, Sun B, Yu X, Hu J, Liu J, Zeng M, Xiong M, Zhou S, Yang J. Role of TGF-β1 in bone matrix production in vascular smooth muscle cells induced by a high-phosphate environment. Nephron Exp Nephrol 115: e60–e68, 2010 [DOI] [PubMed] [Google Scholar]
- 63. Warren BA, Yong JL. Calcification of the aortic valve: its progression and grading. Pathology 29: 360–368, 1997 [DOI] [PubMed] [Google Scholar]
- 64. Watson KE, Bostrom K, Ravindranath R, Lam T, Norton B, Demer LL. TGF-β1 and 25-hydroxycholesterol stimulate osteoblast-like vascular cells to calcify. J Clin Invest 93: 2106–2113, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Willems IE, Havenith MG, Smits JF, Daemen MJ. Structural alterations in heart valves during left ventricular pressure overload in the rat. Lab Invest 71: 127–133, 1994 [PubMed] [Google Scholar]
- 66. Worth NF, Rolfe BE, Song J, Campbell GR. Vascular smooth muscle cell phenotypic modulation in culture is associated with reorganisation of contractile and cytoskeletal proteins. Cell Motil Cytoskeleton 49: 130–145, 2001 [DOI] [PubMed] [Google Scholar]
- 67. Zhao XH, Laschinger C, Arora P, Szaszi K, Kapus A, McCulloch CA. Force activates smooth muscle α-actin promoter activity through the Rho signaling pathway. J Cell Sci 120: 1801–1809, 2007 [DOI] [PubMed] [Google Scholar]