Abstract
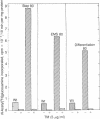
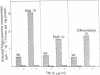
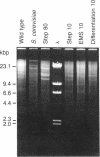
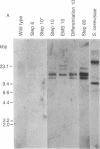
Tunicamycin at 10 micrograms/ml inhibits the growth and infectivity of the parasitic protozoan Leishmania mexicana amazonensis. Tunicamycin-resistant variants of this parasite were produced by gradual acclimatization of cells to increasing concentrations of the drug up to 80 micrograms/ml and a single-step selection of ethyl methanesulfonate-pretreated or differentiating leishmanias with the drug at 10 micrograms/ml. Prolonged exposure to the drug increases stability of drug resistance of those resistant to 10 micrograms/ml. Tunicamycin-resistant cells contain amplified DNA, which hybridizes in proportion to the cells' degree of drug resistance with Alg 7, a cloned DNA probe apparently encoding yeast N-acetylglucosaminyltransferase. This enzyme from all variants remained sensitive to inhibition by tunicamycin, but its specific activity was up to 15-fold higher than that of the wild type. Thus, amplification of the gene encoding this enzyme appears to result in its overproduction in the variants, accounting for their resistance to tunicamycin. The tunicamycin-resistant cells are more virulent to mice than their parental wild type. Thus, leishmanial virulence may be related to amplification or expression of gene(s) encoding enzymes involved in the regulation of N-glycosylation of parasite proteins.
Full text
PDF

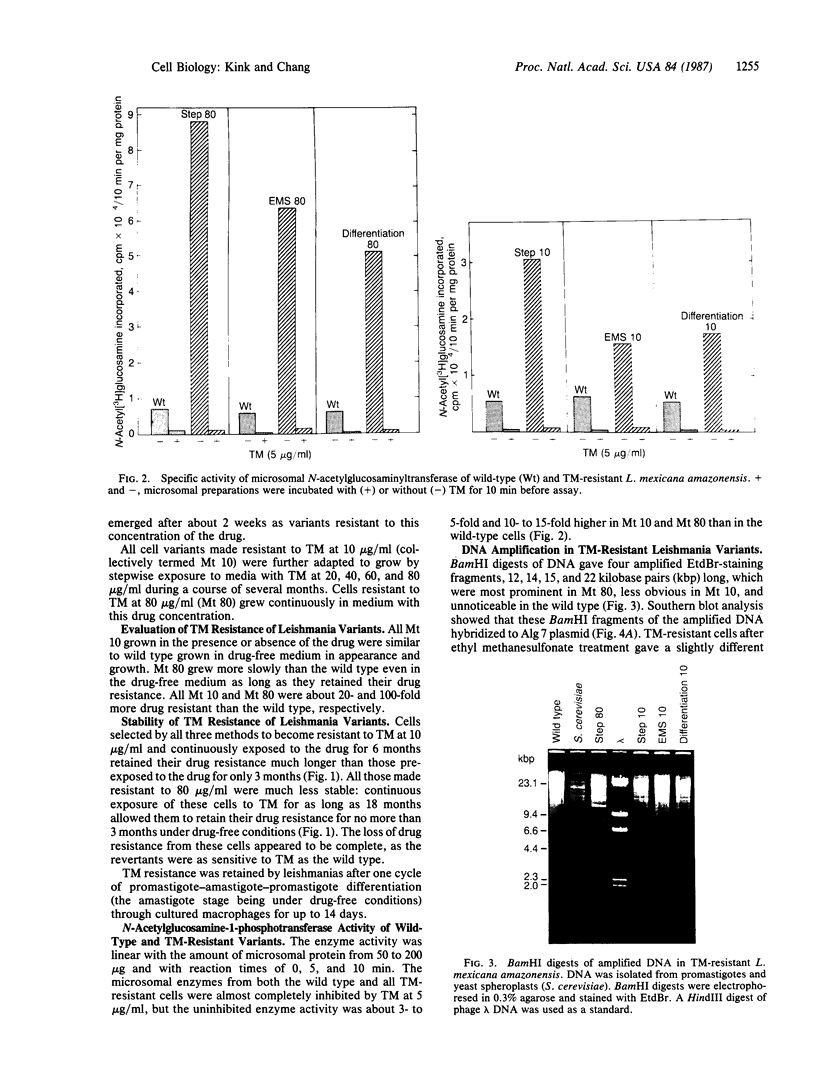


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes G., Hansen W. J., Holcomb C. L., Rine J. Asparagine-linked glycosylation in Saccharomyces cerevisiae: genetic analysis of an early step. Mol Cell Biol. 1984 Nov;4(11):2381–2388. doi: 10.1128/mcb.4.11.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley S. M., Coderre J. A., Santi D. V., Schimke R. T. Unstable DNA amplifications in methotrexate-resistant Leishmania consist of extrachromosomal circles which relocalize during stabilization. Cell. 1984 Sep;38(2):431–439. doi: 10.1016/0092-8674(84)90498-7. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. Cancer genes come of age. Cell. 1983 Apr;32(4):1018–1020. doi: 10.1016/0092-8674(83)90284-2. [DOI] [PubMed] [Google Scholar]
- Chang K. P., Fong D. Cell biology of host-parasite membrane interactions in leishmaniasis. Ciba Found Symp. 1983;99:113–137. doi: 10.1002/9780470720806.ch7. [DOI] [PubMed] [Google Scholar]
- Chang K. P. Human cutaneous lieshmania in a mouse macrophage line: propagation and isolation of intracellular parasites. Science. 1980 Sep 12;209(4462):1240–1242. doi: 10.1126/science.7403880. [DOI] [PubMed] [Google Scholar]
- Coderre J. A., Beverley S. M., Schimke R. T., Santi D. V. Overproduction of a bifunctional thymidylate synthetase-dihydrofolate reductase and DNA amplification in methotrexate-resistant Leishmania tropica. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2132–2136. doi: 10.1073/pnas.80.8.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscuolo B. A., Krag S. S. Selection of tunicamycin-resistant Chinese hamster ovary cells with increased N-acetylglucosaminyltransferase activity. J Cell Biol. 1982 Sep;94(3):586–591. doi: 10.1083/jcb.94.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagger F., Ayesta C., Hernandez A. G. The effect of tunicamycin on Leishmania braziliensis cell growth, cell morphology and ultrastructure. Biol Cell. 1984;50(2):173–189. doi: 10.1111/j.1768-322x.1984.tb00264.x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fong D., Chang K. P. Tubulin biosynthesis in the developmental cycle of a parasitic protozoan, Leishmania mexicana: changes during differentiation of motile and nonmotile stages. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7624–7628. doi: 10.1073/pnas.78.12.7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin J. L., Milbrandt J. D., Heintz N. H., Azizkhan J. C. DNA sequence amplification in mammalian cells. Int Rev Cytol. 1984;90:31–82. doi: 10.1016/s0074-7696(08)61487-4. [DOI] [PubMed] [Google Scholar]
- Hernández A. G. Leishmanial excreted factors and their possible biological role. Ciba Found Symp. 1983;99:138–156. [PubMed] [Google Scholar]
- McKee E. E., Poyton R. O. Mitochondrial gene expression in saccharomyces cerevisiae. I. Optimal conditions for protein synthesis in isolated mitochondria. J Biol Chem. 1984 Jul 25;259(14):9320–9331. [PubMed] [Google Scholar]
- Nolan T. J., Farrell J. P. Inhibition of in vivo and in vitro infectivity of Leishmania donovani by tunicamycin. Mol Biochem Parasitol. 1985 Aug;16(2):127–135. doi: 10.1016/0166-6851(85)90081-7. [DOI] [PubMed] [Google Scholar]
- Olden K., Bernard B. A., White S. L., Parent J. B. Function of the carbohydrate moieties of glycoproteins. J Cell Biochem. 1982;18(3):313–335. doi: 10.1002/jcb.1982.240180306. [DOI] [PubMed] [Google Scholar]
- Pall M. L. Gene-amplification model of carcinogenesis. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2465–2468. doi: 10.1073/pnas.78.4.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rine J., Hansen W., Hardeman E., Davis R. W. Targeted selection of recombinant clones through gene dosage effects. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6750–6754. doi: 10.1073/pnas.80.22.6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovis L., Baekkeskov S. Sub-cellular fractionation of Trypanosoma brucei. Isolation and characterization of plasma membranes. Parasitology. 1980 Jun;80(3):507–524. doi: 10.1017/s0031182000000974. [DOI] [PubMed] [Google Scholar]
- Rovis L., Dube S. Identification and characterisation of two N-acetylglucosaminyltransferases associated with Trypanosoma Brucei microsomes. Mol Biochem Parasitol. 1982 Mar;5(3):173–187. doi: 10.1016/0166-6851(82)90019-6. [DOI] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured animal cells. Cell. 1984 Jul;37(3):705–713. doi: 10.1016/0092-8674(84)90406-9. [DOI] [PubMed] [Google Scholar]
- Schwarz R. T., Datema R. The lipid pathway of protein glycosylation and its inhibitors: the biological significance of protein-bound carbohydrates. Adv Carbohydr Chem Biochem. 1982;40:287–379. doi: 10.1016/s0065-2318(08)60111-0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Wahl G. M. Gene amplification. Annu Rev Biochem. 1984;53:447–491. doi: 10.1146/annurev.bi.53.070184.002311. [DOI] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Verma A. K., Raizada M. K., Schutzbach J. S. Formation of alpha-1,2-mannosyl-mannose by an enzyme preparation from rabbit liver. J Biol Chem. 1977 Oct 25;252(20):7235–7242. [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]