Abstract
Mutations in cardiac troponin T (cTnT), Δ160E and R92Q, have been linked to familial hypertrophic cardiomyopathy (FHC), and some studies have indicated that these mutations can lead to a high incidence of sudden cardiac death in the relative absence of significant ventricular hypertrophy. Alterations in autonomic function have been documented in patients with hypertrophic cardiomyopathy. We hypothesize that alterations in autonomic function may contribute to mutation-specific clinical phenotypes in cTnT-related FHC. Heart rate (HR) variability (HRV) has been used to assess autonomic function from an electrocardiograph. Nontransgenic, Δ160E, or R92Q mice were implanted with radiofrequency transmitters to obtain continuous electrocardiograph recordings during 24-h baseline and 30-min recordings after β-adrenergic receptor drug injections. Although Δ160E mice did not differ from nontransgenic mice for any 24-h HRV measurements, R92Q mice had impaired HR regulation, as measured by a decrease in the SD of the R-R interval, a decrease in the low frequency-to-high frequency ratio, a decrease in normalized low frequency, and an increase in normalized high frequency. β-Adrenergic receptor density measurements and HRV analysis after drug injections did not reveal any significant differences for Δ160E or R92Q mice versus nontransgenic mice. Arrhythmia analysis revealed both an increased incidence of heart block in R92Q mice at baseline and frequency of premature ventricular contractions after isoproterenol injections in Δ160E and R92Q mice. In addition, Δ160E and R92Q mice exhibited a prolonged P duration after drug injections. Therefore, between two independent and clinically severe cTnT mutations within the same functional domain, only R92Q mice exhibited altered autonomic function, whereas both mutations demonstrated abnormalities in conduction and ventricular ectopy.
Keywords: sudden cardiac death, telemetry, β-adrenergic receptors
naturally occurring mutations in cardiac troponin T (cTnT) are associated with familial hypertrophic cardiomyopathy (FHC), an autosomal dominant primary myocardial disorder (20). FHC patients with the cTnT missense mutation Arg92Gln (R92Q) or an in-frame deletion of a single Glu residue (Δ160E) in the TNT1 tail domain have been reported to exhibit significant degrees of sudden cardiac death, often in the absence of massive left ventricular hypertrophy or precedent symptoms (37). Transgenic (TG) murine models with the Δ160E and R92Q cTnT mutations that have been generated in our laboratory recapitulate many aspects of human FHC and have allowed us to longitudinally study these specific FHC mutations in depth (3, 4, 10, 14, 21, 31). Although some phenotypic similarities exist between Δ160E and R92Q, including diastolic dysfunction, a lack of overt cardiac hypertrophy, and cardiomyocyte disarray, the mutations are at opposite ends of the TNT1 tail domain and have been postulated to cause disease via distinct molecular mechanisms (24).
It is unclear how mutations in cardiac sarcomeric proteins and the resulting pathogenic process directly contribute to sudden cardiac death; however, alterations in autonomic function have been proposed as a potential trigger (26). Autonomic function can be assessed using noninvasive Holter monitoring to determine heart rate (HR) variability (HRV) from an ECG. Impaired autonomic function assessed via a decrease in HRV has been demonstrated to be an independent risk factor in both patients with heart failure and myocardial infarction (23). One clinical finding observed in FHC patients that may suggest autonomic dysfunction is an abnormal blood pressure response after exercise and is represented by a failure to appropriately increase blood pressure or a paradoxical drop in blood pressure (28). Heradien et al. (11) recently found that patients with R92W cTnT mutations were predisposed to an abnormal blood pressure response to exercise that may be due to vagal enhancement. Moreover, changes in autonomic function have been previously reported in patients with hypertrophic cardiomyopathy (HCM); however, the authors of these studies found limited value with HRV analysis of this patient population (6, 8, 22, 34, 40). Addressing the obstacles that were faced in HCM studies may help elucidate the potential use of HRV analysis to facilitate risk stratification in patients with FHC.
Our previous characterization of our two TG murine models with the independent cTnT mutations R92Q, and Δ160E, suggested a possible mutation-specific alteration in HR regulation by the autonomic nervous system. The present study was conducted to determine if either of these mutations in cTnT alters autonomic function, which can be assessed from HRV analysis. In addition, we measured β-adrenergic receptor (β-AR) density, measured the response to β-AR drugs in these mice, assessed arrhythmogenesis, and measured ECG parameters to determine possible mechanisms for any changes in HR regulation. Assessment of autonomic function in Δ160E and R92Q mice may help establish a link between genotype and phenotype using noninvasive HR monitoring and may provide a potential prognostic screen in patients with FHC.
METHODS
Study animals.
Δ160E and R92Q mice were generated as previously described and compared against non-TG littermates (4, 31). Δ160E and R92Q were bred on a congenic C57BL/6 background. The Δ160E TG line expressed 70% of its total cTnT as the mutant form, whereas the R92Q TG line had 67% cTnT replacement. To eliminate the potential effect of sex, only male mice were studied for each genotype. Twenty-nine 5 ± 0.5-mo-old mice (11 non-TG, 10 Δ160E, and 8 R92Q mice) weighing 27 ± 2 g at the time of surgery were housed in individual cages at 24°C in 12:12-h light-dark cycles in full compliance with the Public Health Service animal welfare policy and the American Association for the Accreditation of Laboratory Animal Care. The animal research protocol was approved by the Albert Einstein College of Medicine Institute for Animal Studies.
Animal preparation and surgery.
Long-term ECG analysis in conscious, ambulatory mice was obtained with a wireless radiofrequency transmitter (model ETA-F20, Data Sciences, St. Paul, MN) that was implanted in the peritoneal cavity using sterile techniques. Mice were anesthetized with 1.5% inhaled isoflurane (Baxter Healthcare, Deerfield, IL) for the duration of the surgery. A midline skin incision was made on the ventral abdomen, and a smaller incision was made on the right pectoral region. A subcutaneous tunnel from the abdominal incision to the pectoral incision was generated upon removing a trochar from its sleeve to feed through the anodal lead. To generate an ECG lead II configuration, the anodal lead was sutured on the right pectoral muscle, and the cathodal lead was sutured near the apex of the heart to the left of the xyphoid process. The 3.9-g transmitter was inserted into the abdominal cavity and sutured to the abdominal muscles to anchor it in place. The skin incisions were sutured, and a warming lamp was used to maintain body temperature for recovery.
Study protocol.
ECG signal recordings began 8 ± 2 days postsurgery. Mice had unrestricted access to food and water throughout the recordings. Twenty-four-hour baseline recordings of undisturbed mice transpired from 7 PM to 7 PM. To study the effects of various drugs, intraperitoneal injections of 0.9% saline (IVX Animal Health, St. Joseph, MO), 1.0 mg/kg body wt isoproterenol (Sigma-Aldrich, St. Louis, MO) for β-AR stimulation, and 1.0 mg/kg body wt propranolol (Sigma-Aldrich) for β-AR challenge were administered on consecutive but separate days. Drug dosages were similar to those in previous studies (9, 15, 30). A 5-min equilibration period after the injection was given, and the immediate 30-min segment of the ECG signal was used for drug analysis.
Data acquisition and analysis.
ECG signals were recorded using a telemetry receiver (Data Sciences) and digitized with 12-bit precision without a signal filter at a sampling rate of 2 kHz. The Data Sciences data files were then exported and converted to Chart data files and analyzed using HRV module version 1.2 of Chart Pro software version 6.1.2 (AD Instruments, Colorado Springs, CO). Ectopic beats were defined as those having an R-R interval shorter than 70 ms or longer than 150 ms and were excluded from analysis. Excluded beats were not replaced by artificial or interpolated beats. HRV measurement and analysis were carried out based on recommendations from previously published guidelines (9, 32). Measurements of the ECG parameters, P-R interval, P duration, and QRS interval, along with arrhythmia analysis were carried out using ECG module version 2.2 of Chart Pro software. One-second averages of a 10-s segment taken at the beginning of the 30-min postinjection recordings were used for the ECG parameter measurements. A 1-h segment from the 24-h baseline recording beginning at 10 PM and the 30-min postinjection recordings were analyzed for arrhythmias.
Time-domain analysis.
In the time domain, mean HR, mean R-R interval (RRmean), SD of all normal R-R intervals (SDNN), and SD of averages of normal R-R intervals in all 2-min segments of the entire recording (SDANN) were calculated.
Frequency-domain analysis.
In the frequency domain, power spectral density was computed by applying fast Fourier transformation (FFT). FFT was calculated using 512 points and half-overlap with a Hanning window. Frequency band cutoffs were set at 1.5–5 Hz for high frequency (HF), 0.15–1.5 Hz for low frequency (LF), and 0–0.15 for very LF (VLF) as well as 0–5.0 Hz for total power (TP), as recommended by Thireau et al. (32). Normalized HF (nHF) and LF (nLF) were calculated by dividing either LF or HF by the difference between TP and VLF and multiplying by 100 to account for differences in TP (9, 31a). The ratio of LF to HF is represented as LF/HF.
Membrane preparation.
Protocols for myocardial sarcolemmal membrane preparation and β-AR quantification were adapted from protocols by Wolf et al. (38). To generate membrane preparations, hearts were flash frozen after they were extracted and the atria trimmed away. Hearts were then homogenized in ice-cold homogenization buffer [25 mM Tris·HCl (pH 7.4), 5 mM EDTA, 2 mg/ml leupeptin, and 2 mg/ml aprotinin, Sigma-Aldrich] and incubated on ice for 20 min. To remove large tissue fragments and cellular organelles, the homogenate was centrifuged at 750 g for 5 min at 4°C. The supernatant was recentrifuged at 36,600 g for 30 min at 4°C, and the pellet was resuspended in membrane resuspension buffer [75 mM Tris·HCl (pH 7.4), 4 mM EDTA, and 12.5 mM MgCl2, Sigma-Aldrich] to a concentration of 1 mg/ml.
β-AR density binding assay.
Myocardial membranes (20 μg) from 12 mice (5 non-TG, 3 Δ160E, and 4 R92Q mice) were incubated for 60 min at 37°C with 125I-labeled cyanopindolol (ICYP; Perkin-Elmer, Waltham, MA, 80 pM) in a final volume of 400 μl binding buffer [10 mM Tris·HCl (pH 7.4) and 5 mM EDTA, Sigma-Aldrich]. Nonspecific binding of ICYP was measured by adding 200 μM propranolol. The entire assay volume was poured over glass fiber filters (Fisher Scientific, Waltham, MA), and rapid vacuum filtration was used to separate ICYP bound to membrane protein from free, unbound ICYP. Radioactivity in the membranes trapped by the filters was measured in an automatic γ-counter (Wizard 1470, Perkin-Elmer).
Statistics.
Comparisons for all 24-h analysis were done by an unpaired Student's two-tailed t-test except for 1-h baseline heart block and premature ventricular contraction analyses, which were done by a Mann-Whitney test. Comparisons for drug experiments were all done by two-way ANOVA with Bonferroni post hoc analysis (Graphpad Prism version 5.01, La Jolla, CA), and additional post hoc analysis was performed using the QuickCalcs web calculator (www.graphpad.com). Data are expressed as means ± SE. Statistical significance was accepted at the level of P < 0.05.
RESULTS
Twenty-four-hour HR regulation.
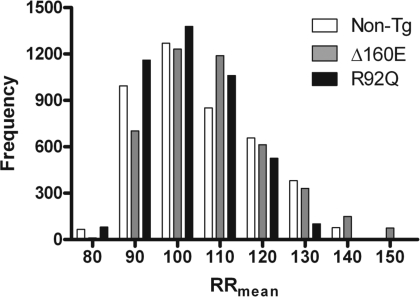
To determine the baseline physiological effects of Δ160E and R92Q cTnT mutations on HR parameters, mice were observed for an entire 24-h period in an undisturbed environment. Δ160E mice showed a trend toward decreased HR (566 ± 10 beats/min) and increased RRmean (107 ± 2 ms), whereas R92Q mice showed the opposite trend with increased HR (591 ± 12 beats/min) and decreased RRmean (102 ± 2 ms) compared with non-TG mice (HR: 579 ± 12 beats/min and RRmean: 105 ± 2 ms). To observe macroscopic HRV among the mice studied, histogram analyses of 2-min averages of RRmean over the same 24-h period showed an increased SD for Δ160E mice but showed a decreased SD for R92Q mice compared with non-TG mice (Fig. 1).
Fig. 1.
Histograms of mean R-R intervals (RRmean) demonstrating differences in macroscopic heart rate (HR) variabilitiy (HRV) of 24-h baseline recordings calculated in 2-min average segments. n = 6 nontransgenic (non-TG), Δ160E, and R92Q mice.
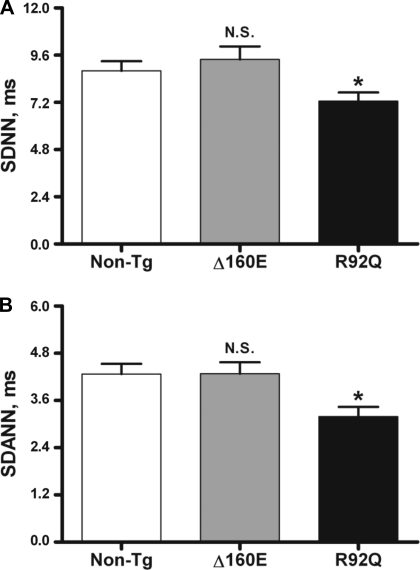
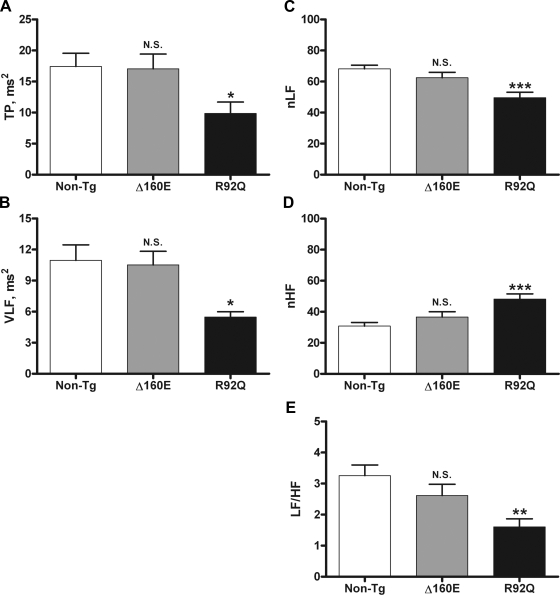
To quantitatively assess beat-to-beat cardiovascular control, time- and frequency-domain measures of HRV were calculated from the R-R interval. Of the time-domain measures of HRV we looked at, SDNN represents the gold standard measurement of HRV, whereas SDANN is used to measure short-term HRV. SDNN and SDANN did not differ between Δ160E (9.4 ± 0.7 and 4.3 ± 0.3 ms, respectively) and non-TG (8.8 ± 0.5 and 4.3 ± 0.3 ms, respectively) mice, whereas R92Q mice had significantly decreased SDNN (7.3 ± 0.4 ms, P < 0.05) and SDANN (3.2 ± 0.2 ms, P < 0.05; Fig. 2). When evaluating frequency-domain measures of HRV, LF is thought to have contributions from both vagal and sympathetic inputs, whereas HF is thought to represent vagal control to the heart; thus, LF/HF is thought to assess the sympathovagal balance. LF and HF were normalized to account for effect that changes in TP have on LF and HF (31a). TP, VLF, LF/HF, nLF, and nHF did not differ between Δ160E (17 ± 2 ms2, 10.5 ± 1 ms2, 2.6 ± 0.4, 62 ± 3, and 37 ± 3, respectively) and non-TG (17 ± 2 ms2, 11 ± 1 ms2, 3.3 ± 0.3, 68 ± 2, and 31 ± 2, respectively) mice. However, R92Q mice had lower TP (10 ± 2 ms2, P < 0.05), VLF (5.5 ± 0.5 ms2, P < 0.05), LF/HF (1.6 ± 0.3, P < 0.01), and nLF (50 ± 3, P < 0.001) and higher nHF (48 ± 3, P < 0.001) compared with non-TG mice (Fig. 3).
Fig. 2.
Time-domain measures of HRV of 24-h baseline recordings. A: SD of all normal R-R intervals (SDNN). B: SD of averages of normal R-R intervals in all 2-min segments of the entire recording (SDANN). Values are means ± SE. N.S., not significant. *P < 0.05 vs. non-TG mice.
Fig. 3.
Frequency-domain measures of HRV of 24-h baseline recordings. A: total power (TP; 0.0–5 Hz). B: very low frequency (VLF; 0.0–0.15 Hz). C: normalized low frequency (nLF). D: normalized high frequency (nHF). E: ratio of low frequency (0.15–1.5 Hz) to high frequency (1.5–5 Hz) (LF/HF). Values are means ± SE. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. non-TG mice.
β-AR density.
Alterations in the autonomic nervous system can lead to alterations in the β-adrenergic signaling cascade, frequently affecting the quantity and distribution of β-ARs on the sarcolemma. To determine if the alterations in the autonomic nervous system that we observed in 24-h baseline recordings resulted in alterations to the β-adrenergic signaling cascade, β-AR density was characterized in myocardial membranes of non-TG, Δ160E, and R92Q mice. Neither Δ160E (53 ± 6 fmol/mg) nor R92Q (63 ± 3 fmol/mg) mice demonstrated a significant change in β-AR density compared with non-TG mice (50 ± 5 fmol/mg).
β-AR drug effects.
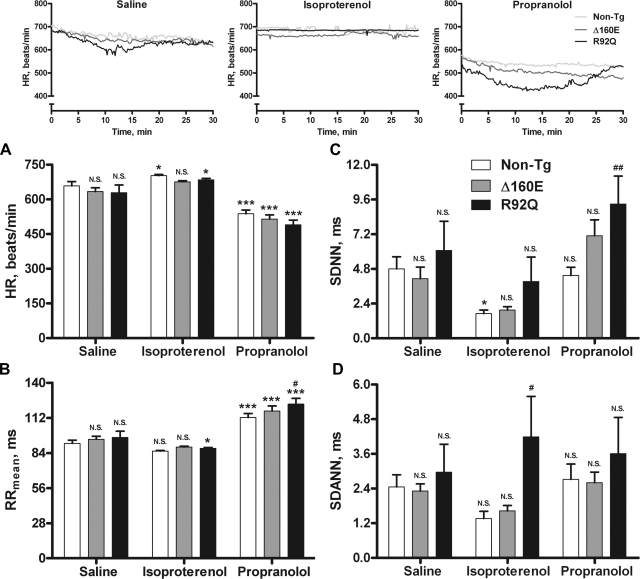
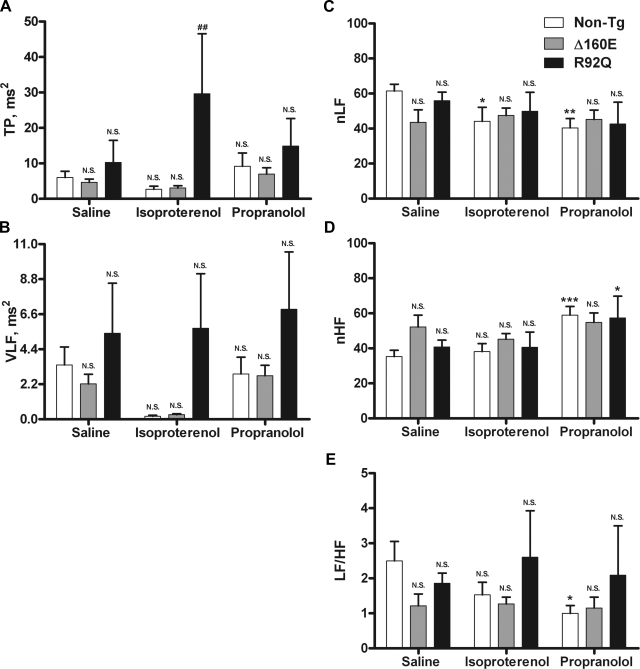
The inset in Fig. 4 shows representative HR tracings for non-TG, Δ160E, and R92Q mice after saline, isoproterenol, and propranolol injections. Each line represents the average HR value for the entire 30-min recording of all animals within a group, illustrating the response to each drug by non-TG, Δ160E, and R92Q mice. Given that the β-adrenergic system is involved in altering HR, transient catecholamine release caused by handling of the animal results in a momentary activation of β-ARs and was assessed via saline sham injections. Continuous activation of β-ARs was achieved via isoproterenol injections, as shown in the HR tracings (Fig. 4, inset). After isoproterenol injections, non-TG mice showed a significant increase in HR with significant decreases in SDNN and nLF, Δ160E mice did not show any significant changes, and R92Q mice showed a significant increase in HR with a significant decrease in RRmean compared with saline injections (Figs. 4 and 5). Compared with non-TG mice, Δ160E mice did not show any significant changes, but R92Q mice showed significant increases in SDANN and TP (Figs. 4 and 5). Inactivation of β-ARs was achieved via propranolol injections, as shown by the reduced HR in the representative tracings (Fig. 4, inset). After propranolol injections, non-TG mice showed a significant increase in RRmean with significant decreases in HR, nLF, and LF/HF, Δ160E mice showed a significant increase in RRmean with a significant decrease in HR, and R92Q mice showed significant increases in RRmean and nHF with a significant decrease in HR compared with saline injections (Figs. 4 and 5). Compared with non-TG mice, Δ160E mice did not show any significant changes, but R92Q mice showed significant increases in RRmean and SDNN (Figs. 4 and 5).
Fig. 4.
HR and time measures of HRV after saline, isoproterenol, and propranolol injections (n = 8 non-TG, 6 Δ160E, and 5 R92Q mice). A: HR. B: RRmean. C: SDNN. D: SDANN. The inset shows averaged representative HR recordings of non-TG, Δ160E, or R92Q mice over the 30-min segment after each saline, isoproterenol, or propranolol injection. Values are means ± SE. *P < 0.05 and ***P < 0.001 vs. the saline counterpart; #P < 0.05 and ##P < 0.01 vs. non-TG mice within drug groups.
Fig. 5.
Frequency-domain measures of HRV after saline, isoproterenol, and propranolol injections. A: TP. B: VLF. C: nLF. D: nHF. E: LF/HF. Values are means ± SE. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. the saline counterpart; ##P < 0.01 vs. non-TG mice within drug groups.
Arrhythmia detection and ECG parameters.
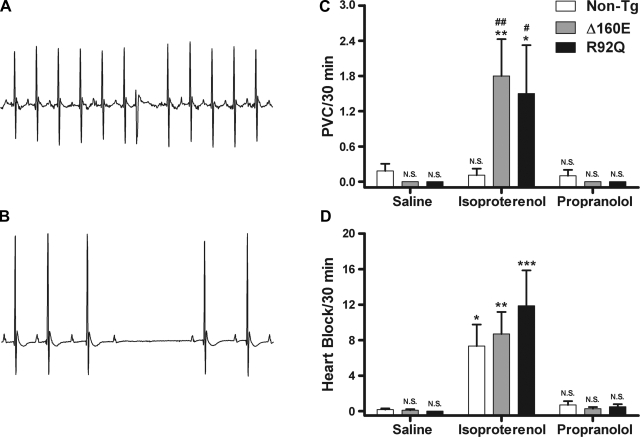
Arrhythmias have been linked to sudden cardiac death in FHC patients. During 1 h of arrhythmia detection in the 24-h baseline recordings, there was no incidence of 2° atrioventricular heart block for non-TG or Δ160E mice; however, R92Q mice showed a significant incidence of heart block compared with non-TG mice (1.5 ± 0.5 vs. 0 heart block/h, P < 0.01). Premature ventricular contractions (PVCs) were sporadically observed at similar rates between non-TG, Δ160E, and R92Q mice (0.3 ± 0.2, 0.1 ± 0.1, and 0.3 ± 0.3 PVCs/h, respectively; Δ160E and R92Q mice were not significant vs. non-TG mice). Representative tracings of heart block and a PVC are shown in Fig. 6, A and B. In the 30-min ECG recordings after saline and propranolol injections, there were sporadic PVCs and/or occasions of heart block between non-TG, Δ160E, and R92Q mice that occurred at similar rates for all three groups (Fig. 6, C and D). However, after isoproterenol injections, all three groups exhibited an increased frequency of PVCs and heart block. Specifically, both Δ160E and R92Q mice showed a significant incidence of PVC events compared with non-TG mice and compared with saline injection alone (Fig. 6C). non-TG, Δ160E, and R92Q mice also showed a significant incidence of heart block events compared with saline injection alone (Fig. 6D). The P-R interval (atrioventricular conduction), P duration (atrial contraction), and QRS interval (ventricular contraction) were measured after injections of saline, isoproterenol, and propranolol. There was a significant increase in the P-R interval after the propranolol injection for non-TG, Δ160E, and R92Q mice compared with the saline injection (Table 1). There was a significant increase in P duration for Δ160E mice compared with non-TG mice after the saline and propranolol injections (Table 1). There was also a significant increase in the P duration for R92Q mice compared with non-TG mice after the isoproterenol and propranolol injections. The only difference observed for the QRS interval was a significant increase in duration after the isoproterenol injection compared with the saline injection for R92Q mice (Table 1).
Fig. 6.
A and B: representative 1-s ECG traces of a premature ventricular contraction (PVC; A) and heart block (B). C and D: PVC and heart block incidence rates after saline, isoproterenol, and propranolol injections. C: PVC/30 min; D: heart block/30 min. Values are means ± SE. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. the saline counterpart; #P < 0.05 and ##P < 0.01 vs. non-TG mice within drug groups.
Table 1.
Effect of drug injections on ECG parameters
| P-R Interval | P Duration | QRS Interval | |
|---|---|---|---|
| non-TG mice | |||
| Saline | 32.5 ± 0.4 | 8.7 ± 0.2 | 10.8 ± 0.2 |
| Isoproterenol | 32.0 ± 0.5 (NS) | 9.0 ± 0.2 (NS) | 11.3 ± 0.2 (NS) |
| Propranolol | 35.1 ± 0.5† | 9.1 ± 0.4 (NS) | 10.7 ± 0.3 (NS) |
| Δ160E mice | |||
| Saline | 32.2 ± 0.4 (NS) | 10.3 ± 0.4‡ | 10.1 ± 0.2 (NS) |
| Isoproterenol | 31.8 ± 1.3 (NS) | 10.3 ± 0.7 (NS) | 10.7 ± 0.1 (NS) |
| Propranolol | 34.3 ± 0.7* | 10.7 ± 0.5‡ | 10.1 ± 0.2 (NS) |
| R92Q mice | |||
| Saline | 33.9 ± 0.6 (NS) | 10.3 ± 0.3 (NS) | 10.3 ± 0.2 (NS) |
| Isoproterenol | 34.4 ± 1.1 (NS) | 11.2 ± 0.7§ | 11.1 ± 0.3* |
| Propranolol | 36.6 ± 0.9* | 10.8 ± 0.4‡ | 10.1 ± 0.3 (NS) |
All values are means ± SE (in ms)
n = 8 nontransgenic (non-TG), 6 Δ160E, and 5 R92Q mice. NS, not significant.
P < 0.05 and
P < 0.01 vs. the saline counterpart
P < 0.05 and
P < 0.01 vs. non-TG mice within drug groups.
DISCUSSION
Alterations in autonomic function are thought to be a trigger for sudden cardiac death, but the underlying mechanism as to how autonomic dysfunction leads to sudden cardiac death remains elusive (26). Clinically, autonomic dysfunction has already been shown to have prognostic significance in patients with heart failure and myocardial infarction (23). If assessment of autonomic function could be used as a noninvasive clinical test to identify patients within FHC cohorts who are most at risk for sudden cardiac death, it would have significant benefits in risk stratification.
In this study, we demonstrated that two independent cTnT mutations, Δ160E and R92Q, which both exhibit clinically severe phenotypes in patients, result in mutation-specific alterations in HR regulation. Δ160E and R92Q mice have been extensively studied and been found to have similar phenotypes as assessed via a broad array of methodologies. In skinned fibers studies, they both demonstrated increased Ca2+ sensitivity at short and long sarcomere length and increased tension-dependent ATP consumption at short sarcomere length (3, 4, 31). Measures of myocellular mechanics and Ca2+ kinetic studies revealed a decreased rate of contraction, decreased percent shortening, decreased peak rate of relaxation, decreased sarcoplasmic reticulum Ca2+ load, decreased baseline Ca2+ levels, decreased peak rate of Ca2+ rise and decline, decreased Ca2+ peak amplitude, and decreased sarco(endo)plasmic reticulum Ca2+-ATPase-to-phospholamban ratios for both mutations (10). Δ160E mice differed from R92Q mice in that ultrastructural analysis revealed extensive Z-band misregistration, myofibrillar lysis, and a disorganized thin filament structure, whereas R92Q mice generally had preserved sarcomeric structure but increased lipid deposition and an increased number of mitochondria. We have determined that of these two independent mutations on opposite ends of the TNT1 tail domain, only R92Q results in alterations in HRV. Specifically, R92Q mice show a decrease in HRV, as indicated by a decrease in SDNN and SDANN, whereas Δ160E mice do not show any changes in these same parameters. A significantly smaller SDNN indicates that the R-R interval for R92Q mice stays within a limited range during a 24-h period compared with non-TG mice. This can be interpreted as a perturbation to the natural physiological variability of autonomic control in the hearts of R92Q mice.
To determine possible contributing factors for a decrease in HRV, we used FFT to look at frequency-domain measures of HRV that can assess sympathetic and parasympathetic nervous system inputs. From previous studies in humans (25) and mice (9, 30), the LF component is believed to be influenced by both the sympathetic and parasympathetic nervous systems, whereas the HF component is mainly influenced by the parasympathetic nervous system. LF/HF is thought to assess sympathovagal balance. In two separate studies, one looking at HCM patients and the other looking at heart failure patients, alterations to the autonomic nervous system were observed in conjunction with alterations to LF and HF of the same magnitude as in our R92Q mice (8, 35). In the study with HCM patients, the authors concluded that there was a decrease in sympathetic drive, whereas the authors of the study with heart failure patients actually measured an increase in sympathetic drive in support of their findings. Interestingly, in canine studies measuring HRV during exercise, where sympathetic drive is known to be increased, a decrease in the LF component was observed in conjunction with a decrease in HF (12). In our experiments, we found that R92Q mice had a significantly reduced nLF component, a significantly elevated nHF, and a significantly reduced LF/HF. It is not entirely understood how or why the LF component decreases in environments where high sympathetic activity is expected and/or observed (i.e., severe heart failure and heavy physical exercise). Van de Borne et al. (35) suggests that while the “traditional paradigm may hold true for a physiological range of autonomic drives,” it may no longer hold true in cases of extreme stress. Ultimately, the increased HR, decreased SDNN, and frequency measures of HRV in R92Q mice are in agreement with previous studies but require further assessment to determine which branch of the autonomic nervous system is involved.
In previous studies using heart failure models, a high sympathetic drive was correlated with downregulation of β-ARs or increased circulating catecholamines (13, 17). However, when we measured β-AR density in our TG mice, we observed no changes in Δ160E or R92Q mice. These results are in agreement with a study using an HCM rat model but are in contrast to studies looking at patients with HCM (5, 16, 29, 39). To further extend our original findings, we performed pharmacological tests that followed previously published guidelines (9, 30, 32). Compared with non-TG mice, R92Q and Δ160E mice had similar responses to time- and frequency-domain measures of HRV after a transient catecholamine release (saline injection), a β-AR agonist (isoproterenol), and a β-AR antagonist (propranolol). If there was β-AR downregulation, we would have expected to see a blunted response to β-AR stimulation or inhibition instead of observing values similar to non-TG mice. These findings would also suggest that if R92Q mice did have increased sympathetic drive, the uncoupling occurs further down the β-adrenergic signaling cascade. Overall, these findings suggest that HR dysregulation in R92Q mice is initiated at the myocellular level.
Alterations in HR regulation have been observed in patients with HCM, suggesting autonomic nervous system dysfunction. There is agreement in many studies that overall HRV is decreased in HCM patients (6, 8, 22, 34, 40). Another common finding among these studies was depressed parasympathetic activity, suggesting unopposed sympathetic activity in HCM patients. Consequently, there has been no clear consensus among these studies as to the predictive value that autonomic function has on risk assessment of HCM patients. However, it is important to note that the clinical profiles and genetic backgrounds for HCM patients in these studies vary considerably, potentially obscuring the benefits of autonomic function assessment. We were able to address these potential obstacles in our highly backcrossed TG mouse models of FHC by comparing two independent cTnT mutations that have been extensively studied in previous studies (3, 4, 10, 14, 18, 19, 21, 31, 33). In doing so, we can now determine that the R92Q cTnT mutation leads to altered HR regulation, and, thus, we can identify a genotype-specific phenotype using a noninvasive technique in mice.
Finally, we also sought to determine the arrhythmogenic potential in our cTnT mutant mice. While ventricular tachyarrhythmias have been posited to be a cause of death in FHC patients, neither nonsustained nor sustained ventricular tachyarrhythmias were observed in R92Q or Δ160E mice in the present study either at baseline or with isoproterenol injections. We did, however, observe significant 2° atrioventricular heart block at a significant rate during a 1-h ECG analysis of the 24-h baseline recording in R92Q mice that was absent in both non-TG and Δ160E mice. In addition, R92Q mice along with Δ160E mice exhibited a significant incidence of PVCs compared with non-TG mice after isoproterenol injections. Increased ventricular ectopy has been linked to mice with myofilament Ca2+ sensitivity, a characteristic also found in both R92Q and Δ160E mice (1). There were no significant changes in P-R and QRS intervals between Δ160E and R92Q mice versus non-TG mice after the drug injections. However, there were significant increases in the P duration in both Δ160E and R92Q mice versus non-TG mice after the drug injections. Coupled with our previous findings of significant atrial enlargement in our R92Q mice (31), these results potentially highlight conduction abnormalities in Δ160E and R92Q mice. Interestingly, all the Δ160E and R92Q mice survived isoproterenol injections, unlike mice in a study by Knollmann et al. (15), where mice carrying the I79N cTnT mutation demonstrated significant mortality as a result of ECG changes and heart block (15). There are also case reports linking bradycardias such as heart block with HCM and with being secondary to underlying conditions such as myocardial ischemia or autonomic dysfunction (7, 36). Another study (27) claimed a pathological interruption of the heart's electrical conduction pathway. Together, these findings highlight the potential proarrhythmogenic nature and conduction abnormalities of our cTnT mutant mice.
Although limitations exist on extrapolating electrophysiological information from murine models due to such differences as having more rapid HRs and shorter action potentials than humans, this is the first study to analyze the role of autonomic regulation in mice with cTnT mutations. Our findings provide the first example of mutations in cTnT leading to autonomic dysfunction. Of the two independent cTnT mutations that were studied, only R92Q mutant mice had significantly altered HRV in 24-h baseline recordings. In addition, R92Q and Δ160E mice did not show a decrease in β-AR density, nor did they have significantly different responses to β-AR drugs compared with non-TG mice. We have found that gene specific, noninvasive physiological measurements of HRV can provide information on autonomic function in mice with cTnT mutations associated with FHC.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants 5F31-HL-085917-04 (to J. Jimenez) and R01-HL-075619-05 (to J. C. Tardiff).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Baudenbacher F, Schober T, Pinto JR, Sidorov VY, Hilliard F, Solaro RJ, Potter JD, Knollmann BC. Myofilament Ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. J Clin Invest 118: 3893–3903, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chandra M, Rundell VL, Tardiff JC, Leinwand LA, De Tombe PP, Solaro RJ. Ca2+ activation of myofilaments from transgenic mouse hearts expressing R92Q mutant cardiac troponin T. Am J Physiol Heart Circ Physiol 280: H705–H713, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Chandra M, Tschirgi ML, Tardiff JC. Increase in tension-dependent ATP consumption induced by cardiac troponin T mutation. Am J Physiol Heart Circ Physiol 289: H2112–H2119, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Choudhury L, Guzzetti S, Lefroy DC, Nihoyannopoulos P, McKenna WJ, Oakley CM, Camici PG. Myocardial beta adrenoceptors and left ventricular function in hypertrophic cardiomyopathy. Heart 75: 50–54, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Counihan PJ, Frenneaux MP, Webb DJ, McKenna WJ. Abnormal vascular responses to supine exercise in hypertrophic cardiomyopathy. Circulation 84: 686–696, 1991 [DOI] [PubMed] [Google Scholar]
- 7. Doven O, Cicek D, Pekdemir H, Camsari A, Parmaksiz T, Cin GV, Akkus NM. Abnormal His-Purkinje system conduction leading to complete atrioventricular block in patients with hypertrophic cardiomyopathy: a report of 3 cases. Jpn Heart J 45: 347–352, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Fei L, Slade AK, Prasad K, Malik M, McKenna WJ, Camm AJ. Is there increased sympathetic activity in patients with hypertrophic cardiomyopathy? J Am Coll Cardiol 26: 472–480, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Gehrmann J, Hammer PE, Maguire CT, Wakimoto H, Triedman JK, Berul CI. Phenotypic screening for heart rate variability in the mouse. Am J Physiol Heart Circ Physiol 279: H733–H740, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Haim TE, Dowell C, Diamanti T, Scheuer J, Tardiff JC. Independent FHC-related cardiac troponin T mutations exhibit specific alterations in myocellular contractility and calcium kinetics. J Mol Cell Cardiol 42: 1098–1110, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Heradien M, Revera M, van der Merwe L, Goosen A, Corfield VA, Brink PA, Mayosi BM, Moolman-Smook JC. Abnormal blood pressure response to exercise occurs more frequently in hypertrophic cardiomyopathy patients with the R92W troponin T mutation than in those with myosin mutations. Heart Rhythm 6: S18–24, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Houle MS, Billman GE. Low-frequency component of the heart rate variability spectrum: a poor marker of sympathetic activity. Am J Physiol Heart Circ Physiol 276: H215–H223, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Igawa A, Nozawa T, Yoshida N, Fujii N, Inoue M, Tazawa S, Asanoi H, Inoue H. Heterogeneous cardiac sympathetic innervation in heart failure after myocardial infarction of rats. Am J Physiol Heart Circ Physiol 278: H1134–H1141, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Javadpour MM, Tardiff JC, Pinz I, Ingwall JS. Decreased energetics in murine hearts bearing the R92Q mutation in cardiac troponin T. J Clin Invest 112: 768–775, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knollmann BC, Blatt SA, Horton K, de Freitas F, Miller T, Bell M, Housmans PR, Weissman NJ, Morad M, Potter JD. Inotropic stimulation induces cardiac dysfunction in transgenic mice expressing a troponin T (I79N) mutation linked to familial hypertrophic cardiomyopathy. J Biol Chem 276: 10039–10048, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Lefroy DC, de Silva R, Choudhury L, Uren NG, Crake T, Rhodes CG, Lammertsma AA, Boyd H, Patsalos PN, Nihoyannopoulos P, et al. Diffuse reduction of myocardial beta-adrenoceptors in hypertrophic cardiomyopathy: a study with positron emission tomography. J Am Coll Cardiol 22: 1653–1660, 1993 [DOI] [PubMed] [Google Scholar]
- 17. Leimbach WN, Jr, Wallin BG, Victor RG, Aylward PE, Sundlof G, Mark AL. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation 73: 913–919, 1986 [DOI] [PubMed] [Google Scholar]
- 18. Lombardi R, Bell A, Senthil V, Sidhu J, Noseda M, Roberts R, Marian AJ. Differential interactions of thin filament proteins in two cardiac troponin T mouse models of hypertrophic and dilated cardiomyopathies. Cardiovasc Res 79: 109–117, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Luckey SW, Walker LA, Smyth T, Mansoori J, Messmer-Kratzsch A, Rosenzweig A, Olson EN, Leinwand LA. The role of Akt/GSK-3β signaling in familial hypertrophic cardiomyopathy. J Mol Cell Cardiol 46: 739–747, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maron BJ, Bonow RO, Cannon RO, 3rd, Leon MB, Epstein SE. Hypertrophic cardiomyopathy. Interrelations of clinical manifestations, pathophysiology, and therapy (1). N Engl J Med 316: 780–789, 1987 [DOI] [PubMed] [Google Scholar]
- 21. Montgomery DE, Tardiff JC, Chandra M. Cardiac troponin T mutations: correlation between the type of mutation and the nature of myofilament dysfunction in transgenic mice. J Physiol 536: 583–592, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morner S, Wiklund U, Rask P, Olofsson BO, Kazzam E, Waldenstrom A. Parasympathetic dysfunction in hypertrophic cardiomyopathy assessed by heart rate variability: comparison between short-term and 24-h measurements. Clin Physiol Funct Imaging 25: 90–99, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Odemuyiwa O, Malik M, Farrell T, Bashir Y, Poloniecki J, Camm J. Comparison of the predictive characteristics of heart rate variability index and left ventricular ejection fraction for all-cause mortality, arrhythmic events and sudden death after acute myocardial infarction. Am J Cardiol 68: 434–439, 1991 [DOI] [PubMed] [Google Scholar]
- 24. Palm T, Graboski S, Hitchcock-DeGregori SE, Greenfield NJ. Disease-causing mutations in cardiac troponin T: identification of a critical tropomyosin-binding region. Biophys J 81: 2827–2837, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pomeranz B, Macaulay RJ, Caudill MA, Kutz I, Adam D, Gordon D, Kilborn KM, Barger AC, Shannon DC, Cohen RJ, Benson H. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol Heart Circ Physiol 248: H151–H153, 1985 [DOI] [PubMed] [Google Scholar]
- 26. Prasad K, Frenneaux MP. Sudden death in hypertrophic cardiomyopathy: potential importance of altered autonomic control of vasculature. Heart 79: 538–540, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rosen KL, Cameron RW, Bigham PJ, Neish SR. Hypertrophic cardiomyopathy presenting with 3rd-degree atrioventricular block. Tex Heart Inst J 24: 372–375, 1997 [PMC free article] [PubMed] [Google Scholar]
- 28. Sadoul N, Prasad K, Elliott PM, Bannerjee S, Frenneaux MP, McKenna WJ. Prospective prognostic assessment of blood pressure response during exercise in patients with hypertrophic cardiomyopathy. Circulation 96: 2987–2991, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Schafers M, Dutka D, Rhodes CG, Lammertsma AA, Hermansen F, Schober O, Camici PG. Myocardial presynaptic and postsynaptic autonomic dysfunction in hypertrophic cardiomyopathy. Circ Res 82: 57–62, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Shusterman V, Usiene I, Harrigal C, Lee JS, Kubota T, Feldman AM, London B. Strain-specific patterns of autonomic nervous system activity and heart failure susceptibility in mice. Am J Physiol Heart Circ Physiol 282: H2076–H2083, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Tardiff JC, Hewett TE, Palmer BM, Olsson C, Factor SM, Moore RL, Robbins J, Leinwand LA. Cardiac troponin T mutations result in allele-specific phenotypes in a mouse model for hypertrophic cardiomyopathy. J Clin Invest 104: 469–481, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31a. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93: 1043–1065, 1996 [PubMed] [Google Scholar]
- 32. Thireau J, Zhang BL, Poisson D, Babuty D. Heart rate variability in mice: a theoretical and practical guide. Exp Physiol 93: 83–94, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Tobacman LS, Lin D, Butters C, Landis C, Back N, Pavlov D, Homsher E. Functional consequences of troponin T mutations found in hypertrophic cardiomyopathy. J Biol Chem 274: 28363–28370, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Uemura S, Tomoda Y, Fujimoto S, Yamamoto H, Matsukura Y, Hashimoto T, Dohi K. Heart rate variability and ventricular arrhythmia in clinically stable patients with hypertrophic cardiomyopathy. Jpn Circ J 61: 819–826, 1997 [DOI] [PubMed] [Google Scholar]
- 35. van de Borne P, Montano N, Pagani M, Oren R, Somers VK. Absence of low-frequency variability of sympathetic nerve activity in severe heart failure. Circulation 95: 1449–1454, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Wang DW, Deng YB. Hypertrophic cardiomyopathy complicated by severe bradycardias: a pedigree report. Clin Cardiol 25: 76–80, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Watkins H, McKenna WJ, Thierfelder L, Suk HJ, Anan R, O'Donoghue A, Spirito P, Matsumori A, Moravec CS, Seidman JG. Mutations in the genes for cardiac troponin T and α-tropomyosin in hypertrophic cardiomyopathy. N Engl J Med 332: 1058–1064, 1995 [DOI] [PubMed] [Google Scholar]
- 38. Wolf MJ, Tachibana H, Rockman HA. Methods for the detection of altered beta-adrenergic receptor signaling pathways in hypertrophied hearts. Methods Mol Med 112: 353–362, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Yamamoto S, Katsume H, Nakagawa M, Kuribayashi T, Hashimoto T, Kuriyama K. The beta-adrenergic receptor/adenylate cyclase system in the cardiac ventricles of a hypertrophic cardiomyopathy rat model. Jpn Circ J 56: 376–383, 1992 [DOI] [PubMed] [Google Scholar]
- 40. Yanagi S, Yoshinaga M, Horigome H, Tanaka Y, Fusazaki N, Matsuoka Y, Shimago A, Fukushige T, Eguchi T, Tokuda K, Nishi J, Kono Y, Nomura Y, Miyata K, Kawano Y. Heart rate variability and ambulatory blood pressure monitoring in young patients with hypertrophic cardiomyopathy. Circ J 68: 757–762, 2004 [DOI] [PubMed] [Google Scholar]