Abstract
Sphingolipids have a variety of important signaling roles in mammalian cells. We tested the hypothesis that certain sphingolipids and neutral sphingomyelinase (N-SMase) can regulate intracellular free magnesium ions ([Mg2+]i) in vascular smooth muscle (VSM) cells. Herein, we show that several sphingolipids, including C2-ceramide, C8-ceramide, C16-ceramide, and sphingosine, as well as N-SMase, have potent and direct effects on content and mobilization of [Mg2+]i in primary cultured rat aortic smooth muscle cells. All of these sphingolipid molecules increase, rapidly, [Mg2+]i in these vascular cells in a concentration-dependent manner. The increments of [Mg2+]i, induced by these agents, are derived from influx of extracellular Mg2+ and are extracellular Ca2+ concentration-dependent. Phospholipase C and Ca2+/calmodulin/Ca2+-ATPase activity appear to be important in the sphingolipid-induced rises of [Mg2+]i. Activation of certain PKC isozymes may also be required for sphingolipid-induced rises in [Mg2+]i. These novel results suggest that sphingolipids may be homeostatic regulators of extracellular Mg2+ concentration influx (and transport) and [Mg2+]i content in vascular muscle cells.
Keywords: intracellular magnesium, magnesium ion membrane transporters, aortic smooth muscle cells, neutral sphingomyelinase, signal transduction
there is now considerable evidence that body (cellular) depletion of Mg2+ is a risk factor for atherogenesis, sudden death ischemic heart disease, congestive heart failure, hypertension, diabetic-vascular disease, inflammatory conditions, preeclampsia-eclampsia, and stroke (see 3–5, 9, 11, 17, 19, 23, 24, 28 33, 38–40, 41, 45, 47). It has long been suspected that it is difficult, if not impossible, to replete certain soft tissue (e.g., heart, blood vessel) stores of Mg2+ (for reviews, see Refs. 3, 4, 17, 40, 41). Four decades ago, it was first demonstrated that the concentration of extracellular Mg2+ ([Mg2+]o) modulates vascular tone and vascular reactivity to vasoconstrictor and vasodilator agonists on peripheral and cerebral blood vessels (1–3). Furthermore, it has been shown that short-term dietary deficiency of Mg in rats resulted in hypertension and narrowing of arterioles and venules in the microcirculation (5). These actions were subsequently demonstrated to be coupled to modulation of membrane, extracellular, and intracellular stores of free calcium ions by [Mg2+]o (3, 4, 6, , 54, 56–59). It is currently believed that Mg2+ influx in mammalian vascular smooth muscle (VSM) cells occurs, primarily, via diffusion from the slightly higher [Mg2+]o, transported through several types of membrane channels (e.g., transient receptor potential melastatin channels, MagT, Na/Mg exchangers/antiporters) (13, 14, 18, 37, 44), promoted by the membrane potential, being negative on the cytosolic side (14, 18). However, the precise molecular basis for cellular uptake of [Mg2+]o and regulation of cytosolic free Mg2+ ([Mg2+]i) and its heterogeneous subcellular distribution (3, 4, 55) in VSM cells still remains to be clearly identified.
We have shown in primary cerebral and peripheral VSM cells, in culture, that a variation in [Mg2+]o causes sustained changes in membrane phospholipids and second messengers (35, 36), as well as truncation and oxidation of membrane fatty acids (35). Decreases in [Mg2+]o produced a fall in membrane sphingomyelin (SM), whereas increases in [Mg2+]o resulted in increases in SM and phosphatidylcholine (36). Intracellular ceramide formation was inversely proportional to Mg2+ (36). It is known that sphingolipids are involved in many cellular signaling processes, such as immune processes, apoptosis, inflammation, cell adhesion, transport systems, ion channels, cell growth processes, membrane receptors, and excitation-contraction coupling (3, 4, 6–8, 21, 25–27, 29, 32, 34–36, 42, 48, 49, 56–59). These lipid molecules behave directly as second messengers or act as precursors and facilitators of several other bioactive molecules (7, 8, 21, 25–27, 30, 34, 49). There is growing evidence, in smooth muscle cells, that sphingolipid release and biosynthesis may be relevant to adequate Ca and Mg levels in animals and humans and regulation of vascular function, per se (7, 8, 21, 35, 36, 42, 48). Extracellular free magnesium has been shown to regulate fatty acid chain length in VSM, oxidation of membrane fatty acids in VSM, some physical properties of VSM membranes, and specific phospholipid signaling pathways in VSM cells (3, 4, 6–8, 10, 35, 36).
We have recently reported in a series of papers that short-term dietary deficiency of Mg in rats results in decreased levels of serum SM, lipid peroxidation, and apoptosis in cardiac and VSM cell levels as well as upregulation of serine palmitoyl transferase and sphingomyelin synthase with de novo synthesis of ceramides in cardiovascular tissues and cells (7, 8). In view of these and the previous findings, we hypothesized that, since both acute and short-term Mg deficiency can upregulate several key enzymes in the sphingolipid pathway in cardiovascular tissues and cells, it could be possible that some of these lipid molecules might be natural modulators of uptake and transport of [Mg2+]o in VSM cells and could, thus, provide a potential mechanism for [Mg2+]-mediated regulation of vascular tone, angiogenesis, and diverse forms of vascular pathology. We, therefore, designed experiments to test the hypothesis that certain sphingolipids (e.g., ceramides, sphingosine) and neutral sphingomyelinase (N-SMase) can regulate [Mg2+]i levels in VSM cells. We report, herein, a novel effect of sphingolipids on cellular transport and regulation of [Mg2+]i in primary cultured rat aortic VSM cells. In addition, insights into how sphingolipids may regulate [Mg2+]o influx and [Mg2+]i in VSM cells are provided.
MATERIALS AND METHODS
Animals, procedures for primary cell culture, and cytosolic free Ca2+ and [Mg2+]i studies in single cultured rat aortic smooth muscle cells.
All experiments were approved by the Animal Use and Care Committee of the State University of New York Downstate Medical Center. The experiments were carried out on adult Wistar male rats (weighing 200–250 g) after rapid decapitation. The procedure employed to isolate and culture single primary aortic VSM cells and the use of digital imaging microscopy with fluorescent indicators has been reported (52, 54–59). Briefly, the primary single cells were cultured in Dulbecco's modified Eagle's medium at 37°C in an humidified atmosphere composed of 95% air-5% CO2 (52, 54–59). For image analysis, cells were cultured in monolayers (52, 54–59). The distribution of cytosolic free Ca2+ ([Ca2+]i) and [Mg2+]i levels in VSM cells was studied using digital imaging microscopy with the fluoresent probes fura 2-AM and mag-fura 2-AM (Molecular Probes, Eugene, OR) (54, 55). To improve loading efficiency, 0.12% pluronic F-127 (Sigma, St. Louis, MO) was used in the loading media (lacking FBS) (52, 54–59). Following dye loading, the labeled cells were washed three times with a modified HEPES buffer solution (in mM: 118 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 KH2PO4, 1.2 MgSO4, 10 glucose, and 5 HEPES). Buffer (control), N-SMase, bioactive ceramide molecules, sphingosine, inactive sphingolipid analogs, and various enzyme inhibitors were added to the monolayers in the above setup for various intervals of time (2–60 min). Because the results at 20 and 60 min were similar, only the results for 20 min are shown. The pH was adjusted to 7.4 with NaOH at 37°C. The cover slips containing the fura 2-loaded or mag-fura 2-loaded cells were affixed to holders that were placed on the temperature-controlled stage of the fluorescence microscope. [Mg2+]i or [Ca2+]i of single cultured VSM cells was calculated by using the following equation (54, 55) [Various concentrations of the lipids were used (10−9 to 10−4 M).].
The dissociation constant (Kd) of 224 nM was used for the fura 2/Ca2+ complex and a Kd of 1.9 mM for the mag-fura 2/Mg2+ complex (52, 54–59). Calibration of Kd requires measurements of the completely ion-free and ion-saturated indicator as well as measurements of the indicator in the presence of known Ca2+ or Mg2+ concentrations; this Kd was based on previous experiments with these cells (52, 54–59). B is the ratio of fluorescence intensity of fura 2 to Ca-bound fura 2 at 380 nm or free mag-fura 2 to Mg-bound mag-fura 2 at 370 nm at 37°C. Calibration showed that our 340:380 and 335:370 ratios fell on the linear portion of the calibration curves. Particular care was taken to minimize photobleaching of the dyes. Experiments were carried out in total darkness, and exposure to excitation light was <2 s in all experiments.
Chemicals and reagents.
N-SMase, neomycin, verapamil, bisindolylmaleimide I, and phosphorylcholine were obtained from Sigma. C2-ceramide, C8-ceramide, C16-ceramide, C8-ceramide 1-phosphate, sphingosine, dl-threo-dihydrosphingosine, C2-dihydroceramide, N,N-dimethylesphingosine, calmidazolium chloride, and U-73122 were purchased from BIOMOL Research Laboratories (Plymouth, PA). Fura 2-AM, mag-fura 2-AM and the acetylmethyl ester of bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA-AM) were purchased from Molecular Probes. Calphostin C was obtained from CALBIOCHEM-NOVABIOCHEM (La Jolla, CA). All other organic and inorganic chemicals were purchased from Fisher Scientific (Fair Lawn, NJ) and were of the highest purity.
Statistical analyses.
Where appropriate, results are expressed as means ± SE. Differences between means were analyzed using nonpaired t-test or ANOVA followed by ANOVA using Scheffé's contrast test. Statistical significance was assumed when P < 0.05.
RESULTS
Influence of sphingolipids and N-SMase on cytosolic free [Ca2+]i and [Mg2+]i in single primary cultured resting rat aortic smooth muscle cells.
The basal [Ca2+]i in resting unstimulated primary cultured aortic VSM cells, estimated from the fluorescence ratio at 340 nm to 380 nm, obtained with digital image analysis and the fluorescent probe fura 2-AM was ∼90.7 ± 7.3 nM (n = 45). C2-ceramide, C8-ceramide, C16-ceramide, C8-ceramide 1-phosphate, and N-SMase did not elicit any significant changes in resting distribution of [Ca2+]i in the single VSM cells (Table 1), but maximal concentrations of sphingosine caused a rapid significant elevation in [Ca2+]i from the resting level to ∼170 ± 8.1 nM, P < 0.01 (Table 1). Upon exposure of the cultured VSM cells to sphingosine, in the absence of [Ca2+]o, the sphingosine-induced rise of [Ca2+]i was inhibited completely (170 ± 8.1 vs. 95.6 ± 6.8, n = 35), suggesting that the mobilization of [Ca2+]i elicited by sphingosine is probably derived from the influx of extracellular Ca2+.
Table 1.
Influence of sphingolipids and N-SMase on cytosolic free [Ca2+]i and [Mg2+]i in single primary cultured resting rat aortic smooth muscle cells ascertained at 20 min of incubation
| Sphingolipids | [Ca2+]i, nM | [Mg2+]i, μM |
|---|---|---|
| Control-placebo | 90.7 ± 7.3 | 530 ± 60.1 |
| C2-ceramide (10−5 M) | 89.9 ± 6.3 | 713 ± 72.3** |
| C8-ceramide (10−5 M) | 93.1 ± 7.9 | 730 ± 55.3** |
| C16-ceramide (10−5 M) | 90.5 ± 6.7 | 829 ± 68.5** |
| C8-Ceramide 1-phosphate (10−5 M) | 85.6 ± 8.2 | 551 ± 49.9 |
| N-SMase (0.1 U/ml) | 97.3 ± 6.8 | 723 ± 60.3** |
| Sphingosine (10−5 M) | 170 ± 8.1** | 776 ± 87.6** |
All values are means ± SE of at least 35-40 cells each. N-SMase, neutral sphingomyelinase; [Ca2+]i, cytosolic free Ca2+; [Mg2+]i, cytosolic free Mg2+. The cover slips containing the fura 2-loaded or mag-fura 2-loaded cells were affixed to holders that were placed on the temperature-controlled stage of the fluorescence microscope. Sphingolipids (i.e., C2-, C8-, and C16-ceramide, C8-ceramide 1-phosphate, sphingosine, and negative analog controls) and N-SMase were incubated with primary cultured VSM cells for 20 min in 37°C. Measurements for [Ca2+]i and [Mg2+]i were undertaken at 20 min after exposure of these cultured cells to sphingolipids and N-SMase. Significantly different from controls (** P < 0.01, paired t-test).
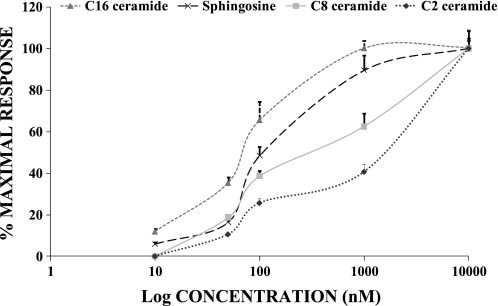
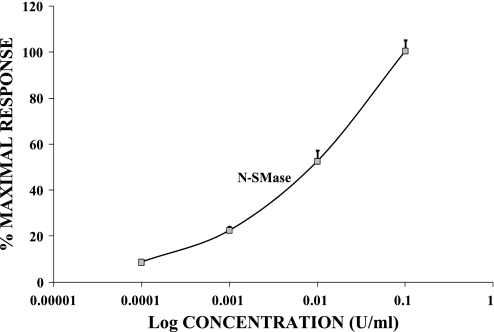
At rest, in normal 1.2 mM Mg2+, [Mg2+]i, as determined by digital image analysis and the fluorescent probe mag-fura 2-AM (55), possess internal free magnesium of ∼530 ± 60 μM (n = 45), similar to values reported previously for these primary VSM cells (55). When the vascular cells were incubated with the diverse biologically active sphingolipids (i.e., C2-ceramide, C8-ceramide, C16-ceramide, and sphingosine) and N-SMase sphingomyelinase for 20 min [Mg2+]i was significantly increased in a concentration-dependent manner and remained constant for the duration of incubation, i.e., 30–60 min (Table 1; Figs. 1 and 2). Sphingosine and C16-ceramide were more potent (P < 0.05) than either C8-ceramide or C2-ceramide in inducing elevation of [Mg2+]i (Fig. 1). dl-Threo-dihydrosphingosine, C2-dihydroceramide, N,N-dimethylsphingosine, and phosphorylcholine were tested widely as negative controls, as well as ceramide 1-phosphate, but none of these molecules altered resting levels of [Mg2+]i (n ≥ 35 cells each, P > 0.05, e.g., Table 2).
Fig. 1.
Relative concentration-dependent effects of sphingosine, C2-ceramide, C8-ceramide, and C16-ceramide on cytosolic free Mg2+ ([Mg2+]i) in single primary cultured rat aortic smooth muscle cells. Sphingosine (10−8 to 10−5 M), C2-ceramide (10−8 to 10−5 M), C8-ceramide (10−8 to 10−5 M), and C16-ceramide (10−8 to 10−5 M) were incubated with primary cultured vascular smooth muscle cells for 20 min at 37°C. Results are means ± SE from 4–6 different cover slips. Mean values for 100% maximal responses are given in Table 1.
Fig. 2.
Concentration-dependent effects of neutral sphingomyelinase on [Mg2+]i in single primary cultured rat aortic smooth muscle cells. Experimental conditions are identical to those in Fig. 1. Neutral sphingomyelinase (0.001–0.1 U/ml) was incubated with primary cultured vascular smooth muscle cells for 20-min periods at 37°C. Results are means ± SE from 4–6 different cover slips. Mean values for 100% maximal responses are given in Table 1.
Table 2.
Failure of dl-threo-dihydrosphingosine, C2-dihydroceramide, N,N-dimethylsphingosine, and PH to alter [Mg2+]i levels in primary cultured resting aortic smooth muscle cells
| Sphingolipid-PH | [Mg2+]i, μM |
|---|---|
| Control-placebo | 550 ± 65.3 |
| dl-threo-dihydrosphingosine (10−5 M) | 576 ± 72.5 |
| C2-dihyroceramide (10−5 M) | 527 ± 68.6 |
| N,N-dimethylsphingosine (10−5 M) | 585 ± 72.8 |
| PH (10−4 M) | 538 ± 63.5 |
All values are means ± SE. PH, phosphorylcholine. See Table 1 for protocol.
Effects of BAPTA-AM, [Ca2+]o-free media, verapamil, and calmidazolium on sphingolipid- and N-SMase-induced rises of [Mg2+]i in single primary cultured rat aortic smooth muscle cells.
To determine the trigger for [Mg2+]i elevation, induced by sphingolipids, and the relationships with [Ca2+]o and [Ca2+]i in relation to [Mg2+]i, primary cultured VSM cells were incubated, acutely, with sphingolipids in either BAPTA-AM [a chelator of intracellular free Ca2+ (5 × 10−6 M)] alone in Ca2+-containing media or Ca2+-free media (with 2 mM EDTA added), an L-type Ca2+ channel blocker (verapamil, 10−5 M) alone (in Ca2+-containing media), or in Ca2+-free media (with 2 mM EDTA) with BAPTA-AM (5 × 10−6 M) added, as well as in Mg2+-free incubation media for 20 min. C2-ceramide (10−5 M)-, C8-ceramide (10−5 M)-, C16-ceramide (10−5 M)-, and sphingosine (10−5 M)-induced elevations of [Mg2+]i were inhibited completely (n = 45) by BAPTA-AM (with [Ca2+]o present) (Table 3). However, N-SMase-induced rises in [Mg2+]i were not inhibited by BAPTA-AM (n = 45, with [Ca2+]o present). In [Ca2+]o-free media, verapamil alone (in Ca2+-containing media) or in [Ca2+]o-free media with BAPTA-AM, C2-ceramide-, N-SMase-, and sphingosine-induced changes of [Mg2+]i were inhibited completely, but C8-ceramide and C16-ceramide changes in [Mg2+]i were only inhibited partially by verapamil (22–30%) (Table 3). These results suggest that C2-ceramide, C8-ceramide, N-SMase-, and sphingosine-induced transport of [Mg2+]o and mobilization of [Mg2+]i in rat aortic smooth muscle cells is [Ca2+]o-dependent, whereas those changes induced by C8- and C16-ceramide appear to be partially [Ca2+]o-dependent.
Table 3.
Effects of BAPTA-AM, [Ca2+]o-free media, verapamil, and calmidazolium on sphingolipid- and N-SMase-induced rises of [Mg2+]i in single primary cultured rat aortic smooth muscle cells
| [Mg2+]i, μM |
|||||||
|---|---|---|---|---|---|---|---|
| Sphingolipids | Control (placebo) | Sphingolipid Alone | BAPTA-AM With SPs | BAPTA-AM [Ca2+]o-free With SPs | [Ca2+]o-free With SPs | Verapamil With SPs | Calmidazolium With SPs |
| C2-ceramide (10−5 M) | 598 ± 52.2 | 713 ± 72.3** | 598 ± 49.9 | 573 ± 52.5 | 560 ± 48.1 | 564 ± 47.7 | 596 ± 50.2 |
| C8-ceramide (10−5 M) | 585 ± 49.2 | 730 ± 55.3** | 645 ± 51.9 | 594 ± 68.5 | 579 ± 52.1 | 698 ± 58.3* | 712 ± 55.1** |
| C16-ceramide (10−5 M) | 594 ± 50.8 | 818 ± 84 | 606 ± 66 | 612 ± 58.2 | 602 ± 68 | 744 ± 68.2* | 810 ± 78** |
| N-SMase (0.1 U/ml) | 570 ± 42.6 | 723 ± 60.3** | 795 ± 68.1** | 593 ± 51.1 | 552 ± 50.3 | 597 ± 52.1 | 689 ± 59.1* |
| Sphingosine (10−5 M) | 585 ± 55.9 | 776 ± 87.6** | 608 ± 45.9 | 578 ± 52.9 | 564 ± 49.3 | 589 ± 51.1 | 558 ± 52.3 |
Values are means ± SE of at least 35–40 cells each. [Mg2+]i measurements were made under conditions identical to those in Table 1. Cells were incubated with sphingolipids (SP) and N-SMase in either BAPTA-AM (5×10−6 M) alone ( in Ca2+-containing media), BAPTA-AM in [Ca2+]o-free media, Ca2+-free media (with 2 mM EDTA), verapamil (10−5 M) alone ( in Ca2+-containing media), as well as in calmidazolium (5 × 10−6 M) ( in Ca2+-containing media). Measurements of [Mg2+]i were undertaken at 20 min after exposure of these cultured cells to sphingolipids and N-SMase. Significantly different from controls (*P < 0.05, ** P < 0.01, paried t-test).
Calmidazolium (5 × 10−6 M), a specific inhibitor of calmodulin-dependent phosphodiesterase and Ca2+-ATPase, significantly attenuated the rises of [Mg2+]i elicited by maximal concentrations (10−5 M) of C2-ceramide and sphingosine but not that induced by either N-SMase, C8-ceramide, or C16-ceramide (Table 3). These results suggest that the transport of [Mg2+]o and mobilization of [Mg2+]i induced by C2-ceramide and sphingosine may be Ca2+/calmodulin/Ca2+-ATPase-dependent. Inhibition of both calmidazolium-dependent phosphodiesterase and Ca-ATPase would seem to support the idea offered here that [Mg2+]o uptake and [Mg2+]i are Ca-dependent since both enzymes regulate Ca2+ metabolism. Moreover, all sphingolipid-induced increases of [Mg2+]i were completely inhibited in Mg2+-free media (as well as following the addition of 1.2 mM [Mg2+]o 20 min after exposure to 0 mM [Mg2+]o) and also after exposure to 0.3 or 0.6 mM extracellular Mg2+ (n = 30 cells each, data not shown), suggesting that the rises of [Mg2+]i are derived from Mg2+ influx across the plasma membrane; these data also indicate that [Mg2+]o transport across the aortic VSM cell membrane requires an extracellular Mg2+ >0.6 mM.
Effects of inhibitors of PKC on [Mg2+]i in single primary cultured rat aortic smooth muscle cells.
It has been suggested, previously, that activation of PKC may be involved in hormone-induced increases in Mg2+ influx and in contractions of arterial smooth muscle induced by low [Mg2+]o (50, 52). To consider the possibility that PKC activation might also be involved in [Mg2+]i mobilization, induced by sphingolipids in VSM cells, we compared the effects of four different PKC inhibitors. A specific PKC inhibitor, calphostin C (2 × 10−6 M), that is Ca2+/phospholipid independent and that competes with 1,2-diacylglycerol binding sites was chosen first. Interestingly, we found that calphostin C did not attenuate [Mg2+]i alterations induced by either C2-ceramide, C8-ceramide, C16-ceramide, or sphingosine but did significantly abolish the rise of [Mg2+]i induced by N-SMase (∼70%) (P < 0.05) (Table 4), suggesting that the action of certain ceramides and N-SMase-induced mobilization of [Mg2+]i might involve PKC activation. Moreover, another potent and selective inhibitor of PKC, viz., chelerythrine (a competitive inhibitor with respect to the phosphate acceptor), was also used. The latter, however, could not inhibit either N-SMase- or sphingosine-induced rises of [Mg2+]i but did inhibit C2-ceramide-, C8-ceramide-, and C16-ceramide-induced elevations in [Mg2+]i (Table 4). We also examined two highly selective cell-permeable but phospholipid/Ca2+-dependent PKC inhibitors, namely staurosporine (5 × 10−7 to 5 × 10−6 M) and Gö-6976 (1 × 10−6 to 3 × 10−6 M), that selectively inhibit PKC-α and PKC-β1 (52). These phospholipid/Ca2+-dependent PKC inhibitors, i.e., Gö-6976 (3 × 10−6 M) and staurosporine (5×10−6 M), inhibited all sphingolipid-induced elevation of [Mg2+]i, except for sphingosine (Table 4).
Table 4.
Effects of inhibitors of PKC on [Mg2+]i in single primary cultured rat aortic smooth muscle cells
| [Mg2+]i, μM |
||||
|---|---|---|---|---|
| Sphingolipids | Calphostin C | Chelerythrine | Staurosporine | Gö-6976 |
| Control-placebo | 563 ± 58.9 | 765 ± 59.2 | 786 ± 51.6 | 742 ± 69.6 |
| C2-ceramide (10−5 M) | 683 ± 57.4** | 758 ± 51.4 | 791 ± 75.3 | 737 ± 76.1 |
| C8-ceramide (10−5 M) | 884 ± 63.4** | 820 ± 63.7 | 819 ± 60.5 | 729 ± 73.4 |
| C16-ceramide (10−5 M) | 926 ± 88.2** | 798 ± 80.4 | 822 ± 78 | 804 ± 76.2 |
| N-SMase (0.1 U/ml) | 587 ± 48.1 | 914 ± 69.8** | 816 ± 72.4 | 751 ± 78.3 |
| Sphingosine (10−5 M) | 667 ± 45.9** | 913 ± 67.1** | 860 ± 70.9* | 859 ± 80.2** |
Values are means ± SE of at least 35–40 cells each. Cells were incubated with different PKC inhibitors [i.e., calphostin C (2 × 10−6 M), chelerythrine (10−6 M), staurosporine (5 × 10−6 M), and Gö;-6976 (3 × 10−6 M)] for 15 min before addition of sphingolipids and N-SMase at 37°C. Measurements of [Mg2+]i were made at 20 min after exposure of these cultured cells to sphingolipids and N-SMase. Control values for the respective sphingolipids (alone) are found in Table 3). Significantly different from controls (**P < 0.01 and *P < 0.05).
Effects of phospholipase C inhibitors on [Mg2+]i in single primary cultured rat aortic smooth muscle cells.
Some sphingolipids, for example, sphingosine, have been demonstrated to stimulate polyphosphoinositide breakdown through activation of phospholipase C (PLC) (25, 26). To gain a better understanding of the possible role of PLC activation in sphingolipid-induced [Mg2+]i responses, two PLC inhibitors (neomycin and U-73122) were next used in our experiments. Both neomycin and U-73122 inhibited, completely, the C2-ceramide-, N-SMase-, and sphingosine-induced increases of [Mg2+]i in the cultured single vascular cells; neomycin and U-73122, however, did not attenuate the rises in [Mg2+]i induced by C8-ceramide and C16-ceramide (Table 5).
Table 5.
Effects of phospholipase C inhibitors on [Mg2+]i in single primary cultured rat aortic smooth muscle cells
| [Mg2+]i, μM |
|||
|---|---|---|---|
| Sphingolipids | Sphingolipid Alone | Neomycin (With SPs) | U-73122 (With SPs) |
| Control-placebo | 530 ± 60.1 | 548 ± 44.7 | 570 ± 53.1 |
| C2-ceramide (10−5 M) | 713 ± 72.3** | 550 ± 42.2 | 577 ± 48.2 |
| C8-ceramide (10−5 M) | 730 ± 55.3** | 681 ± 53.8** | 702 ± 58.2** |
| C16-ceramide (10−5 M) | 818 ± 84** | 716 ± 56.4 | 704 ± 62.2 |
| N-SMase (0.1 U/ml) | 723 ± 60.3** | 559 ± 50.1 | 569 + 50.0 |
| Sphingosine (10−5 M) | 776 ± 87.6** | 566 + 49.3 | 589 + 51.3 |
Values are means ± SE of at least 35–40 cells each. Cells were incubated with neomycin (2 × 10−5 M) or U-73122 (1 × 10−5 M) for 15 min before addition of sphingolipids and N-SMase at 37°C. Measurements of [Mg2+]i were made at 20 min after exposure of these cultured cells to sphingolipids and N-SMase. Significantly different from controls (**P < 0.01).
DISCUSSION
Collectively, we present, herein, the first observations that: 1) C2-ceramide, C8-ceramide, C16-ceramide, and sphingosine, as well as N-SMase, increase [Mg2+]i in single rat aortic cultured VSM cells in a concentration-dependent manner; 2) the increments in [Mg2+]i induced by these lipid-putative second messengers are derived from influx of extracellular Mg2+; 3) the increases of [Mg2+]i induced by C2-ceramide, C8-ceramide, C16-ceramide, sphingosine, and N-SMase are all [Ca2+]o-dependent; 4) PLC and Ca2+/calmodulin/Ca2+-ATPase activity appear to be important in the mobilization of [Mg2+]i induced by ceramides, N-SMase, and sphingosine; and 5) activation of several PKC isozymes, varying with the precise sphingolipid, may also be required for sphingolipid-induced rises in [Mg2+]i. These differential responses to the diverse PKC inhibitors suggest that different PKC isozymes are probably stimulated by the different sphingolipid agonists. These new results raise the possibility that novel and classical PKC isozymes may perform different and potentially important roles in sphingolipid modulation of Mg2+ uptake, transport, and content in VSM cells. We believe this hypothesis merits further study.
Because the elevation of [Mg2+]i caused by the major potential sphingolipid product of sphingomyelinase, namely ceramide, was not inhibited consistently by the PKC inhibitors, we cannot as yet pinpoint the specific sphingolipid molecule that may be the bioactive species causing elevation of [Mg2+]i upon addition of N-SMase. However, because the naturally occurring ceramide, viz, C16-ceramide (21, 49), is clearly the most potent in inducing Mg2+ uptake, we are tempted to implicate this ceramide in regulation of Mg2+ uptake and transport, at least in rat aortic smooth muscle cells. It cannot be phosphorylcholine, since the latter exerts neither any effect on contraction, vascular tone (Ref. 59 and unpublished observations), nor [Mg2+]i mobilization in these VSM cells (e.g., Table 2). Although ceramide kinase is present in aortic smooth muscle (21), and could form ceramide 1-phosphate, the latter was found (10−8 to 10−5 M) not to exert either any relaxant action on vasomotor tone in these cells (unpublished findings) or any action on [Mg2+]i (e.g., Table 2). An important question that immediately arises from our findings is concerned with the molecular mechanism(s) whereby sphingolipids bind to their direct targets. It has long been known that numerous lipid molecules function as structural components of all cell membranes and can act as second messengers (21, 25, 30, 32, 34, 43, 46, 49). Moreover, many of these molecules are vital in very complex intracellular cell signaling pathways (21, 25, 26, 30, 34, 49). A number of these molecules have been demonstrated to bind to target proteins and induce conformational changes in proteins important in cell membrane functions, e.g., opening ion channels (21, 25, 26, 30, 32, 34, 43, 49).
Cholesterol and sphingolipids serve as major components of lipid rafts and related microdomains in animal cell membranes (21, 32). Lipid raft domains are believed to be involved in the regulation of numerous cellular functions, such as protein functions, lipid trafficking, and signal transduction (20, 31, 43). Membrane sphingolipids demonstrate an extensive range of headgroup structures, varying from ceramide to glycososphingolipids exhibiting rather diverse and often complex oligosaccharide residues. Membrane partitioning of sphingolipids into ordered domains is fostered, in part, by their ceramide moieties, which become enriched in molecular species having long (i.e., >C12) saturated N-acyl chains (15, 43). Ceramide is now known to, preferentially, partition into lipid raft domains and displace cholesterol from the lipid rafts (31, 53). It is believed that, “when ceramide is generated in situ by a SMase, instead of being premixed with the other lipids gel-like domain formation occurs” (22). Surprisingly, it is unlikely that ceramide 1-phosphate would interact in this latter manner (22), which would support our observations of a lack of effect of this molecule on uptake and transport of [Mg2+]o across the VSM cell membranes. Very little, however, is known regarding the effects of various headgroups on the affinities of diverse sphingolipids for ordered membrane lipid domains and lipid rafts (15, 26, 43). Ceramides, surprisingly, exhibit higher affinities for ordered membrane domains than do other sphingolipids (46) and appear to be dependent on the length of the saturated N-acyl chains. In this regard, it is clear from our concentration-response data presented in Fig. 1 that there is a significant relative potency of the ceramides, where C16- > C8- > C2-ceramide in promoting the transport of Mg2+ across the VSM membranes of the primary cultured aortic smooth muscle cells (ANOVA, P < 0.05). Such membrane partitioning could be responsible, at least in part, for the increased uptake of [Mg2+]o in our experiments. In this regard, it might be of considerable interest to determine whether, with both cholesterol-free and cholesterol-containing lipid bilayers, diverse ceramides increase Mg2+ uptake in the relative order predicted by our present studies.
Because we have demonstrated in living rats, made Mg-deficient, that serum SM levels are altered significantly (i.e., decreased, see Ref. 7), that such Mg-deficient diets result in de novo synthesis of ceramides (Refs. 10 and 11 and unpublished observations), and that primary cerebral and peripheral VSM cells also demonstrate de novo synthesis of ceramides when exposed to low [Mg2+]o (36), we hypothesize there is a natural feedback mechanism that attempts to restore VSM cell [Mg2+]i balance by stimulating de novo synthesis of ceramides to facilitate uptake and transport of Mg2+ in the Mg2+-depleted VSM cells. Although the present investigation does not define the specific transport mechanism, it is distinctly possible that the de novo generation of the ceramides stimulates transporter molecules like the transient receptor potential melastatin ion channel molecules and/or other recently discovered transporter molecules to facilitate Mg2+ uptake and membrane transport in VSM cells (18, 37, 42, 44, 48).
The precise mechanism(s) involved, and the specific isoforms, in the contribution of PKC activation/inhibition to sphingolipid-induced increases of [Mg2+]i will remain unknown, pending further investigation. In view of our present novel findings, it may be entertained that sphingolipid-induced relaxation of intact and isolated blood vessels reported elsewhere (4, 21, 29, 56–58) may, in part, be a consequence of the direct rise of cytosolic free Mg2+ that has recently been shown to result in biosynthesis and release of nitric oxide in the microcirculation (51).
A few additional words with respect to Mg2+ and N-SMase appear to be in order. Recently, it has been demonstrated that N-SMase relaxant actions in rat aortic VSM are Mg2+-dependent (58). This raises an interesting question in relation to the present findings, which show that N-SMase is a potent stimulator of [Mg2+]i mobilization. The question is, since N-SMase relaxant action is Mg2+-dependent, can this enzyme bring about and perpetuate its own actions by mobilization of [Mg2+]i?
These new observations, shown herein, lead us to speculate that sphingolipid-induced [Mg2+]i mobilization in rat aortic VSM cells probably involves an activation of PLC. The precise relationship(s) between the three various molecular signaling pathways identified by our new studies (i.e., Ca2+/calmodulin, PKC, and PLC), however, remains to be uncovered. It should be pointed out that these three signaling pathways are known to be intimately involved in various activities and functions of sphingolipids (6, 21, 25, 30, 34, 49). Although it is clear that further studies will be necessary to determine the precise molecular pathways through which sphingolipids regulate the influx, membrane transport, and cytosolic content of Mg2+ in VSM cells, our new data provide the first clear-cut evidence that sphingolipids may be prime physiological (homeostatic?) regulators of Mg2+ transport and content in vascular muscle cells. We believe that, since nanomolar concentrations of all of the sphingolipids induce significant increases in Mg uptake and content of the vascular cells (see Fig. 1), the use of the term “physiological” here is justified. In addition, the results in this report could be used to suggest a new therapeutic approach for elevating cellular and tissue [Mg2+]i in clinical states of hypomagnesemia, particularly those known to be refractory to acute oral and systemic administration of Mg salts (6, 9, 10, 33, 40, 41). Last, it must be entertained that a great deal of the cellular tissue deficits in Mg2+ noted recently in diverse cardiovascular pregnancy-vascular aberrations, gestational diabetes, and type 1 and 2 diabetic-related vascular diseases (for specifics, see Refs. 3, 4, 11, 12, 17, 23, 24, 40, 41) may be, in large measure, due to disturbances in vascular and endothelial cell membrane N-SMase and the inability of these cells to generate and release sphingolipids needed for magnesium transport and homeostasis, rather than renal loss of magnesium, per se. In view of the latter clinical possibilities, we believe this hypothesis deserves further investigation.
GRANTS
This study was supported, in part, by National Institute on Alcohol Abuse and Alcoholism Grant AA-08674 (to B. M. Altura).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Altura BM, Altura BT. Influence of magnesium on drug-induced contractions and ion content in rabbit aorta. Am J Physiol 220: 938–944, 1971 [DOI] [PubMed] [Google Scholar]
- 2. Altura BM, Altura BT. Magnesium and contraction of arterial smooth muscle. Microvascular Res 7: 145–155, 1974 [DOI] [PubMed] [Google Scholar]
- 3. Altura BM, Altura BT. Magnesium and cardiovascular biology: an important link between cardiovascular risk factors and atherogenesis. Cell Mol Bio Res 41: 347–359, 1995 [PubMed] [Google Scholar]
- 4. Altura BM, Altura BT. Magnesium: forgotten mineral in cardiovascular biology and atherogenesis. In: New Perspectives in Magnesium Research, edited by Nishizawa N, Morii H, Durlach J. New York, NY: Springer, 2007, p. 239–260 [Google Scholar]
- 5. Altura BM, Altura BT, Gebrewold A, Ising H, Gunther T. Magnesium deficiency and hypertension: correlation between magnesium deficient diets and microcirculatory changes in situ. Science 223: 1315–1317, 1984 [DOI] [PubMed] [Google Scholar]
- 6. Altura BM, Kostellow AB, Zhang A, Li W, Morrill GA, Gupta RK, Altura BT. Expression of the nuclear factor kB and proto-oncogenes c-fos and c-jun are induced by low extracellular Mg2+ in aortic and cerebral vascular smooth muscle cells: possible links to hypertension, atherogenesis and stroke. Am J Hypertens 16: 701–707, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Altura BM, Shah NC, Jiang XC, Li Z, Perez-Albela JL, Altura BT. Short-term magnesium deficiency results in decreased levels of serum sphingomyelin, lipid peroxidation, and apoptosis in cardiovascular tissues. Am J Physiol Heart Circ Physiol 297: H86–H92, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Altura BM, Shah NC, Li Z, Jiang XC, Perez-Albela JL, Altura BT. Magnesium deficiency upregulates serine palmitoyl transferase(SPT1 and SPT2) in cardiovascular tissues: relationship to serum ionized Mg and cytochrome c. Am J Physiol Heart Circ Physiol 299: H932–H938, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Altura BT, Altura BM. Measurement of ionized magnesium in whole blood, plasma and serum with a new ion-selective electrode in healthy and diseased human subjects. Magnesium Trace Elem 10: 90–98, 1991 [PubMed] [Google Scholar]
- 10. Altura BT, Brust M, Barbour RL, Bloom S, Stempak J, Altura BM. Magnesium dietary intake modulates blood lipid levels and atherogenesis. Proc Natl Acad Sci USA 87: 1840–1844, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Apostol A, Apostol R, Ali E, Choi A, Ehsumi N, Hu B, Li L, Altura BT, Altura BM. Cerebral spinal fluid and serum ionized magnesium and calcium levels in preeclamptic women during administration of magnesium sulfate. Fertil Steril 94: 276–282, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Bardicef M, Bardiceff O, Sorokin Y, Altura BM, Altura BT, Cotton DB, Resnick LM. Extracellular and intracellular magnesium depletion in pregnancy and gestational diabetes. Am J Obst Gynecol 172: 1009–1013, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Beech DJ. Emerging functions of 10 types of TRP cationic channel in vascular smooth muscle. Clin Exp Pharmacol Physiol 32: 597–603, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beyenbach KW. Transport of magnesium across biological membranes. Magnes Trace Elem 9: 233–234, 1990 [PubMed] [Google Scholar]
- 15. Brown DA, London E. Functions of lipid rafts in biological membranes. Ann Rev Cell Dev Biol 14: 111–136, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Dietrich A, Chubanov V, Kalwwa H, Rost BR, Gudermann T. Cation channels of the transient receptor potential superfamily: their role in physiological and pathophysiological processes of smooth muscle cells. Pharmacol Ther 112: 744–760, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Djurhuus S, Henriksen JE, Klitgaard NAH, Blaabjerg O, Thye-Ron P, Altura BM, Altura BT, Beck-Nielsen H. Effects of moderate improvement in metabolic control on magnesium and lipid concentrations in patients with type 1 diabetes. Diabetes Care 22: 546–554, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Ebel H, Gunther T. Na+/Mg2+ antiport in erythrocytes of spontaneously hypertensive rats: role of Mg2+in the pathogenesis of hypertension. Magnes Res 18: 175–185, 2005 [PubMed] [Google Scholar]
- 19. Eisenberg MI. Magnesium deficiency and sudden death. Am Heart J 124: 544–549, 1992 [DOI] [PubMed] [Google Scholar]
- 20. Field KA, Holowka D, Baird B. Compartmentalized activation of the high affinity immunoglobulin E receptor within membrane domains. J Biol Chem 272: 4276–4280, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Futerman AH. Ceramide Signaling. New York, NY: Kluwer, 2002 [Google Scholar]
- 22. Goni FM, Alonso A. Effects of ceramide and other simple sphingolipids on membrane lateral structure. Biochim Biophys Acta 1788: 169–177, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Handwerker SM, Altura BT, Royo B, Altura BM. Ionized magnesium and calcium levels in umbilical cord blood of pregnant women with transient hypertension during labor. Am J Hypertens 6: 542–545, 1993 [DOI] [PubMed] [Google Scholar]
- 24. Handwerker SM, Altura BT, Royo B, Altura BM. Ionized serum magnesium and potassium levels in pregnant women with preeclampsia. J Reprod Med 40: 201–208, 1995 [PubMed] [Google Scholar]
- 25. Hannun YA. Functions of ceramide in coordinating response to stress. Science 274: 1855–1859, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Holtis JCM, Pomorowski T, Raggers RJ, Sprong H, Van Meer G. The organizing potential of sphingolipids in intracellular membrane transport. Physiol Rev 81: 1689–1723, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Jiang XC, Paultre F, Pearson TA, Reed RG, Francis CK, Lin Berglund L, Tall AR. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscl Thromb Vasc Biol 20: 2614–2618, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Joffres MR, Reed DM, Yano K. Relation of magnesium intake and other dietary factors to blood pressure: the Honolulu Heart Study. Am J Clin Nutr 118: 114–120, 1987 [DOI] [PubMed] [Google Scholar]
- 29. Johns DG, Webb RC. Ceramide: a novel cell signaling mechanism for vasodilation. Biochem Biophys Res Commun 237: 95–97, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Kolesnick R. Signal transduction through the sphingomyelin pathway. Mol Chem Neuropathol 21: 287–297, 1994 [DOI] [PubMed] [Google Scholar]
- 31. London M, London E. Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): implications for lipid raft structure and function. J Biol Chem 279: 9997–10004, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Masserini M, Ravaski D. Role of sphingolipids in the biogenesis of membrane domains. Biochim Biophys Acta 1532: 149–161, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Mazur A, Maier JA, Rock E, Gueux E, Nowacki W, Rayssiguier Y. Magnesium and the inflammatory response: potential pathophysiological implications. Arch Biochem Biophys 458: 48–56, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Merrill AH., Jr De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J Biol Chem 277: 25843–25846, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Morrill GA, Gupta RK, Kostellow AB, Ma GY, Zhang A, Altura BT, Altura BM. Mg2+ modulates membrane lipids in vascular smooth muscle: a link to atherogenesis. FEBS Lett 408: 191–194, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Morrill GA, Gupta RK, Kostellow AB, Ma GY, Zhang A, Altura BT, Altura BM. Mg2+ modulates membrane sphingolipids and lipid second messenger levels in vascular smooth muscle cells. FEBS Lett 440: 167–171, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Quamme GA. Molecular identification of ancient and modern mammalian magnesium transporters. Am J Physiol Cell Physiol 298: C407–C429, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Rayssiguier Y, Gueux E. Magnesium and lipids in cardiovascular disease. J Am Coll Nutr 5: 507–519, 1986 [DOI] [PubMed] [Google Scholar]
- 39. Resnick LM, Bardicef D, Altura BT, Alderman MH, Altura BM. Serum ionized magnesium: relation to blood pressure and racial factors. Am J Hypertens 10: 1420–1424, 1997 [DOI] [PubMed] [Google Scholar]
- 40. Saris NE, Mervaala E, Karppanen H. An update on physiological, clinical and analytical aspects. Clin Chem Acta 294: 1–26, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Seelig MS, Rosanoff A. The Magnesium Factor. New York, NY: Penguin Group, 2003 [Google Scholar]
- 42. Simard JM, Gerzanich V. Sphingolipids and transient receptor potential channels: Evolutionarily ancient families now joined. Circ Res 98: 1347–1348, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Simons K, Ikonen E. Functional rafts in cell membranes. Nature 387: 569–572, 1997 [DOI] [PubMed] [Google Scholar]
- 44. Touyz RM. Transient receptor potential melastatin 6 and 7 channels, magnesium transport and vascular biology: implications in hypertension. Am J Physiol Heart Circ Physiol 294: H1103–H1118, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Turlapaty PDMV, Altura BM. Magnesium deficiency produces spasms of coronary arteries: relationship to etiology of sudden death ischemic heart disease. Science 208: 198–200, 1980 [DOI] [PubMed] [Google Scholar]
- 46. Wang TY, Silvius JR. Sphingolipid partitioning into ordered domains in cholesterol-free and cholesterol-containing lipid bilayers. Biophys J 84: 367–378, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weglicki WB, Phillips TM, Freedman AM, Cassidy MM, Dickens BF. Magnesium deficiency elevates circulating levels of inflammatory cytokines and endothelin. Mol Cell Biochem 118: 105–111, 1992 [DOI] [PubMed] [Google Scholar]
- 48. Xu SZ, Muraki K, Zeng F, Li j Sukumar P, Shah S, Dedman AM, Flemming PK, McHugh D, Naylor J, Cheong A, Bateson AN, Munsch CM, Porter KE, Beech DJ. A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle motility. Circ Res 98: 1381–1389, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wymann M, Schneiter R. Lipid signaling in disease. Nat Rev Mol Cell Biol 9: 162–176, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Yang ZW, Altura BT, Altura BM. Low eztracellular magnesium contractions of arterial muscle: role of protein kinase C and protein tyrosine phosphorylation. Eur J Pharmacol 378: 273–281, 1999 [DOI] [PubMed] [Google Scholar]
- 51. Yang ZW, Gebrewold A, Nowakowski M, Altura BT, Altura BM. Mg2+-induced endothelial -dependent relaxation of blood vessels and blood pressure lowering: role of NO. Am J Physiol Regul Integr Comp Physiol 278: R628–R639, 2000 [DOI] [PubMed] [Google Scholar]
- 52. Yang ZW, Wang J, Zheng T, Altura BT, Altura BM. Low extracellular Mg induces contraction and [Ca2+]i rises in cerebral arteries: roles of Ca2+, PKC and PI 3-kinases. Am J Physiol Heart Circ Physiol 279: H2898–H2907, 2000 [DOI] [PubMed] [Google Scholar]
- 53. Yu C, Alterman M, Dobrowsky RT. Ceramide displaces cholesterol from lipid rafts and decreases the association of the cholesterol binding protein caveolin-1. J Lipid Res 46: 1678–1691, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Zhang A, Cheng TP, Altura BM. Magnesium regulates intracellular free ionized calcium concentration and cell geometry in VSMC. Biochim Biophys Acta 1134: 25–29, 1992 [DOI] [PubMed] [Google Scholar]
- 55. Zhang A, Cheng TPO, Altura BT, Altura BM. Extracellular magnesium regulates intracellular free Mg2+ in vascular smooth muscle cells. Pflug Archiv Eur J Physiol 421: 391–393, 1992 [DOI] [PubMed] [Google Scholar]
- 56. Zheng T, Li W, Altura BT, Altura BM. C2-ceramide attenuates prostaglandin F2-induced vasoconstriction and elevation of [Ca2+]I in canine cerebral vascular smooth muscle. Neurosci Lett 256: 113–116, 1998 [DOI] [PubMed] [Google Scholar]
- 57. Zheng T, Li W, Wang J, Altura BT, Altura BM. C2-ceramide attenuates phenylephrine-induced vasoconstriction and elevation in [Ca2+]i in rat aortic smooth muscle. Lipids 24: 689–695, 1999 [DOI] [PubMed] [Google Scholar]
- 58. Zheng T, Li W, Wang J, Altura BT, Altura BM. Effects of neutral-sphingomyelinase on phenylephrine-induced vasoconstriction and Ca2+ mobilization in rat aortic smooth muscle. Eur J Pharmacol 191: 127–135, 1999 [DOI] [PubMed] [Google Scholar]
- 59. Zheng T, Li W, Wang J, Altura BT, Altura BM. Sphingomyelinase and ceramide analogs induce contraction and rises of [Ca2+]i in canine cerebral vascular muscle. Am J Physiol Heart Circ Physiol 278: H1421–H1428, 2000 [DOI] [PubMed] [Google Scholar]