Abstract
Calsequestrin 2 (CSQ2) is generally regarded as the primary Ca2+-buffering molecule present inside the sarcoplasmic reticulum (SR) in cardiac cells, but findings from CSQ2 knockout experiments raise major questions about its role and necessity. This study determined the absolute amount of CSQ2 present in cardiac ventricular muscle to gauge its likely influence on SR free Ca2+ concentration ([Ca2+]) and maximal Ca2+ capacity. Ventricular tissue from hearts of freshly killed sheep was examined by SDS-PAGE without any fractionation, and CSQ2 was detected by Western blotting; this method avoided the >90% loss of CSQ2 occurring with usual fractionation procedures. Band intensities were compared against those for purified CSQ2 run on the same blots. Fidelity of quantification was verified by demonstrating that CSQ2 added to homogenates was detected with equal efficacy as purified CSQ2 alone. Ventricular tissue from sheep (n = 8) contained 24 ± 2 μmol CSQ2/kg wet wt. Total Ca2+ content of the ventricular tissue, measured by atomic absorption spectroscopy, was 430 ± 20 μmol/kg (with SR Ca2+ likely <250 μmol/kg) and displayed a linear correlation with CSQ2 content, with gradient of ∼10 Ca2+ per CSQ2. The large amount of CSQ2 bestows the SR with a high theoretical maximal Ca2+-binding capacity (∼1 mmol Ca2+/kg ventricular tissue, assuming a maximum of ∼40 Ca2+ per CSQ2) and would keep free [Ca2+] within the SR relatively low, energetically favoring Ca2+ uptake and reducing SR leak. In mice with CSQ2 ablated, histidine-rich Ca2+-binding protein was upregulated ∼35% in ventricular tissue, possibly in compensation.
Keywords: excitation-contraction coupling, sarcoplasmic reticulum, calcium content, calcium buffering, catecholaminergic polymorphic ventricular tachycardia, calsequestrin 2 knockout mice
calsequestrin 2 (CSQ2) is the sole calsequestrin isoform present in the sarcoplasmic reticulum (SR) in cardiac muscle (2, 6, 9) and is thought to be predominantly localized to the junctional SR (11, 14, 44). CSQ2 can bind up to ∼35–40 Ca2+ per CSQ2 molecule (30), or perhaps more (34), with an apparent dissociation constant of ∼0.5 mM in typical in vitro conditions (30, 39). CSQ2 is widely regarded as the principal Ca2+-buffering molecule present within the SR in adult cardiac muscle cells, although other Ca2+-binding proteins are also present (6). Acute or chronic overexpression of CSQ2 results in an increase in SR Ca2+ content (21, 43), and conversely acute reduction in expression of CSQ2 by gene silencing results in a decrease in SR Ca2+ content (43), although the relative contributions of alterations in SR Ca2+ storage capacity and in Ca2+ leakage out of the SR are uncertain. Ablation of CSQ2 in mice caused catecholaminergic polymorphic ventricular tachycardia (CPVT) (25). Surprisingly, the complete loss of cardiac CSQ2 in this mouse model resulted in only an apparently minor reduction in SR Ca2+ content in basal conditions (as measured by rapid application of caffeine), with the only evident compensation being an ∼50% increase in total SR volume (11, 25). These findings were interpreted as showing that the contribution of CSQ2 to SR Ca2+ storage and release during excitation-contraction coupling is largely dispensable, raising major questions about the assumed importance of CSQ2 in buffering Ca2+ within the SR (24).
In deciding whether or not CSQ2 is likely to have a major role in SR Ca2+ buffering and movements in cardiac muscle, one important consideration has to be how much CSQ2 is actually present (3, 18). However, this has not been determined previously, and, to date, there are only inexact estimates of the amount of CSQ2 in cardiac muscle (see page 171 in Ref. 3). These estimates (∼80–160 mg/kg wet wt) are extrapolations from measurements of CSQ2 in isolated SR preparations (7), based on the assumption that the yield of total CSQ2 was 25–50% (3). However, even with such assumptions, this presumed CSQ2 content could only bind a maximum of ∼140 μmol Ca2+/kg, which is threefold lower than some estimates of the maximal SR Ca2+ content (∼400 μmol Ca2+/kg wet wt) (3, 20, 38).
In the present study, we first show that SR preparations actually contain a much lower proportion of the total CSQ2 than assumed in previous calculations (3). We then use our recently developed quantitative Western blotting technique (31, 32) to determine the absolute amount of CSQ2 present in adult cardiac muscle using unfractionated samples of ventricular tissue. Finally, we demonstrate that, when unfractionated samples are examined, the histidine-rich Ca2+-binding protein (HRC) is upregulated ∼35% or more in cardiac SR from CSQ2 knockout (KO) compared with wild-type (WT) mice. Our key finding, that there is a comparatively large absolute amount of CSQ2 present in normal adult cardiac muscle, strongly suggests that CSQ2 is indeed likely to be the predominant Ca2+ buffer in the SR, and likely substantially influences the intraluminal SR free Ca2+ concentration ([Ca2+]) and Ca2+ uptake and release.
MATERIALS AND METHODS
Purification of CSQ2.
Sheep cardiac SR vesicles were isolated as described in Laver et al. (26), as approved by the Australian National University Animal Ethics Committee. CSQ2 was purified by gel elution from large vertical slab SDS polyacrylamide gels (46), with minor changes. In brief, sheep cardiac SR was loaded on a 10% SDS polyacrylamide gel and separated electrophoretically. Bands of CSQ2 (resolving at > 50 kDa) were excised and eluted from the gel matrix by resuspension in a buffer containing 20 mM MOPS and 150 mM NaCl (pH 7.4) for 24–36 h at either room temperature or 37°C, with gentle agitation. Purification was enhanced by immunoselection with anti-CSQ.
Protein assay.
Protein content of the purified sheep CSQ2 was measured using both Bradford and Lowry protein assays against standard curves of BSA. For the former, 1 ml of Bradford reagent (catalog no. B6916; Sigma) was added to a cuvette with 25 μl of CSQ2 sample, and absorbance was read at 595 nm. Concentration was calculated by comparing with a standard curve of known amounts of BSA (0–250 μg). For the Lowry assay, a microplate was used, and 25 μl of DC protein assay Reagent A (catalog no. 500–0110; Bio-Rad, Hercules, CA) were added to a well with 5 μl of CSQ2 sample. After mixing, 200 μl of reagent B (Folin's reagent) were added, and following 15 min incubation at room temperature the absorbance was read at 650 nm. The estimate of the CSQ2 concentration was slightly higher using the Lowry assay (0.44 mg/ml) compared with the Bradford assay (0.34 mg/ml). The value obtained using the Bradford assay was used for consequent calculations, which, if anything, would result in underestimation of the amount of CSQ2 present. CSQ2 protein content was also further verified by using silver staining (see below) to directly compare the purified sheep CSQ2 with a range of known amounts of human recombinant CSQ2 (e.g., see Fig. 2D).
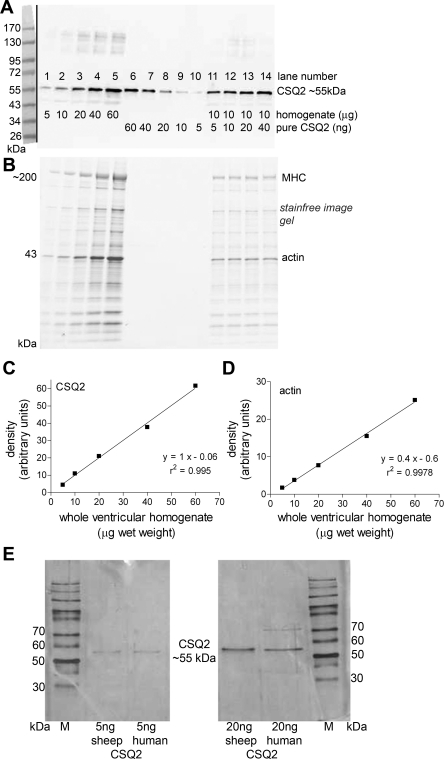
Fig. 2.
CSQ2 in sheep cardiac ventricular muscle. A: Western blot of CSQ2 in homogenate samples of sheep cardiac ventricular tissue (5–60 μg tissue, lanes 1–5) compared with purified sheep cardiac CSQ2 (60–5 ng, lanes 6–10) and with homogenate samples (10 μg of tissue) with added purified CSQ2 (5–40 ng, lanes 11–14). B: MHC and actin were identified in gels before transfer (see materials and methods) to verify relative loading in each lane. Band intensities for CSQ2 (Western blot, C) and actin (pretransfer, D) in homogenate samples (lanes 1–5 in A and B, respectively) are shown with linear regressions (y = mx + c), and r2 values for lines of best fit are indicated. E: silver gels showing a single band for recombinant human CSQ2 and CSQ2 purified from sheep heart. Gels also compare the same amounts (5 and 20 ng) of CSQ2 purified from sheep hearts and from cells recombinantly expressing human CSQ2.
Silver staining.
The amount and purity of the sheep cardiac CSQ2 was assessed by silver staining, resolving samples of purified CSQ2 on 8 or 10% SDS polyacrylamide gels and detecting proteins with the GelCode SilverSNAP Stain Kit II (Pierce) as described previously (45) and per the manufacturer's instructions. Molecular mass standards used were PageRuler protein ladder (catalog no. SM0661; Fermentas, Burlington, Canada).
Ventricular homogenates.
Whole hearts from freshly killed adult sheep (n = 8, 10–12 mo old) were obtained from an abattoir and placed on dry ice while still warm. A portion (∼200 mg) of the ventricular tissue was cut into small pieces and homogenized (1:10 wt/vol) in buffer 1 (Na-EGTA solution) consisting of (in mM) 165 Na+, 50 EGTA, 90 HEPES, 1 free Mg2+ (10.3 total Mg2+), 8 total ATP, 10 creatine phosphate, pH 7.10, with addition of protease inhibitor cocktail (PIC, COMplete; Roche Diagnostics, Sydney, Australia). Homogenates were further diluted 1:40 with buffer 1 and incubated on ice for 40 min. They were then subsequently added (2:1 vol/vol) to SDS loading buffer (0.125 M Tris·HCl, 10% glycerol, 4% SDS, 4 M urea, 10% mercaptoethanol, and 0.001% bromophenol blue, pH 6.8) to give a final concentration of ∼1.7 μg muscle/μl. Preparations were stored at −20°C until analyzed by Western blotting.
In the case of the rat ventricular tissue, with approval of the La Trobe University Animal Ethics Committee, male Long-Evans hooded rats (∼5–8 mo old) were killed by overdose with isoflurane (4% vol/vol), the heart was rapidly excised, and a portion of the ventricular muscle was homogenized using the same protocol as above.
Homogenate fractionation procedures.
Crude SR preparations were obtained from rat ventricular tissue, but, importantly, fractions that are typically discarded were collected to establish how much CSQ2 was present in those fractions. Muscle was homogenized (1:10 wt/vol) in buffer 2 containing (in mM) 300 sucrose and 2.5 NaOH, and two aliquots of equal volume were taken. The first aliquot was immediately prepared for Western blotting (1:2 vol/vol with SDS loading buffer), whereas the matching second aliquot was instead subjected to the fractionation procedures. It was first centrifuged (10,000 g, 3 min); the resulting pellet was resuspended for Western blotting (in a volume equal to that of the whole homogenate in SDS loading buffer), whereas the supernatant was further centrifuged (14,100 g, 30 min). The supernatant was collected for Western blotting (again in the same final volume as for the whole homogenate in SDS loading buffer), and the pellet (crude SR) was resuspended in a solution containing (in mM) 150 KCl and 20 MES and centrifuged (14,100 g, 30 min). Last, both the supernatant and the final pellet (crude SR) were prepared for Western blotting, again each in the same final volume as for the whole homogenate in SDS loading buffer. This procedure allowed direct comparison of CSQ2 band intensities for the various fractions and total homogenate (see Fig. 1).
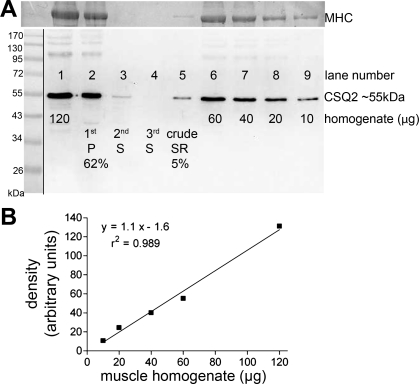
Fig. 1.
Loss of calsequestrin 2 (CSQ2) during fractionation when preparing crude sarcoplasmic reticulum (SR). A: Western blot of whole homogenate samples of sheep cardiac ventricular tissue (lanes 1 and 6-9, loaded with 120, 60, 40, 20, and 10 μg of tissue, respectively) and fractions (lanes 2-5) collected at each step during production of crude SR vesicles from another 120-μg sample of the same whole homogenate. Each fraction was made up to the same final volume as for the 120-μg whole homogenate in lane 1, enabling direct comparison of band intensity between lane 1 and lanes 2-5. More than 60% of the total CSQ2 was lost in the first pellet (lane 2), and only 5% of the total CSQ2 was recovered in the final crude SR preparation (lane 5). Homogenization was performed in buffer 1 (Na-EGTA solution). Myosin heavy chain (MHC) in the posttransferred gel was detected with Coomassie blue staining and used to verify the relative amount of muscle tissue loaded in each lane. Of the total MHC, 78% was found in the first pellet (lane 2) and <2% was found in the crude SR preparation (lane 5). Molecular mass markers are shown in position on left (see materials and methods). B: calibration curve derived by plotting CSQ2 band intensity (in arbitrary units) vs. the amount of whole homogenate loaded for lanes 1 and 6-9 in A, with the linear regression (y = mx + c) and the r2 value for the line of best fit indicated. Such calibration on the same gel is required to accurately relate the band intensity for a given fraction sample to the proportion of CSQ2 present. P, pellet; S, supernatant.
Additionally, portions of sheep ventricular tissue were homogenized and then fractionated using each of three different types of buffer to test whether the buffer composition affected the CSQ2 yield [buffer 1 (as above): Na-EGTA solution but without PIC; buffer 2 (as above): 0.3 M sucrose and 2.5 mM NaOH; or buffer 3: 10 mM NaHCO3, 50 mM NaF, and 5 mM Na4P2O7 at pH 7.4]. In each case, one or more aliquots of whole homogenate that had not been subjected to any centrifugation was prepared (1:2 vol/vol) in SDS loading buffer. A separate equal volume of whole homogenate was centrifuged at 10,000 g for 20 min. The supernatant and pellets were separated and collected into a volume of SDS loading buffer equal to the final volume of that used for the whole homogenate sample. Equal volumes of each sample were loaded on SDS-PAGE for Western blotting.
CSQ2 KO mice.
CSQ2 KO mice used have been described previously (25). The mice are bred on a background of C57BL/6 and were 3–4 mo of age. CSQ2 KO and WT mice of either sex were killed by exsanguination under general anesthesia with isoflurane vapor at Vanderbilt University as approved by the Vandrbilt University Animal Care and Use Committee, and the hearts were excised and sent frozen on dry-ice to La Trobe University, Australia. Western blotting was used to assess the relative content of HRC protein in cardiac tissue from CSQ2 KO (n = 7) and WT (n = 4) mice. Portions (∼15–20 mg) of ventricular tissue from different KO and WT mice were homogenized individually in Na-EGTA solution (buffer 1, above, 1:20 wt/vol). Unfractionated samples were added (2:1 vol/vol) to SDS loading buffer at a final concentration of ∼5 μg muscle/μl. Preparations were stored at −20°C until analyzed in their entirety by Western blotting. For Western blotting, equal volumes of ventricular preparations from KO and WT mice were mixed together and used to generate a signal calibration curve on each gel.
Western blotting.
Entire ventricular homogenates (5–60 μg muscle tissue), and subfractions where required, were analyzed for CSQ2 and/or HRC protein contents by Western blotting using a similar protocol to that described previously (32). Protein from muscle preparations, as well as pure CSQ2 protein samples, was separated on 8% SDS-polyacrylamide gels or 10% Criterion Stainfree gels (Bio-Rad) and probed with antibodies against CSQ2 (1 in 2,000, rabbit affinity isolated polyclonal, ab 3516; Abcam) or HRC [1 in 1,000, rabbit polyclonal (15, 22), kindly provided by Dr. Woo Jin Park] both diluted in 1% BSA in PBS with 0.025% Tween. In some circumstances, following transfer, the SDS polyacrylamide gel was stained with BioSafe Coomassie Stain (Bio-Rad) for detection of myosin heavy chain (MHC). Although this staining step had to be performed following transfer due to Coomassie staining interfering with Western blotting, we have previously shown it to be a valid indicator of the relative amount of protein loaded when only small amounts of muscle are loaded on a gel, e.g., in fiber segments (32). In some circumstances, samples were loaded on a new formulation of gel that allows a digital image of the SDS polyacrylamide gel to be collected after electrophoresis where the proteins are observed following ultraviolet-induced modification of tryptophan residues present in the proteins (Bio-Rad Criterion Stain Free system, Bio-Rad tech note 5782). The relative amounts of myosin and actin present in the samples were determined following activation of the gels, collection of the image, and analyses, all using Image Lab software (Bio-Rad), and used as an indicator of the amount of protein loaded. Following image collection, proteins were transferred to nitrocellulose, and Western blotting continued as described above. Chemiluminescent substrate (SuperSignal West Femto; Pierce) was applied to the membrane, and Western blot images were taken with ChemiDoc XRS fitted with a CCD camera using Quantity One software (Bio-Rad). With the membrane position unchanged, the white light source was switched on to obtain an image of the prestained molecular mass markers on the membrane, and this image was then overlayed in its correct position on the Western blot image (i.e., Figs. 1A, 2A, and 5A, left). Densitometry was performed with the Quantity One software.
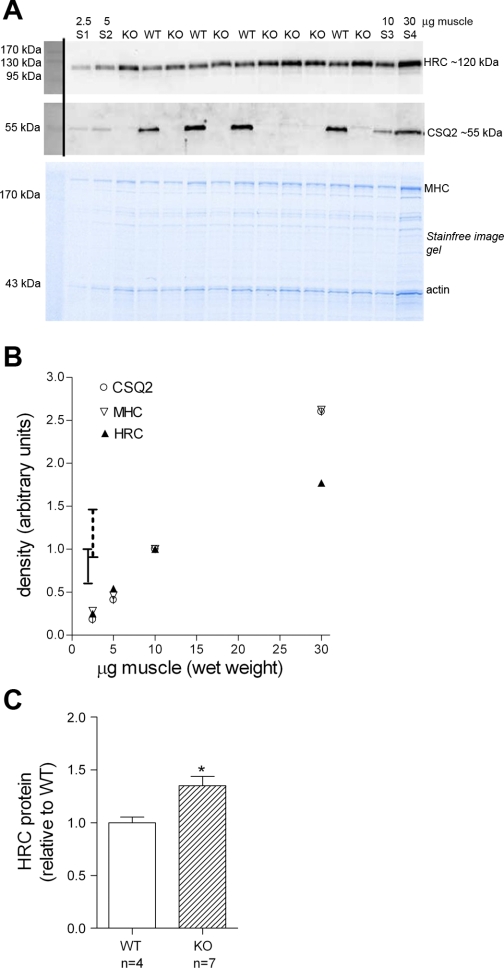
Fig. 5.
Histidine-rich Ca2+-binding protein (HRC) is upregulated in ventricular muscle from CSQ2 knockout (KO) mice. A: 10-μg samples of ventricular muscle from CSQ2 KO mice (n = 7) and wild-type (WT) mice (n = 4) were separated on 10% Stainfree gel (see materials and methods). Calibration curve was generated using 2.5, 5, 10, and 30 μg muscle (S1-S4) of a mixture made from equal portions of all homogenates. Blot was cut at ∼72 kDa, and separate parts were probed for HRC (top) and CSQ2 (middle). The relative amount of protein loaded in each lane was apparent on the pretransfer Stainfree image (bottom). B: band intensities for HRC and CSQ2 (Western blot) and MHC (Stainfree gel) for homogenate standards (lanes S1, S2, S3, and S4), normalized to respective amount in S3. Range of HRC densities in homogenate samples for CSQ2 HRC KO and WT mice is indicated by vertical lines (WT range: 0.59–1.02, solid line; KO range: 0.90–1.48, broken line). C: mean (+SE) of HRC density in WT and KO homogenate samples; each HRC band was normalized to density of corresponding MHC band, and the value is expressed relative to the average of that for all WT samples on the same gel. Ventricular tissue from KO mice had ∼35% more HRC protein than WT mice (*P < 0.05, 2-tailed unpaired t-test, average of triplicate runs of same samples) (see text).
Staining of Ca2+-binding proteins.
Stains-all is a cationic carbocyanine dye that stains Ca2+-binding proteins blue or red depending on the conditions (8). Homogenate samples with 1.5 mg muscle wet weight of sheep or rat ventricle or rat soleus muscle and 3 μg of sheep purified CSQ2 were separated in different lanes on an 8% SDS polyacrylamide gel and, following washes in 25% isopropanol to remove SDS (4 × 30 min), exposed to Stains-all solution (0.025% Stains-All, 7.5% formamide, 25% isoproponal, 30 mM Tris base, pH 8.8) overnight in a container protected from light. Following staining, the gel was washed extensively in 25% isopropanol to destain. Stains-all-stained gels were photographed with a digital camera (Panasonic Lumix).
Atomic absorption spectroscopy.
When determining total Ca2+ content, ventricular samples from the different hearts were all prepared and run in parallel. Portions (∼1 g) of frozen sheep ventricular tissue were cut into small pieces and sat at room temperature ∼15 min to fully thaw. Pieces were then blotted onto filter paper, weighed accurately, and placed in an oven at 70°C for ∼64 h. Samples then underwent nitric acid/perchloric acid (PCA) digestion where 7.5 ml of acid solution (4 parts nitric acid to 1 part PCA, analytical grade) were added to each sample, and the samples went through a series of heating stages (4.5–230°C) for various time periods (20 min to ∼3 h). Following digestion, 10 ml reverse osmosis water were added to each sample, and they were vortexed vigorously, heated ∼100°C for 5 min, and vortexed again until PCA was fully dissolved. Ca2+ standard solutions in the [Ca2+] range 0–5 μg/ml were used. Following the addition of 0.5 ml of a 20 mg/ml Sr2+ solution to 5 ml of all samples and standards, [Ca2+] was determined by atomic absorption spectroscopy using a Varian AA-1475 series instrument and Ca2+ lamp at 422.7 nm with 0.2 nm slit and an air-acetylene reducing flame.
Statistical analyses.
Differences between CSQ2 KO and WT animals were analyzed using a Student's t-test (unpaired, 2-tailed). To compare differences between the amount of CSQ2 in tissue from mouse, rat, and sheep, a one-way ANOVA was performed, with Newman-Keuls post hoc analysis. Statistics and linear regression equations were calculated using GraphPad software version 4.01. Data are expressed as means ± SE. Statistical significance was accepted if P < 0.05.
RESULTS
Loss of CSQ2 during fractionation.
We first examined the effects of tissue fraction on the yield of CSQ2. We determined the percentage retention of CSQ2 in the various fractions produced during the preparation of crude SR vesicles by typical methods (9, 23, 42). Ventricular muscle was homogenized in one of three different buffers (see materials and methods), with similar results found irrespective of the buffer used. Western blotting was used to quantify the amount of CSQ2 present in the whole homogenate and in fractions of that homogenate. The surprising result, as seen in Fig. 1, is that the great majority of the CSQ2 is actually lost to the pellet during the initial spin typically used to remove nuclei, mitochondria, and cell debris (∼10,000 g for 20 min). In the case where samples prepared in each of the three types of buffer were compared on the same gel, the proportion of total CSQ2 found in the first pellet was 84, 92, and 90% for buffers 1 to 3, respectively. After the usual further centrifugation and fractionation steps, the final crude SR preparation was found to contain only ∼5% of the CSQ2 that had been present in the original ventricular tissue (e.g., compare lane 5 with lane 1 in Fig. 1A). Thus it is clear that the CSQ2 amount present in ventricular muscle needs to be quantified using whole homogenates rather than SR vesicles or other subfractions.
Measurement of absolute amounts of CSQ2 present in ventricular homogenates.
The absolute amount of CSQ2 present in sheep ventricular tissues was determined by comparing the Western blot band intensities for homogenate samples with those for a range of amounts of purified CSQ2 obtained from sheep heart (Fig. 2). Silver gels were used to verify that this sheep CSQ2 was relatively pure and in its full-length 55-kDa form (e.g., Fig. 2E). Also, comparing sheep CSQ2 and recombinant human CSQ2 by silver staining gave similar density values when putatively the same amounts of CSQ2 were loaded side by side (Fig. 2E). For accurate quantification of the CSQ2 in the homogenate samples, it was crucial to make comparison of Western blot signals between homogenate samples and purified CSQ2 present on the same gel; such direct comparison was done for every measurement reported in the present study. Furthermore, it was crucial to use relatively small samples of tissue to avoid possible saturation problems and also to examine the Western blot signals over a whole range of purified CSQ2 amounts to obtain a full and accurate calibration curve for converting the band intensity values of the homogenate samples to CSQ2 amounts (31). Finally, it was vital to demonstrate the fidelity of the quantification method by verifying that purified CSQ2 added to homogenate samples was detected with equal efficacy as when purified CSQ2 was run in lanes by itself. An example of this is shown in Fig. 3 by the fact that band intensities for the homogenate sample with added CSQ2 increased in parallel with the calibration curve for CSQ2 alone; similar results were seen on four separate Western blots. Thus one can have confidence that the overall procedure provides an accurate estimate of the absolute CSQ2 content of the ventricular tissue.
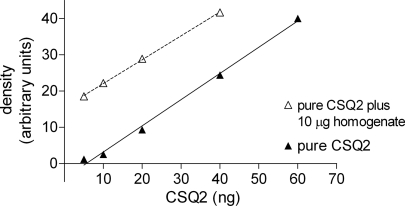
Fig. 3.
Validation of directly comparing signals for purified CSQ2 and homogenate samples. Western blot CSQ2 band intensities for various amounts of purified CSQ2 (lanes 6–10 in Fig. 2A, closed triangles) and for 10 μg ventricular homogenate samples with added CSQ2 (lanes 11–14 in Fig. 2A, open triangles). CSQ2 present in the homogenate samples was detected with similar efficacy as purified CSQ2 by itself. Linear regressions for lines of best fit are y = 0.73x − 4.0, r2 = 0.995 (pure CSQ2) and y = 0.66x − 15.5, r2 = 0.999 (pure CSQ2 + homogenate). Similar results were obtained on 4 separate Western blots.
Using these procedures, the CSQ2 content in ventricular samples from eight different adult sheep was found to be 1.1 ± 0.1 ng/μg wet wt of tissue (mean ± SE, n = 8, with each sample run 2–6 times across 6 Western blots). Based on a deduced MW of 45,269 Da (36), this equates to 24 ± 2 μmol of CSQ2/kg ventricular tissue.
Ca2+ content of ventricular samples.
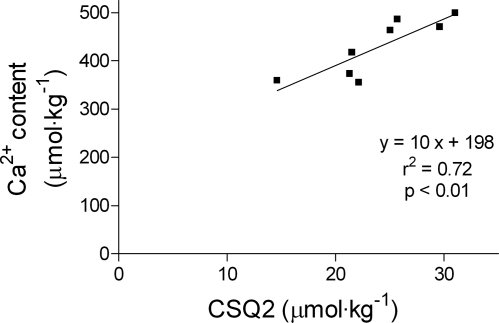
The total Ca2+ content in other portions of the same eight ventricular samples was found by atomic absorption spectroscopy to be 430 ± 20 μmol/kg tissue (mean ± SE). Total water content of the samples was 73 ± 2% (mean ± SD, n = 8, range 70–76%). The Ca2+ content values were plotted against the matching CSQ2 content for the same ventricular samples (Fig. 4). Linear regression analysis indicated a linear correlation between total Ca2+ content and CSQ2 content in the samples, with a gradient of ∼10 Ca2+ per CSQ2 molecule and an intercept of ∼200 μmol Ca2+/kg tissue (r2 = 0.72, P < 0.01). There was no such linear relationship between the Ca2+ contents and the relative amounts of MHC (r2 = 0.34, P = 0.13), indicating that the correlation between CSQ2 and Ca2+ content was not just the result of differences in myocardial cell density in the different samples.
Fig. 4.
Correlation of total Ca2+ content and CSQ2 content in ventricular samples from hearts of 8 sheep. Ca2+ content was measured by atomic absorption spectroscopy, and CSQ2 content was measured by Western blotting as in Fig. 2; values/kg wet wt of muscle. Linear regression was performed, and r2, P value, and the line of best fit are indicated. The fit values were 10 ± 2.5 (slope) and 198 ± 60 (y-intercept).
Comparison of HRC protein content in cardiac muscle from CSQ2 KO and WT mice.
Western blotting was also used to assess the relative content of HRC protein in ventricular tissue from CSQ2 KO (n = 7) and WT (n = 4) mice to test whether any upregulation of this SR Ca2+-binding protein could be detected in the KO mice tissue if total samples were examined rather than fractionated samples as in a previous examination (25). Samples were analyzed in their entirety (see materials and methods), with samples from all the animals compared together on each of three separate Western blots (e.g., see Fig. 5A). It was readily apparent from the Western blots that there was substantially more HRC in the CSQ2 KO tissue than in the WT tissue. The HRC measurements for the samples from WT mice all fell within the range where the HRC band density was close to directly proportional to the amount of tissue loaded (see values for calibration standards run on same gel; Fig. 5B). Some of the HRC measurements for the CSQ2 KO mice, however, fell in the range where the HRC band intensity seemingly deviated below such proportionality (see Fig. 5B). Rather than attempt any interpolation of the calibration data in that range (i.e., for HRC density for samples between 10 and 30 μg), we calculated the amount of HRC present in the CSQ2 KO tissue samples assuming the proportional relationship still held, which likely slightly underestimated the amount of HRC actually present in the KO samples. This analysis indicated that the ventricular tissue of the CSQ2 KO mice on average contained ∼35% more HRC protein than that of the WT mice (Fig. 5C), which might reflect compensatory upregulation of HRC to provide additional Ca2+ buffering in the absence of CSQ2.
Comparison of rat, mouse, and sheep ventricular homogenates.
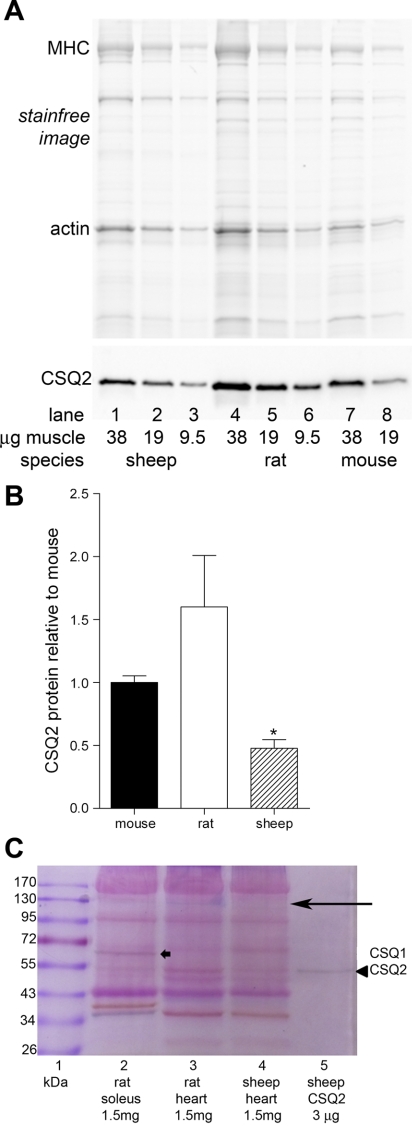
It was not possible to make a precise estimate of the amount of CSQ2 in rat cardiac tissue using the quantitative Western blotting method because sufficient amounts of appropriately purified CSQ2 could not be obtained from rat hearts. To address the question of whether the amount of CSQ2 is similar between rat, sheep, and mouse cardiac muscle, whole ventricular preparations were examined side by side by Western blotting and also by Stains-all staining. These approaches were undertaken because a limitation of the Western blotting approach is that it could not be assumed that the CSQ2 antibody would detect rat, mouse, and sheep CSQ2 with equal efficacy. A major limitation of the Stains-all approach is that quantitative analysis is not possible due to nonlinearity and nonspecificity of the staining. Nevertheless, useful data could be obtained using a combination of the two methods. Taking into account the relative amounts of tissue loaded (as indicated by densities of actin and MHC on Stainfree gel; Fig. 6A, top), analysis of the Western blotting band intensities suggests that the amount of CSQ2 present in ventricular muscle was broadly similar in rat and mouse but that it was significantly lower in sheep than in rat (Fig. 6, A and B). The Stains-all staining had to be performed on whole homogenate samples despite the problems with background staining and the relatively low intensities of the relevant bands, owing to the major uncontrolled loss of CSQ2 occurring when attempting any subfractionation or purification of the preparation (Fig. 1). Even in partially purified preparations, Stains-all staining of CSQ2 may appear purple or even dark red rather than blue (e.g., see Fig. 3D in Ref. 32). If it is assumed that the staining band at ∼55 kDa in lanes 3 and 4 in the Stains-all image in Fig. 6 is predominantly due to CSQ2, it again appears that rat ventricular tissue (lane 3) contained more CSQ2 than sheep ventricular tissue (lane 4). In addition, a blue band was apparent at ∼120–130 kDa [most prominent in the rat heart homogenate (lane 3); Fig. 6C, arrow on top], which is probably HRC (22) (see Fig. 5A). If this is the case, comparison of its intensity with the purified CSQ2 band at 55 kDa (lane 5) suggests that the amount of Ca2+ binding to HRC is quite appreciable, at least in the rat ventricular tissue.
Fig. 6.
Comparison of CSQ2 amounts in sheep, rat, and mouse ventricular muscle. Homogenized ventricular samples (without any fractionation) separated on 10% (A) or 8% (C) SDS-PAGE. A: pretransfer Stainfree image of gel (top) and corresponding Western blot of CSQ2 (bottom) for indicated amounts (μg wet wt muscle) of ventricular tissue from sheep (lanes 1–3), rat (lanes 4–6), and mouse (lanes 7 and 8). B: relative amount of CSQ2 in ventricular tissue of different species found by Western blotting. CSQ2 band density for a given sample was first normalized to amount of tissue loaded (gauged by density of corresponding actin band on Stainfree image) and then expressed relative to the average for all mouse samples run on the same gel. Mean data were derived from 3 gels. *P < 0.05 different from rat, 1-way ANOVA, Newman-Keuls post hoc analyses. C: gel of separated proteins stained with Stains-all (see materials and methods). Purified CSQ2 was seen as a single dark band at ∼55 kDa (arrowhead in lane 5); a band was apparent at the same molecular mass in sheep and rat ventricular tissue and also in rat soleus muscle tissue. The blue band at ∼120–130 kDa (long arrow), most apparent in rat ventricular tissue (lane 3), is likely to be HRC. CSQ1 is seen as a dark band in soleus muscle at ∼63 kDa (thick arrow in lane 2). Molecular mass markers are on left (lane 1). An equal amount of unfractionated muscle tissue (1.5 mg wet wt) was loaded in lanes 2, 3, and 4, and 3 μg pure CSQ2 prepared from sheep heart were loaded in lane 5.
DISCUSSION
This study provides the first direct measurement of the total amount of CSQ2 present in cardiac ventricular tissue. The amount found, ∼1.1 g/kg wet wt, is quite a high value given its considerable Ca2+-buffering properties and makes it clear that CSQ2 normally must have a major effect in buffering Ca2+ within the SR. The CSQ2 content measured here is much larger than the only previous estimate in the literature (∼80–160 mg/kg) (3); that estimate had been based on the assumption that a relatively large percentage of the total CSQ2 present in cardiac muscle is recovered in SR vesicle preparations. In contrast to that assumption, it was shown here that only ∼5% of the total CSQ2 is typically recovered in the crude SR vesicle fraction (Fig. 1).
Maximum Ca2+ capacity of CSQ2 and SR.
The literature to date on ventricular tissue contains a very wide spectrum of estimates of maximum SR Ca2+ content, and normal SR Ca2+ content and cycling (see Table 21 in Ref. 3), and it has been unclear whether this large range solely reflects genuine differences between conditions and animal species, or in part also reflects problems with the accuracy of different experimental assays. The amount of CSQ2 found here in adult sheep ventricular tissue (∼24 μmol/kg) indicates that the maximum SR Ca2+ content is likely to be at the upper end of those previous estimates. This CSQ2 could bind a theoretical maximum of ∼960 μmol Ca2+/kg tissue if each CSQ2 molecule can bind up to ∼40 Ca2+ (30), but the maximum functional Ca2+ capacity of the CSQ2 must be somewhat lower than this theoretical maximum because full saturation of CSQ2 with Ca2+ only occurs at very high levels of SR free Ca2+ (30, 39), higher than can be obtained even with optimal SR Ca2+ pumping (3). The maximum Ca2+-binding capacity of the CSQ2 implied by the present findings is generally consistent with upper estimates of the maximum Ca2+ capacity of the SR in cardiac cells, such as the value of ∼430 μmol Ca2+/kg wet wt found with rat ventricular myocytes by Shannon and Bers (38). The latter value in fact may well be an underestimate of the true maximum SR Ca2+ capacity because Ca2+ leakage out of the SR through the pump itself was not blocked in those experiments. Such Ca2+ leakage may have been considerable because there was no creatine phosphate present to help prevent local accumulation of ADP, which is a potent stimulus to pump leakage, at least in skeletal muscle (27, 28, 32).
It is assumed above that all or virtually all of the CSQ2 found in the ventricular homogenate samples here had been present within the SR. Immunolocalization experiments in normal mouse or rat ventricular cells found that the CSQ2 signal was predominantly colocalized with cardiac ryanodine receptors, presumably inside the junctional SR (12, 37).
Normal SR Ca2+ content and CSQ2 occupancy in sheep ventricular tissue.
The total amount of Ca2+ present endogenously in the sheep ventricular tissue here was found to be ∼430 μmol/kg wet wt. A substantial proportion of this total Ca2+ must have been located extracellularly rather than in the ventricular cells themselves. The total water was found to be 73% of overall mass, and so if ∼20% of this was extracellular fluid, with a free ionized [Ca2+] similar to that of plasma (1.2 mmol/l) (47), it would account for ∼180 μmol/kg of the total Ca2+. Furthermore, an appreciable amount of total Ca2+ was likely to have been bound on the outside of the sarcolemma. However, the precise amount bound is uncertain, since the only value seemingly available in the literature is for sarcolemmal vesicles from cultured neonatal heart cells (4), which is unlikely to be directly comparable with the adult tissue here. Even ignoring the contribution of such sarcolemmal-bound Ca2+, as well as any Ca2+ present within the mitochondria [which is likely to be a comparatively low amount in healthy cells (19)], it is apparent that the total Ca2+ actually within the SR in the tissue here was almost certainly less than ∼250 μmol/kg wet wt. Thus, given that some of the Ca2+ within the SR was presumably bound to other binding molecules such as HRC and sarcalumenin (6, 15), and that a small portion of the total SR Ca2+ remains free, it can be concluded that the CSQ2 present in the ventricular tissue here on average must have been loaded with at most ∼10 Ca2+/CSQ2 molecule. This would mean it was loaded at ≤25% of its maximum capacity if the saturating levels are indeed ∼40 Ca2+ per CSQ2 (30) or higher as in the most recent estimate (34). It may be possible, nevertheless, that the CSQ2 in the junctional SR has a higher level of Ca2+ occupancy than any CSQ2 located in the longitudinal SR; the value calculated here is simply the overall average, and local luminal factors in the various regions of the SR may differentially affect CSQ2 polymerization and Ca2+ binding.
In the context of the preceding calculations, it is interesting to note that the total amount of Ca2+ in the different ventricular samples was correlated to the CSQ2 content of those samples (Fig. 4). One interpretation of the linear relationship seen in Fig. 4 is that the extrapolated intercept with the ordinate axis (∼200 μmol Ca2+/kg tissue) indicates the total of all non-SR Ca2+ present in the ventricular samples (likely predominantly being the Ca2+ from extracellular fluid, including that bound to the outside of the ventricular cells) and that the slope of the relationship indicates that on average there were ∼10 Ca2+ bound on each CSQ2 molecule. These values are quite similar to those estimated independently above from comparison of the total Ca2+ and CSQ2 content. These estimates of the number of Ca2+ per CSQ2 molecule (∼10, which is ∼25% of the theoretical maximum) should represent the peak occupancy of the CSQ2 during diastole, when most of the Ca2+ has been returned to the SR, and the level of occupancy during systole would be even lower.
CSQ2 polymerization and Ca2+ buffering.
Based on in vitro measurements (34), it appears that cardiac CSQ2 exists primarily in its monomeric form when it has ≤10 Ca2+ bound. If those findings apply to the CSQ2 inside the SR in situ, it would mean that CSQ2 normally remains in its monomeric form right through the entire excitation-contraction coupling cycle, as the occupancy values discussed above represent the peak occupancy during diastole when virtually all of the Ca2+ is back in the SR. It is possible that there is some advantage to having CSQ2 normally remaining primarily in its monomeric form at all times, such as faster or more homogeneous binding or unbinding of Ca2+. This might be one reason why CSQ2 content is relatively high in cardiac muscle. Another possible reason of course could be that it bestows the SR with a relatively high maximum Ca2+-binding capacity, which would enable the SR to cope more easily with periods of vigorous activity where there is a large net influx of extracellular Ca2+. Furthermore, the relatively high CSQ2 amounts would keep the free [Ca2+] within the SR comparatively low during normal activity, reducing any background and spontaneous SR Ca2+ leak and energetically favoring Ca2+ reuptake during diastole while still providing a large pool of releasable Ca2+ for systole.
In skeletal muscle, where the CSQ is primarily or exclusively the CSQ1 isoform (2, 32), electron microscopy of the junctional SR reveals structures consistent with the presence of CSQ polymers (14). The electron microscopic evidence for the existence of such CSQ2 polymers in normal cardiac muscle, however, is less clear, with it being clearly seen only with overexpression of CSQ2 or associated SR proteins (14, 44). As discussed recently (46), the material condensed near the junctional SR membrane in normal cardiac muscle may be primarily due to CSQ2 monomers accumulating near the membrane rather than reflecting the presence of CSQ2 polymers. It is significant that in vitro experiments attempting to cross-link calsequestrin in solutions with normal ionic strength and a free [Ca2+] close to the reported normal SR levels (∼1 mM) (3, 10, 16) found clear evidence of CSQ1 polymer formation but little or no indication that the CSQ2 was polymerized under those conditions (46). This also agrees with other studies using light scattering, which found CSQ2 polymerization occurred appreciably only at a free [Ca2+] of ∼3 mM and above (34, 35), suggesting that it only occurs when the SR is loaded with Ca2+ substantially above normal levels. Nevertheless, it is worth reiterating that such in vitro experiments may not accurately mimic all the conditions pertaining inside the SR in situ. Further in this regard, we note that, since the SR volume is only ∼3% of the total cardiac cell volume (3), the relatively high CSQ2 content of ventricular tissue found here (∼24 μmol/kg tissue) equates to the CSQ2 being present within the SR at a concentration of ∼800 μM, much higher than the concentration examined in in vitro experiments, and at a level that may well affect its polymerization and other properties.
Although the present results strongly support that CSQ2 has a major role in the storage and buffering of Ca2+ within the SR in cardiac muscle, they in no way preclude CSQ2 from having other important additional roles. In particular, other work suggests that CSQ2 has a major role in sensing levels of SR Ca2+, modulating activity of the cardiac ryanodine receptor (RyR2) in response to changes in luminal Ca2+ concentration (17). CSQ2 seemingly aids in the high rate of Ca2+ release during systole by increasing the gain of RyR2 (46). The physiological role of even subtle changes in CSQ2 protein are illustrated by studies of heterozygous CSQ null mice, which exhibit a 25% reduction in CSQ2 protein with no detectable compensatory changes (e.g., no endoplasmic reticulum remodeling). The consequence of the 25% loss of CSQ was an increased diastolic SR Ca2+ leak, which occurred independent of any change in intra-SR free [Ca2+]. These results illustrate the need for accurate CSQ assessment in future studies.
Relation to CSQ2 ablation findings.
Finally, the conclusion here that CSQ2 must normally play a major role in buffering Ca2+ within the SR is by no means inconsistent with the finding that complete ablation of CSQ2 in murine heart resulted in an apparent reduction in total Ca2+ content of only ∼11% (25). First, we report an ∼35% increase in the content of another SR luminal Ca2+ binding protein, HRC, in ventricular tissue from KO mice compared with WT mice. No apparent compensatory upregulation of any other SR luminal Ca2+-binding proteins (such as HRC and sarcalumenin) had been noted previously in these CSQ2 KO mice (25). This could have been due to loss of much of the HRC during the fractionation procedures because that study examined SR vesicle preparations, and it was found here that such fractionation results in very considerable loss of CSQ2 itself (Fig. 1). A later study on the same CSQ2 KO mouse model (11), using unfractionated ventricular samples as here, found no upregulation in the adult tissue of calreticulin, an endoplasmic reticulum Ca2+-binding protein expressed at high levels during embryogenesis. The latter finding is in marked contrast to that of Song et al. (41) in a different CSQ2 KO model, which used fractionated samples. Another possible reason for the differences in findings in the two CSQ2 KO models is that Song et al. (41) studied older mice that exhibited significant cardiac hypertrophy. Thus the increased calreticulin may simply reflect evidence of reexpression of fetal genes as part of the hypertrophic gene program. Because calreticulin protein is present in very low amounts in adult cardiac muscle (33) and calreticulin is not present in the junctional SR (1), increased calreticulin expression is unlikely to provide significant compensation for the loss of Ca2+ buffering resulting from CSQ2 ablation. Accordingly, SR Ca2+ content was likely reduced to a similar degree in both CSQ2 KO mouse models (25, 41).
Even though higher HRC levels were not observed to give increased SR Ca2+ content in HRC overexpression animals where CSQ2 was still present (15), the increased levels of HRC protein observed here in the CSQ2 KO mice could be expected to have enhanced the SR Ca2+ storage capacity above the level that would have otherwise prevailed in the absence of all CSQ2 (22). However, full understanding of the functional significance of the increase in HRC protein would require knowledge of the absolute amounts present, which was not determined here. If the blue band at ∼120–130 kDa in the Stains-all gel (Fig. 6C) arises from HRC (6, 22, 32) (and see Fig. 5A), its relative intensity would suggest that, at least in rat ventricular tissue, the Ca2+-binding capacity of HRC is not insignificant compared with that of CSQ2. We do not suggest, however, that the observed HRC upregulation alone could have increased SR Ca2+-buffering capacity enough to compensate for the complete loss of CSQ2.
A second reason why complete ablation of CSQ2 in murine heart resulted in only a small apparent reduction in total Ca2+ content could be that the increased SR membrane area may also have further increased the total intraluminal Ca2+-binding sites; even though the ∼50% increase in SR volume occurring in the CSQ KO mice would by itself provide only a negligible increase in total Ca2+ content, the SR membrane typically contains a high proportion of negative phospholipids that can bind Ca2+ (13). Third, alterations in the properties of the SR Ca2+ pumps in the CSQ2 KO mice, or of the associated modulatory proteins phospholamban and sarcolipin (5, 29), may have increased the free Ca2+ inside the SR, helping reduce decline in the total SR Ca2+. Fourth, given that application of 10 mM caffeine to cardiac cells may not necessarily fully deplete the SR of all of its Ca2+ content (40), it is possible that the assay of total SR Ca2+ content in the CSQ2 KO myocytes was compensated to some extent by an increase in the proportion of SR Ca2+ released, because of the absence of the normal modulation of the RyR2 occurring when CSQ2 is present (17, 18, 46).
In conclusion, it should be stressed that, even with any compensatory changes that did occur, the cardiac myocytes from the CSQ2 KO animals were definitely far from normal, and the mice were rendered susceptible to CPVT, emphasizing the importance of CSQ2 to normal cardiac cell function.
GRANTS
This work was supported by National Health and Medical Research Council of Australia Grant 541938 and Fellowship to R. M. Murphy (380842) and National Heart, Lung, and Blood Institute Grant HL-088635 to B. C. Knollmann.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Joe Edwards, La Trobe University, for assistance with the atomic absorption spectroscopy. We thank Woo Jin Park, Kwangju Institute of Science and Technology, South Korea, for the HRC antibody.
REFERENCES
- 1. Allen BG, Katz S. Calreticulin and calsequestrin are differentially distributed in canine heart. J Mol Cell Cardiol 32: 2379–2384, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Beard NA, Laver DR, Dulhunty AF. Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Prog Biophys Mol Biol 85: 33–69, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Bers D. Excitation-Contraction Coupling and Cardiac Contractile Force. The Netherlands: Kluwer, 2001 [Google Scholar]
- 4. Bers DM, Langer GA. Uncoupling cation effects on cardiac contractility and sarcolemmal Ca2+ binding. Am J Physiol Heart Circ Physiol 237: H332–H341, 1979 [DOI] [PubMed] [Google Scholar]
- 5. Bhupathy P, Babu GJ, Periasamy M. Sarcolipin and phospholamban as regulators of cardiac sarcoplasmic reticulum Ca2+ ATPase. J Mol Cell Cardiol 42: 903–911, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cala SE, Scott BT, Jones LR. Intralumenal sarcoplasmic reticulum Ca(2+)-binding proteins. Semin Cell Biol 1: 265–275, 1990 [PubMed] [Google Scholar]
- 7. Campbell KP. Protein components and their roles in sarcoplasmic reticulum function. In: Sarcoplasmic Reticulum in Muscle Function, edited by Entman ML, Van Winkle WB. Boca Raton, FL: CRC, 1986, p. 65–99 [Google Scholar]
- 8. Campbell KP, MacLennan DH, Jorgensen AO. Staining of the Ca2+-binding proteins, calsequestrin, calmodulin, troponin C, and S-100, with the cationic carbocyanine dye “Stains-all.” J Biol Chem 258: 11267–11273, 1983 [PubMed] [Google Scholar]
- 9. Campbell KP, MacLennan DH, Jorgensen AO, Mintzer MC. Purification and characterization of calsequestrin from canine cardiac sarcoplasmic reticulum and identification of the 53,000 dalton glycoprotein. J Biol Chem 258: 1197–1204, 1983 [PubMed] [Google Scholar]
- 10. Chen W, Steenbergen C, Levy LA, Vance J, London RE, Murphy E. Measurement of free Ca2+ in sarcoplasmic reticulum in perfused rabbit heart loaded with 1,2-bis(2-amino-5,6-difluorophenoxy)ethane-N,N,N′,N′-tetraacetic acid by 19F NMR. J Biol Chem 271: 7398–7403, 1996 [PubMed] [Google Scholar]
- 11. Chopra N, Kannankeril PJ, Yang T, Hlaing T, Holinstat I, Ettensohn K, Pfeifer K, Akin B, Jones LR, Franzini-Armstrong C, Knollmann BC. Modest reductions of cardiac calsequestrin increase sarcoplasmic reticulum Ca2+ leak independent of luminal Ca2+ and trigger ventricular arrhythmias in mice. Circ Res 101: 617–626, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Chopra N, Yang T, Asghari P, Moore ED, Huke S, Akin B, Cattolica RA, Perez CF, Hlaing T, Knollmann-Ritschel BE, Jones LR, Pessah IN, Allen PD, Franzini-Armstrong C, Knollmann BC. Ablation of triadin causes loss of cardiac Ca2+ release units, impaired excitation-contraction coupling, and cardiac arrhythmias. Proc Natl Acad Sci USA 106: 7636–7641, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dalton KA, Pilot JD, Mall S, East JM, Lee AG. Anionic phospholipids decrease the rate of slippage on the Ca(2+)-ATPase of sarcoplasmic reticulum. Biochem J 342: 431–438, 1999 [PMC free article] [PubMed] [Google Scholar]
- 14. Franzini-Armstrong C. Architecture and regulation of the Ca2+ delivery system in muscle cells. Appl Physiol Nutr Metab 34: 323–327, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Gregory KN, Ginsburg KS, Bodi I, Hahn H, Marreez YM, Song Q, Padmanabhan PA, Mitton BA, Waggoner JR, Del Monte F, Park WJ, Dorn GW, 2nd, Bers DM, Kranias EG. Histidine-rich Ca binding protein: a regulator of sarcoplasmic reticulum calcium sequestration and cardiac function. J Mol Cell Cardiol 40: 653–665, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Guo T, Ai X, Shannon TR, Pogwizd SM, Bers DM. Intra-sarcoplasmic reticulum free [Ca2+] and buffering in arrhythmogenic failing rabbit heart. Circ Res 101: 802–810, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Gyorke I, Hester N, Jones LR, Gyorke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J 86: 2121–2128, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gyorke S, Stevens SC, Terentyev D. Cardiac calsequestrin: quest inside the SR. J Physiol 587: 3091–3094, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ho R, Fan D, Somlyo AV, Somlyo AP. Calcium content of peripheral and central mitochondria in the guinea pig myocardium: electron probe analysis. Cell Calcium 33: 247–256, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Hove-Madsen L, Bers DM. Passive Ca buffering and SR Ca uptake in permeabilized rabbit ventricular myocytes. Am J Physiol Cell Physiol 264: C677–C686, 1993 [DOI] [PubMed] [Google Scholar]
- 21. Jones LR, Suzuki YJ, Wang W, Kobayashi YM, Ramesh V, Franzini-Armstrong C, Cleemann L, Morad M. Regulation of Ca2+ signaling in transgenic mouse cardiac myocytes overexpressing calsequestrin. J Clin Invest 101: 1385–1393, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim E, Shin DW, Hong CS, Jeong D, Kim DH, Park WJ. Increased Ca2+ storage capacity in the sarcoplasmic reticulum by overexpression of HRC (histidine-rich Ca2+ binding protein). Biochem Biophys Res Commun 300: 192–196, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Kirchhefer U, Jones LR, Begrow F, Boknik P, Hein L, Lohse MJ, Riemann B, Schmitz W, Stypmann J, Neumann J. Transgenic triadin 1 overexpression alters SR Ca2+ handling and leads to a blunted contractile response to beta-adrenergic agonists. Cardiovasc Res 62: 122–134, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Knollmann BC. New roles of calsequestrin and triadin in cardiac muscle. J Physiol 587: 3081–3087, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, Knollmann BE, Horton KD, Weissman NJ, Holinstat I, Zhang W, Roden DM, Jones LR, Franzini-Armstrong C, Pfeifer K. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest 116: 2510–2520, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laver DR, Roden LD, Ahern GP, Eager KR, Junankar PR, Dulhunty AF. Cytoplasmic Ca2+ inhibits the ryanodine receptor from cardiac muscle. J Membr Biol 147: 7–22, 1995 [DOI] [PubMed] [Google Scholar]
- 27. Macdonald WA, Stephenson DG. Effect of ADP on slow-twitch muscle fibres of the rat: implications for muscle fatigue. J Physiol 573: 187–198, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Macdonald WA, Stephenson DG. Effects of ADP on sarcoplasmic reticulum function in mechanically skinned skeletal muscle fibres of the rat. J Physiol 532: 499–508, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. MacLennan DH, Asahi M, Tupling AR. The regulation of SERCA-type pumps by phospholamban and sarcolipin. Ann NY Acad Sci 986: 472–480, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Mitchell RD, Simmerman HK, Jones LR. Ca2+ binding effects on protein conformation and protein interactions of canine cardiac calsequestrin. J Biol Chem 263: 1376–1381, 1988 [PubMed] [Google Scholar]
- 31. Mollica JP, Oakhill JS, Lamb GD, Murphy RM. Are genuine changes in protein expression being overlooked? Reassessing Western blotting. Anal Biochem 386: 270–275, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Murphy RM, Larkins NT, Mollica JP, Beard NA, Lamb GD. Calsequestrin content and SERCA determine normal and maximal Ca(2+) storage levels in sarcoplasmic reticulum of fast- and slow-twitch fibres of rat. J Physiol 587: 443–460, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakamura K, Robertson M, Liu G, Dickie P, Nakamura K, Guo JQ, Duff HJ, Opas M, Kavanagh K, Michalak M. Complete heart block and sudden death in mice overexpressing calreticulin. J Clin Invest 107: 1245–1253, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Park H, Park IY, Kim E, Youn B, Fields K, Dunker AK, Kang C. Comparing skeletal and cardiac calsequestrin structures and their calcium binding: a proposed mechanism for coupled calcium binding and protein polymerization. J Biol Chem 279: 18026–18033, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Qin J, Valle G, Nani A, Nori A, Rizzi N, Priori SG, Volpe P, Fill M. Luminal Ca2+ regulation of single cardiac ryanodine receptors: insights provided by calsequestrin and its mutants. J Gen Physiol 131: 325–334, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scott BT, Simmerman HK, Collins JH, Nadal-Ginard B, Jones LR. Complete amino acid sequence of canine cardiac calsequestrin deduced by cDNA cloning. J Biol Chem 263: 8958–8964, 1988 [PubMed] [Google Scholar]
- 37. Scriven DR, Dan P, Moore ED. Distribution of proteins implicated in excitation-contraction coupling in rat ventricular myocytes. Biophys J 79: 2682–2691, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shannon TR, Bers DM. Assessment of intra-SR free [Ca] and buffering in rat heart. Biophys J 73: 1524–1531, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Slupsky JR, Ohnishi M, Carpenter MR, Reithmeier RA. Characterization of cardiac calsequestrin. Biochemistry 26: 6539–6544, 1987 [DOI] [PubMed] [Google Scholar]
- 40. Smith GL, Steele DS. Measurement of SR Ca2+ content in the presence of caffeine in permeabilised rat cardiac trabeculae. Pflugers Arch 437: 139–148, 1998 [DOI] [PubMed] [Google Scholar]
- 41. Song L, Alcalai R, Arad M, Wolf CM, Toka O, Conner DA, Berul CI, Eldar M, Seidman CE, Seidman JG. Calsequestrin 2 (CASQ2) mutations increase expression of calreticulin and ryanodine receptors, causing catecholaminergic polymorphic ventricular tachycardia. J Clin Invest 117: 1814–1823, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Suko J, Hasselbach W. Characterization of cardiac sarcoplasmic reticulum ATP-ADP phosphate exchange and phosphorylation of the calcium transport adenosine triphosphatase. Eur J Biochem 64: 123–130, 1976 [DOI] [PubMed] [Google Scholar]
- 43. Terentyev D, Viatchenko-Karpinski S, Gyorke I, Volpe P, Williams SC, Gyorke S. Calsequestrin determines the functional size and stability of cardiac intracellular calcium stores: Mechanism for hereditary arrhythmia. Proc Natl Acad Sci USA 100: 11759–11764, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tijskens P, Jones LR, Franzini-Armstrong C. Junctin and calsequestrin overexpression in cardiac muscle: the role of junctin and the synthetic and delivery pathways for the two proteins. J Mol Cell Cardiol 35: 961–974, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Verburg E, Murphy RM, Stephenson DG, Lamb GD. Disruption of excitation-contraction coupling and titin by endogenous Ca2+-activated proteases in toad muscle fibres. J Physiol 564: 775–990, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wei L, Hanna AD, Beard NA, Dulhunty AF. Unique isoform-specific properties of calsequestrin in the heart and skeletal muscle. Cell Calcium 45: 474–484, 2009 [DOI] [PubMed] [Google Scholar]
- 47. Wills MR, Lewin MR. Plasma calcium fractions and the protein-binding of calcium in normal subjects and in patients with hypercalcaemia and hypocalcaemia. J Clin Pathol 24: 856–866, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]