Abstract
Large-conductance Ca2+-activated K+ (BK) channels are composed of pore-forming α-subunits and accessory β1-subunits that modulate Ca2+ sensitivity. BK channels regulate arterial myogenic tone and renal Na+ clearance/K+ reabsorption. Previous studies using indirect or short-term blood pressure measurements found that BK channel β1-subunit knockout (BK β1-KO) mice were hypertensive. We evaluated 24-h mean arterial pressure (MAP) and heart rate in BK β1-KO mice using radiotelemetry. BK β1-KO mice did not have a higher 24-h average MAP when compared with wild-type (WT) mice, although MAP was ∼10 mmHg higher at night. The dose-dependent peak declines in MAP by nifedipine were only slightly larger in BK β1-KO mice. In BK β1-KO mice, giving 1% NaCl to mice to drink for 7 days caused a transient (5 days) elevation of MAP (∼5 mmHg); MAP returned to pre-saline levels by day 6. BK β1-KO mesenteric arteries in vitro demonstrated diminished contractile responses to paxilline, increased reactivity to Bay K 8644 and norepinephrine (NE), and maintained relaxation to isoproterenol. Paxilline and Bay K 8644 did not constrict WT or BK β1-KO mesenteric veins (MV). BK β1-subunits are not expressed in MV. The results indicate that BK β1-KO mice are not hypertensive on normal or high-salt intake. BK channel deficiency increases arterial reactivity to NE and L-type Ca2+ channel function in vitro, but the L-type Ca2+ channel modulation of MAP is not altered in BK β1-KO mice. BK and L-type Ca2+ channels do not modulate murine venous tone. It appears that selective loss of BK channel function in arteries only is not sufficient to cause sustained hypertension.
Keywords: large-conductance calcium-activated potassium channel β1-knockout, salt-sensitive, L-type calcium channel, arterial and venous tone
large-conductance ca2+-activated K+ channels (BK channels) are activated by membrane depolarization and increases in cytosolic Ca2+ concentration (17, 27). BK channels provide “negative feedback” control of vascular tone as BK channel activation causes hyperpolarization of vascular smooth muscle cell (VSMC) membrane potential, closure of voltage-gated Ca2+ channels, and muscle relaxation. In VSMCs, BK channels are composed of pore-forming α-subunits and accessory β1-subunits that modulate Ca2+ sensitivity (17, 43). The BK channel β1-subunit is enriched in VSMC and copurifies with the BK pore-forming α-subunit. Deletion of the gene for the β1-subunit decreases Ca2+ sensitivity of BK channels and reduces coupling of Ca2+ sparks to BK channel activation (2, 32). BK channel β1-subunit knockout (BK β1-KO) mice have been used to study the role of BK channel function in physiological regulation of VSMC contractility and as models of human diseases, such as asthma (41), erectile dysfunction (47), and overactive bladder (29). Results from these studies indicate that BK channels could be therapeutic targets for treatments of human disease (8, 9, 18).
Studies in arteries from BK β1-KO mice have shown higher arterial myogenic tone and contractility (2, 31), endothelial dysfunction, and increased O2− formation via increased activity and expression of NADPH oxidase (28). It is unclear if BK β1-KO mice also have higher venous tone and contractility, although BK channels were shown to modulate venous capacitance in vivo in pigs (49) and in vitro in some experimental animals (26, 43) and humans (14, 22, 23, 37). It has been reported that mean arterial blood pressure (MAP) in BK β1-KO mice is elevated ∼10–15 mmHg when measured acutely using carotid arterial catheterization (2, 32). Measurements made using tail cuff plethysmography revealed much higher MAP values in BK β1-KO mice (>20 mmHg) compared with control mice (11, 12). BK channel β1-subunits are also expressed in renal glomerular cells, in the collecting tubule, and in the aldosterone-sensitive distal nephron, where the β1-subunit associates with BK channels to regulate K+ secretion (11, 12, 13, 33, 34). Hypertension in BK β1-KO mice has been linked to alterations in K+ secretion, Na+ clearance, and aldosteronism (11, 12, 13, 33, 34).
Blood pressure measurement using tail cuff plethysmography or carotid arterial catheterization requires that mice be restrained; the associated stress can alter blood pressure (31). In BK β1-KO mice, effects of stress on blood pressure may be amplified because of higher vascular myogenic tone, endothelial dysfunction, increased O2− formation, and aldosteronism (2, 11, 12, 32, 28). Therefore, it is crucial to determine blood pressure in BK β1-KO mice using telemetry, which minimizes stress-related influences on blood pressure measurements (15, 24, 31, 48). Disruption of BK channels leads to activation of L-type Ca2+ channels in bladder smooth muscle cells by membrane potential (Em) depolarization and increased L-type Ca2+ current density (42). No studies have directly assessed if L-type Ca2+ channels contribute to elevated blood pressure in BK β1-KO mice. BK β1-KO mice may exhibit increased salt sensitivity of blood pressure, since they have elevated plasma aldosterone, Na+, and osmolality caused by impaired Na+ clearance (11). Elevated blood pressure in BK β1-KO was reduced by a low-Na+ diet when measured using tail cuff plethysmography (11). In the present studies, we compared blood pressure in BK β1-KO and wild-type (WT) control mice using radiotelemetry. We also assessed the contribution of vascular L-type Ca2+ channel function and the effects of high salt intake on blood pressure in BK β1-KO mice. The role of BK channel function in arterial contractility and β-adrenergic receptor (AR)-mediated relaxation was assessed in vitro in mesenteric arteries (MA). Finally, early studies have suggested that impairment of BK channels in vivo not only increases MAP but also decreases venous capacitance (49). Because we and others have suggested that regulation of venous smooth muscle tone contributes to regulation of arterial pressure (10, 16, 21, 35), we also compared BK and L-type Ca2+ channel function in MA and mesenteric veins (MV) from WT and BK β1-KO mice in vitro.
MATERIALS AND METHODS
Animals.
Homozygous breeder BK β1-KO mice were provided by Dr. Robert Brenner, Department of Physiology, University of Texas Health Science Center at San Antonio (2, 41), and the mice were bred in the Animal Care Facility at Michigan State University. BK β1-KO mice used in our studies are congenic as a result of seven generations of inbreeding to the C57BL/6 line and maintained as homozygous lines in Dr. Brenner's laboratory (41). Control animals used in these studies were C57BL/6 (WT) mice from Charles River Laboratories. Animal protocols were approved by the Institutional Animal Care and Use Committee at Michigan State University. BK β1-KO mice were weaned at 3 wk. All mice were fed normal diet and were studied at 10–12 wk of age (male, 25–30 g).
Measurements of MAP and heart rate in WT and BK β1-KO mice using telemetry.
Under isoflurane anesthesia, a catheter attached to a radiotelemetry transmitter (Data Sciences International, St. Paul, MN) was inserted in the abdominal aorta via the femoral artery. The transmitter was placed subcutaneously. Mice were maintained on a 12:12-h light-dark cycle. After mice recovered from surgery (at least 3 days), blood pressure and heart rate (HR) were sampled for 10 s every 10 min. Data are reported as 1- or 24-h averages during the experimental period.
L-type Ca2+ channels and blood pressure in WT and BK β1-KO mice.
MAP data were collected 2 h before and 6 h after subcutaneous administration of nifedipine. Nifedipine was initially dissolved in 100% cremophor EL as a stock solution and then slowly diluted with saline. The final concentration of cremophor EL in nifedipine solution was <5%. In control studies, subcutaneous injection of 5% cremophor EL (0.3 ml) without nifedipine did not change MAP. Nifedipine was given subcutaneously at doses of 1.25, 2.5, 5, and 10 mg/kg. The doses of nifedipine were chosen based on preliminary studies that showed a dose of 0.6 mg/kg did not change MAP while a maximum drop in MAP occurred after a 10 mg/kg dose. Nifedipine was given at ∼10:00 A.M.; baseline MAP was similar in control and BK β1-KO mice. There was at least a 24-h interval between subsequent nifedipine treatments in a given mouse.
Salt sensitivity of blood pressure in BK β1-KO mice.
Salt sensitivity was tested in WT and BK β1-KO mice by supplying mice with 1% NaCl in the drinking water with normal diet for 7 days. MAP and HR were continuously measured starting 2 days before high salt, during 7 days of high salt, and for 2 days after returning the mice to normal drinking water.
Measurement of heart-to-body weight ratio.
Hearts were collected from 10- to 12-wk-old (25–30 g) WT and BK β1-KO mice. The ratio of heart weight to body weight was calculated.
Measurement of arterial and venous BK and L-type Ca2+ channel function, arterial contractility, and β-AR-mediated relaxation in isolated and pressurized vessels from BK β1-KO and control mice.
MA and MV [pressurized inner diameter (ID) ≈150–250 μm] were mounted to a custom-made pressure myograph apparatus with Krebs solution equilibrated with compressed air (5% CO2-21% O2-74% N2, 37°C) as previous reported (4). Pressures were 2 mmHg in MV and 60 mmHg in MA, the normal MV and MA pressures in vivo. Changes in ID were recorded. MA and MV BK and L-type Ca2+ channel function were tested by treatment with paxilline (0.5 μmol/l, a nonpeptide selective BK channel blocker) and Bay K 8644 (0.1 μmol/l, a selective dihydropyridine Ca2+ channel agonist).
Arterial contractility was tested using responses to norepinephrine (NE) and KCl in pressurized MA as described above. β-AR-mediated MA relaxations were tested by isoproterenol (ISP)-induced relaxation in phenylephrine (PE, 1 μmol/l)-precontracted MA. β1- or β2-AR-mediated relaxations were distinguished by making measurements with and without ICI-118551 (1 μmol/l, a selective β2-AR antagonist) treatment. These data were normalized to the maximal relaxation caused by papaverine (0.1 mmol/l). Endothelial function was tested by measuring relaxations caused by acetylcholine (10 μmol/l) in PE-preconstricted vessels.
Detection of LacZ gene expression in MA and MV from BK β1-KO mice.
The β-galactosidase reporter (LacZ) was used as gene-targeted to the β1-subunit translation initiation site in BK β1-KO mice, which permitted examination of the cell types that normally express the β1-subunit (2, 41). We measured tissue-specific expression of the β1-subunit promoter in arteries and veins by detection of LacZ expression as described earlier (2, 41).
Statistical analysis.
Data are reported as means ± SE from n rats. Unpaired t-tests were used to make single-point comparisons. Comparisons of multiple time points of MAP and HR in vivo and MA contractility and relaxation in vitro were accomplished using a two-way ANOVA with repeated measures followed by Bonferroni posttest. Comparisons of multiple points generated in MAP in each group before and after the high-salt treatment were accomplished using a one-way ANOVA with repeated measures followed by Dunnett's posttest. Tests were performed using GraphPad Prism software. P < 0.05 was considered statistically significant.
RESULTS
BK β1-KO mice are not hypertensive.
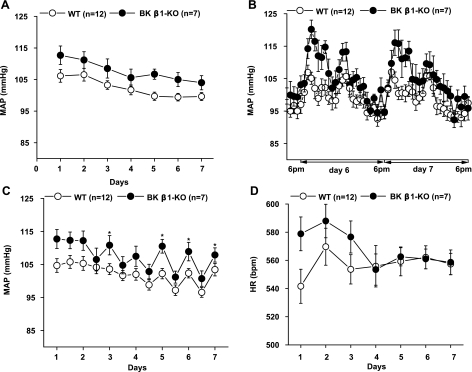
Daily (24 h average) MAP in BK β1-KO mice was not different from that in WT mice (Fig. 1A). Because physical activity affects MAP (41), MAP was also determined at night when mice are most active. Five days after telemeter implantation, when mice had regained their normal circadian rhythm, MAP in BK β1-KO mice was ∼10 mmHg higher than in WT mice at night (6:00 P.M.-6:00 A.M.), whereas there was no difference during daylight hours (6:00 A.M.-6:00 P.M.) (Fig. 1B). The average nighttime and daytime MAP measurements are shown in Fig. 1C.
Fig. 1.
Continuous measurement of mean arterial pressure (MAP) in wild-type (WT) and large-conductance Ca2+-activated K+ channel (BK) β1-knockout (KO) mice for 7 days after telemetry implantation. MAP was sampled for 10 s every 10 min. A: daily MAP calculated as the average of 24 hourly measurements. B: hourly MAP calculated as the average of 6 measurements each hour at day 6 and day 7. C: averaged MAP at night (6:00 P.M.-6:00 A.M.) and during the day (6:00 A.M.-6:00 P.M.). D: daily heart rate (HR) in WT and BK β1-KO mice measured for 7 days after telemetry implantation. HR was sampled for 10 s every 10 min. Daily HR was calculated as the average of 24 hourly measurements. *Significantly different from WT mice (P < 0.05).
From day 3, when mice regained circadian rhythm of blood pressure, there was no difference in HR between WT and BK β1-KO mice (Fig. 1D). BK β1-KO mice also did not have cardiac hypertrophy as indicated by similar values for the heart-to-body weight ratio (WT, 4.8 ± 0.1 mg/g; BK β1-KO, 4.9 ± 0.1 mg/g, n = 14 for each group, P > 0.05).
Depressor effects of nifedipine are similar in WT and BK β1-KO mice.
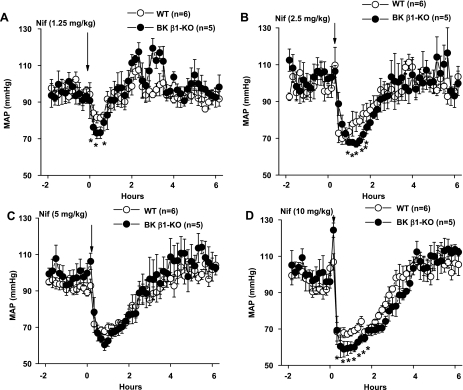
Nifedipine caused a rapid onset and dose-dependent decrease in MAP in WT and BK β1-KO mice (Fig. 2, A–D). The threshold dose of nifedipine in both groups was 1.25 mg/kg, with a maximum decrease in MAP occurring at 10 mg/kg. The fall in MAP to nifedipine showed only modest dose dependency but was slightly larger in BK β1-KO mice compared with WT mice at each dose of nifedipine. There was no difference in MAP recovery time in BK β1-KO mice compared with WT mice.
Fig. 2.
A–D: nifedipine (Nif)-induced depressor responses in WT and BK β1-KO mice. MAP is displayed at 10-min intervals for 2 h before and 4 h after nifedipine administration. *Significantly different from WT mice (P < 0.05).
Blood pressure in BK β1-KO mice may not be salt sensitive.
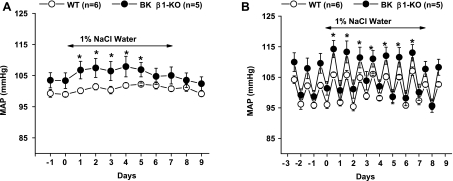
Salt water (1% NaCl) was given for 7 days to WT and BK β1-KO mice. In BK β1-KO mice, MAP immediately increased in response to elevated salt intake (Fig. 3A). However, 24-h average MAP was only increased by 3–5 mmHg over a 5-day period compared with baseline levels of MAP (Fig. 3A) and then returned to baseline levels by day 6. There was no difference in 24-h average MAP between WT and BK β1-KO mice during salt water administration. MAP was significantly higher in BK β1-KO only during the nighttime hours (Fig. 3, B and C). High salt intake did not change MAP in WT mice at any time.
Fig. 3.
Effects of 1% NaCl intake on MAP in WT and BK β1-KO mice. A: daily MAP measured before, during, and after 1% NaCl water intake. B: average MAP at night and during the day while mice received high salt intake. *Significantly different from control levels (P < 0.05).
Deletion of the β1-subunit impairs arterial BK channel function and increases L-type Ca2+ channel function.
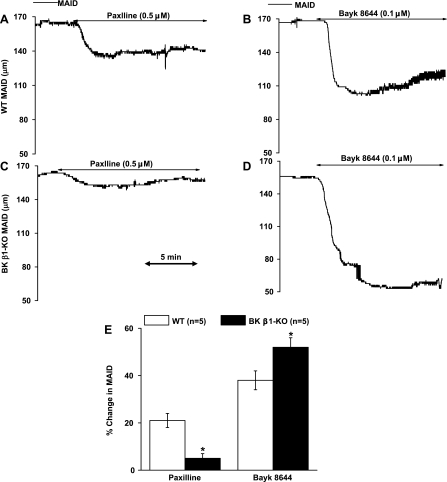
BK and L-type Ca2+ channel function was tested with paxilline and Bay K 8644 in isolated and pressurized MA (Fig. 5). In WT MA, paxilline and Bay K 8644 reduced ID by 21 ± 4 and 38 ± 5% from the baseline levels, respectively (Fig. 4, A, B, and E). In BK β1-KO MA, paxilline constricted MA by only 4 ± 2%, a 75% reduction compared with WT MA (Fig. 4, C and E). Bay K 8644-induced MA constriction was 52 ± 4%, a 30% increase compared with WT MA (Fig. 4, D and E).
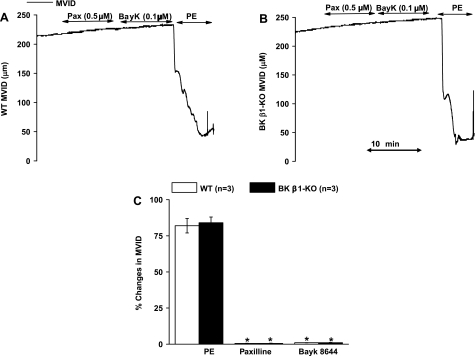
Fig. 5.
Effects of paxilline, Bayk 8644, and phenylephrine (PE) on WT and BK β1-KO MV in vitro. A and B: trace recordings in WT and BK β1-KO mesenteric veins (MV). C: summarized data from WT and BK β1-KO MV. MVID, MV inner diameter. *Significantly different from WT MV.
Fig. 4.
Effects of paxilline and Bayk 8644 on WT and BK β1-KO mesenteric arteries (MA) in vitro. Trace recording of contractile responses to paxilline and Bayk 8644 in WT MA (A and B) and BK β1-KO MA (C and D). E: averaged data from WT and BK β1-KO MA. MAID, MA inner diameter. *Significantly different from WT MA.
PE caused a very large and similar MV constriction in preparations from WT and BK β1-KO mice while neither paxilline nor Bay K 8644 constricted MV (Fig. 5, A–C).
BK channel deficiency increases MA contractility but does not change β-AR-mediated MA relaxation.
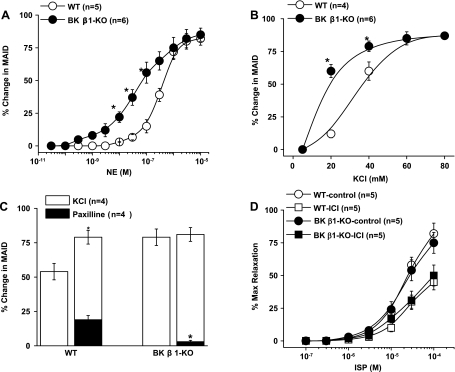
To determine if BK channel deficiency alters arterial contractility, we compared the contractile responses to NE and KCl in BK β1-KO and WT MA (Fig. 6). NE concentration-response curves were left-shifted in BK β1-KO MA (Fig. 6A). pD2 values were 7.2 ± 0.3 for BK β1-KO and 6.3 ± 0.4 for WT MA (n = 5–6, P < 0.05). Maximal constrictions were similar, and peak constriction was at 1 μmol/l of NE in both preparations. KCl concentration-response curves were also left-shifted in BK β1-KO compared with WT MA (Fig. 6B). At 40 mmol/l of KCl, the contractile responses in BK β1-KO MA were larger than in WT MA (72 ± 5 vs. 52 ± 5%, P < 0.05) (Fig. 6C). Paxilline eliminated the difference between WT and BK β1-KO MA preparations (Fig. 6C). Paxilline constricted WT MA by 21 ± 3%, but paxilline did not constrict BK β1-KO MA.
Fig. 6.
Comparison of contractile and dilatory responses in WT and BK β1-KO MA in vitro. A and B: concentration-response curves of NE and KCl in WT and BK β1-KO MA. C: contractile responses to KCl (40 mmol/l) with or without pretreatment of paxilline (0.5 μmol/l). D: concentration-responses curves of isoproterenol (ISP) in PE (1 μmol/l)-precontracted WT and BK β1-KO MA with or without ICI-118551 (1 μmol/l) pretreatment. *Significantly different from WT MA.
To determine if β-AR function is upregulated in BK β1-KO mice, ISP-induced MA relaxations were tested with or without ICI-118551 treatment. Relaxation of preconstricted WT and BK β1-KO preparations to ISP was similar, with or without ICI-118551 treatment (Fig. 6D).
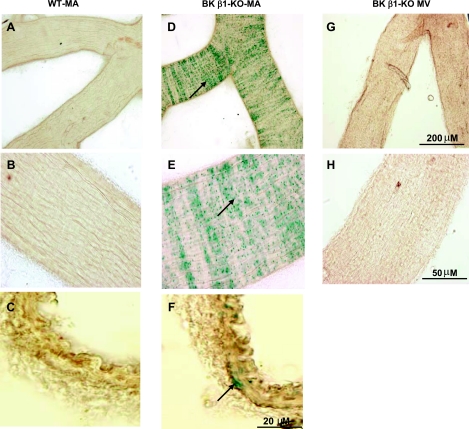
BK β1-subunits are not expressed in murine veins.
The expression of BK β1-subunits was determined by LacZ gene staining in vessels from BK β1-KO mice (2, 41). β1-Subunit expression was readily detected in MA smooth muscle cells (Fig. 7, D–F) but not in MV preparations (Fig. 7, G and F). We also compared the BK β1-subunit expression in other arteries and veins [see supplemental data (Supplemental data for this article may be found on the American Journal of Physiology: Heart and Circulatory Physiology website.)]. BK β1-subunits were detected in the entire arterial system, but it was only detected in the main trunk of portal vein and pulmonary veins.
Fig. 7.
LacZ gene staining in MA and MV from WT and BK β1-KO mice. Positive staining is shown in the BK β1-KO MA segment (D and E), and in the smooth muscle cells in the cross section of MA (F), but not in the segment and cross section from WT MA (A-C). Staining is negative in the segment of BK β1-KO MV. Arrows indicate the positive staining.
DISCUSSION
BK β1-KO mice have been used extensively as a tool to probe the contribution of BK channel function to smooth muscle cell contractility in animal models of human diseases (29, 41, 47). Previous studies have found that BK β1-KO mice are hypertensive, possibly because of increased vascular myogenic tone and contractility, oxidative stress, and abnormal Na+-K+ absorption/clearance by the kidney (2, 11, 12, 28, 32). However, we report that, when blood pressure is measured using telemetry, and hypertension is defined as a significantly elevated 24-h average MAP, BK β1-KO mice are not hypertensive. BK β1-KO mice do have slightly higher MAP than WT mice during the night-time hours when mice are most active, but the night-time increase in MAP is modest and does not lead to significantly increased 24-h average MAP compared with that of WT mice.
In the current studies, blood pressure was measured by telemetry. This technique provides more reliable measurements because they can be made continuously and in the absence of the restraint stress required for tail cuff plethysmography or direct measurement using intra-arterial catheters in tethered animals (15, 24, 31, 48). MAP in our C57BL6 BK β1-KO mice was elevated by <10 mmHg at night when sympathetic nervous system activity would be highest. MAP was not different between BK β1-KO and WT mice during daylight hours when sympathetic activity would be lower. Overall, 24-h average blood pressure measurements were not different between the two groups of mice. These data support our conclusion that BK β1-KO mice are not hypertensive. Our data contrast with results from previous studies showing that MAP is elevated when measured using tail cuff plethysmography or carotid artery catheterization (2, 11, 12, 13, 32). Hypertension was previously reported in a mixed 129 svj + C57BL/6 BK β1-KO strain of mice (∼20 mmHg higher) (2), a strain that has higher blood pressure than any other mouse strain (7), and in 4- to 8-mo-old C57BL/6 BK β1-KO mice (∼13 mmHg higher) (32), measured by catheters. Interestingly, early studies by Grimm et al. (11, 12) also showed that the 3- to 4-mo-old C57BL/6 BK β1-KO mice (from Dr. Brenner's Laboratory) were hypertensive. Blood pressure was ∼20 mmHg higher (measured by tail cuff) compared with C57BL/6 control mice that were purchased from Charles River Laboratory (11, 12). In our studies, we used BK β1-KO and control mice of exactly the same age and genetic background. Therefore, different age and genetic background are not explanations for our observations. The behavioral stress associated with nontelemetric blood pressure measurements may increase sympathetic nerve activity, and this could account for the increased MAP observed in earlier experiments. In our studies, isolated MA from BK β1-KO mice exhibited increased contractility to NE. These data are similar to previous studies in aorta and cerebral arteries (2, 28, 32), i.e., those arteries exhibited higher myogenic tone due to Em depolarization and higher levels of reactive oxygen species (2, 28, 32). However, the increased contractility in BK β1-KO arteries is mediated by Em depolarization, not by specifically increased sensitivity to NE, since responses to KCl were also enhanced, and this enhancement was diminished by a BK channel blocker (32). Therefore, sympathetically mediated blood pressure responses to restraint-induced stress may be increased in BK β1-KO mice because of increased vascular reactivity. This explanation is supported by our telemetry measurements showing BK β1-KO mice had higher MAP during their active phase. We did not test the MAP responses to exogenous NE in vivo. However, “hypertensive” C57BL/6 BK β1-KO mice responded normally to α1-AR activation (PE) in vivo when MAP was measured by implanted catheters in early studies (32). Different responses to α1-adrenergic vasoconstriction in vivo and in vitro may be explained by restraint-induced stress in vivo, i.e., the baseline blood pressure was likely increased by sympathetically mediated vasoconstriction in BK β1-KO mice in earlier studies. We found that the threshold for responses to NE was lower in BK β1-KO arteries, but the maximal responses to NE were similar in BK β1-KO and WT arteries. Interestingly, the baseline levels of HR were significantly higher in tethered BK β1-KO than in control mice (680 ± 8 vs. 617 ± 20 beats/min) (32), whereas the HR was similar in the two groups of animals when measured by telemetry in our studies (560 ± 15 beats/min in both groups). These results indicate that stress and sympathetically mediated vasoconstriction likely contributed to the higher blood pressure observed in the earlier studies. Although it is unlikely that BK β1-subunit mutation would directly increase sympathetic outflow, the role of sympathetic activity in the regulation of blood pressure in these mice needs to be studied further.
Sustained hypertension causes cardiac hypertrophy, and BK β1-KO mice would be expected to exhibit cardiac hypertrophy if their MAP was chronically elevated. However, we did not detect cardiac hypertrophy in BK β1-KO mice, consistent with our conclusion that the mice are not hypertensive. This result differs from early reports of cardiac hypertrophy in 129 svj + C57BL6 BK β1-KO mice that have a genetic background of higher blood pressure (2, 7). Our results are supported by early reports on C57BL/6 BK β1-KO mice, in which cardiac hypertrophy was not evident even though blood pressure was reported to be ∼13–20 mmHg higher than in control mice. The age of mice ranged from 12 wk to 8 mo (11, 12, 32). Thus early studies do not suggest that C57BL/6 BK β1-KO mice become hypertensive as they age (46), since there was no cardiac hypertrophy even in 8-mo-old mice (32). Our results are also supported by studies in BK channel α-subunit KO mice. BK channel function is globally depleted in these mice, which leads to a small but significant elevation of blood pressure (∼6 mmHg) as measured by telemetry (38). The modest increase in blood pressure results from hyperaldosteronism accompanied by decreased serum K+ levels, as well as increased vascular tone in small arteries. Cardiac hypertrophy was not detected in those mice. Furthermore, the authors concluded that the systemic blood pressure phenotype caused by complete BK channel deletion is relatively mild, milder than expected, at least under resting conditions. Nevertheless, a contribution of BK channel function to regulation of vascular tone and blood pressure in “emergency” situations, such as endotoxin and hemorrhagic shock, hypoxia, and ischemia (8, 9), is very possible.
Arteries from BK β1-KO mice exhibited increased myogenic tone and contractility in vitro in previous (2, 28, 32), and our current, studies. In similar arteries, myogenic tone in response to intravascular pressure was absent when L-type Ca2+ channels were inactivated specifically in smooth muscle (25). In our in vitro studies, we found that BK β1-KO MA exhibited a significantly impaired BK channel function and a moderately increased L-type Ca2+ channel function when tested using paxilline and Bay K 8644, respectively. This suggests that the increased myogenic tone in BK β1-KO arteries is mediated by enhanced Ca2+ influx through L-type Ca2+ channels, presumably because of the less negative membrane potential of arterial smooth muscle cells and an attendant increase of the L-type Ca2+ window current. If so, blockade of L-type Ca2+ channel function should cause a larger fall of blood pressure in BK β1-KO compared with WT mice. Surprisingly, in our study, depressor responses to nifedipine were only slightly (∼10 mmHg at peak) larger in BK β1-KO and WT mice. A much larger augmentation of nifedipine-induced depressor responses was expected based on in vitro findings. Nevertheless, only moderate increases in L-type Ca2+ channel-mediated effects on blood pressure are consistent with our finding that the mice are normotensive. The unexpectedly modest blood pressure dependence on L-type Ca2+ channels could reflect compensatory downregulation in BK β1-KO, but that idea is not supported by our in vitro data. It is unclear if the L-type Ca2+ channels are expressed in murine MV; however, a lack of functional L-type Ca2+ channels in MV is supported by current studies showing that Bay K 8644 does not contract WT and BK β1-KO MV. Presumably then, there was no nifedipine-induced venodilation in vivo in WT and BK β1-KO mice. Our results are consistent with early studies showing that L-type Ca2+ channels are expressed in veins, but the channels are “silenced” by intracellular Ca2+ (45). We cannot rule out abnormalities in other mechanisms that may contribute to regulating arterial tone in BK β1-KO mice; for example, T-type Ca2+ channels may also be associated with BK channels (5, 19). Measurement of cardiac output and calculation of total peripheral resistance are necessary to evaluate overall arterial constrictor tone in intact BK β1-KO mice.
The expected hypertensive phenotype in BK β1-KO mice also could be masked by adaptive upregulation of compensatory signaling pathways, leading to reduced Ca2+ influx through voltage-operated Ca2+ channels in vivo. For example, β-AR-cAMP-protein kinase A (PKA) signaling is associated with BK and L-type Ca2+ channel function in smooth muscle cells (19, 20). Mutation of the BK channel α-subunit leads to upregulation of expression of PKA and enhanced β-AR/cAMP-mediated relaxation in tracheas and bladder (3, 39, 42). However, when we tested β-AR-mediated arterial vasodilation in our studies, we did not find any alteration in the β-AR (β1- or β2-AR)-mediated arterial relaxation. Alternatively, vascular myogenic tone may be increased in vivo in BK β1-KO mice, but hypertension is prevented by suppression of other (e.g., neurohumoral) mechanisms regulating blood pressure. Earlier studies indicated that there is little compensation for β1-subunit mutation; however, more dramatic compensatory mechanisms are evident in tracheas and bladder in mice with α-subunit mutation (3, 29, 39, 41).
Recent studies indicate that BK β1-subunit channels are associated with K+ secretion, Na+ clearance, and aldosterone sensitivity in the distal nephron. Disruption of the BK β1-subunit is reported to cause altered K+ secretion, Na+ clearance, hyperaldosteronism, and hypertension (11, 12, 13). In published studies, BK β1-KO mice (3–4 mo old) were reported to have higher plasma volume, Na+ concentration, and osmolality caused by impaired sodium clearance (11, 12). Blood pressure in BK β1-KO mice was reduced by a low-salt diet (11), suggesting that blood pressure in these mice is salt sensitive. In our studies, BK β1-KO and control mice were the same age and genetic background as in these earlier studies, but 7 days of 1% NaCl intake caused only a transient increase in MAP of 5 mmHg for 5 days, indicating that blood pressure in these animals is not strongly salt sensitive. Although we show that BK β1-KO mice do not develop sustained hypertension when provided a high-salt intake, we cannot rule out that BK β1-KO mice may become hypertensive with higher levels of salt intake (12), or over a longer period of high-salt diet.
In earlier studies, functional BK channels were detected in portal veins from rat (6), rabbit (1), guinea pig (30), and BALB/c mice (36), but also in MV from rats (26, 43). BK channels contribute to modulation of human saphenous (14, 23, 37) and forearm venous tone (22, 40). In the current studies, we found that BK β1-subunits are expressed in arteries but not veins of mice (except for the portal and pulmonary veins, i.e., large-conductance veins that contribute little to venous capacitance). BK channel α-subunits can function without β1-subunits. Generally, this makes the BK channel harder to open; there is about a 30- to 40-mV positive shift in the voltage required to open BK channel lacking β1-subunits in smooth muscle cells (2). It is still unclear if BK channel α-subunits are expressed in murine veins, but the lack of functional BK channels in murine veins is supported by our data showing that paxilline did not contract MV. Currently, we do not know if the absence of functional BK channels in veins only occurs in C57BL/6 mice. The role, if any, of venous BK channel function in regulation of blood pressure is still unknown, but our data suggest that isolated increases in arterial tone caused by defective BK channel function may not have a large effect on blood pressure. Therefore, these mice may be a useful tool to study BK channel function in modulation of vascular tone, since C57BL mice per se are venous BK β1-subunit “knocked out.”
In conclusion, BK β1-KO mice are not hypertensive based on continuous 24-h blood pressure measurements. Although L-type Ca2+ channel function is enhanced in vitro in arterial smooth muscle from BK β1-KO mice, this does not increase blood pressure dependence on L-type Ca2+ channels in vivo. Functional BK channels do not exist in murine venous smooth muscle cells. Although BK β1-KO mice have abnormal renal handling of Na+ and K+, and increased plasma aldosterone, they do not exhibit strong salt sensitivity of blood pressure when assessed by telemetry. Either arterial BK channels do not have a critical role in blood pressure regulation in vivo under resting situation, or permanent loss of BK channel function is effectively compensated by other cellular or integrative blood pressure control mechanisms.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant P01 HL-070687 and by a Michigan State University Health and Biomedical Research Initiative Award for H. Xu.
DISCLOSURES
None.
ACKNOWLEDGMENTS
We thank Dr. Robert Brenner (Department of Physiology, University of Texas Health Science Center at San Antonio, TX) for a gift of the BK β1-KO mice and advice for the manuscript.
REFERENCES
- 1. Beech DJ, Bolton TB. Properties of the cromakalim-induced potassium conductance in smooth muscle cells isolated from the rabbit portal vein. Br J Pharmacol 98: 851–864, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brenner R, Peréz GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature 407: 870–876, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Brown SM, Bentcheva-Petkova LM, Liu L, Hristov KL, Chen M, Kellett WF, Meredith AL, Aldrich RW, Nelson MT, Petkov GV. Beta-adrenergic relaxation of mouse urinary bladder smooth muscle in the absence of large-conductance Ca2+-activated K+ channel. Am J Physiol Renal Physiol 295: F1149–F1157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burns WR, Cohen KD, Jackson WF. K+-induced dilation of hamster cremasteric arterioles involves both the Na+/K+-ATPase and inward-rectifier K+ channels. Microcirculation 11: 279–293, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen CC, Lamping KG, Nuno DW, Barresi R, Prouty SJ, Lavoie JL, Cribbs LL, England SK, Sigmund CD, Weiss RM, Williamson RA, Hill JA, Campbell KP. Abnormal coronary function in mice deficient in alpha1H T-type Ca2+ channels. Science 302: 1416–1418, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Cogolludo AL, Pérez-Vizcaíno F, López-López G, Ibarra M, Zaragozá-Arnáez F, Tamargo J. Propafenone modulates potassium channel activities of vascular smooth muscle from rat portal veins. J Pharmacol Exp Ther 299: 801–810, 2001 [PubMed] [Google Scholar]
- 7. Desai KH, Sato R, Schauble E, Barsh GS, Kobilka BK, Bernstein D. Cardiovascular indexes in the mouse at rest and with exercise: new tools to study models of cardiac disease. Am J Physiol Heart Circ Physiol 272: H1053–H1061, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Eichhorn B, Dobrev D. Vascular large conductance calcium-activated potassium channels: functional role and therapeutic potential. Naunyn Schmiedebergs Arch Pharmacol 376: 145–155, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Félétou M. Calcium-activated potassium channels and endothelial dysfunction: therapeutic options? Br J Pharmacol 156: 545–562, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fink GD, Johnson RJ, Galligan JJ. Mechanisms of increased venous smooth muscle tone in desoxycorticosterone acetate-salt hypertension. Hypertension 35: 464–469, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Grimm PR, Irsik DL, Liu L, Holtzclaw JD, Sansom SC. Role of BKβ1 in Na+ reabsorption by cortical collecting ducts of Na+-deprived mice. Am J Physiol Renal Physiol 297: F420–F428, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grimm PR, Irsik DL, Settles DC, Holtzclaw JD, Sansom SC. Hypertension of Kcnmb1−/− is linked to deficient K secretion and aldosteronism. Proc Natl Acad Sci USA 106: 11800–11805, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grimm PR, Foutz RM, Brenner R, Sansom SC. Identification and localization of BK-β subunits in the distal nephron of the mouse kidney. Am J Physiol Renal Physiol 293: F350–F359, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Gruhn N, Boesgaard S, Eiberg J, Bang L, Thiis J, Schroeder TV, Aldershvile J. Effects of large conductance Ca(2+)-activated K(+) channels on nitroglycerin-mediated vasorelaxation in humans. Eur J Pharmacol 446: 145–150, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Huetteman DA, Bogie H. Direct blood pressure monitoring in laboratory rodents via implantable radio telemetry. Methods Mol Biol 573: 57–73, 2009 [DOI] [PubMed] [Google Scholar]
- 16. King AJ, Fink GD. Chronic low-dose angiotensin II infusion increases venomotor tone by neurogenic mechanisms. Hypertension 48: 927–933, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Knaus HG, Garcia-Calvo M, Kaczorowski GJ, Garcia ML. Subunit composition of the high conductance calsium-activated potassium channel from smooth muscle, a representative of the mslo and slowpoke family of potassium channels. J Bio Chem 269: 3921–3924, 1994 [PubMed] [Google Scholar]
- 18. Kun A, Matchkov VV, Stankevicius E, Nardi A, Hughes AD, Kirkeby HJ, Demnitz J, Simonsen U. NS11021, a novel opener of large-conductance Ca(2+)-activated K(+) channels, enhances erectile responses in rats. Br J Pharmacol 158: 1465–1476, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu G, Shi J, Yang L, Cao L, Park SM, Cui J, Marx SO. Assembly of a Ca2+-dependent BK channel signaling complex by binding to beta2 adrenergic receptor. EMBO J 23: 2196–2205, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu R, Alioua A, Kumar Y, Eghbali M, Stefani E, Toro L. MaxiK channel partners: physiological impact. J Physiol 570: 65–72, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martin DS, Rodrigo MC, Appelt CW. Venous tone in the developmental stages of spontaneous hypertension. Hypertension 31: 139–144, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Martinez-León JB, Segarra G, Medina P, Vila JM, Lluch P, Peiró M, Otero E, Lluch S. Ca2+-activated K+ channels mediate relaxation of forearm veins in chronic renal failure. J Hypertens 21: 1927–1934, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Mauricio MD, Serna E, Cortina B, Novella S, Segarra G, Aldasoro M, Martínez-León JB, Vila JM. Role of Ca2+-activated K+ channels on adrenergic responses of human saphenous vein. Am J Hypertens 20: 514–519, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Mills PA, Huetteman DA, Brockway BP, Zwiers LM, Gelsema AJ, Schwartz RS, Kramer K. A new method for measurement of blood pressure, heart rate, and activity in the mouse by radiotelemetry. J Appl Physiol 88: 1537–1544, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Moosmang S, Schulla V, Welling A, Feil R, Feil S, Wegener JW, Hofmann F, Klugbauer N. Dominant role of smooth muscle L-type calcium channel Cav1.2 for blood pressure regulation. EMBO J 22: 6027–6034, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nagakawa T, Yamazaki M, Hatakeyama N, Stekiel TA. The mechanisms of propofol-mediated hyperpolarization of in situ rat mesenteric vascular smooth muscle. Anesth Analg 97: 1639–1645, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science 270: 633–637, 1995 [DOI] [PubMed] [Google Scholar]
- 28. Oelze M, Warnholtz A, Faulhaber J, Wenzel P, Kleschyov AL, Coldewey M, Hink U, Pongs O, Fleming I, Wassmann S, Meinertz T, Ehmke H, Daiber A, Münzel T. NADPH oxidase accounts for enhanced superoxide production and impaired endothelium-dependent smooth muscle relaxation in BKbeta1−/− mice. Arterioscler Thromb Vasc Biol 26: 1753–1759, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol 537: 443–452, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pfründer D, Kreye VA. Tedisamil blocks single large-conductance Ca(2+)-activated K+ channels in membrane patches from smooth muscle cells of the guinea-pig portal vein. Pflugers Arch 418: 308–312, 1991 [DOI] [PubMed] [Google Scholar]
- 31. Plehm R, Barbosa ME, Bader M. Animal models for hypertension/blood pressure recording. Methods Mol Med 129: 115–126, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Plüger S, Faulhaber J, Fürstenau M, Löhn M, Waldschütz R, Gollasch M, Haller H, Luft FC, Ehmke H, Pongs O. Mice with disrupted BK channel beta1 subunit gene feature abnormal Ca(2+) spark/STOC coupling and elevated blood pressure. Circ Res 87: E53–E60, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Pluznick JL, Sansom SC. BK channels in the kidney: role in K+ secretion and localization of molecular components. Am J Physiol Renal Physiol 291: F517–F529, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Pluznick JL, Wei P, Grimm PR, Sansom SC. BK-β1 subunit: immunolocalization in the mammalian connecting tubule and its role in the kaliuretic response to volume expansion. Am J Physiol Renal Physiol 288: F846–F854, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Ricksten SE, Yao T, Thoren P. Peripheral, and central vascular compliances in conscious normotenisve and spontaneously hypertensive rats. Acta Physiol Scand 112: 169–177, 1981 [DOI] [PubMed] [Google Scholar]
- 36. Saleh SN, Angermann JE, Sones WR, Leblanc N, Greenwood IA. Stimulation of Ca2+-gated Cl- currents by the calcium-dependent K+ channel modulators NS1619 {1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-2H-benzimidazol-2-one} and isopimaric acid. J Pharmacol Exp Ther 321: 1075–1084, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Sanz E, Monge L, Fernández N, Martínez MA, Martínez-León JB, Diéguez G, García-Villalón AL. Relaxation by urocortin of human saphenous veins. Br J Pharmacol 136: 90–94, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sausbier M, Arntz C, Bucurenciu I, Zhao H, Zhou XB, Sausbier U, Feil S, Kamm S, Essin K, Sailer CA, Abdullah U, Krippeit-Drews P, Feil R, Hofmann F, Knaus HG, Kenyon C, Shipston MJ, Storm JF, Neuhuber W, Korth M, Schubert R, Gollasch M, Ruth P. Elevated blood pressure linked to primary hyperaldosteronism and impaired vasodilation in BK channel-deficient mice. Circulation 112: 60–68, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Sausbier M, Zhou XB, Beier C, Sausbier U, Wolpers D, Maget S, Martin C, Dietrich A, Ressmeyer AR, Renz H, Schlossmann J, Hofmann F, Neuhuber W, Gudermann T, Uhlig S, Korth M, Ruth P. Reduced rather than enhanced cholinergic airway constriction in mice with ablation of the large conductance Ca2+-activated K+ channel. FASEB J 21: 812–822, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Segarra G, Lluch P, Mauricio MD, Vila JM, Medina P, Martinez-León JB, Aldasoro M, Lluch S. Contractile hyporesponsiveness to norepinephrine of forearm veins in chronic renal failure. Am J Hypertensi 19: 818–822, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Semenov I, Wang B, Herlihy JT, Brenner R. BK channel beta1-subunit regulation of calcium handling and constriction in tracheal smooth muscle. Am J Physiol Lung Cell Mol Physiol 291: L802–L1810, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Sprossmann F, Pankert P, Sausbier U, Wirth A, Zhou XB, Madlung J, Zhao H, Bucurenciu I, Jakob A, Lamkemeyer T, Neuhuber W, Offermanns S, Shipston MJ, Korth M, Nordheim A, Ruth P, Sausbier M. Inducible knockout mutagenesis reveals compensatory mechanisms elicited by constitutive BK channel deficiency in overactive murine bladder. FEBS J 276: 1680–1697, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stekiel TA, Contney SJ, Kokita N, Bosnjak ZJ, Kampine JP, Stekiel WJ. Mechanism of isoflurane-mediated hyperpolarization of vascular smooth muscle in chronically hypertensive and normotensive conditions. Anesthesiology 94: 496–506, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Tanaka Y, Meera P, Song M, Knaus HG, Toro L. Molecular constitutents of maxi Kca channels in human coronary smooth muscle: predominat α + β subunit complexes. J Physiol 502: 545–575, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thakali KM, Kharade SV, Sonkusare SK, Rhee SW, Stimers JR, Rusch NJ. Intracellular Ca2+ silences L-type Ca2+ channels in mesenteric veins: mechanism of venous smooth muscle resistance to calcium channel blockers. Circ Res 106: 739–747, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang YX, Fitch RM. Vascular stiffness: measurements, mechanisms and implications. Curr Vasc Pharmacol 2: 379–384, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Werner ME, Knorn AM, Meredith AL, Aldrich RW, Nelson MT. Frequency encoding of cholinergic- and purinergic-mediated signaling to mouse urinary bladder smooth muscle: modulation by BK channels. Am J Physiol Regul Integr Comp Physiol 292: R616–R624, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Whitesall SE, Hoff JB, Vollmer AP, D'Alecy LG. Comparison of simultaneous measurement of mouse systolic arterial blood pressure by radiotelemetry and tail-cuff methods. Am J Physiol Heart Circ Physiol 286: H2408–H2415, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Zanzinger J, Czachurski J, Seller H. Role of Calcium-dependent K+ channels in the regulation of arterial and venous tone by nitric oxide in pigs. Pflugers Arch 432: 671–677, 1996 [DOI] [PubMed] [Google Scholar]