Abstract
Diamide is a membrane-permeable, thiol-oxidizing agent that rapidly and reversibly oxidizes glutathione to GSSG and promotes formation of protein-glutathione mixed disulfides. In the present study, the acute effect of diamide on free cytosolic Ca2+ concentration ([Ca2+]i) was examined in fura-2-loaded bovine aortic endothelial cells. At low concentrations (50, 100 μM), diamide reversibly increased spontaneous, asynchronous Ca2+ oscillations, whereas, at higher concentrations (250, 500 μM), diamide caused an immediate synchronized Ca2+ oscillation in essentially all cells of the monolayer, followed by a time-dependent rise in basal [Ca2+]i. The effects of diamide on [Ca2+]i dynamics were independent of extracellular Ca2+. Inhibition of phospholipase C by U-73122 prevented the observed changes in [Ca2+]i. Additionally, the diamide-induced oscillations, but not the rise in basal [Ca2+]i, were blocked by inhibition of the inositol-1,4,5-trisphosphate (IP3) receptor (IP3R) by 2-aminoethyl diphenyl borate. However, diamide failed to alter the plasmalemmal distribution of a green fluorescent protein-tagged phosphatidylinositol-4,5-bisphosphate binding protein, demonstrating that diamide does not activate phospholipase C. Inhibition of glutathione reductase by N,N′-bis(2-chloroethyl)-N-nitrosourea or depletion of glutathione by l-buthionine-sulfoximine enhanced the effects of diamide, which, under these conditions, could only be reversed by addition of dithiothreitol to the wash buffer. Biochemical assays showed that both the IP3R and the plasmalemmal Ca2+-ATPase pump could be reversibly glutathionylated in response to diamide. These results demonstrate that diamide promotes Ca2+ release from IP3-sensitive internal Ca2+ stores and elevates basal [Ca2+]i in the absence of extracellular Ca2+, effects that may be related to a diamide-induced glutathionylation of the IP3R and the plasmalemmal Ca2+-ATPase Ca2+ pump, respectively.
Keywords: Ca2+ oscillations, oxidative stress, diamide, glutathione, Ca2+ channels
the vascular endothelium is susceptible to a diverse range of oxidative insults arising from the reduction of molecular oxygen, the activation of neutrophils, the products of drug metabolism, and the generation of reactive species, such as nitric oxide. Oxidative stress has also been implicated in a wide range of disease processes, ranging from acute events, such as ischemia-reperfusion injury, to chronic conditions, such as diabetes, atherosclerosis, and hypertension. Oxidative stress is defined as an imbalance between the amount of reactive oxygen and reactive nitrogen species (ROS/RNS) and the normal antioxidant defense mechanisms of the cell, leading to abnormal changes in molecular signaling, and/or damage to cellular proteins, resulting in a pathological state (33). Glutathione, along with its associated enzyme networks, serves as the primary cellular antioxidant defense system (45). Under basal conditions, glutathione is present in the cytoplasm of most cells in its reduced form (GSH) at a concentration of 1–10 mM, and at a ratio of ∼100:1 relative to its oxidized form (GSSG) (37). During conditions of oxidative stress, GSH functions as a scavenger of ROS/RNS and as a substrate for glutathione peroxidase-dependent reduction of hydrogen peroxide (H2O2). Conditions favoring oxidation of GSH to GSSG can also lead to the formation of protein-glutathione mixed disulfides (P-SSG), i.e. protein S-glutathionylation, the reversible posttranslational modification of sensitive cysteine thiol groups by GSH (10, 11, 46). Although the precise molecular mechanism(s) regulating P-SSG remains controversial, it is well established that glutathionylation of intracellular proteins can serve to protect against the irreversible oxidation of cysteine thiols and/or to regulate protein function during an oxidative insult.
Exogenous application of ROS/RNS to tissues and cells in culture has been used for decades as an experimental tool to tip the redox balance toward oxidative stress. In addition, specific inhibitors of the glutathione redox pathway have also been used to alter the antioxidant defenses of the cell. Collectively, these studies have provided a wealth of information related to both how cells normally handle oxidants and how a shift in the redox balance can lead to pathological changes. However, it is often difficult to identify the molecular events associated with exogenous application or even endogenous generation of ROS/RNS, because these reactive species not only modify proteins, but also affect membrane lipids and nucleic acids. Additionally, it is becoming increasingly evident that small changes in redox balance can lead to modification of specific signaling pathways, which, in turn, elicit a physiological response, i.e., redox signaling (26, 27, 33). These reversible molecular switches appear to be flipped by nitrosylation and/or glutathionylation of cellular proteins. How these modifications alter protein function and the molecular events associated with conversion from physiological signaling to pathological changes is an area of active investigation.
One of the earliest events in endothelial cells associated with an oxidative insult is a disruption of Ca2+ homeostasis (52). Ca2+ signal transduction plays an essential role in vascular endothelial cell function. Receptor-mediated changes in cytosolic free Ca2+ concentration ([Ca2+]i) are important for controlling the production and release of paracrine factors critical to the regulation of vascular permeability and tone, platelet aggregation and thrombosis, fibrinolysis, angiogenesis, mechanoreception, and inflammation. Physiologically occurring oxidants known to decrease GSH, such as superoxide, H2O2, and peroxynitrite, can significantly affect both basal and agonist-mediated changes in endothelial [Ca2+]i (12, 14, 22, 30, 31). Previous studies have shown that oxidant-induced changes in endothelial cell [Ca2+]i are related to the oxidation of intracellular GSH to GSSG (13, 15, 25). Additionally, oxidative stress is known to increase the formation of P-SSG mixed disulfides (55), but the role of P-SSG in Ca2+ homeostasis and signaling of vascular endothelial cells has not been explored.
Diamide [diazenedicarboxylic acid bis (N,N-dimethylamide)] is a widely used, cell-permeable, thiol-oxidizing agent first described by Kosower and colleagues (38, 39). In a stoichiometric two-step reaction, diamide rapidly and reversibly oxidizes intracellular GSH to GSSG. In vitro, diamide preferentially oxidizes GSH compared with other physiological reducing equivalents, such as NADH, NADPH, and coenzyme A (36). Likewise, studies on cultured cells have shown that diamide treatment causes a decrease in GSH, an increase in GSSG, and an increase in the formation of P-SSG mixed disulfides (24, 55). The ability of diamide to promote the formation of P-SSG has been exploited to identify protein targets of glutathionylation and to examine the effect of glutathionylation on protein and/or cellular function (for recent examples, see Refs. 6, 17, 20, 28, 32, 41, 62). Since the cytosolic concentration of GSH is normally in the millimolar range, low concentrations of diamide can be used to alter the redox balance in a graded fashion and thus to interrogate the consequences of P-SSG formation over both the physiological and pathological range of oxidative challenge, without the added complexities associated with direct application or generation of ROS/RNS.
To determine whether protein S-glutathionylation alters the function of ion channels and pumps associated with Ca2+ signaling, the acute effect of diamide on [Ca2+]i of cultured bovine aortic endothelial cells (BAEC) was examined in the present study at the single-cell level using the fluorescent Ca2+ indicator fura-2 and time-lapse video microscopy. Our results demonstrate that diamide produces a dramatic increase in single-cell [Ca2+]i oscillation frequency and a significant increase in basal [Ca2+]i both in the presence and, importantly, in the absence of extracellular Ca2+. The effects of diamide were dependent on both GSH and the capacity of the cell to regenerate GSH from GSSG. Furthermore, the effects were reversed by exogenous application of dithiothreitol (DTT), implicating thiol modification in the effects seen. Lastly, pull-down assays using biotinylated-GSH were consistent with a diamide-induced glutathionylation of both the inositol-1,4,5-trisphosphate (IP3) receptor (IP3R) and the plasmalemmal Ca2+-ATPase (PMCA). Overall, the results suggest that IP3R channel activity is enhanced by glutathionylation, whereas PMCA pump activity is inhibited. A preliminary report of this study has been published in abstract form (42).
EXPERIMENTAL PROCEDURES
Cell culture.
The isolation, culture, and characterization of the BAEC line used in this study were extensively described in previous reports (9, 53, 54). Briefly, BAECs were cultured as monolayers on 100-mm plastic cell culture dishes in low-glucose DMEM containing l-glutamine (Invitrogen) and supplemented with 10% heat-inactivated fetal bovine serum, 15 mM HEPES, 28.6 mM sodium bicarbonate, and 1% penicillin-streptomycin-neomycin solution (Gibco). BAEC monolayers, which exhibited a cobblestone appearance typical of contact-inhibited endothelial cell cultures, were used for experimentation between passages 13 and 20. LLC-PK1 cells stably expressing the green fluorescent protein (GFP)-tagged pleckstrin homology domain of phospholipase C (PLC)-γ (GFP-PH) were a generous gift of Dr. Jeffrey Schelling (MetroHealth Medical Center, Cleveland, OH). LLC-PK1 cells were cultured in DMEM-F12 supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin-neomycin solution, and 400 μg/ml G418.
Solutions and reagents.
Normal Ca2+ extracellular solution (Ca2+-ECS) contains the following in mM: 10 HEPES, 140 NaCl, 5.4 KCl, 1 MgCl2, 1.8 CaCl2, and 10 glucose, pH 7.4, at 37°C. Zero Ca2+ buffer (zero Ca2+-ECS) contains the following in mM: 10 HEPES, 140 NaCl, 5.4 KCl, 1 MgCl2, 0.3 EGTA, and 10 glucose, pH 7.4, at 37°C. Phosphate-buffered saline (PBS) contained 137 mM NaCl, 2.7 mM KCl, 10.1 mM Na2HPO4, 1.8 mM KH2PO4, pH adjusted to 7.4 or 8.0 with NaOH, as indicated. Diamide [diazenedicarboxylic acid bis (N,N-dimethylamide)], 2-APB (2-aminoethyl diphenyl borate), BCNU [N,N′-bis(2-chloroethyl)-N-nitrosourea], BSO (l-buthionine-sulfoximine), and DMSO (dimethyl sulfoxide) were purchased from Sigma-Aldrich. U73122 and U73343 were obtained from Calbiochem. Fura-2 acetoxymethyl ester (fura-2 AM), and pluronic F-127 were obtained from Invitrogen. Biotinylated-GSH ethyl ester (BioGEE) was synthesized as previously described (16). The 5F10 mouse monoclonal anti-PMCA antibody was from Affinity Bioreagents (catalog no. MA3–914). The anti-IP3R type I antibody was from Millipore (catalog no. 07–514). Stock solutions of 2-APB (50 mM), U-73122 (10 mM), and U-73343 (10 mM) were prepared in DMSO. BCNU stock (37.5 mM) was prepared in an aqueous solution with 10% ethanol and was stored at −20°C (up to 1 mo). BSO was prepared as an aqueous 100 mM stock solution and was stored at 4°C (up to 1 mo). Fura-2 AM was reconstituted using DMSO and 10% pluronic F-127 at a 1:1 ratio to yield a 1 mM stock solution. Diamide stock solutions were prepared in either Ca2+-ECS or zero Ca2+-ECS at a final concentration of 10 mM; both fura-2 AM and diamide were prepared fresh each day of experimentation.
Ca2+ imaging.
Time-dependent changes in [Ca2+]i were measured in BAEC monolayers as previously described (21). Briefly, BAEC monolayers grown on glass coverslips were loaded with fura-2, mounted in a temperature-controlled perfusion chamber, and placed on the stage of a Leica DMIRE2 inverted microscope. At 6-s intervals, excitation wavelength alternated between 340 and 380 nm, and emission was recorded at 510 nm using filters appropriate for fura-2. Epifluorescence was recorded using a SPOT-RT camera (Diagnostic Instruments, Sterling Heights, MI), and images were acquired and analyzed using SimplePCI imaging software (Compix, Cranberry Township, PA). Solutions were perfused into the recording chamber via an inline heater; all fura-2 imaging experiments were performed at 37°C.
Data analysis.
Over the course of these experiments, slight variation in the sensitivity of the BAECs to diamide was noted. For this reason, controls were always performed in parallel for each experimental protocol. For example, the effect of 250 μM diamide on Ca2+ oscillations is reported in Figs. 3D, 7D, and 8D; these represent independent data sets. The figures show [Ca2+]i responses from individual cells (40–80 cells/field of view) as different gray-scale lines. Oscillation frequency was determined for each individual cell and subsequently binned into three frequency categories based on the number of oscillations observed during the test period: no oscillations, one to two oscillations, or three or more oscillations (expressed as a percentage of the total cells counted per monolayer). Histograms of oscillation frequencies show the average values from multiple monolayers under each condition reported as mean ± SE, with n equal to the number of monolayers examined under each condition. The total number of cells and the total number of monolayers counted for each condition are given in the figure legend. Statistical analysis was performed using the paired Student's t-test with Bonferroni's correction for multiple comparisons; P value < 0.05 was considered to be statistically significant.
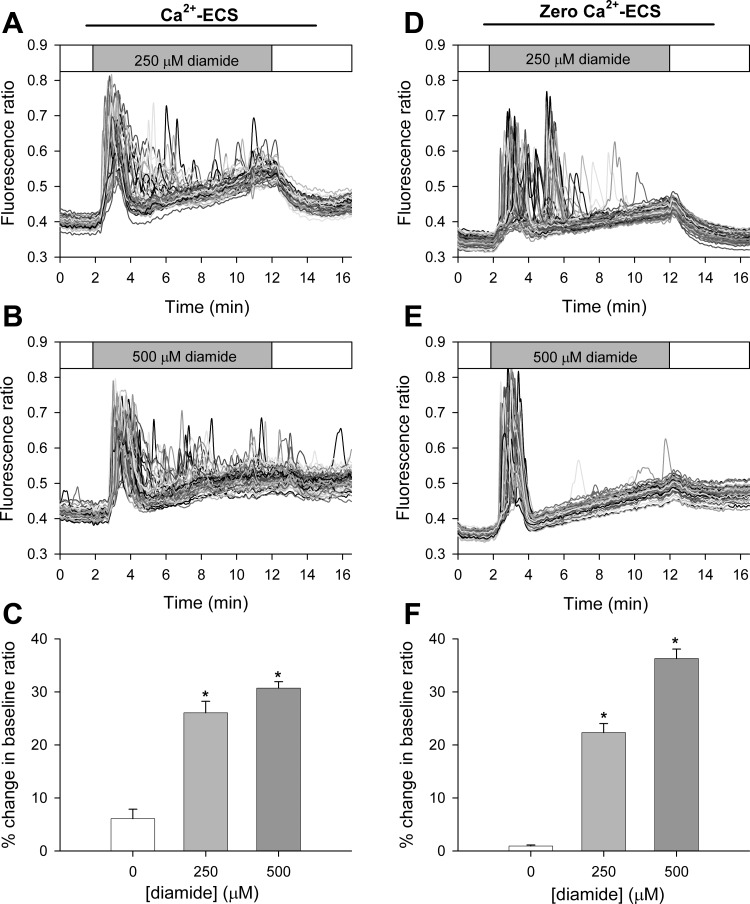
Fig. 3.
High concentrations of diamide elevates basal [Ca2+]i when measured in both the presence and absence of extracellular Ca2+. Fura-2 fluorescence ratio (340 nm/380 nm) was recorded from single BAECs in normal Ca2+ containing buffer (Ca2+-ECS) or in the absence of extracellular Ca2+ (zero Ca2+-ECS), in the presence of 250 μM (A and D) or 500 μM diamide (B and E). Basal [Ca2+]i (C and F) was quantified as the percent change in the baseline fluorescence ratio at the end of the 10-min treatment period for each experimental condition. C: values represent means ± SE of 5–6 experiments; 261 (untreated), 354 (250 μM), and 281 (500 μM) cells were analyzed per treatment, with 43–64 cells analyzed per experiment. F: values represent means ± SE of 4–5 experiments; 263 (untreated), 258 (250 μM), and 332 (500 μM) cells were analyzed per treatment, with 54–74 cell analyzed per experiment. *P < 0.0001 compared with matched untreated controls.
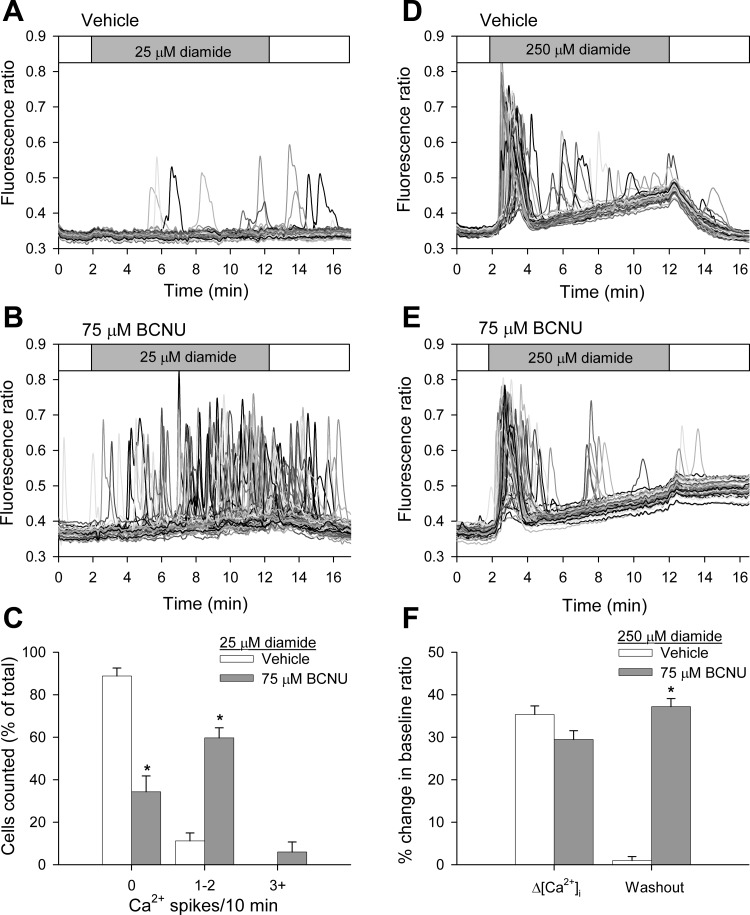
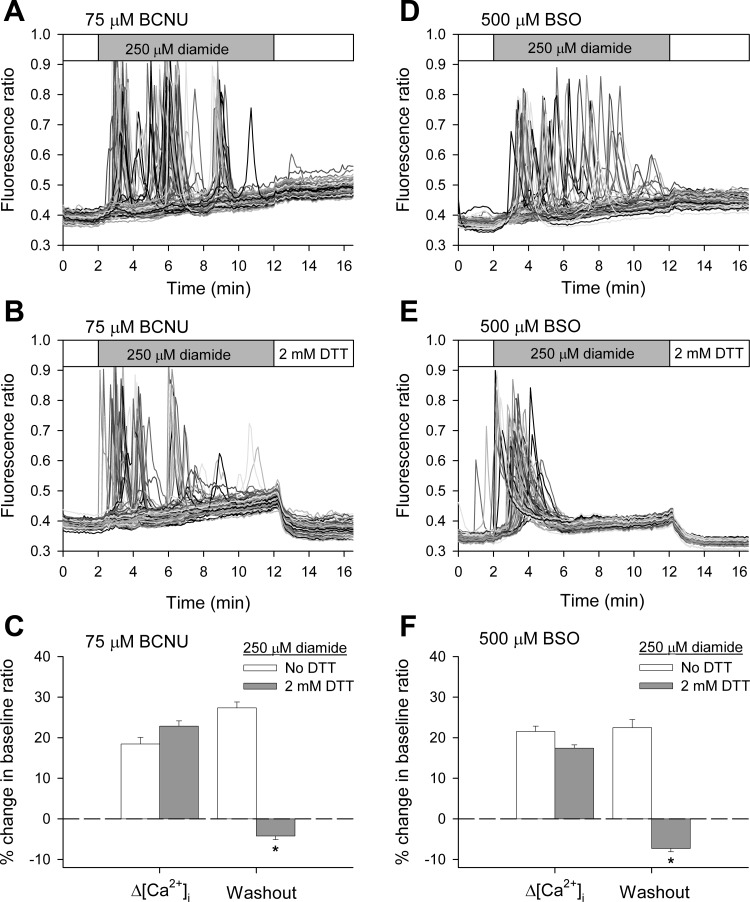
Fig. 7.
Inhibition of glutathione reductase by N,N′-bis(2-chloroethyl)-N-nitrosourea (BCNU) potentiates diamide-induced changes when measure in the absence of extracellular Ca2+. BAECs in normal Ca2+-ECS were pretreated for either 30 min with vehicle or 75 μM BCNU before challenging with diamide. Fura-2 fluorescence ratio was recorded from single BAECs in zero Ca2+-ECS in the presence of 25 μM (A and B) or 250 μM diamide (D and E), as indicated by the horizontal bar at the top of each panel. C: single-cell [Ca2+]i oscillation frequency was quantified as described in the legend to Fig 1. Values represent means ± SE of 4 experiments; 260 (vehicle) and 261 (75 μM BCNU) cells were counted per experimental condition, with 54–76 cells counted per experiment. *P value < 0.001 compared with matched controls. F: the change in basal [Ca2+]i was quantified as the percent change in the baseline fluorescence ratio at the end of the 10-min treatment period (Δ[Ca2+]i) and at the end of a 5-min washout period (Washout). Values represent means ± SE of 3–4 experiments; 169 (vehicle) and 236 (75 μM BCNU) cells were analyzed per experimental condition, with 51–64 cells analyzed per experiment. *P < 0.001 compared with matched controls.
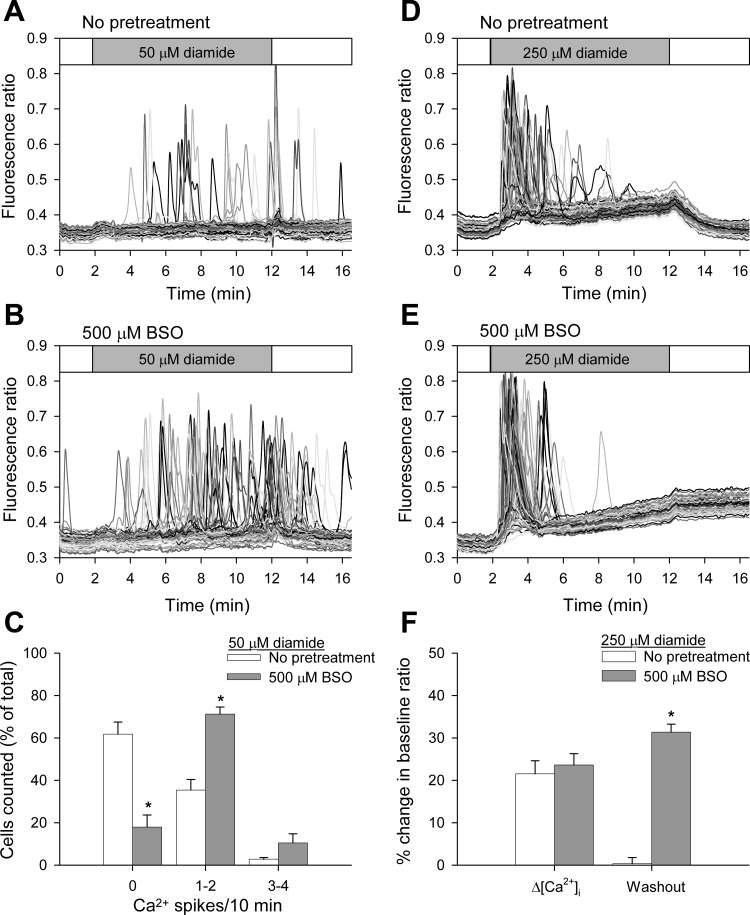
Fig. 8.
Inhibition of γ-glutamylcysteine synthetase by l-buthionine-sulfoximine (BSO) enhances diamide-induced changes in [Ca2+]i when measured in the absence of extracellular Ca2+. BAECs were pretreated for 24 h with 500 μM BSO before challenge with diamide. Fura-2 fluorescence ratio was recorded from single BAECs in zero Ca2+-ECS in the presence of 50 μM (A and B) or 250 μM diamide (D and E), as indicated by the horizontal bar at the top of each panel. C: single-cell [Ca2+]i oscillation frequency was quantified, as described in the legend to Fig 1. Values represent means ± SE of 4–5 experiments; 246 (no treatment) and 296 (500 μM BSO) cells were counted per experimental condition, with 59–65 cells counted per experiment. *P value < 0.001 compared with matched controls. F: the change in basal [Ca2+]i was quantified as described in Fig 7. Values represent means ± SE of 3–4 experiments; 244 (no treatment) and 196 (500 μM BSO) cells were analyzed per experimental condition, with 58–66 cells analyzed per experiment. *P < 0.001 compared with matched controls.
Isolation of membranes from BAECs.
Membranes were isolated as previous described (56). Briefly, confluent BAEC monolayers were harvested from the culture dishes by scrapping, subjected to centrifugation at 500 g for 5 min, and resuspended in lysis buffer containing 20 mM Tris·Cl, 5 mM EDTA, 1 mM EGTA, and protease inhibitor mixture. The cell suspension was sonicated on ice using a sonic dismembranator (Fisher) on a power setting of 2.5. The cell suspension was sonicated three times for 10 s, with a 10-s rest between pulses. The cell lysate was subjected to centrifugation at 6,000 g for 10 min at 4°C. The resulting pellet was discarded, and the supernatants were centrifuged at 50,000 g for 30 min. The microsomal pellets were resuspended in lysis buffer at a protein concentration of 5–10 mg/ml.
Synthesis of biotinylated GSH.
GSH was labeled with sulfo-link-NHS-LC-biotin (Pierce). The reaction was performed by combining 10 mM GSH with 10 mM of biotin reagent in PBS (pH 8.0). After 1 h at room temperature, 50 mM Tris was added to remove any excess sulfo-link reagent.
Glutathionylation of the IP3R and PMCA in BAEC microsomal membranes.
Membrane proteins were glutathionylated by incubating microsomes with 125 μM biotin-GSH and 100 μM diamide for 10 min at room temperature in PBS (pH 8.0 or 7.4). Some samples were treated with DTT for an additional 15 min at room temperature. Following incubation, membranes were pelleted by centrifugation and washed three times with PBS to remove excess reagents. Washed membrane pellets were solubilized in PBS containing 1% Triton X-100 for 30 min on ice. Lysates were cleared by centrifugation at 50,000 g for 60 min at 4°C, and glutathionylated proteins were extracted by overnight incubation with streptavidin-agarose beads (Pierce).
Glutathionylation of IP3R and PMCA in vivo.
BAECs, harvested by scraping, were washed and resuspended in 1.5 ml Ca2+-ECS without BSA (pH 7.4). The cells were incubated with 250 or 500 μM BioGEE for 60 or 180 min at 37°C. BioGEE-loaded cells were washed with Ca2+-ECS and incubated for 15 min at room temperature in the absence or presence of 500 μM diamide. Following incubation, the cells were washed with diamide-free Ca2+-ECS, and membrane preparations were generated as described above. Membrane aliquots were solubilized in PBS containing 1% Triton X-100 for 30 min on ice. Lysates were cleared by centrifugation, and biotinylated proteins were extracted with streptavidin-agarose beads. Where indicated, DTT (20 mM) was added before pull-down.
Immunoblots.
Following in vitro or in vivo glutathionylation reactions, proteins captured on streptavidin beads were fractionated by SDS-PAGE and electrotransfered to polyvinylidene difluoride membrane (100 V for 1 h) in 3-(cyclohexylamino)-1-propanesulfonic acid-methanol buffer. Blots were probed with anti-PMCA or anti-IP3R antibody and detected, following incubation with horseradish peroxidase-conjugated IgG, by SuperSignal West Pico chemiluminescent substrate (Pierce). For comparison and statistical analysis, band intensities from avidin pull-down samples were quantified by densitometry and normalized to input controls.
RESULTS
Diamide increases Ca2+ oscillations and elevates basal [Ca2+]i.
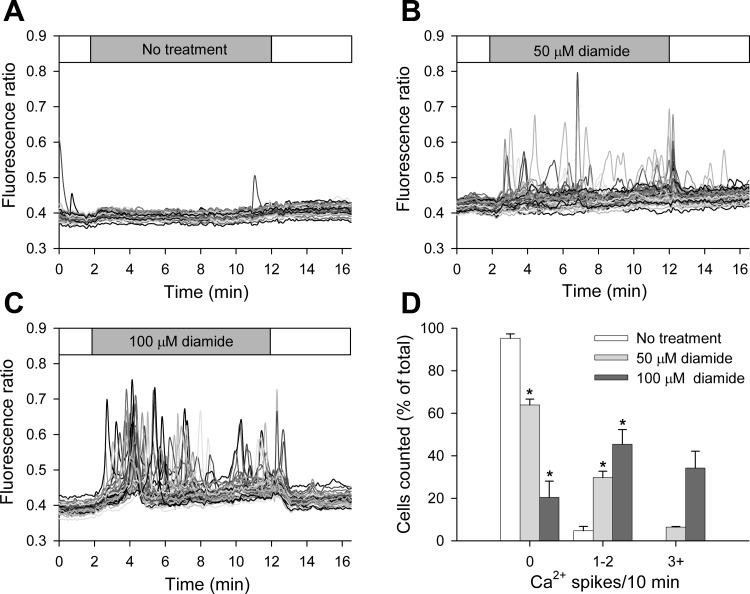
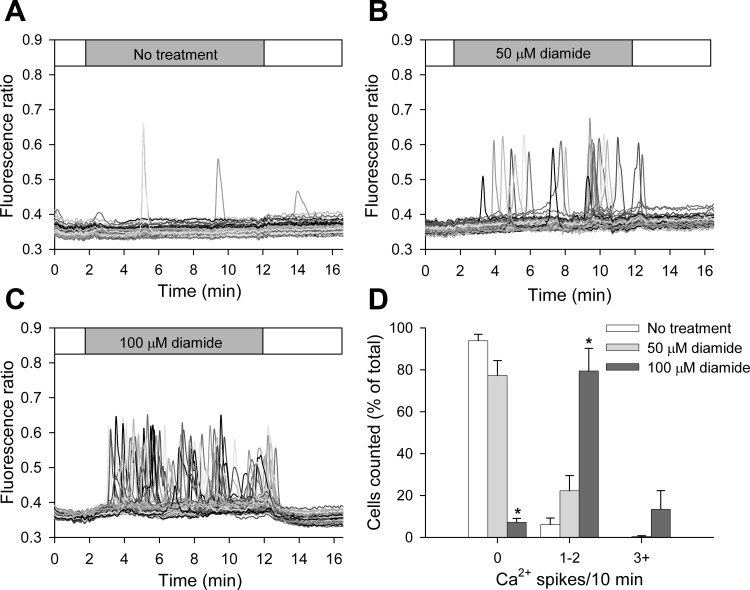
The acute effect of diamide on [Ca2+]i of BAECs was examined by single-cell Ca2+ imaging. Low concentrations of diamide (50 μM, 100 μM) progressively increased the number of cells exhibiting spontaneous asynchronous Ca2+ oscillations and increased the oscillation frequency of individual cells. As seen in Fig. 1, A–C, and quantified in Fig. 1D, 95% of cells failed to exhibit even a single Ca2+ oscillation during the recording period in the absence of diamide. However, addition of 50 μM diamide to the extracellular solution caused ∼30% of the cells to exhibit one or two Ca2+ spikes, and 12% of cells to exhibit three or more Ca2+ oscillations during the treatment period. Raising the concentration of diamide to 100 μM caused ∼80% of the cells to oscillate, with ∼40% of the cells showing three or more oscillations. Upon washout of diamide, there was an immediate cessation of Ca2+ oscillations and a return of [Ca2+]i to basal levels, demonstrating that the effects of diamide are rapidly reversible under control conditions. To determine the source of Ca2+ responsible for the oscillations observed, the experiments were repeated in the absence of extracellular Ca2+ (zero Ca2+-ECS). As seen in Fig. 2, A–C, and quantified in Fig. 2D, ∼20% of cells treated with 50 μM diamide responded with one or two Ca2+ oscillations, whereas exposure to 100 μM diamide produced a more robust response, with over 90% of cells exhibiting at least one Ca2+ spike during the treatment period. Once again this response was reversed on washout of diamide. These results demonstrate that the diamide-induced Ca2+ oscillations primarily reflect the release of Ca2+ from internal stores. The only notable difference in the diamide response observed in the absence vs. the presence of extracellular Ca2+ was the number of cells exhibiting higher frequency oscillations; e.g., ∼10 vs. ∼40% at 100 μM diamide. Thus, in the absence of extracellular Ca2+, the internal stores will eventually deplete, and oscillations will cease. For comparison, diamide-induced Ca2+ oscillations in both the presence and absence of extracellular Ca2+ were similar in magnitude to the Ca2+ transients observed in response to bradykinin, a potent endothelial-dependent vasodilator (Supplemental Fig. S1; The online version of this article contains supplemental data).
Fig. 1.
Low concentrations of diamide cause asynchronous Ca2+ oscillations when measured normal Ca2+ extracellular solution (Ca2+-ECS). A–C: fura-2 fluorescence ratio (340 nm/380 nm) was recorded from single bovine aortic endothelial cells (BAECs) in the absence (A) and presence of 50 μM (B) or 100 μM (C) diamide, as indicated by the horizontal bar at the top of each panel. Individual cells are shown as different gray-scale traces. D: single-cell free cytosolic Ca2+ concentration ([Ca2+]i) oscillation frequency was quantified as the number of cells exhibiting 0, 1–2, and 3+ Ca2+ spikes during the 10-min treatment period. Values represent means ± SE of 3–4 experiments; 259 (untreated), 204 (50 μM), and 197 (100 μM) cells were counted per treatment, with 42–63 cells counted per experiment. *P < 0.0005 compared with matched untreated controls.
Fig. 2.
Low concentrations of diamide cause asynchronous Ca2+ oscillations when measured in the absence of extracellular Ca2+. The protocol was the same as in Fig 1, with the exception that 0.3 mM EGTA was added and CaCl2 was omitted from the extracellular buffer (zero Ca2+-ECS). Fura-2 fluorescence ratio (340 nm/380 nm) was recorded from BAECs in the absence (A) and presence of 50 μM (B) or 100 μM (C) diamide. D: single-cell [Ca2+]i oscillation frequency was quantified as the number of cells exhibiting 0, 1–2, and 3+ Ca2+ spikes during the 10-min treatment period. Values represent means ± SE of 3–5 experiments; 336 (untreated), 205 (50 μM), and 177 (100 μM) cells were counted per treatment, with 54–80 cells counted per experiment. *P < 0.0001 compared with matched untreated controls.
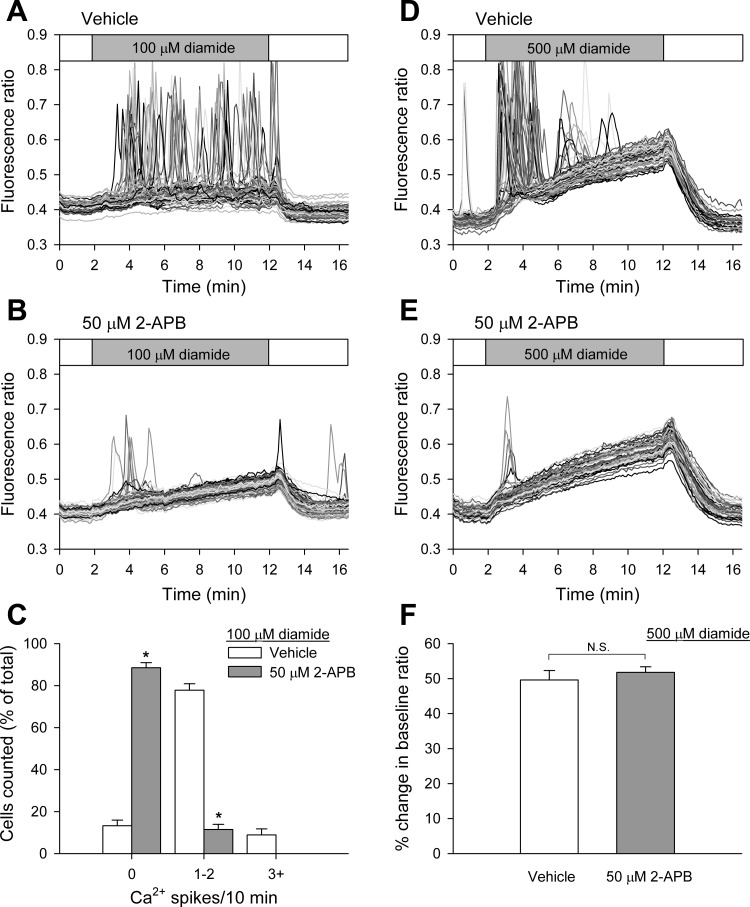
Higher concentrations of diamide (250 μM, 500 μM) effectively synchronized the observed Ca2+ oscillations (Fig. 3). Upon exposure to the oxidant, nearly all cells monitored exhibited a single Ca2+ oscillation, followed by a steady rise in basal [Ca2+]i throughout the remainder of the treatment period. This response was observed in both the presence (Ca2+-ECS) and absence (zero Ca2+-ECS) of extracellular Ca2+. In the presence of extracellular Ca2+, the [Ca2+]i of some cells remained elevated or continued to oscillate after the initial spike, but the oscillations, if present, occurred in an asynchronized fashion. At the end of the 10-min treatment period, basal [Ca2+]i of diamide-treated BAECs was significantly elevated compared with matched untreated controls. In the presence of extracellular Ca2+ (Fig. 3C), cells challenged with either 250 or 500 μM diamide exhibited a 25 and 30% increase in their baseline fluorescence ratio at the end of the treatment period, respectively. Likewise, in the absence of extracellular Ca2+ (Fig. 3F), cells treated with 250 or 500 μM diamide produced a 22 and 35% increase in baseline fluorescence ratio, respectively. The rise in basal [Ca2+]i was completely reversed following washout of the reagent from cells treated with 250 μM diamide. However, following removal of 500 μM diamide, 170 of 281 cells (in Ca2+-ECS) and 140 of 322 cells (in zero Ca2+-ECS) failed to return to baseline levels during the 5-min washout period.
The products of the reaction of diamide with GSH are GSSG and a hydrazine byproduct of diamide (38, 39). A number of control experiments were, therefore, performed to determine the specificity of the diamide response. First, cell-free experiments confirmed that the changes in [Ca2+]i were not due to a direct effect of diamide or the hydrazine byproduct on fura-2 fluorescence per se (data not shown). Second, the diamide-induced changes in [Ca2+]i of BAECs were prevented by premixing diamide with GSH (at 1:4 molar ratio) to inactivate diamide, demonstrating that the thiol-oxidizing effect of diamide, and not the hydrazine byproduct, is responsible for the observed changes in Ca2+ dynamics (Supplemental Fig S2). Since BAECs express purinergic receptors, which, when stimulated, initiate a robust Ca2+ response, we considered the possibility that diamide may cause the release of ATP. However, addition of 5 U/ml of apyrase to the bath solution during treatment with 100 μM diamide did not affect the observed Ca2+ oscillation frequency compared with matched controls, indicating that a diamide-induced ATP release is not responsible for the changes seen in [Ca2+]i (Supplemental Fig S3). Collectively, these results show that diamide at low concentrations dynamically alters the release of Ca2+ from internal stores, presumably via activation of IP3R, and, at higher concentrations, inhibits Ca2+ efflux from the cytosol. Since BAECs lack a Na+/Ca2+ exchanger (52), the rise in basal [Ca2+]i in the absence of extracellular Ca2+ presumably reflects inhibition of the PMCA pump.
Diamide-induced Ca2+ oscillations are attenuated by the IP3R inhibitor 2-APB.
To determine the role of the IP3R in the diamide-induced Ca2+ oscillations, the effect of 2-APB, a receptor antagonist (44), was examined in the absence of extracellular Ca2+. As shown in Fig. 4, A and B, and quantified in Fig. 4C, pretreatment of BAECs for 10 min with 50 μM 2-APB, immediately before challenge with 100 μM diamide, significantly attenuated Ca2+ oscillations compared with matched vehicle-pretreated controls. Pretreatment of the cells with 2-APB also blocked the synchronized oscillation seen upon exposure to 500 μM diamide (Fig. 4, D and E). These results confirm that diamide-induced Ca2+ oscillations are due to Ca2+ release from internal stores via the IP3R. Interestingly, 2-APB did not block the effect of diamide on the reversible rise in basal [Ca2+]i (Fig. 4F), suggesting that this phase is independent of the IP3R.
Fig. 4.
Inhibition of the inositol-1,4,5-trisphosphate receptor (IP3R) by 2-aminoethyl diphenyl borate (2-APB) attenuates diamide-induced Ca2+ oscillations when measured in the absence of extracellular Ca2+. Protocol was the same as in Fig 2, with the exception that BAECs were pretreated for 10 min with vehicle (A), or 50 μM 2-APB (B) immediately before challenge with 100 μM diamide in zero Ca2+-ECS. C: diamide-induced single-cell [Ca2+]i oscillation frequency was quantified as described in the legend to Fig 1 for each experimental condition. Values represent means ± SE of 3–5 experiments; 314 (vehicle) and 222 (50 μM 2-APB) cells were counted per treatment, with 58–82 cells counted per experiment. *P < 0.00001 compared with matched vehicle controls. D and E: same protocol as in A and B, with 500 μM diamide added during the time indicated. F: basal [Ca2+]i from experiments shown in D and E were quantified as the percent change in the baseline fluorescence ratio at the end of the 10-min treatment period. Values represent means ± SE of 3–4 experiments; 201 (vehicle) and 261 (50 μM 2-APB) cells were counted per treatment, with 53–75 cells per experiment. NS, not significant.
Diamide-induced Ca2+ oscillations are prevented by inhibition of PLC.
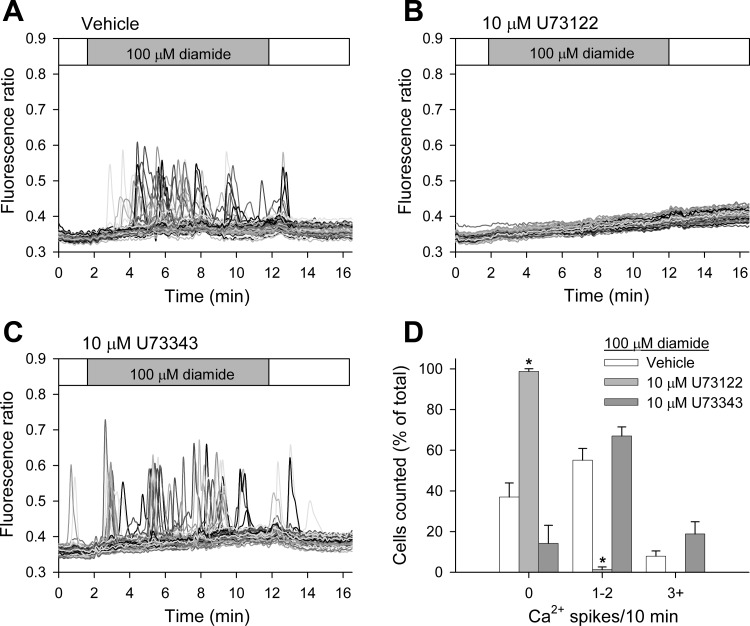
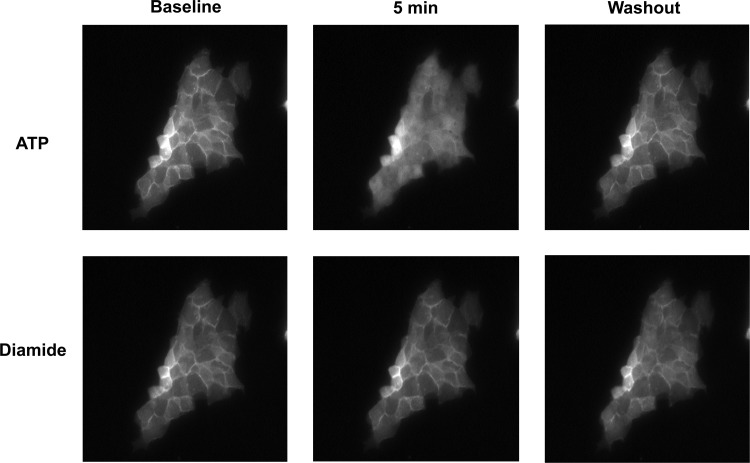
Previous studies have shown that the thiol-reagent thimerosal stimulates Ca2+ oscillations by sensitizing the IP3R to basal levels of IP3, i.e., without increasing the formation of IP3 (5). To evaluate the role of IP3 in the diamide-induced changes in [Ca2+]i, we monitored Ca2+ oscillations in BAECs pretreated with U-73122 (10 μM), an inhibitor of PLC (4), or its inactive analog U-73343 (10 μM) before challenge with 100 μM diamide. As seen in Fig. 5, diamide-induced Ca2+ oscillations measured in zero Ca2+-ECS were essentially eliminated by U-73122, but not by U-73343, compared with matched vehicle-pretreated controls. To determine whether diamide activates PLC and increases phosphoinositide hydrolysis at the single-cell level, we examined fluorescence distribution of the GFP-PH. This reporter protein binds to phosphatidylinositol 4,5-bisphosphate (PIP2) and associates with the plasmalemma under basal conditions, but redistributes to the cytosol following activation of PLC and hydrolysis of PIP2 (61). These experiments were performed on an LLC-PK1 cell line stably expressing GFP-PH. As seen in Fig. 6 (top), stimulation of the LLC-PK1 cells with ATP (100 μM), which activates PLC via P2Y purinergic receptors and increases [Ca2+]i (2, 29, 64), caused the rapid and reversible redistribution of GFP-PH from the plasma membrane to the cytosol, but the subsequent application of diamide (500 μM; Fig. 6, bottom) had no effect on the distribution of GFP-PH, demonstrating that diamide does not stimulate the hydrolysis of PIP2. In parallel experiments, a second application of ATP again produced a redistribution of the probe (not shown). Control experiments also showed that diamide increased Ca2+ oscillations in the LLC-PK1 cells similar to that observed in BAECs (not shown). Together these results demonstrate that diamide increases Ca2+ release from IP3-sensitive internal Ca2+ stores without increasing PIP2 hydrolysis.
Fig. 5.
Inhibition of phospholipase C (PLC) by U-73122 prevents diamide-induced Ca2+ oscillations when measured in the absence of extracellular Ca2+. Protocol was the same as in Fig 2, with the exception that BAECs were pretreated for 4 min with vehicle (A), 10 μM U-73122 (B), or 10 μM U-73343 (C) immediately before challenge with 100 μM diamide in zero Ca2+-ECS. D: diamide-induced single-cell [Ca2+]i oscillations were quantified as described in Fig 1 for each experimental condition. Values represent means ± SE of 4–5 experiments; 331 (vehicle), 279 (U-73122), and 252 (U-73343) cells were counted per treatment, with 56–77 cells counted per experiment. *P < 0.001 compared with matched vehicle controls.
Fig. 6.
Diamide does not stimulate phosphatidylinositol 4,5-bisphosphate (PIP2) hydrolysis. Fluorescence images were acquired from LLC-PK1 cells stably expressing the green fluorescent protein (GFP)-tagged pleckstrin homology domain of PLC-γ (GFP-PH). In the representative experiment shown, the cells, perfused with normal Ca2+-ECS, were challenged with ATP (100 μM) for 10 min, followed by a washout period. The cells were then challenged with diamide (500 μM) for 10 min, followed by a final wash period. Approximately 10 min elapsed between the end of the ATP wash and the beginning of the challenge with diamide. Selected images are shown before, 5 min after each agent, and 5 min after initiation of the wash, as indicated at the top. Diamide had no effect on the distribution of GFP-PH when tested on naive cells (n = 3) or when applied after ATP (n = 3).
Inhibition of glutathione reductase by BCNU potentiates diamide-induced changes in [Ca2+]i.
Diamide rapidly converts GSH into GSSG. However, when diamide is added to cells at low concentrations, the GSSG produced is rapidly converted back to GSH by glutathione reductase at the expense of NADPH. Thus diamide-induced changes in the [Ca2+]i of BAECs may reflect a change in the GSH-to-GSSG ratio (GSH/GSSG) or a decrease in cellular NADPH. Inhibition of glutathione reductase would be expected to augment the effects of diamide, if they are related to a change in the GSH/GSSG rather than the loss of NADPH. BCNU, a well-established inhibitor of glutathione reductase, has previously been shown to increase the sensitivity of a variety of cells, including endothelial cells (15), to oxidative stress. As shown in Fig. 7, the effects of both low and high concentrations of diamide on the [Ca2+]i of BAECs are enhanced following pretreatment of cells with 75 μM BCNU compared with matched vehicle-pretreated controls. Cells challenged with 25 μM diamide pretreated with vehicle only had no effect on oscillation frequency, i.e., ∼95% of the cells failed to oscillate, a value similar to controls shown in Fig. 2. Pretreatment of the cells with BCNU also had no effect on oscillation frequency; however, the subsequent addition of 25 μM diamide to BAECs pretreated with 75 μM BCNU caused ∼55% of the cells to oscillate (Fig. 7, A and B, and quantified in Fig. 7C). Interestingly, in some experiments, the BCNU-treated cells continued to exhibit Ca2+ oscillations, even during the washout period, suggesting that reversal of the diamide effect requires reduced GSH.
BAECs challenged with 250 μM diamide pretreated with vehicle only exhibited a rise in basal [Ca2+]i similar to that shown in Fig. 3, which, upon washout of the reagent, returned back to basal levels. However, although BCNU-pretreated cells displayed a rise in basal [Ca2+]i similar to that observed in non-BCNU treated cells when challenged with 250 μM diamide, the elevated [Ca2+]i failed to reverse upon washout of the oxidant (Fig. 7, D and E, and quantified in 7F). These results suggest that the diamide-induced changes in [Ca2+]i are not related to changes in NADPH, but rather are linked to the GSH/GSSG. Furthermore, the ability of the cells to recover from the diamide-induced insult (both Ca2+ release from intracellular stores and the elevation of basal [Ca2+]i) requires reduced GSH, which presumably is needed to reverse protein S-glutathionylation.
Inhibition of γ-glutamylcysteine synthetase by BSO also enhances diamide-induced changes in [Ca2+]i.
The generation of γ-glutamylcysteine is the first and rate-limiting step in the de novo synthesis of GSH. The enzyme controlling this reaction, γ-glutamylcysteine synthetase, can be inhibited by BSO (23). Previous studies in cultured cells have shown BSO reduces total cellular GSH (5, 25) without affecting GSSG (20, 35). As shown in Fig. 8, the effects of both low and high concentrations of diamide on the [Ca2+]i of BAECs are enhanced following pretreatment of cells with 500 μM BSO compared with matched untreated controls. Untreated cells challenged with 50 μM diamide exhibited oscillation frequencies similar to those shown in Fig. 2, i.e., ∼30% of the cells exhibit one or two oscillations during the treatment period in zero Ca2+-ECS. However, the addition of 50 μM diamide to BAECs pretreated with 500 μM BSO caused ∼80% of cells to oscillate (Fig. 8, A and B, and quantified in Fig. 8C). Although BSO-pretreated cells displayed a rise in basal [Ca2+]i in response to 250 μM diamide, similar to that observed in non-BSO treated cells, [Ca2+]i did not return to baseline following washout of the oxidant (Fig. 8, D and E, and quantified in Fig. 8F). These results provide additional evidence that a decrease in GSH renders BAECs more susceptible to diamide-induced changes in [Ca2+]i and emphasize the importance of the reduced GSH in reversal of the diamide response. To demonstrate that the changes observed indeed reflect a thiol modification, we examined the effect of DTT added during the washout of diamide from BCNU-and BSO-pretreated cells. As seen in Fig. 9, A and B, and D and E, and as quantified in Fig. 9, C and F, the rise in basal [Ca2+]i seen at the higher diamide concentration was rapidly reversed when the wash buffer contained 2 mM DTT, supporting the hypothesis that the effects of diamide reflect a reversible thiol modification.
Fig. 9.
Effects of diamide are reversed by dithiothreitol (DTT). A and B: BAECs in normal Ca2+-ECS were pretreated for 30 min with 75 μM BCNU before challenge with diamide, as described in the legend to Fig 7. DTT (2 mM) was added to the wash buffer in B. C: the change in basal [Ca2+]i was quantified as described in Fig 7. Values represent means ± SE of 3 experiments; 169 (No DTT) and 236 (2 mM DTT) cells were analyzed per treatment, with 51–64 cells analyzed per experiment. *P < 0.001 compared with no DTT. D and E: BAECs were pretreated with 500 μM BSO before challenge with diamide, as described in the legend to Fig 8. DTT (2 mM) was added to the wash buffer in E. F: the change in basal [Ca2+]i was quantified as described in Fig 7. Values represent means ± SE of 3 experiments; 190 (No DTT) and 207 (2 mM DTT) cells were analyzed per treatment, with 55–85 cells analyzed per experiment. *P < 0.0002 compared with no DTT.
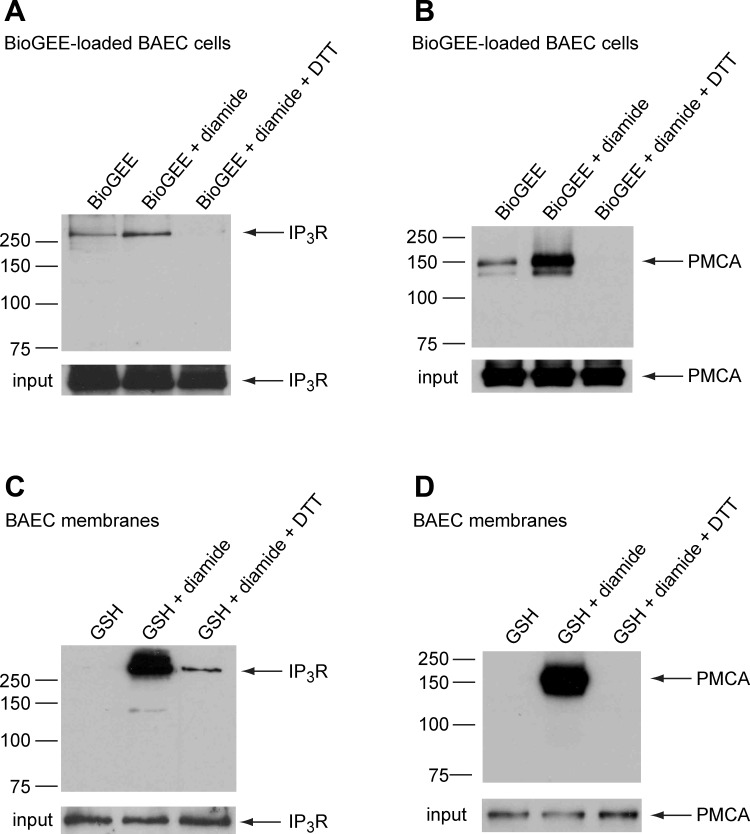
Diamide increases glutathionylation of the IP3R and the PMCA both in vivo and in vitro.
To determine whether diamide causes glutathionylation of the PMCA and/or the IP3R, BAECs were loaded intracellularly with BioGEE, a membrane-permeable ethyl-ester form of biotin-GSH that is trapped within the cell by the action of cellular esterases (59). Following treatment with diamide, membranes isolated from BioGEE-loaded cells were solubilized in lysis buffer, and glutathionylated proteins were extracted using avidin-conjugated agarose beads, as described in experimental procedures. A small amount of each protein was present in pull-downs from untreated control cells, indicative of some basal level of glutathionylation. However, following treatment of the cells for 10 min with 500 μM diamide, the amount of recovered IP3R (Fig. 10A) and PMCA (Fig. 10B) significantly increased 2.83 ± 0.54-fold (P < 0.03) and 7.99 ± 1.1-fold (P < 0.001), respectively, consistent with increased glutathionylation. To determine whether the IP3R and the PMCA could also be glutathionylated in vitro, membrane preparations isolated from BAECs were treated with biotin-GSH alone or biotin-GSH plus diamide. As seen in Fig. 10, C and D, no apparent glutathionylation was observed in the absence of diamide, whereas a substantial amount of both the PMCA and the IP3R were recovered in the avidin pull-downs following treatment of isolated membranes with biotin-GSH plus diamide. Consistent with thiol modification, both the in vivo and in vitro glutathionylation were reversed by addition of excess DTT before pull-down.
Fig. 10.
Diamide increases glutathionylation of IP3R and plasmalemmal Ca2+-ATPase (PMCA), both in vivo and in vitro. A and B: BAECs, loaded with the cell-permeable form of biotinylated-GSH [biotinylated-GSH ethyl ester (BioGEE)], were suspended in normal Ca2+-ECS and divided into three aliquots, as indicated above each lane. One aliquot was left untreated (BioGEE alone), and the remaining two aliquots were incubated with diamide (500 μM) for 10 min. The membrane lysate from one aliquot of diamide-treated cells was incubated with DTT before pull-down. Biotinylated proteins were extracted from the cleared lysates with avidin-agarose beads and probed for either the IP3R or PMCA, as described in experimental procedures. C and D: membranes isolated from BAECs were treated with glutathionylation reagents, as indicated above each lane (125 μM biotin-GSH alone, 100 μM diamide plus 125 μM biotin-GSH, and diamide plus biotin-GSH, followed by 20 mM DTT before pull-down). Biotinylated proteins were extracted from the cleared membrane lysates with avidin-agarose beads and probed for either the IP3R or PMCA. Samples of membrane lysates were reserved before pull-down (input). Results shown are representative of 3–6 independent experiments.
DISCUSSION
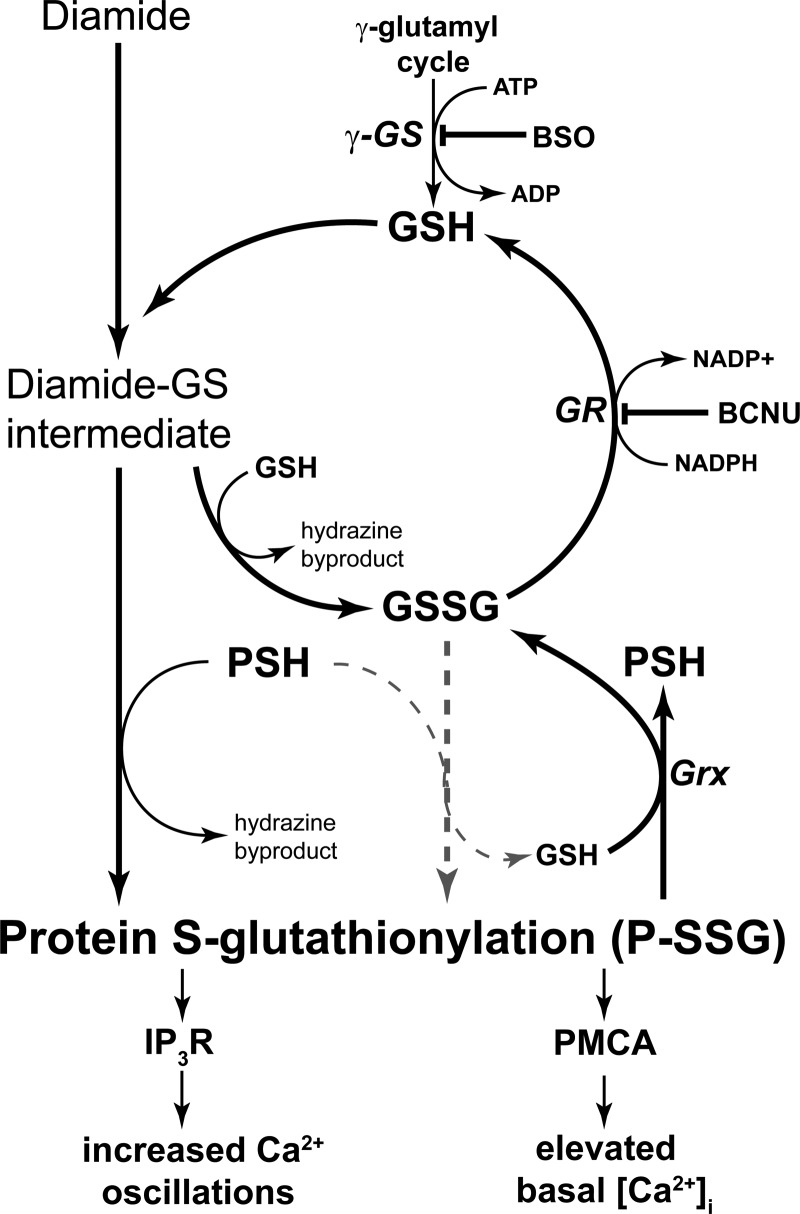
Diamide produced two major effects on Ca2+ homeostasis in BAECs. First, diamide caused a dramatic, concentration-dependent increase in Ca2+ oscillation frequency, and second, diamide increased basal [Ca2+]i. The later effect became particularly obvious at higher diamide concentrations. Diamide is a membrane-permeable thiol-oxidizing agent that has been used for over 40 yr to alter the redox balance in a variety of cell types. Diamide rapidly and reversibly oxidizes intracellular GSH to GSSG and causes formation of P-SSG. It is thought that most, if not all, of the effects of diamide at the cellular level reflect thiol modifications, but the actual mechanism by which diamide increases formation of P-SSG is not entirely clear. The oxidation of GSH by diamide occurs by a well-studied two-step reaction (38). The first step involves the formation of a diamide-glutathione intermediate (diamide-GS), which then rapidly reacts with a second molecule of GSH, yielding GSSG plus the hydrazine metabolite of diamide (Fig. 11). As the concentration of GSSG increases in response to diamide, P-SSG might occur via a disulfide exchange reaction (shaded dashed lines in Fig. 11). However, as discussed by Gallogly et al. (18, 19), for most protein thiols, the cellular GSH/GSSG must decrease from the normal value of ∼100 to <1 for significant disulfide exchange to occur. In this regard, previous studies have shown that the GSH/GSSG was only reduced to ∼35 following treatment of human umbilical vein endothelial cells with 200 μM diamide for 15 min (55). Thus it seems unlikely that diamide causes P-SSG formation via GSSG disulfide exchange. Since diamide preferentially reacts with cellular GSH and exhibits limited reactivity toward protein thiol groups (38), it seems likely that the diamide-induced glutathionylation occurs by an exchange reaction between the diamide-GS intermediate and accessible cysteine groups on cellular proteins (Fig. 11). The lifetime of the P-SSG formed by this reaction is dependent on the activity of cellular glutaredoxins, which de-glutathionylate P-SSG at the expense of GSH (18). The GSSG product of this reaction, and the GSSG directly produced by diamide, is then rapidly reduced back to GSH by the action of glutathione reductase at the expense of NADPH. Thus, by adding low concentrations of diamide to the cell, the equilibrium is shifted toward P-SSG. It is easy to see how inhibition of glutathione reductase by BCNU or inhibition of GSH synthesis would enhance the effect of diamide; both maneuvers decrease the availability of cellular GSH and thus limit the ability of the cell to de-glutathionylate the modified protein, i.e., the equilibrium is shifted even further toward P-SSG. Indeed, in the present study, the effects of diamide on Ca2+ oscillations and basal [Ca2+]i were greatly enhanced by inhibition of glutathione reductase by BCNU or by inhibition of GSH synthesis by BSO. Furthermore, the effects of diamide were irreversible when the cellular capacity to regenerate reduced GSH was compromised, but, importantly, could be reversed under these conditions by DTT. Taken together, these results provide strong support for the hypothesis that the effect of diamide on Ca2+ homeostasis reflects thiol modification. The reversal by DTT also shows that diamide does not cause irreversible damage to the cellular mechanism responsible for returning [Ca2+]i to the normal resting level.
Fig. 11.
Metabolism of diamide leads to increased protein S-glutathionylation (P-SSG). The schematic diagram shows the enzymatic pathways responsible for generation and maintenance of cellular GSH and two pathways by which diamide increases P-SSG formation. The GSSG disulfide exchange reaction (shaded arrows) is unlikely to occur at low concentrations of diamide (see text for details). The γ-glutamylcysteine synthetase (γ-GS) catalyzes the rate-limiting step in GSH synthesis and is inhibited by BSO. Glutathione reductase (GR) catalyzes the conversion of GSSG to GSH at the expense of NADPH and is inhibited by BCNU. Glutaredoxin (Grx) catalyzes protein deglutathionylation at the expense of GSH.
What is the molecular mechanism by which diamide-induced gluthathionylation increases Ca2+ oscillation frequency? The diamide-induced change in Ca2+ oscillation frequency was 1) unaffected by removal of extracellular Ca2+; 2) inhibited by the IP3R antagonist 2-APB; and 3) blocked by inhibition of PLC with U-73122. Clearly, diamide stimulates the release of Ca2+ from IP3-sensitive internal Ca2+ stores, but, since diamide had no effect on PIP2 hydrolysis, it seems unlikely that diamide stimulates PLC in a fashion analogous to receptor stimulation. However, it is well-established that the IP3R is sensitive to Ca2+ concentration within the lumen of the endoplasmic reticulum, and that spontaneous release can occur when the endoplasmic reticulum becomes overloaded with Ca2+ (48, 49). Previous studies have shown that peroxynitrite activates the SERCA pump via glutathionylation of cysteine-674 (1, 8, 40). Thus it seems possible that a diamide-induced glutathionylation of SERCA in the BAECs might elevate luminal Ca2+ and thus increase spontaneous Ca2+ release.
Another possibility is that diamide may sensitize the IP3R to basal levels of IP3. Consistent with this hypothesis, diamide did not itself activate PLC, and the effects of diamide were completely blocked by inhibition of PLC by U-73122, showing that some basal level of IP3 generation is required for the diamide effects. These results are reminiscent of the effects of thimerosal, which has been shown to initiate Ca2+ oscillations without stimulating phosphoinositide hydrolysis (5). Thimerosal causes an increase in affinity of the IP3R, such that channel activation occurs at the basal resting levels of IP3. The effects of thimerosal are also observed in partially purified IP3R preparations reconstituted into either lipid vesicles or planar lipid bilayers, consistent with direct modification of critical sulfhydryl groups of the IP3R (34, 60). Ca2+ release via the IP3R has also been observed in permeabilized cells challenged with high (millimolar) concentrations of GSSG (48, 49). The effect of GSSG may also be related to a change in IP3R affinity for IP3 (43), but GSSG appears to be much less effective compared with thimerosal, and the effects may be indirect (47, 51). To examine the possibility that diamide promotes the direct glutathionylation of the IP3R, we probed for the presence of the receptor in avidin-pull-down assays from BAECs loaded intracellularly with biotin-GSH and from lysate of BAEC microsomal membranes reacted with diamide in the presence of biotin-GSH. Indeed, the IP3R was present in the pull-downs both in vivo and in vitro, and, in both assays, the glutathionylation was reversed by excess DTT. These results suggest that diamide stimulates the reversible glutathionylation of the IP3R or some tightly associated regulatory protein. Whether or not glutathionylation changes affinity of the receptor for IP3 remains to be determined, but, given the well-known effects of thimerosal, this seems to be a plausible mechanism.
Higher concentrations of diamide caused a significant time-dependent increase in basal resting [Ca2+]i. The increase in [Ca2+]i was seen in both the presence and, importantly, in the complete absence of extracellular Ca2+. As mentioned above, the BAECs used in the present study do not express the Na+/Ca2+ exchanger. Thus the rise in basal [Ca2+]i can only be explained by a diamide-induced inhibition of the PMCA. A similar rise in basal [Ca2+]i was previously reported in aortic and pulmonary artery endothelial cells treated with tert-butyl-hydroperoxide (14, 15). It is well established that the PMCA is sensitive to oxidant stress. Specifically, it has been shown that ROS inhibit Ca2+ pump function, leading to inhibition of Ca2+ extrusion across the plasma membrane (for reviews, see Refs. 58, 63). At least in part, the effects of ROS on PMCA may be attributable to changes in calmodulin (CaM). The PMCA is a Ca2+-CaM-regulated protein (7). In the absence of CaM, the affinity of the PMCA for Ca2+ is 10–20 μM, whereas, in the presence of CaM, the affinity of the pump for Ca2+ is greatly increased to 0.4–0.5 μM. Oxidative stress will inactivate CaM through modification of highly conserved methionine residues (3). The oxidized form of CaM essentially acts as a dominant negative, preventing the binding of native CaM to the PMCA, blocking activation of the pump. Thus oxidative stress appears to have both direct effects on the PMCA and indirect effects via CaM, both of which will attenuate Ca2+ pump function, contributing to the rise in basal [Ca2+]i. The ability of glutathionylation to alter PMCA pump function, however, has never been described. To determine whether the PMCA is glutathionylated in response to diamide, we probed the avidin-pull-downs from biotin-GSH-loaded BAECs for the PMCA. Diamide increased the PMCA captured in these pull-downs, consistent with increased direct glutathionylation of the PMCA or a tightly associated regulatory protein. The rise in basal [Ca2+]i and the glutathionylation of the PMCA were reversed by excess DTT, again consistent with thiol modification. Our recent studies have shown that specific PMCA catalytic activity in isolated membrane preparations is reversibly inhibited by glutathionylation (Ref. 57 and unpublished observations), consistent with the results of the present study.
In summary, diamide enhances Ca2+ oscillations and increases basal [Ca2+]i in the absence of receptor stimulation or phosphoinositide hydrolysis. To our knowledge, these are the first experiments showing that glutathionylation alters PMCA and IP3R function, possibly via direct protein modification. At least over the time frame examined, the effects of diamide were reversible, suggesting that the change in oscillation frequency observed at the lower concentrations and the rise in basal [Ca2+]i seen at the higher concentrations of diamide may be indicative of physiological redox signaling mechanisms rather than pathological oxidative stress. Distinct information is encoded by frequency vs. amplitude modulation of Ca2+ signals (50). Thus redox signaling via glutathionylation may differentially activate multiple downstream pathways responsive to even small changes in cytosolic Ca2+ dynamics.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute (NHLBI) Grant HL097355. J. T. Lock was supported in part by training Grant HL007887 from the NHLBI.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- 1. Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C, Cohen RA. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med 10: 1200–1207, 2004. [DOI] [PubMed] [Google Scholar]
- 2. Anderson RJ, Breckon R, Dixon BS. ATP receptor regulation of adenylate cyclase and protein kinase C activity in cultured renal LLC-PK1 cells. J Clin Invest 87: 1732–1738, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bigelow DJ, Squier TC. Redox modulation of cellular signaling and metabolism through reversible oxidation of methionine sensors in calcium regulatory proteins. Biochim Biophys Acta 1703: 121–134, 2005. [DOI] [PubMed] [Google Scholar]
- 4. Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ, Bunting S. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutorphils. J Pharmacol Exp Ther 255: 756–768, 1990. [PubMed] [Google Scholar]
- 5. Bootman MD, Taylor CW, Berridge MJ. The thiol reagent, thimerosal, evokes Ca2+ spikes in HeLa cells by sensitizing the inositol 1,4,5-trisphosphate receptor. J Biol Chem 267: 25113–25119, 1992. [PubMed] [Google Scholar]
- 6. Caplan JF, Filipenko NR, Fitzpatrick SL, Waisman DM. Regulation of annexin A2 by reversible glutathionylation. J Biol Chem 279: 7740–7750, 2004. [DOI] [PubMed] [Google Scholar]
- 7. Carafoli E. Calcium pump of the plasma membrane. Physiol Rev 71: 129–153, 1991. [DOI] [PubMed] [Google Scholar]
- 8. Cohen RA, Adachi T. Nitric-oxide-induced vasodilatation: regulation by physiologic oxidation of the sarcoplasmic reticulum calcium ATPase. Trends Cardiovasc Med 16: 109–114, 2006. [DOI] [PubMed] [Google Scholar]
- 9. Colden-Stanfield M, Schilling WP, Ritchie AK, Eskin SG, Navarro LT, Kunze DL. Bradykinin-induced increases in cytosolic calcium and ionic currents in cultured bovine aortic endothelial cells. Circ Res 61: 632–640, 1987. [DOI] [PubMed] [Google Scholar]
- 10. Dalle-Donne I, Milzani A, Gagliano N, Colombo R, Giustarini D, Rossi R. Molecular mechanisms and potential clinical significance of S-glutathionylation. Antioxid Redox Signal 10: 445–473, 2008. [DOI] [PubMed] [Google Scholar]
- 11. Dalle-Donne I, Rossi R, Giustarini D, Colombo R, Milzani A. S-glutathionylation in protein redox regulation. Free Radic Biol Med 43: 883–898, 2007. [DOI] [PubMed] [Google Scholar]
- 12. Elliott SJ. Peroxynitrite modulates receptor-activated Ca2+ signaling in vascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 270: L954–L961, 1996. [DOI] [PubMed] [Google Scholar]
- 13. Elliott SJ, Doan TN, Henschke PN. Reductant substrate for glutathione peroxidase modulates oxidant inhibition of Ca2+ signaling in endothelial cells. Am J Physiol Heart Circ Physiol 268: H278–H287, 1995. [DOI] [PubMed] [Google Scholar]
- 14. Elliott SJ, Eskin SG, Schilling WP. Effect of t-butyl-hydroperoxide on bradykinin stimulated changes in cytosolic calcium in vascular endothelial cells. J Biol Chem 264: 3806–3810, 1989. [PubMed] [Google Scholar]
- 15. Elliott SJ, Schilling WP. Carmustine augments the effects of tert-butyl-hydroperoxide on calcium signaling in cultured pulmonary artery endothelial cells. J Biol Chem 265: 103–107, 1990. [PubMed] [Google Scholar]
- 16. Figtree GA, Liu CC, Bibert S, Hamilton EJ, Garcia A, White CN, Chia KKM, Cornelius F, Geering K, Rasmussen HH. Reversible oxidative modification: a key mechanism of Na+-K+ pump regulation. Circ Res 105: 185–193, 2009. [DOI] [PubMed] [Google Scholar]
- 17. Fratelli M, Demol H, Puype M, Casagrande S, Eberini I, Salmona M, Bonetto V, Mengozzi M, Duffieux F, Miclet E, Bachi A, Vandekerckhove J, Gianazza E, Ghezzi P. Identification by redox proteomics of glutathionylated proteins in oxidatively stressed human T lymphocytes. Proc Natl Acad Sci U S A 99: 3505–3510, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gallogly MM, Mieyal JJ. Mechanism of reversible protein glyutathionylation in redox signaling and oxidative stress. Curr Opin Pharmacol 7: 381–391, 2007. [DOI] [PubMed] [Google Scholar]
- 19. Gallogly MM, Starke DW, Mieyal JJ. Mechanistic and kinetic details of catalysis of thiol-disulfide exchange by glutaredoxins and potential mechanism of regulation. Antioxid Redox Signal 11: 1059–1081, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gilge JL, Fisher M, Chai YC. The effect of oxidant and the non-oxidant alteration of cellular thiol concentration on the formation of protein mixed-disulfides in HEK 293 cells. PLoS One 3: e4015, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goel M, Schilling WP. Role of TRPC3 channels in ATP-induced Ca2+ signaling in principal cells of the inner medullary collecting duct. Am J Physiol Renal Physiol 299: F225–F233, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Graier WF, Hoebel BG, Paltauf-Doburzynska J, Kostner GM. Effects of superoxide anions on endothelial Ca2+ signaling pathways. Arterioscler Thromb Vasc Biol 18: 1470–1479, 1998. [DOI] [PubMed] [Google Scholar]
- 23. Griffith OW, Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem 254: 7558–7560, 1979. [PubMed] [Google Scholar]
- 24. Hansen RE, Roth D, Winther JR. Quantifying the global cellular thiol-disulfide status. Proc Natl Acad Sci U S A 106: 422–427, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Henschke PN, Elliott SJ. Oxidized glutathione decreases luminal Ca2+ content of the endothelial cell Ins(1,4,5)P3-sensitive Ca2+ store. Biochem J 312: 485–489, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 6: 150–166, 2005. [DOI] [PubMed] [Google Scholar]
- 27. Hidalgo C, Donoso P. Crosstalk between calcium and redox signaling: from molecular mechanisms to health implications. Antioxid Redox Signal 10: 1275–1312, 2008. [DOI] [PubMed] [Google Scholar]
- 28. Hill BG, Higdon AN, Dranka BP, Darley-Usmar VM. Regulation of vascular smooth muscle cell bioenergetic function by protein glutathionylation. Biochim Biophys Acta 1797: 285–295, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Himpens B, De Smedt H, Casteels R. Intracellular Ca2+ signaling induced by vasopressin, ATP, and epidermal growth factor in epithelial LLC-PK1 cells. Am J Physiol Cell Physiol 265: C966–C975, 1993. [DOI] [PubMed] [Google Scholar]
- 30. Hu Q, Corda S, Zweier JL, Capogrossi MC, Ziegelstein RC. Hydrogen peroxide induces intracellular calcium oscillations in human aortic endothelial cells. Circulation 97: 268–275, 1998. [DOI] [PubMed] [Google Scholar]
- 31. Hu Q, Zheng G, Zweier JL, Deshpande S, Irani K, Ziegelstein RC. NADPH oxidase activation increases the sensitivity of intracellular Ca2+ stores to inositol 1,4,5-trisphosphate in human endothelial cells. J Biol Chem 275: 15749–15757, 2000. [DOI] [PubMed] [Google Scholar]
- 32. Humphries KM, Juliano C, Taylor SS. Regulation of cAMP-dependent protein kinase activity by glutathionylation. J Biol Chem 277: 43505–43511, 2002. [DOI] [PubMed] [Google Scholar]
- 33. Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol 295: C849–C868, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kaplin AL, Ferris CD, Voglmaier SM, Snyder SH. Purified reconstituted inositol 1,4,5-trisphosphate receptors. Thiol reagents act directly on receptor protein. J Biol Chem 269: 28972–28978, 1994. [PubMed] [Google Scholar]
- 35. Khamaisi M, Kavel O, Rosenstock M, Porat M, Yuli M, Kaiser N, Rudich A. Effect of inhibition of glutathione synthesis on insulin action: in vivo and in vitro studies using buthionine sulfoximine. Biochem J 349: 579–586, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kosower EM, Correa W, Kinon BJ, Kosower NS. Glutathione. VII. Differentiation among substrates by the thiol-oxidizing agent, diamide. Biochim Biophys Acta 264: 39–44, 1972. [DOI] [PubMed] [Google Scholar]
- 37. Kosower NS, Kosower EM. The glutathione status of cells. Int Rev Cytol 54: 109–160, 1978. [DOI] [PubMed] [Google Scholar]
- 38. Kosower NS, Kosower EM. Diamide: an oxidant probe for thiols. Methods Enzymol 251: 123–133, 1995. [DOI] [PubMed] [Google Scholar]
- 39. Kosower NS, Kosower EM, Wertheim B. Diamide, a new reagent for the intracellular oxidation of glutathione to disulfide. Biochem Biophys Res Commun 37: 593–596, 1969. [DOI] [PubMed] [Google Scholar]
- 40. Lancel S, Zhang J, Evangelista A, Trucillo MP, Tong XY, Siwik DA, Cohen RA, Colucci WS. Nitrosyl activates SERCA in cardiac myocytes via glutathiolation of cysteine 674. Circ Res 104: 720–723, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lind C, Gerdes R, Hamnell Y, Schuppe-Koistinen I, Brockenhuus von Lowenhielm H, Holmgren A, Cotgreave IA. Identification of S-glutathionylated cellular proteins during oxidative stress and constitutive metabolism by affinity purification and proteomic analysis. Arch Biochem Biophys 406: 229–240, 2002. [DOI] [PubMed] [Google Scholar]
- 42. Lock JT, Schilling WP. Effect of diamide-induced oxidative stress on Ca2+ signaling in cultured bovine aortic endothelial cells (BAEC) (Abstract). FASEB J 24: 1048.6, 2010. [Google Scholar]
- 43. Lopez-Colome AM, Lee I. Pharmacological characterization of inositol-1,4,5,-trisphosphate binding to membranes from retina and retinal cultures. J Neurosci Res 44: 149–156, 1996. [DOI] [PubMed] [Google Scholar]
- 44. Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem 122: 498–505, 1997. [DOI] [PubMed] [Google Scholar]
- 45. Meister A, Anderson ME. Glutathione. Annu Rev Biochem 52: 711–760, 1983. [DOI] [PubMed] [Google Scholar]
- 46. Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal 10: 1941–1988, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Missiaen L, De Smedt H, Parys JB, Sienaert I, Valingen S, Casteels R. Threshold for inositol 1,4,5-trisphosphate action. J Biol Chem 271: 12287–12293, 1996. [DOI] [PubMed] [Google Scholar]
- 48. Missiaen L, Taylor CW, Berridge MJ. Spontaneous calcium release from inositol trisphosphate-sensitive calcium stores. Nature 352: 241–244, 1991. [DOI] [PubMed] [Google Scholar]
- 49. Missiaen L, Taylor CW, Berridge MJ. Luminal Ca2+ promoting spontaneous Ca2+ release from inositol trisphosphate-sensitive stores in rat hepatocytes. J Physiol 455: 623–640, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Parekh AB. Decoding cytosolic Ca2+ oscillations. Trends Biochem Sci. In press. [DOI] [PubMed] [Google Scholar]
- 51. Renard-Rooney DC, Joseph SK, Seitz MB, Thomas AP. Effect of oxidized glutathione and temperature on inositol 1,4,5-trisphosphate binding in permeabilized hepatocytes. Biochem J 310: 185–192, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schilling WP, Elliott SJ. Ca2+ signaling mechanisms of vascular endothelial cells and their role in oxidant-induced endothelial cell dysfunction. Am J Physiol Heart Circ Physiol 262: H1617–H1630, 1992. [DOI] [PubMed] [Google Scholar]
- 53. Schilling WP, Rajan L, Strobl-Jager E. Characterization of the bradykinin-stimulated calcium influx pathway of cultured vascular endothelial cells: saturability, selectivity and kinetics. J Biol Chem 264: 12838–12848, 1989. [PubMed] [Google Scholar]
- 54. Schilling WP, Ritchie AK, Navarro LT, Eskin SG. Bradykinin-stimulated calcium influx in cultured bovine aortic endothelial cells. Am J Physiol Heart Circ Physiol 255: H219–H227, 1988. [DOI] [PubMed] [Google Scholar]
- 55. Schuppe I, Moldeus P, Cotgreave IA. Protein-specific S-thiolation in human endothelial cells during oxidative stress. Biochem Pharmacol 44: 1757–1764, 1992. [DOI] [PubMed] [Google Scholar]
- 56. Sinkins WG, Estacion M, Prasad V, Goel M, Shull GE, Kunze DL, Schilling WP. Maitotoxin converts the plasmalemmal Ca2+ pump into a Ca2+-permeable non-selective cation channel. Am J Physiol Cell Physiol 297: C1533–C1543, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sinkins WG, Schilling WP. Reversible glutathionylation of the plasmalemmal Ca2+-ATPase/pump by the thiol-oxidizing agent diamide (Abstract). FASEB J 24: 607.1, 2010. [Google Scholar]
- 58. Squier TC, Bigelow DJ. Protein oxidation and age-dependent alterations in calcium homeostasis. Front Biosci 5: 504–526, 2000. [DOI] [PubMed] [Google Scholar]
- 59. Sullivan DM, Wehr NB, Fergusson MM, Levine RL, Finkel T. Identification of oxidant-sensitive proteins: TNF-a induces protein glutathionylation. Biochemistry 39: 11121–11128, 2000. [DOI] [PubMed] [Google Scholar]
- 60. Thrower EC, Duclohier H, Lea EJA, Molle G, Dawson AP. The inositol 1,4,5-trisphosphate-gated Ca2+ channel: effect of the protein thiol reagent thimerosal on channel activity. Biochem J 318: 61–66, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Varnai P, Tamas B. Live cell imaging of phosphoinositides with expressed inositide binding protein domains. Methods 46: 167–176, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang W, Oliva C, Li G, Holmgren A, Lillig CH, Kirk KL. Reversible silencing of CFTR chloride channels by glutathionylation. J Gen Physiol 125: 127–141, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Waring P. Redox active calcium ion channels and cell death. Arch Biochem Biophys 434: 33–42, 2005. [DOI] [PubMed] [Google Scholar]
- 64. Weinberg JM, Davis JA, Shayman JA, Knight PR. Alterations of cytosolic calcium in LLC-PK1 cells induced by vasopressin and exogenous purines. Am J Physiol Cell Physiol 256: C967–C976, 1989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.