Abstract
Hydrogen sulfide (H2S) is a gaseous signaling molecule that appears to be involved in numerous biological processes, including regulation of blood pressure and vascular tone. The present study is designed to address the hypothesis that H2S is a functionally significant, endogenous dilator in the newborn cerebrovascular circulation. In vivo experiments were conducted using newborn pigs with surgically implanted, closed, cranial windows. Topical application of H2S concentration-dependently (10−6 to 2 × 10−4 M) dilated pial arterioles. This dilation was blocked by glibenclamide (10−6 M). l-Cysteine, the substrate of the H2S-producing enzymes cystathionine γ-lyase (CSE) and cystathionine β-synthase (CBS), also dilated pial arterioles. The dilation to l-cysteine was blocked by the CSE inhibitor d,l-propargylglycine (PPG, 10 mM) but was unaffected by the CBS inhibitor amino-oxyacetate (AOA, 1 mM). Western blots detected CSE, but not CBS, in cerebral microvessels, whereas CBS is detected in brain parenchyma. Immunohistological CSE expression is predominantly vascular while CBS is expressed mainly in neurons and astrocytes. l-Cysteine (5 mM) increased H2S concentration in cerebrospinal fluid (CSF), measured by GC-MS, from 561 ± 205 to 2,783 ± 818 nM before but not during treatment with PPG (1,030 ± 70 to 622 ± 78 nM). Dilation to hypercapnia was inhibited by PPG but not AOA. Hypercapnia increased CSF H2S concentration from 763 ± 243 to 4,337 ± 1789 nM before but not during PPG treatment (357 ± 178 vs. 425 ± 217 nM). These data show that H2S is a dilator of the newborn cerebral circulation and that endogenous CSE can produce sufficient H2S to decrease vascular tone. H2S appears to be a physiologically significant dilator in the cerebral circulation.
Keywords: cerebrovascular circulation, gasotransmitters, pial arterioles, cystathionine γ-lyase, cystathionine β-synthase
disorders of the perinatal cerebral circulation are the most prominent cause of mortality and morbidity in newborns and often result in lifelong disabilities in survivors (8, 11). The single most prominent cause of death and chronic disability in newborns is hypoxic-ischemic brain injury (8). The pathophysiological mechanisms of neonatal ischemic brain injury can be distinct from those of the adult and include different circulatory control mechanisms in a system not fully developed (8, 11). Newborn animals are used to study mechanisms involved in regulation of blood flow to and within the newborn brain because, while there can be commonalities, mechanisms involved in regulation of cerebrovascular circulation are often different in newborn pigs and presumably newborn infants from those in older pigs, adult rodents, and adult humans (3, 9, 15, 41, 48, 49, 55). The present experiments measure piglet pial arterioles that are critical for control of cerebral blood flow (5, 6, 14, 16, 17).
Hydrogen sulfide (H2S) is a gaseous, endogenously produced, signaling molecule joining NO and CO as gasotransmitters. Data are accumulating that endogenous H2S is involved in control of blood pressure and tone of arteries from certain vascular beds (3, 26, 40, 51, 53). While there are reports linking H2S to neuronal function (18, 20, 22), nothing is known about H2S in regulation of blood flow in the brain.
Knowledge of the role of H2S in regulation of newborn cerebrovascular circulation and effects of exogenous H2S on cerebral arteriolar tone is particularly important because of potential therapeutic applications of H2S and H2S-releasing molecules (34, 35). H2S is being postulated to be of value for attenuating neuronal injury in cerebral ischemia and vascular dementia because H2S can be anti-apoptotic, reduce cellular metabolism, and produce a hibernation-like state (22, 32, 37, 46, 52).
H2S is produced by catabolism of l-cysteine to H2S, NH4, and pyruvate, primarily by cystathionine γ-lyase (CSE, EC 4.4.1.1) and cystathionine β-synthase (CBS, EC 4.2.1.22), reminiscent of l-arginine metabolism to NO. In general, CSE expression has been detected in blood vessels, and CBS and 3-mercaptopyruvate sulfur transferase (EC2.8.1.2), which can also catalyze H2S production, have been detected in the brain parenchyma (1, 20, 22).
Although there are reports on H2S in adult peripheral arteries, no data exist in the cerebral circulation, arterioles, or in the newborn period. The decrease in blood pressure caused by intravascular H2S (54) and the hypertension of CSE knockout mice (51) suggest H2S has a role in the regulation of resistance vessels.
The mechanism causing H2S-induced dilation of various peripheral arteries has been reported to involve ATP-dependent K+ (KATP) channels (36, 54), a Cl−/HCO3− exchanger (24), and/or endothelium-dependent dilators (10, 53). In adult rat aortic rings, Ca2+-dependent K+ (KCa) and voltage-gated K+ channel inhibitors had no effect on relaxation to H2S, but the relaxation was blocked by glibenclamide (54), an inhibitor of KATP channels (4, 43). Electrophysiological studies of single aortic myocytes detected H2S-induced stimulation of KATP channel currents and hyperpolarization, both of which were blocked by glibenclamide (54). A mechanism reported to be involved in cerebral vascular dilation to acidosis, which is caused by hypercapnia, is KATP channel activation (12, 35, 42, 44, 47).
The present study is designed to address the hypothesis that H2S is a functionally significant, endogenous dilator in the newborn cerebrovascular circulation. Here we report data that newborn pig cerebral arterioles in vivo dilate to H2S and l-cysteine, dilation is blocked by the KATP channel inhibitor glibenclamide, CSE is expressed in cerebral vessels and CBS in the brain parenchyma, inhibition of CSE reduces dilation to l-cysteine and hypercapnia, and the brain produces H2S from endogenous and exogenous substrate in sufficient amounts to produce dilation.
METHODS
All experiments involving animals were reviewed and approved by the University of Tennessee Health Science Center Animal Care and Use Committee.
Cranial windows in vivo.
These experiments were conducted in vivo using α-chloralose-anesthetized, ventilated, newborn pigs equipped with surgically implanted, closed, cranial windows to allow direct observation of the cerebral arterioles leading to the capillaries of the brain parenchyma (26). Briefly, a cranial window with a glass pane was implanted in a craniotomy with retracted dura mater. The space under the window was filled with artificial cerebrospinal fluid (aCSF) (pH ∼7.35 and Pco2 and Po2 ∼43 mmHg) via ports on the sides of the window frame. Pial arteriolar diameter measurements of one ∼50-μm arteriole per piglet were used for analyses (piglet cerebral arterioles penetrate at ∼30 μm). Blood pressure, body temperature, blood gases, and pH were monitored and kept within normal limits except when altered blood gases were the treatments.
Investigation of effects of H2S and l-cysteine on pial arteriolar diameter.
A saturated H2S-in-water solution (10−1 M) was diluted in aCSF (10−6, 10−5, 10−4, and 2 × 10−4 M) and injected under the cranial window. Ascending concentration-response curves were produced by replacing each concentration with the higher concentration at 5-min intervals.
For l-cysteine concentration-response curves (1, 3, 5, and 7 mM), each concentration placed under the cranial window was replaced by the higher concentration at 10-min intervals.
Effects of glibenclamide on dilation to H2S and l-cysteine.
The effect of the KATP channel inhibitor glibenclamide on dilation of newborn pig pial arterioles to H2S was determined by measuring arteriolar diameter changes to topical H2S before and in the presence of glibenclamide (10−6 M). Efficacy of glibenclamide was evaluated by blockade of dilation to the KATP channel agonist pinacidil (10−5 M) and selectivity by absence of effects on dilations to the KCa channel agonist NS-1619 (2 × 10−6 M), and the β−adrenergic agonist isoproterenol (10−6 M).
Effects of CSE and CBS inhibition on responses to l-cysteine, hypercapnia, hypoxia, sodium nitroprusside, and isoproterenol.
Responses to the CSE/CBS substrate l-cysteine, as above, were measured before and in the presence of the CSE inhibitor d,l-propargylglycine (PPG, 10 mM) and, in separate piglets, the CBS inhibitor amino-oxyacetate (AOA, 1 mM) (33, 40).
Hypercapnia was produced by ventilating for 5 min with 10% CO2, 21% O2, and 69% N2 to produce PaCO2 ∼75 mmHg. Hypoxia was produced by 5 min ventilation with 10% O2, 90% N2 to reduce PaO2 to ∼35 mmHg, while maintaining constant PaCO2. Isoproterenol (10−6 M) and sodium nitroprusside (SNP, 2 × 10−7 M) were applied topically under the cranial window (5 min).
Determination of the cellular localizations of CSE and CBS in cerebral tissue.
For Western immunoblotting, cerebral microvessels (arteriolar branches of the middle cerebral artery, 60–300 μm in diameter) were dissected from the brain and gently cleaned of adjacent tissue. Vessel-free brain parenchyma was prepared by consecutive filtration of the piglet cortex homogenate through 300- and 60-μm nylon mesh filters; the filtrate contains neurons and astrocytes (brain parenchyma), whereas cerebral vessels (60 μm and larger) are retained on the filters. Dissected cerebral microvessels and brain parenchyma were lysed in Laemmli buffer, resolved by 12% SDS-PAGE, and electrotransferred to polyvinylidene difluoride membranes. The membranes were probed with CSE antibody and CBS antibody (1:2,000; Novus Biological, Littleton, CO) followed by peroxidase-anti-mouse IgG (1:20,000; Sigma) and visualized with Western Lightning-ECL (Perkin-Elmer, Shelton, CT).
Immunohistochemistry was performed on the slices of formalin-fixed/paraffin-embedded brain cortex using the avidin-biotin-peroxidase complex technique, as we described previously (26). CSE and CBS immunohistochemical detection was performed using monoclonal anti-human antibodies highly selective to CSE or CBS (1:100 dilution; Novus Biological). The brain sections were then counterstained with hematoxylin and viewed with an Olympus BX50 light microscope.
Measurement of H2S in cerebrospinal fluid.
Collections of cerebrospinal fluid (CSF) from under cranial windows were accomplished as we have described for another gasotransmitter, CO (21, 26). CSF was collected after being under the cranial window for 5 min and the vial sealed. H2S in headspace gas was measured by gas chromatography/mass spectrometry (GC-MS).
Standards and samples (400 μl) were collected in N2-filled 2-ml vials with Teflon/rubber septum caps containing three 20 × 4-mm plastic rods to reduce the liquid volume. An N2-saturated solution of 2% ascorbic acid was added to favor gaseous H2S over the water-soluble ion (HS−) and thus accumulation in the headspace gas.
GC-MS analysis of the headspace gas (80 μl) was performed using a Varian 3400cx GC connected to a Varian Saturn 3 mass spectrometer. GC was accomplished on a 30-m fused silica capillary column (0.32 mm ID) coated with SilicaPLOT (Varian CP8567) with H2 as the carrier gas and a temperature ramp of 32 to 50°C over 3.0 min, followed by 2 min of 50°C isocratic and a transfer tube temperature of 50°C. The H2S concentration in unknown samples was calculated based on standard curves diluted from saturated stock (10−1 M at 24°C).
Also, 10−5 M H2S was injected under the cranial window and immediately collected. Recoveries from under the cranial window were 70–80%.
Statistical analyses.
Comparisons among three or more populations were made using ANOVA for repeated measures and Bonferroni post hoc tests. Comparisons between two groups used paired t-tests. Data are presented as means ± SE.
RESULTS
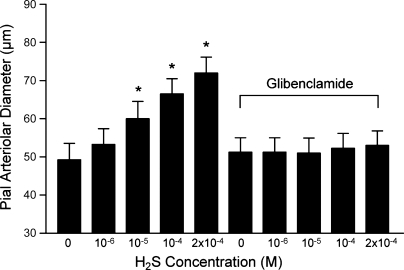
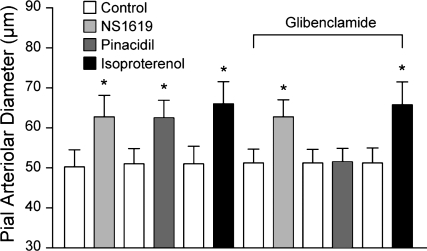
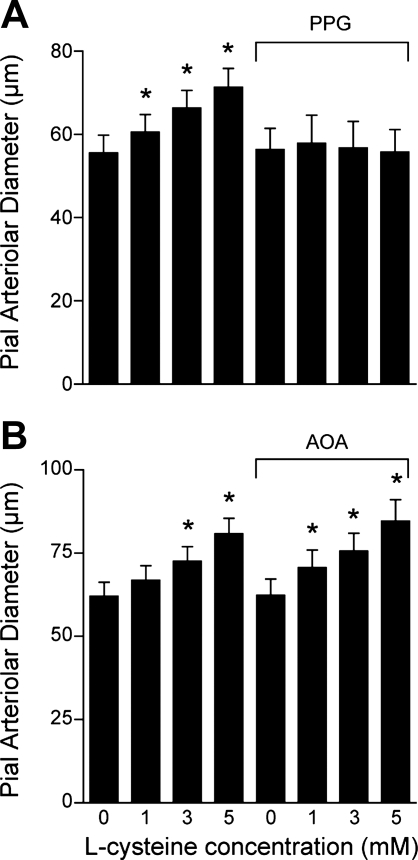
Exogenous H2S and the CSE/CBS substrate l-cysteine dilated newborn pig pial arterioles in vivo. Topical application of H2S in aCSF concentration-dependently dilated pial arterioles (Fig. 1). The dilation to H2S was blocked by glibenclamide (10−6 M), which alone did not change pial arteriolar diameter (Fig. 1). Glibenclamide also blocked the dilation to the KATP channel activator pinacidil (10−5 M) but not to the KCa channel activator NS-1619 (2 × 10−6 M) or isoproterenol, a β-adrenergic agonist (10−6 M) (Fig. 2). The CSE/CBS substrate l-cysteine also dilated piglet pial arterioles (Fig. 3). The dilation to exogenous l-cysteine was blocked by the CSE inhibitor PPG (10 mM) but was unaffected by the CBS inhibitor AOA (1 mM). In addition to H2S, l-cysteine metabolism produces ammonium and pyruvate. Therefore, we applied ammonium and pyruvate under the window at 2 × 10−4 M, the maximum concentration of H2S used. Ammonium and pyruvate had apparent, minimal, dilator effects on pial arteriolar diameters (data not shown). CSE appears to be the enzyme causing H2S production from exogenous substrate leading to pial arteriolar dilation.
Fig. 1.
The effect of topical hydrogen sulfide (H2S) on newborn pig pial arteriolar diameters before and in the presence of glibenclamide (10−6 M). Data are means ± SE; n = 4 piglets. *P < 0.05 compared with zero H2S.
Fig. 2.
Effects of glibenclamide (10−6 M) on dilation of pial arterioles to the Ca2+-dependent K+ (KCa) channel agonist NS-1619 (2 × 10−6 M), the ATP-dependent K+ (KATP) channel agonist pinacidil (10−5 M), and the β-adrenergic agonist isoproterenol (10−6 M). Values are means ± SE. Each agonist was applied after washout of the previous agonist in the order presented; n = 4 piglets. *P < 0.05 compared with preceding control.
Fig. 3.
Dilation of newborn pig pial arterioles to topical application of the cystathionine γ-lyase (CSE) and cystathionine β-synthase (CBS) substrate l-cysteine at the concentrations shown on the abscissa. The dilation to l-cysteine is shown before and following either the CSE inhibitor d,l-propargylglycine (PPG, 5 mM) (n = 6) (A), or the CBS inhibitor amino-oxyacetate (AOA, 1 mM) (n = 4) (B). Data are means ± SE. *P < 0.05 compared with zero l-cysteine.
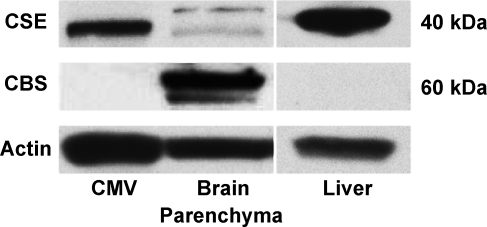
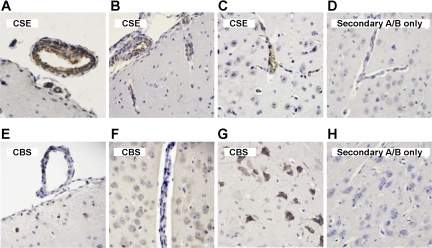
To determine the cellular localization of H2S synthesis in the brain, we used highly specific antibodies against human recombinant CSE and CBS proteins (Novus Biologicals). To test the specificity of the antibodies on Western immunoblotting, we used the liver tissue known to express CSE as the major H2S-producing enzyme (20, 34). CSE, but not CBS, is highly detectable in newborn pig liver (Fig. 4). In isolated cerebral microvessels (300–60 μm), Western blots detected CSE but not CBS (Fig. 4). Conversely, CBS is the predominant enzyme in freshly isolated parenchyma (Fig. 4). CSE and CBS distribution was also detected immunohistochemically in newborn pig cerebral cortex. CSE was expressed predominantly in blood vessels, including pial and penetrating arterioles (Fig. 5). Conversely, CBS was expressed in neurons and astrocytes but was not detectable in penetrating arterioles (Fig. 5). CBS also was minimally detectable in larger pial arterioles. Overall, in the newborn brain, CSE is preferentially expressed in the vasculature, whereas CBS is the major isoform in neurons and astrocytes.
Fig. 4.
Representative Western immunoblots of CSE and CBS expression in isolated cerebral microvessels (CMV) and vessel-free brain parenchyma.The liver that expresses mainly CSE is shown as a control for the antibody specificity.
Fig. 5.
Immunohistochemistry of CSE (A–D) and CBS (E–H) in newborn pig cerebral cortex detected by the avidin-biotin-peroxidase complex technique. A and B: CSE-positive (brown) pial arterioles on the cerebral surface. B and C: CSE-positive (brown) penetrating cerebral vessels. E: CBS-negative pial arteriole on the cerebral cortical surface and an adjacent CBS-negative venule. F: CBS-negative parenchymal vessel. F and G: abundant CBS-positive (brown) neurons and astrocytes, including pyramidal cells (G). D and H: control slices treated with secondary antibody only. Counterstaining: hematoxylin. A/B, antibody.
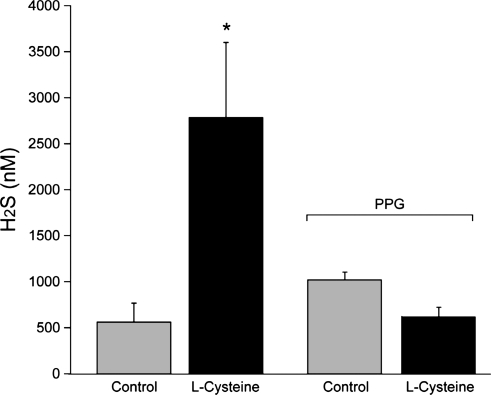
H2S production was measured using GC-MS in CSF from the brain surface in vivo. Basal H2S concentration was ∼600 nM. Elevation of l-cysteine (5 mM) in the CSF under the cranial window increased H2S concentration nearly fivefold (Fig. 6), coincident with pial arteriolar dilation (Fig. 3).
Fig. 6.
H2S concentration in cerebrospinal fluid (CSF) collected from under the cranial window in control and during application of l-cysteine (5 mM, topically). Data are means ± SE; n = 10 before PPG and n = 2 during PPG (5 mM). *P < 0.05 compared with control.
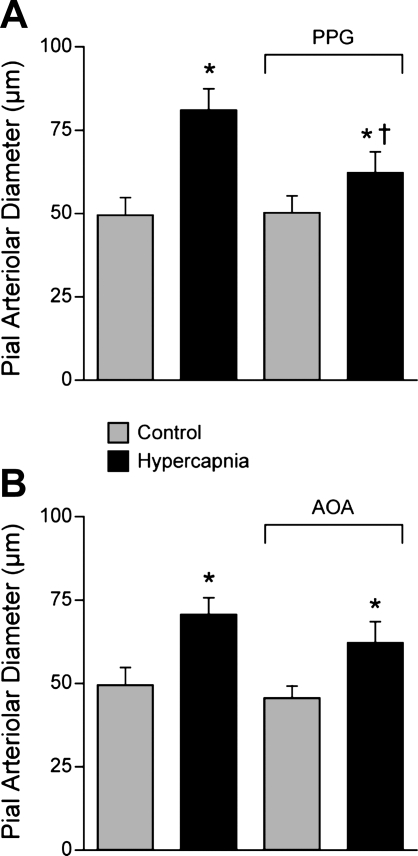
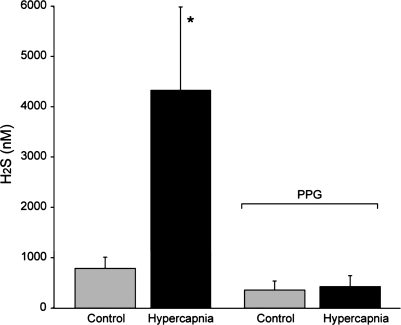
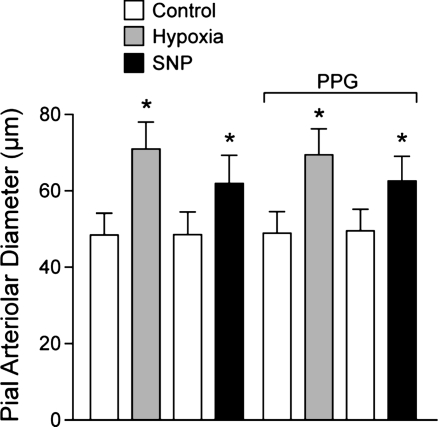
We measured dilation of newborn pig pial arterioles in vivo to ventilation with 10% CO2, 10% O2, and to topical SNP (2 × 10−7 M), an NO donor, before and in the presence of PPG or AOA. Pial arteriolar dilation in response to 10% CO2 was inhibited by PPG but not AOA (Fig. 7). Hypercapnia also increased H2S concentration in CSF, an effect that was blocked by PPG (Fig. 8). In contrast, dilations to hypoxia and SNP were unaffected by PPG (Fig. 9).
Fig. 7.
The effects of hypercapnia on piglet pial arteriolar diameter before and in the presence of PPG (5 mM) (n = 4) (A) or AOA (1 mM) (n = 5) (B). Data are means ± SE. *P < 0.05 compared with preceding control. +P < 0.05 compared with hypercapnia without inhibitor, PPG.
Fig. 8.
H2S in cortical artificial CSF (aCSF) from under cranial windows during normocapnia and hypercapnia before (n = 9) and in the presence of (5 mM) (n = 4) PPG. Data are means ± SE. *P < 0.05 compared with previous control.
Fig. 9.
Effects of PPG (5 mM) on pial arteriolar dilations to hypoxia (10% O2 ventilation, PaO2 ∼35 mmHg) and sodium nitroprusside (SNP, 2 × 10−7 M). In 8 piglets, pial arteriolar diameter measurements were made during normoxia and then hypoxia, and, after return to normoxia, SNP was given topically, and diameter measurements were taken. SNP was removed from the aCSF, and PPG was added to aCSF for all subsequent measurement periods with responses to hypoxia and SNP repeated. Data are means ± SE. *P < 0.05 compared with preceding control.
DISCUSSION
The new findings of this study of newborn pigs include: 1) H2S concentration-dependently dilates cerebral cortical pial arterioles, 2) dilation to H2S is blocked by glibenclamide, 3) topical application of l-cysteine dilates pial arterioles, 4) dilation to l-cysteine is blocked by the CSE inhibitor PPG but not by the CBS inhibitor AOA, 5) CSE is predominately expressed in cerebral vessels, 6) CBS is located mainly in neurons and astrocytes, 7) l-cysteine increases H2S in cortical CSF coincident with vasodilation, 8) pial arteriolar dilation to hypercapnia is inhibited by PPG but not by AOA, and 9) hypercapnia increases cortical CSF H2S concentration, and this increase is also inhibited by PPG. These data show for the first time a potential regulatory role of the CSE/H2S system in the cerebrovascular circulation. These data suggest that H2S is a dilator of newborn cerebral arterioles and that endogenous CSE is capable of producing sufficient H2S to decrease vascular tone.
We detected CSE in cerebral vessels and CBS in astrocytes and neurons (Figs. 4 and 5). For detection of enzyme expression in tissues, Western blot and immunocytochemistry each have limitations and strengths. With Western blot, the detected protein can be isolated to a specific molecular weight known to be the weight of the protein of interest, but the localization of the protein in the intact tissue cannot be visualized. By immunocytochemistry, the cellular localizations in the intact tissue can be visualized, but the molecular weight cannot be confirmed. Use of the same antibodies and the two methods provides greater confidence that accurate interpretation is obtained. In the present study, by Western blot, the CSE antibodies stain at the appropriate CSE molecular weight in microvessels but not brain parenchyma, which is principally neurons and glia. With the use of the same CSE antibody, prominent staining was seen in pial and penetrating microvessels in situ but not in the brain parenchyma. Conversely, in brain parenchyma, the CBS antibody bound to a protein at the appropriate molecular weight for CBS. In cerebral microvessels, no binding was detected at the appropriate molecular weight for CBS. With the use of the same antibody, CBS immunostaining was in neurons and glia in situ but not detected in cerebral microvessels. Thus, because we found consistent localizations of CSE and CBS using the same antibodies with two different methods, each with its own strengths and limitations, we feel confident in concluding that CSE is localized to the microvessels and CBS in neurons and glia. The vascular CSE expression in newborn pig brain is consistent with mouse mesenteric arteries (51).
The effectiveness of CSE blockade was evaluated by determining the effects of PPG on the pial arteriolar responses to topical l-cysteine. l-Cysteine produced dose-dependent vasodilation but had no effect on pial arteriolar diameter in the presence of PPG (Fig. 3), consistent with effective inhibition of H2S production. Conversely, AOA, a CBS inhibitor (33), had no effect on pial arteriolar diameter or dilation to l-cysteine (Fig. 3), suggesting the entire vasodilatory response to l-cysteine may result from metabolism catalyzed by CSE.
PPG and AOA were applied at concentrations purportedly selective for CSE and CBS, respectively (33, 40). High concentrations of l-cysteine are required in intact cells and in vivo presumably because l-cysteine does not readily permeate intact cell membranes, and both CSE and CBS are intracellular. Similarly, high concentrations of l-arginine and l-arginine methyl ester are required to cause NO-induced dilation inhibited by NG-nitro-l-arginine methyl ester (l-NAME; see Refs. 7 and 23). The evidence that l-cysteine must be metabolized to cause dilation is provided by blockade of the dilator response to l-cysteine by PPG (Fig. 3) and that l-cysteine is metabolized to H2S is supported by a large increase in CSF H2S concentration measured for the first time by GC-MS (Fig. 6). The lack of effect of AOA does not confirm CBS cannot be involved in regulation of cerebrovascular tone. Even though dilation to exogenous l-cysteine was blocked by PPG, CBS could still be active in cells that are not involved in producing the dilation to topical l-cysteine.
CSE activity is substrate-dependent (Fig. 3). Exogenous l-cysteine increased H2S production (Fig. 6) and dilated newborn pig pial arterioles in vivo (Fig. 3). The CSE inhibitor PPG blocked this dilation. CSE catalytic activity also can be increased by a Ca2+/calmodulin-dependent mechanism (51). Two other gasotransmitters, NO and CO, can also affect H2S production. NO increases CSE activity in rat aorta and mesenteric arteries (52), but both NO and CO inhibit CBS activity (36). H2S is metabolized via thiosulfate reductase and the sulfite oxidized to sulfate by sulfite oxidase (20). Further experimentation will be necessary to determine if such interactions are functionally significant in regulation of neonatal cerebrovascular circulation.
H2S dissociates to H+ and HS− in solution with more as H2S in acidic solutions and more as HS− at higher pH. The actual form of H2S causing the physiological effect is uncertain (18) with the possibility of gaseous H2S diffusing between cells while an ionic form may interact with channels or second messenger enzymes within cells. Of importance to the present discussion, respiratory acidosis would increase the proportion that is H2S. Increasing the proportion as H2S would allow the gas to diffuse among cells and have gasotransmitter function.
Concentrations of H2S detected in tissues and fluids depend upon methods employed and whether those methods measure only gaseous H2S, the total amount of sulfur ions in a liquid, or total tissue sulfur ions. The H2S electrode used by Yang et al. (51) employs a buffer that converts H2S and HS− to S2− that is detected, thereby measuring all biological forms of unbound H2S. Yang et al. (51) detected basal H2S concentration of 4 μM in serum and 60 μM in aorta (51). Furne et al. (13) used GC with a chemiluminescence sulfur detector of headspace gas above homogenized mouse brain and liver. They concluded that whole tissue concentrations were very low [∼15 nM in the tissue (13)]. Conversely, our measurements of H2S in headspace gas by GC-MS gave considerably higher H2S levels in piglet cortical periarachnoid CSF (∼0.5–1 μM; Figs. 6 and 8) and severalfold increases with l-cysteine and hypercapnia (Figs. 6 and 8). We use acidification after sample collection and in quantification standards to promote the gaseous form of H2S. H2S concentration has not been measured before in CSF or intact brain so direct comparison of the present report with studies of others is not possible.
The potential functional significance of each gasotransmitter needs not be correlated with the absolute concentrations needed to cause dilation when applied topically in vivo. Instead, functional significance would be related to the concentration needed to dilate relative to the in vivo endogenous vascular smooth muscle concentration. The difference between aCSF concentration and concentration near production sites of CO will be less than the differences for H2S and NO. CO is a stable molecule that can be produced, diffuse some distance through tissue, and accumulate in extracellular fluid. Exogenous CO causes dilation with a threshold of ∼50 nM and the basal CSF concentration is ∼20–50 nM. In contrast, concentrations of H2S and NO around the cerebrovascular smooth muscle certainly are much higher than the concentration in CSF. Neither H2S nor NO can diffuse far before reacting in biological systems. In vivo, tissue NO concentrations are not known. Similarly, in the case of H2S, that the spillover H2S concentration in CSF (basal ∼600 nM) is near the threshold (1–10 μM) indicates to us that H2S concentrations near points of production are in the vasodilatory range and these sites of production may be on the brain surface, for example, endothelium of pial arterioles (Fig. 5).
H2S and pinacidil, a KATP channel activator, caused dilation of piglet pial arterioles that was blocked by glibenclamide (Figs. 1 and 2). H2S may affect many ion channels and receptors, including KATP channels (45, 53). Recently, H2S was reported to activate recombinant KATP channels expressed in HEK-293 cells by interaction with the sulfonylurea receptor (SUR) 1 extracellular NH2 terminus (19). These data may provide a mechanism by which H2S activates KATP channels to cause vasodilation, although vascular smooth muscle cell KATP channels contain SUR2B, indicating that the mechanisms may be different (2). The KATP channel mechanism for H2S-induced cerebrovascular dilation might have applicability to our finding that H2S appears to be involved in pial arteriolar dilation to hypercapnia in piglets (Figs. 7 and 8). The probable H2S effectors, KATP channels, are less sensitive to pharmacological activation by lemakalim in newborn than in adult cerebral arteries in vitro (42). Therefore, maturational increases in KATP channel activity could contribute to age-related changes in cerebrovascular contractility (41), which could involve H2S.
In an earlier study, we reported that glibenclamide did not inhibit dilation of piglet cerebral arterioles in vivo to hypercapnia (28). These data do not appear to be consistent with our interpretation of the current data that glibenclamide inhibits dilation to H2S (Fig. 1), and the CSE inhibitor PPG, which blocks dilation to the CSE substrate l-cysteine, inhibits dilation to hypercapnia. We have reexamined the earlier data and concluded that experiments on the effects of glibenclamide on piglet cerebrovascular responses to hypercapnia should be repeated and expanded. While incomplete, these new data suggest glibenclamide attenuates, but does not block, dilation to hypercapnia (38), similarly to PPG in the present study (Fig. 7).
The present results suggest cerebrovascular dilation to hypoxia does not involve CSE/H2S (Fig. 9). However, only a single level and duration of hypoxia was employed so that additional experiments to determine whether involvement of H2S depends on the degree and length of hypoxia must be performed. Even though PPG completely blocks dilation to l-cysteine, we cannot exclude the possibility that conversion of endogenous l-cysteine to H2S catalyzed by CBS contributes to dilation to hypoxia. H2S is involved in fish hypoxic vascular responses (39, 40). In addition, the question of whether other mechanisms are compensating for loss of H2S responses in the dilation will require further experimentation to exclude a role for H2S.
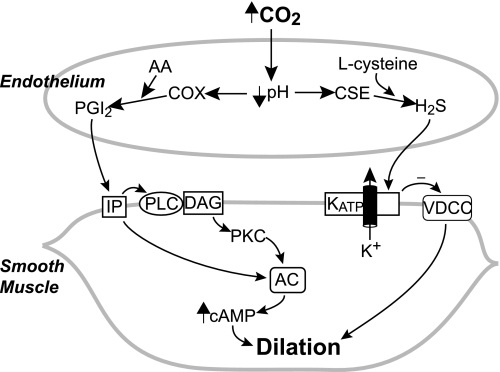
Control of cerebrovascular circulation is complicated with multiple interacting, sometimes apparently redundant, pathways. Figure 10 is a simplified schematic diagram of two systems that appear to be involved in pial arteriolar dilation to hypercapnia in newborn pigs, CSE/H2S (present results) and prostaglandin cyclooxygenase/prostanoids (28, 29, 30). Regarding the role of endothelium and prostacyclin in cerebrovascular dilation to hypercapnia in the newborn, what initially appeared simple and straightforward turned out to be more complicated. Endothelial dependence of dilation to hypercapnia can be explained by a permissive role of prostacyclin via a protein kinase C-dependent mechanism, even though hypercapnia increases prostacyclin production in vivo and by endothelial cells in vitro (28). Even though the permissive role of prostacyclin can explain its role in dilation to hypercapnia, this role may not be dominant in the intact system. The permissive role may be contributory, secondary, or backup when prostanoids are allowed to increase rather than being held constant. Hypercapnia elevates dilator prostanoids in the newborn pig cortical CSF sufficiently that higher levels at sites of production could explain the pial arteriolar dilation to hypercapnia via elevation of cAMP (29, 30). Whether or not the elevation of prostacyclin and PGE2 plays a direct role in causing hypercapnia-induced dilation when prostanoid production is not inhibited is not known. If they do, it is not known why dilation to CO2 is not accentuated in the intact system compared with when prostacyclin is held constant. Of importance to the present discussion about H2S, the final mediator mechanism in the cerebrovascular smooth muscle cell that is enabled when prostacyclin is held constant is not known. H2S would potentially fill a critical mechanistic gap if it were to be a downstream mediator by activating KATP channels. In contrast to NO in adult rats (50), in the newborn piglet, we have consistently found no role for NO or CO in dilation to hypercapnia (25, 55). Data are consistent with H2S being involved, but interactions with prostacyclin and other pathways will require further investigation (interactions between the two systems are not shown in Fig. 10 because they are speculative and possibilities numerous).
Fig. 10.
Simplified schematic diagram of contributions of the CSE/H2S and cyclooxygenase/prostanoid pathways in arteriolar dilation to hypercapnia in newborn brain. COX, prostaglandin cyclooxygenase; AA, arachidonic acid; PGI2, prostacyclin; IP, prostacyclin receptor; PLC, phospholipase C; DAG, diacylglycerol; AC, adenylyl cyclase; PKC, protein kinase C; VDCC, voltage-dependent Ca2+ channel.
Our data strongly suggest that CSE-derived H2S is a component of regulation of neonatal cerebrovascular circulation. These in vivo data demonstrate that H2S dilates newborn pial arterioles. H2S is detectable in cortical CSF under control conditions and increases during elevation of a dominant regulator of cerebral blood flow, CO2. A blocker of CSE inhibits the dilation to hypercapnia. These data indicate that H2S is a physiologically significant dilator in the cerebral circulation.
GRANTS
These studies were supported National Institutes of Health Grants HL-042851, HL-34059, HL-67061, HL-94378, and NS-046385.
DISCLOSURES
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1. Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16: 1066–1071, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adebiyi A, McNally EM, Jaggar JH. Sulfonylurea receptor-dependent and -independent pathways mediate vasodilation induced by ATP-sensitive K+ channel openers. Mol Pharmacol 74: 736–743, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Armstead WM, Zuckerman SL, Shibata M, Parfenova H, Leffler CW. Different pial arteriolar responses to acetylcholine in the newborn and juvenile pig. J Cerebral Blood Flow Metab 14: 1088–1095, 1994 [DOI] [PubMed] [Google Scholar]
- 4. Beech DJ, Zhang H, Nakao K, Bolton TB. K channel activation by nucleotide diphosphates and its inhibition by glibenclamide in vascular smooth muscle cells. Br J Pharmacol 110: 573–582, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Busija DW, Heistad DD. Factors involved in the physiological regulation of the cerebral circulation. Rev Physiol Biochem Pharmacol 101: 161–211, 1984 [DOI] [PubMed] [Google Scholar]
- 6. Busija DW. Cerebral autoregulation. In: The Regulation of Cerebral Blood Flow, edited by Phyllis LW. Boca Raton, FL: CRC, 1993, p. 45–65 [Google Scholar]
- 7. Busija DW, Leffler CW. Dilator effects of amino acid neurotransmitters on piglet pial arterioles. Am J Physiol Heart Circ Physiol 257: H1200–H1203, 1989 [DOI] [PubMed] [Google Scholar]
- 8. Calvert JW, Zhang JH. Pathophysiology of an hypoxic-ischemic insult during the perinatal period. Neurol Res 27: 246–260, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Charles SM, Zhang L, Cipolla MJ, Buchholz JN, Pearce WJ. The roles of cytosolic Ca2+ concentration and myofilament Ca2+ sensitization in age-dependent cerebrovascular myogenic tone. Am J Physiol Heart Circ Physiol 299: H1034–H1044, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol 287: H2316–H2323, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Du Plessis AJ, Volpe JJ. Perinatal brain injury in preterm and term newborn. Curr Opin Neurol 15: 151–157, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Faraci FM, Breese KR, Heistad DD. Cerebral vasodilation during hypercapnia. Stroke 25: 1679–1683, 1994 [DOI] [PubMed] [Google Scholar]
- 13. Furne J, Saeed A, Levitt MD. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am J Physiol Regul Integr Comp Physiol 295: R1479–R1485, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Heistad DD, Kontos HA. Cerebral circulation. In: Handbook of Physiology. The Cardiovascular System. Peripheral Circulation and Organ Blood Flow. Bethesda, MD: Am Physiol Soc, 1983, sect. 2, vol. III, pt. 1, chapt. 5, p. 137–182 [Google Scholar]
- 15. Holt DC, Fedinec AL, Vaughn AN, Leffler CW. Age and species dependence of pial arteriolar responses to topical carbon monoxide in vivo. Exptl Biol Med 232: 1465–1469, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nature Rev Neurosci 5: 347–360, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Iadecola C. Regulation of the cerebral microcirculation during neuronal activity: is nitric oxide the missing link? Trends Neurosci 16: 206–214, 1993 [DOI] [PubMed] [Google Scholar]
- 18. Ishigami M, Hiraki K, Umemura K, Ogasawara Y, Ishii K, Kimura H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid Redox Signal 11: 205–214, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Jiang B, Tang G, Cao K, Wu L, Wang R. Molecular mechanism for H2S-inducted activation of KATP channels. Antioxid Redox Signal 12: 1167–1178, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Kamoun P. Endogenous production of hydrogen sulfide in mammals. Amino Acids 26: 243–254, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Kanu A, Whitfield J, Leffler CW. Carbon monoxide contributes to hypotension-induced cerebrovascular dilation in piglets. Am J Physiol Heart Circ Physiol 291: H2409–H2414, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kimura H. Hydrogen sulfide: its production, release, functions. Amino Acids 12: 1–13, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Knecht KR, Milam S, Wilkinson DA, Fedinec AL, Leffler CW. Time-dependent action of carbon monoxide on newborn cerebrovascular circulation. Am J Physiol Heart Circ Physiol 299: H70–H75, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee SW, Cheng Y, Moore PK, Bian JS. Hydrogen sulphide regulates intracellular pH in vascular smooth muscle cells. Biochem Biophys Res Commun 358: 1142–1147, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Leffler CW, Nasjletti A, Yu C, Johnson RA, Fedinec AL, Walker N. Carbon monoxide and cerebral microvascular tone in newborn pigs. Am J Physiol Heart Circ Physiol 276: H1641–H1646, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Leffler CW, Parfenova H, Fedinec AL, Basuroy S, Tcheranova D. Contributions of astrocytes and CO to pial arteriolar dilation to glutamate in newborn pigs. Am J Physiol Heart Circ Physiol 291: H2897–H2904, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leffler CW, Parfenova H, Jaggar JH, Wang R. Carbon monoxide and hydrogen sulfide: gaseous messengers in cerebrovascular circulation. J Appl Physiol 100: 1065–1076, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leffler CW. Prostanoids: intrinsic modulators of cerebral circulation. NIPS 12: 72–77, 1997 [Google Scholar]
- 29. Leffler CW, Busija DW. Prostanoids in cortical subarachnoid cerebrospinal fluid and pial artery diameter in newborn pigs. Circ Res 57: 689–694, 1985 [DOI] [PubMed] [Google Scholar]
- 30. Leffler CW, Busija DW. Prostanoids and pial arteriolar diameter in hypotensive newborn pigs. Am J Physiol Heart Circ Physiol 252: H687–H691, 1987 [DOI] [PubMed] [Google Scholar]
- 31. Leffler CW, Smith JS, Edrington JL, Zuckerman SL, Parfenova H. Mechanisms of hypoxia-induced cerebrovascular dilation in the newborn pig. Am J Physiol Heart Circ Physiol 272: H1323–H1332, 1997 [DOI] [PubMed] [Google Scholar]
- 32. Leslie M. Nothing rotten about hydrogen sulfide's medical promise. Science 320: 1155–1157, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Li L, Moore PK. Putative biological roles of hydrogen sulfide in health and disease: a breath of not so fresh air? Trends Pharmacol Sci 29: 84–90, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Li L, Salto-Tellez M, Tan CH, Whiteman M, Moore PK. GYY4137, a novel hydrogen sulfide-releasing molecule, protects against endotoxic shock in the rat. Free Radic Biol Med 47: 103–113, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Lindauer U, Vogt J, Schuh-Hofer S, Dreier JP, Dirnagl U. Cerebrovascular dilation to extralumenal acidosis occurs via combined activation of ATP-sensitive and Ca2+- activated potassium channels. J Cerebral Blood Flow Metab 23: 1227–1238, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Lowicka E, Bełtowski J. Hydrogen sulfide (H2S): the third gas of interest for pharmacologists. Pharmacol Rep 59: 4–24, 2007 [PubMed] [Google Scholar]
- 37. Mancardi D, Penna C, Merlino A, Del Soldato P, Wink DA, Pagliaro P. Physiological and pharmacological features of the novel gasotransmitter: Hydrogen sulfide. Biochim Biophys Acta 1787: 864–872, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nnorom CC, Fedinec AL, Leffler CW. Role of ATP sensitive potassium channels in dilation of newborn cerebral arterioles to hypercapnia (Abstract). Pediatr Res In press [Google Scholar]
- 39. Olson KR, Whitfield NL, Bearden SE, St Leger J, Nilson E, Gao Y, Madden JA. Hypoxic pulmonary vasodilation: a paradigm shift with a hydrogen sulfide mechanism. Am J Physiol Regul Integr Comp Physiol 298: R51–R60, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Olson KR, Dombkowski RA, Russell MJ, Doellman MM, Head SK, Whitfield NL, Madden JA. Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation. J Exp Biol 209: 4011–4023, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Pearce WJ, Elliott SR. Maturation enhances the sensitivity of ovine cerebral arteries to the ATP sensitive potassium channel activator lemakalim. Pediatr Res 35: 729–732, 1994 [DOI] [PubMed] [Google Scholar]
- 42. Phillis JW. Adenosine and adenine nuceotides as regulators of cerebral blood flow: roles of acidosis, cell swelling, and KATP channels. Crit Rev Neurobiol 16: 237–270, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Quayle JM, Standen NB. KATP channels in vascular smooth muscle. Cardiovasc Res 28: 797–804, 1994 [DOI] [PubMed] [Google Scholar]
- 44. Santa N, Kitazono T, Ago T, Ooboshi H, Kamouchi M, Wakisada M, Ibayashi S, Iida M. ATP-sensitive potassium channels mediate dilation of basilar artery to intracellular acidification in vivo. Stroke 34: 1276–1280, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Tang G, Wu L, Wang R. Interaction of hydrogen sulfide with different ion channels. Clin Exp Pharmacol Physiol 37: 753–763, 2010 [DOI] [PubMed] [Google Scholar]
- 46. Wagner F, Asfar P, Calzia E, Radermacher P, Szabó C. Bench-to-bedside review: Hydrogen sulfide-the third gaseous transmitter: applications for critical care (Abstract). Crit Care 13: 213, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang X, Wu J, Chen F, Wang R, Jiang C. Hypercapnic acidosis activates KATP channels in vascular smooth muscles. Circ Res 92: 1225–1232, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Willis AP, Leffler CW. Nitric oxide and prostanoids: age-dependence of hypercapnic- and histamine-induced dilations of pig pial arterioles. Am J Physiol Heart Circ Physiol 277: H299–H307, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Willis AP, Leffler CW. Endothelial NO and prostanoid involvement in newborn and juvenile pig pial arteriolar responses. Am J Physiol Heart Circ Physiol 281: H2366–H2377, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Xu HL, Koenig HM, Ye S, Feinstein DL, Pelligrino DA. Influence of glia limitans on pial arteriolar relaxation in the rat. Am J Physiol Heart Circ Physiol 2 87: H331–H339, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 322: 587–590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhao W, Ndisang JF, Wang R. Modulation of endogenous production of H2S in rat tissues. Can J Physiol Pharmacol 81: 848–853, 2003 [DOI] [PubMed] [Google Scholar]
- 53. Zhao W, Wang R. H2S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol Heart Circ Physiol 283: H474–H480, 2002 [DOI] [PubMed] [Google Scholar]
- 54. Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous KATP channel opener. EMBO J 20: 6008–6016, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zuckerman SL, Armstead WM, Hsu P, Shibata M, Leffler CW. Age dependence of cerebrovascular response mechanisms in the domestic pig. Am J Physiol Heart Circ Physiol 271: H535–H540, 1996 [DOI] [PubMed] [Google Scholar]