Abstract
Our earlier finding that the activity of protein phosphatase 2A from rat brain is inhibited by micromolar concentrations of the dithiol cross-linking reagent phenylarsine oxide (PAO) has encouraged the hypothesis that the catalytic subunit (PP2Ac) of PP2A contains one or more pairs of closely-spaced (vicinal) thiol pairs that may contribute to regulation of the enzyme. The results of the present study demonstrate using immobilized PAO-affinity chromatography that PP2Ac from rat brain formed stable DTT-sensitive adducts with PAO with or without associated regulatory subunits. In addition, a subset of the PAO-binding vicinal thiols of PP2Ac was readily oxidized to disulfide bonds in vitro. Importantly, a small fraction of PP2Ac was still found to contain disulfide bonds after applying stringent conditions designed to prevent protein disulfide bond formation during homogenization and fractionation of the brains. These findings establish the presence of potentially regulatory and redox-active PAO-binding vicinal thiols on the catalytic subunit of PP2A and suggest that a population of PP2Ac may contain disulfide bonds in vivo.
Keywords: Protein phosphatase 2A, Disulfide bonds, Phenylarsine oxide, Oxidative stress
Introduction
Protein Phosphatase 2A (PP2A) is a ubiquitous serine/threonine protein phosphatase that is particularly abundant in the brain [1]. PP2A activity regulates protein kinase cascades [2], apoptosis [3], neuronal transmission [4], cytoskeleton dynamics [5], and down-regulation of the enzyme has been linked to Alzheimer's disease [6]. The PP2A holoenzyme is expressed in cells as a heterotrimer comprised of a catalytic (PP2Ac) subunit, a scaffolding/regulatory (A) subunit, and a second regulatory (B) subunit that is highly variable [7].
PP2Ac contains 10 cysteine residues [8, 9] that are conserved among mammals but not considered to be essential for catalysis [10]. Nevertheless, findings that thiol-reactive agents [11–13] and oxidants [14–16] can inhibit PP2A activity in vitro suggest that modification of one or more of the thiols of PP2A is sufficient to impair enzyme activity via a presumed conformational change. The roles of PP2A thiol groups in the regulation and dysregulation of PP2A activity are, however, unknown.
In an earlier study [17], we demonstrated that micromolar concentrations of the vicinal1 dithiol cross-linking reagent phenylarsine oxide (PAO) inhibited the activity of PP2A in a soluble extract from rat brain. This previous finding has encouraged the hypothesis that PP2Ac contains one or more PAO-binding vicinal thiol pairs that may be of regulatory significance for the enzyme. Vicinal thiols may facilitate the regulation of protein function by reversible disulfide bond formation in response to oxidative stress [18] and by contributing to the binding of proteins by phosphate ion/phosphoryl groups [19] and by Zn2+ ion [20].
We have recently improved an immobilized PAO-affinity chromatography method that enables the capture from tissues and analysis of proteins containing PAO-binding vicinal thiols and the fraction of these proteins containing vicinal thiols that have been oxidized reversibly to disulfide bonds [21]. PAO and other trivalent arsenicals form stable dithioarsine rings with low-molecular-weight and protein dithiols but not with monothiols [22, 23]. The key to the enhanced PAO-affinity method is the use of the disulfide reducing agent tris(2-carboxyethyl)-phosphine (TCEP) [24]. Unlike traditional disulfide reducing agents such as DTT, TCEP does not contain thiol groups and so does not compete with protein thiols for binding to the immobilized PAO. By reversing disulfide bond formation and maintaining protein thiols in the reduced state necessary to bind PAO, TCEP promotes a much more efficient capture of vicinal thiol proteins and the fraction of these containing disulfide bonds than had been achieved in an earlier study [25].
In the present investigation, we have employed the immobilized PAO-affinity method to examine directly the binding of PP2Ac from rat brain to PAO with and without associated PP2A regulatory subunits and the possibility that the postulated PAO-binding vicinal thiols of PP2Ac may undergo ready oxidation to form disulfide bonds.
Experimental Procedures
Preparation of the S100 Fraction from Rat Brain
Whole brains from 7–8 week-old Sprague–Dawley rats were obtained on dry ice from Pel-Freez Biologicals (Rogers, AZ) and were stored until use at −80°C. For each S100 fraction prepared, one whole brain was partially thawed and homogenized in a glass-Teflon homogenizer in 15 mL of Tris–EDTA buffer (50 mM Tris, 1 mM EDTA, 1 mM benzamidine, pH 7.4) to which was added 50 μL of mammalian protease inhibitor cocktail (Sigma Chemical) per brain. Homogenates were centrifuged at 100,000g for 65 min at 4°C. The resulting supernatant was designated the S100 fraction and was diluted to 1 mg/mL. Protein concentrations were determined by the Coomassie blue assay (Pierce Chemical).
Dissociation of the Catalytic and Regulatory Subunits of PP2A
Dissociation of the catalytic (PP2Ac) and regulatory subunits of PP2A was achieved by ethanol precipitation by minor modification of the method described by Hue et al. [26]. Briefly, S100 fractions were prepared as described above from three whole brains homogenized with 9 mL of the Tris–EDTA buffer containing 10 mM β-mercaptoethanol. Ammonium sulfate was added to the concentrated S100 fraction to achieve a 25% saturated solution and stirred slowly at 4°C. Following a 30 min incubation period, the solution was centrifuged for 10 min at 4°C at 14,000g. Additional ammonium sulfate was added to the resulting supernatant to achieve a 50% saturated solution that was allowed to stir slowly at 4°C for 30 min. The supernatant was discarded and the pellet was resuspended with 0.4 mL of the Tris–EDTA-mercaptoethanol buffer. The resuspended pellet was combined with five volumes of 95% ethanol at room temperature to produce a precipitate which formed immediately. The precipitated protein was collected by centrifugation at 4,200g for 10 min 4°C. The pellet was extracted twice by resuspending with 3 mL of the Tris–EDTA-mercaptoethanol buffer and centrifuging at 6,400g for 5 min. The supernatants from each extraction were combined and ammonium sulfate was added to achieve a 65% saturated solution. The precipitated protein was collected in multiple aliquots by centrifugation for 10 min at 4°C at 14,000g following incubation of the ammonium sulfate solution for 30 min while slowly stirring at 4°C. The resulting pellets containing the free catalytic subunit of PP2A were designated the EtOH-AS65 fraction and were stored at −80°C until use.
Dissociation of PP2A subunits by the ethanol precipitation step was assessed by separation of protein from either the S100 (3.0 mg protein) or the EtOH-AS65 (0.15 mg protein) fractions by size exclusion chromatography on a 1.5 × 75 cm column containing 100 mL of packed Sephacryl 300HR pre-equilibrated in Tris–EDTA buffer containing 10 mM β-mercaptoethanol and 100 mM NaCl. The column was run at 0.15 mL/min and 2 mL fractions were collected. The molecular weights of PP2Ac in these fractions were estimated by combining 225 μL of each fraction with 25 μL of the phosphothreonine-containing peptide substrate and measuring the phosphatase activity of the column fractions following a 10 min incubation at 37°C as described below. γ-Globulin, ovalbumin, and myoglobin were employed as molecular weight standards.
On the day of use, the EtOH-AS65 pellets were resuspended with 1 mL of Tris-ETDA buffer. Residual β-mercaptoethanol and ammonium sulfate were removed by centrifugal gel filtration through three successive spin columns containing 8 mL of packed Biogel P6 that had been equilibrated in Tris–EDTA buffer.
Alkylation of Protein Thiols for Analysis of Disulfide Bonds Formed In Vitro
Following resuspension in Tris–EDTA buffer of the EtOH-AS65 pellets and removal by gel filtration of residual ammonium sulfate and β-mercaptoethanol, the EtOH-AS65 fraction was diluted to 0.25 mg protein/mL with Tris–EDTA buffer and warmed for 15 min at 37°C to permit oxidation of PP2Ac thiols. Following the warming period, the EtOH-AS65 fraction was brought to 25 mM iodoacetamide, which was found in preliminary experiments to be sufficient to block PP2Ac binding to the immobilized PAO, and alkylation was allowed to proceed in the dark for 1 h at room temperature. Unreacted iodoacetamide was removed prior to the PAO-affinity chromatography by centrifuging 1 mL of the alkylated fractions through two successive spin columns containing 8 mL of packed Bio-Gel P-6 (Bio-Rad) that had been pre-equilibrated with Tris-ETDA buffer containing 10% glycerol.
Modifications Designed to Prevent Disulfide Bond Formation In Vitro
In order to assess the possible occurrence of disulfide bond formation in vivo, several steps were taken to prevent thiol oxidation that would otherwise occur during brain fractionation procedures as a result of exposure to dissolved oxygen and transition metals. First, alkylation was initiated at the point of homogenization by homogenizing partially thawed brains at 1 g/mL in Tris–EDTA buffer containing dissolved iodoacetamide and 1% v/v Triton X-100. Second, the iodoacetamide concentration was increased to 500 mM and the pH of the buffer increased to 8.0 to maximize the rate of thiol alkylation [27]. In doing so, the concentration of Tris in the buffer was increased to 500 mM to prevent a drop in pH resulting from the greater extent of alkylation expected owing to the higher protein and iodoacetamide concentrations. Third, the buffer was purged with N2 gas to remove dissolved O2 just prior to homogenization. Fourth, the EDTA concentration was increased to 10 mM to ensure complete binding of transition metals. Following a 1 h alkylation period in the dark at room temperature, the alkylated homogenates were centrifuged at 100,000g for 65 min at 4°C. The resulting alkylated S100 fraction was diluted to 5 mg protein/mL while also bringing to 10% v/v glycerol. Protein concentrations were determined by the 660 nm assay (Pierce Chemical).
Preparation of the Immobilized Phenylarsine Oxide
p-Aminophenylarsine oxide (amino-PAO) was prepared by reduction of arsanilic acid (Acros Organics) and coupled via the amine group to an N-hydroxysuccinimide ester derivative of agarose (Affi-Gel 10; Bio-Rad), as described by us previously [21]. Any remaining activated esters of the Affi-Gel 10 were blocked by the addition of excess ethanolamine. Control columns were prepared by reacting the Affi-Gel 10 with ethanolamine only.
Phenylarsine Oxide-Affinity Chromatography
PAO-affinity chromatography was performed as described by us previously [21]. Briefly, 600 μL of the protein fractions under study and described above were combined with 400 μL of packed immobilized PAO gel in microspin columns and incubated while rotating for 2 h at 4°C in the absence or presence of 5 mM TCEP. Unbound (flow-through; FT), protein was collected by centrifugation. The microspin columns were washed by the repeated addition of 600 μL Tris–EDTA buffer and re-centrifuged until no protein was detected eluting from the columns. Five washes were found to be sufficient. Bound protein was removed by centrifugation following resuspension of the immobilized PAO with 600 μL Tris-ETDA buffer containing 25 mM DTT and incubation for 10 min at 4°C. In some experiments, bound protein was eluted in step-wise manner by successive additions of buffer containing 500 mM NaCl, 1 mM β-mercaptoethanol, 1 mM DTT, and 10 mM DTT. PAO-affinity fractions were stored at −80°C until analyzed.
Measurement of PP2A Activity
PP2A activity was measured as described by us previously [17]. Briefly, 225 μL of the size-exclusion fractions or 25 μL of the PAO-affinity fractions were combined in a final volume of 250 μL with Tris–EDTA buffer and 0.4 mM of the phosphopeptide substrate KRpTIRR (Biomol Research Laboratories). Following 10 min (for the size-exclusion fractions) or 4 min (for the PAO-affinity fractions) incubation periods at 37°C, reactions were terminated by addition of 2 mL of Biomol Green reagent (Biomol Research Laboratories) and absorbances at 620 nm were measured. Inorganic phosphate was quantified from a standard curve prepared using Na2HPO4. We have shown previously that greater than 95% of the phosphothreonine peptide phosphatase activity in crude extracts from rat brain is attributable to PP2A under these assay conditions [12, 16, 17].
Gel Electrophoresis and Western Blotting
The FT and DTT fractions from the immobilized PAO-affinity chromatography experiments were diluted 1:1 with reducing Laemmli sample buffer containing 20 mM DTT and heated for 5 min at 95°C. For TPI blots, PAO affinity fractions were diluted fivefold with Tris–EDTA buffer prior to the 1:1 dilution with reducing sample buffer. Equal volumes (20 μL unless stated otherwise) of the diluted fractions were separated by discontinuous SDS–polyacrylamide gel electrophoresis employing a 4.5% stacking gel and a 10% running gel. Proteins were stained with Imperial stain (Pierce Chemical) or transferred to nitrocellulose blotting membranes. Blots were blocked overnight at 4°C, incubated at room temperature with anti-PP2AC antibody (Millipore, product no. 07–324, diluted 1:1,000) for 2 h followed by washing and incubation with secondary antibody conjugated to horse radish peroxidase (Bio-Rad Laboratories, diluted 1:10,000). PP2AC was detected on film following the addition of HRP ECL substrate (Pierce Chemical). The intensities of PP2Ac bands were quantified were appropriate by densitometric analysis using Total Lab TL100 software (Nonlinear Dynamics) under conditions in which the measured intensity on the blots was proportional to the amount of protein loaded onto the gels.
Results
PP2Ac Binds Immobilized PAO in a Specific and DTT-Reversible Manner
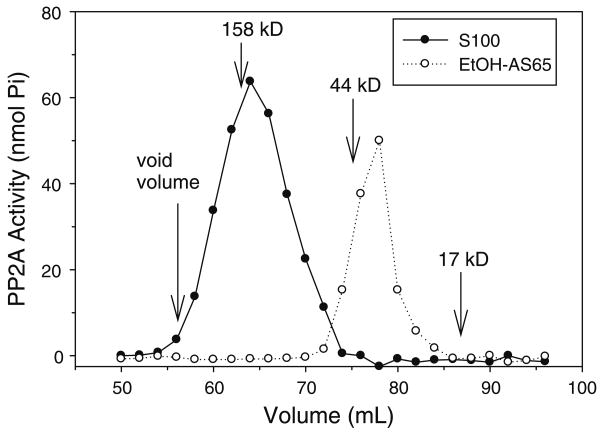
PP2Ac has a molecular weight of about 36 kDa [7]. We have previously shown that PP2Ac from the S100 fraction of rat brain migrates by size exclusion chromatography with an apparent molecular weight of about 155 kDa [16] in agreement with the expected association of PP2Ac with the PP2A regulatory subunits. To examine the binding of PP2Ac to immobilized PAO in the present study, PP2Ac was dissociated from the regulatory subunits by ethanol precipitation [26]. Figure 1 shows the migration on a size exclusion chromatography column of PP2Ac, monitored by measuring the phosphatase activity of the enzyme, in the S100 fraction and the S100 fraction following ethanol precipitation, extraction, and ammonium sulfate precipitation to yield the EtOH-AS65 fraction. As expected, ethanol precipitation promoted a shift in the apparent molecular weight of PP2Ac from about 155 kDa to about 40 kDa consistent with a dissociation of PP2Ac from the regulatory subunits.
Fig. 1.
Ethanol Precipitation Dissociates the Catalytic Subunit from the Regulatory Subunits of PP2A. The effect of ethanol precipitation on the molecular form of PP2A was determined following size exclusion chromatography in Tris–EDTA buffer containing 10 mM β-mercaptoethanol and 100 mM NaCl on a Sephacryl 300HR column of the crude S100 fraction (3.0 mg protein) and S100 fraction following ethanol precipitation, extraction, and ammonium sulfate precipitation to form the EtOH-AS65 fraction (0.15 mg protein). PP2Ac in the fractions was determined by measuring the phosphatase activity toward a phosphothreonine peptide. Phosphatase activity is reported as nmol Pi produced during a 10 min incubation period per 225 μL of the column fractions. γ-Globulin (158 kDa), ovalbumin (44 kDa), and myoglobin (17 kDa) were employed as molecular weight markers
β-mercaptoethanol (10 mM) was included in the experimental buffer during the PP2A subunit dissociation steps to prevent formation of interprotein disulfide bonds that might interfere with the dissociation and recovery of PP2Ac. The β-mercaptoethanol was removed by gel filtration prior to PAO-affinity chromatography since it would be expected to compete at this high concentration with protein/PP2Ac thiols for binding to the PAO.
Following removal of β-mercaptoethanol, the dissociated and partially purified PP2Ac was incubated with immobilized PAO in microspin columns for 2 h at 4°C in the absence and presence of the disulfide reducing agent TCEP. Unbound protein was collected by centrifugation in the flow-through (FT) fraction and microspin columns were subjected to repeated washing to remove any residual unbound protein. Bound protein was removed by centrifugation following resuspension of the immobilized PAO for 10 min at 4°C with buffer containing 25 mM DTT. The presence of PP2Ac in the FT (unbound) and DTT (bound) fractions was determined by western blotting.
As shown in Fig. 2a, PP2Ac bound the immobilized PAO quantitatively and in a DTT-sensitive manner in either the absence or presence of TCEP. PP2Ac from the S100 fraction, which as shown in Fig. 1 is associated with the PP2A regulatory subunits, also bound quantitatively to the immobilized PAO (Fig. 2b). Therefore, access of the immobilized PAO to the PAO-binding sites was not impaired by association of PP2Ac with the regulatory subunits. Importantly, no PP2Ac bound to control columns containing ethanolamine-blocked Affi-Gel 10 that had not been linked to PAO (Fig. 2c).
Fig. 2.
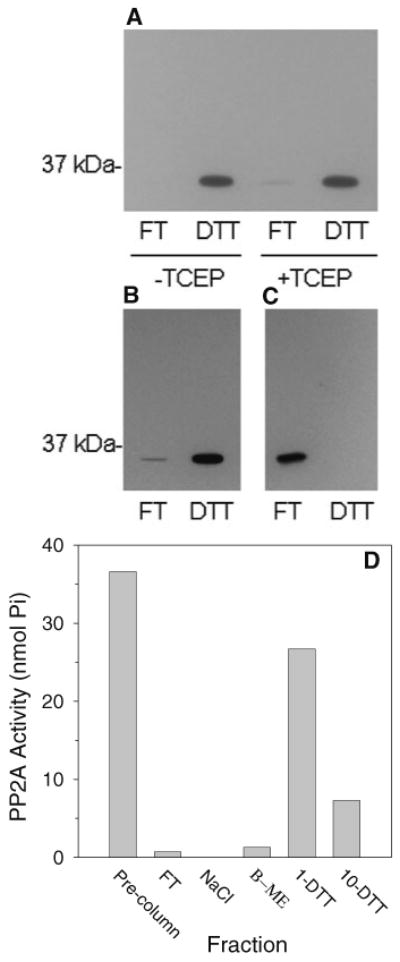
PP2Ac Binds Immobilized PAO in a DTT-Sensitive and Specific Manner. The EtOH-AS65 fraction (a) or S100 fractions (b, c, d) were incubated in microspin columns in the presence and, where specified, the absence of 5 mM TCEP for 2 h at 4°C with immobilized PAO (a, b, d) or with a control polymer that was not coupled to PAO (c). Unbound (flow-through; FT) proteins were collected by centrifugation. Bound proteins were removed by centrifugation following repeated washing to remove residual unbound protein and resuspension of the immobilized PAO in buffer containing 25 mM DTT (a, b, c). In (d), bound protein was eluted in step-wise fashion with 500 mM NaCl, 1 mM β-mercaptoethanol, 1 mM DTT, and 10 mM DTT. The presence of PP2Ac in the PAO-affinity fractions was determined by western blotting (a, b, c) or by measuring the phosphothreonine peptide phosphatase activity (d). Results shown are representative of at least three separate experiments
Figure 2d shows the elution from the immobilized PAO spin columns of PP2A, monitored by measuring PP2A phosphatase activity, as a function of changing the nature and concentration of low-molecular-weight thiol. The results show that, at 1 mM concentrations, DTT was more effective than was β-mercaptoethanol at displacing PP2A from the immobilized PAO in agreement with the higher affinity of dithiols than monothiols for PAO. A 10-fold higher concentration of β-mercaptoethanol was found in related experiments to be sufficient to elute PP2A (result not shown) thus demonstrating the need to remove the β-mercaptoethanol from the free PP2Ac fraction prior to PAO-affinity chromatography. Comparison of the activity in the pre-PAO sample to the sum of the activity in the eluted fractions demonstrates that PP2A activity was completely recovered from the immobilized spin PAO columns. This result is in agreement with our earlier finding that inhibition of PP2A activity by PAO was reversed by DTT [16].
A Substantial Fraction of PP2Ac Forms Disulfide Bonds Readily In Vitro
In order to determine whether or not the PAO-binding thiols of PP2Ac or a subset of these thiols were redox-active, the EtOH-AS65 fraction containing the free PP2Ac was subjected to a 15 min warming period at 37°C following removal of β-mercaptoethanol to allow the oxidation of any redox-active PAO-binding vicinal thiols on PP2Ac that would be expected to have been reduced in the presence of the disulfide reducing agent. Subsequently, reduced protein thiols in this fraction were alkylated with iodoacetamide. Alkylation prevents further oxidation of protein thiols and is essential to prevent the binding of proteins containing only reduced thiols to the immobilized PAO [21]. Following alkylation, proteins containing vicinal thiols that had been reversibly oxidized to disulfide bonds are captured selectively by PAO-affinity chromatography upon reduction of these disulfides by TCEP to regenerate the vicinal thiols. After removal of unbound proteins, the bound proteins (formerly containing disulfide bonds) are displaced by DTT which competes with the protein vicinal thiols for binding to the PAO.
Following removal of unreacted iodoacetamide, the dissociated PP2Ac fraction was incubated with immobilized PAO for 2 h at 4°C in the absence and presence of TCEP. Figure 3a shows that, as expected, all of the PP2Ac eluted in the FT fraction in the absence of TCEP. This lack of PP2Ac in the DTT fraction in the absence of TCEP shows that all of the PAO-binding thiols of PP2Ac were either alkylated or oxidized and unavailable for binding the immobilized PAO. Importantly, however, an average of 51% (with a high of 75%) of the PP2Ac bound to the immobilized PAO in the presence of TCEP (Fig. 3a, c) demonstrating that a substantial fraction of PP2Ac contained PAO-binding thiols that had been reversibly oxidized. A moderate but non-significant decrease in the percent of PP2Ac associated with reversibly oxidized vicinal thiols was obtained when the S100 fraction rather than the EtOH-AS65 fraction was used as a source of PP2Ac (Fig. 3b, c).
Fig. 3.
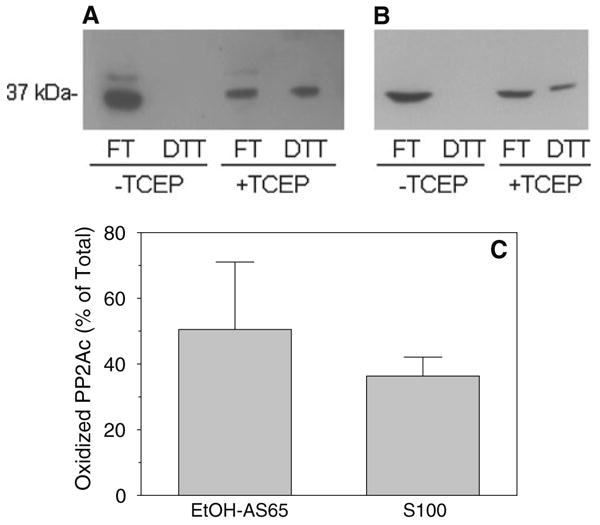
A substantial fraction of PP2Ac forms disulfide bonds readily in vitro. Protein thiols in the EtOH-AS65 fraction (a, c) were alkylated with 25 mM iodoacetamide following removal of β-mercaptoethanol and a subsequent 15 min warming period at 37°C to introduce mild oxidative stress. In related experiments, protein thiols were alkylated also with 25 mM iodoacetamide but immediately following generation of the S100 fraction (b, c). Proteins from these fractions were subjected to PAO-affinity chromatography as described in the legend to Fig. 2 in both the absence and the presence of 5 mM TCEP. The presence of PP2Ac in the FT (reduced) and DTT (oxidized) fractions was determined by western blotting (a, b) and the relative intensities in these fractions was measured by densitometric analysis. The mean ± SD of the percent of PP2Ac in the oxidized form for n = 3–4 is shown in (c)
A Small Fraction of PP2Ac Contains Disulfide Bonds under Conditions Designed to Block Protein Thiol Oxidation In Vitro
The findings reported above that a fraction of PP2Ac contained disulfide bonds in the absence of added oxidants demonstrates a high propensity of one or more pairs of vicinal pairs of PP2Ac towards disulfide bond formation. In the case of the EtOH-AS65 fraction, simply incubating PP2Ac in the experimental buffer following removal of β-mercaptoethanol was sufficient to induce disulfide bond formation. In the case of the S100 fraction, disulfide bonds were present despite the absence of explicit experimental oxidative stress. To determine whether or not PP2Ac may contain disulfides in vivo, very stringent conditions were applied to prevent or to greatly minimize protein thiol oxidation that may occur during brain homogenization and fractionation. Specifically, brains were homogenized in buffer containing dissolved iodoacetamide to initiate alkylation concomitant with homogenization. Furthermore, while 25 mM iodoacetamide was sufficient to block the reduced vicinal thiols of PP2Ac from binding to the PAO (Fig. 3a, b), the iodoacetamide concentration was increased to 500 mM and the pH of the buffer was increased to 8.0 to increase the rate of alkylation [27]. In addition, the buffer was purged with N2 to remove dissolved O2 just prior to homogenization and the concentration of EDTA was increased to 10 mM to ensure the removal of transition metals.
Following application of these restrictions on protein disulfide bond formation, essentially no PP2Ac was detected in the DTT fractions in either the absence or the presence of TCEP by the chemiluminescence western blotting procedure used by us at a standard film exposure of 1 min (Fig. 4a). Importantly, however, a small amount of PP2Ac could be detected on the blots in the presence but not the absence of TCEP when films were (over)exposed for 5 min (Fig. 4b). We estimate that the amount of PP2Ac in the DTT fractions was up to 10% of the total PP2Ac applied to the PAO-affinity column.
Fig. 4.
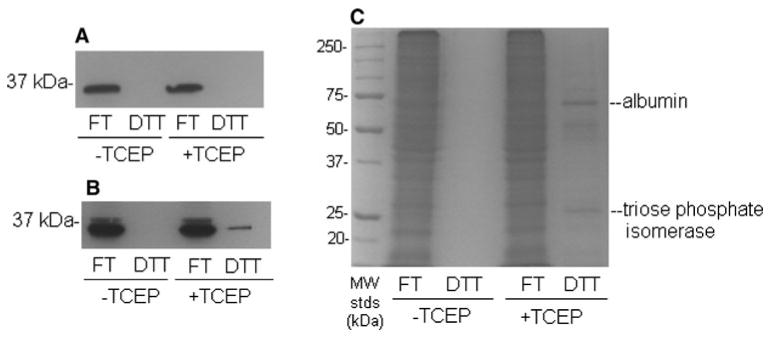
A small fraction of PP2Ac contains disulfide bonds under conditions designed to block protein thiol oxidation in vitro. Alkylation of protein thiols was initiated at the start of homogenization of brains with 500 mM iodoacetamide, in buffer that had been purged with N2 and that contained an elevated (10 mM) concentration of EDTA. Following alkylation, homogenates were centrifuged at 100,000g and proteins were subjected to PAO-affinity chromatography as described in the legend to Fig. 2 in both the absence and the presence of 5 mM TCEP. The presence in the FT (reduced) and DTT (oxidized) fractions of PP2Ac was determined by western blotting following 1 min (a) and 5 min (b) film exposures. Total protein was determined by Coomassie blue staining (c). Results are representative of three separate experiments
To place the findings regarding PP2Ac in some context, it is important to point out that only 3–5% of the total protein from rat brain that had been applied to the PAO-affinity columns was recovered in the DTT fractions in the presence of TCEP (Fig. 4c). We have identified previously the protein components of the enriched bands at 70 and 27 kDa as albumin and triose phosphate isomerase, respectively [21]. The extracellular protein albumin contains 17 disulfide bonds [28] and serves as an important positive control for the PAO-affinity method.
Discussion
The present study examined the interaction of PP2Ac with the vicinal thiol cross-linking reagent PAO using an efficient PAO-affinity method developed recently by us [21]. The major findings reported here are that PP2Ac (1) bound to immobilized PAO quantitatively with or without associated PP2A regulatory subunits and (2) exhibited a high propensity to form apparent intrachain disulfide bonds in vitro.
These results are the first to demonstrate the formation of disulfide bonds in PP2Ac or, indeed, any subunit of PP2A. In addition, the specific and DTT-sensitive binding of PP2Ac to PAO demonstrated here provides an explanation for the inhibition by micromolar PAO of the phosphatase activity of PP2A reported by us previously [17]. That PAO binding inhibits PP2A activity suggests a potential regulatory significance of the PAO binding vicinal thiols of PP2Ac that may go beyond the formation of disulfide bonds. In fact, protein vicinal thiols are believed to contribute to the binding of phosphate ion/phosphoryl groups [19] and to Zn2+ ions [20], both of which may regulate PP2A [29, 30]. Further, the present results together with our earlier report [17] demonstrate clearly that phosphatase interaction with and inhibition by PAO is not unique to the tyrosine phosphatases [31].
The observations in the present study that the binding to immobilized PAO of non-alkylated PP2Ac did not require the presence of the reducing agent TCEP while the binding of alkylated PP2Ac was strictly dependent on TCEP argues strongly for the presence on PP2Ac of two subsets of PAO binding sites, which we will classify here as “oxidation-sensitive” and “oxidation-resistant”. Accordingly, while a fraction of the PAO-binding thiols of PP2Ac are readily oxidized, PP2Ac is able to bind PAO in the absence of TCEP provided that the oxidation-resistant thiols have not been alkylated.
The specific cysteine residues of PP2Ac that comprise the redox-active and redox-resistant vicinal thiol pairs remain to be identified. As vicinal thiols may exist in a protein by virtue of either primary or tertiary structure, they are not easily predicted from sequence alone. Nevertheless, it may be highly significant that the amino acid sequence of PP2Ac [8, 9] reveals only two segments that can be considered sequentially vicinal thiol motifs. These reside at C266-C269 and C50-C55 giving rise to C(X)2C and C(X)4C motifs, respectively, and are the strong candidates for the PAO binding sites on PP2Ac described here. The importance of these cysteine residues for the structure, function, and regulation of PP2A are largely unknown although, significantly, the C266-C269 pair appears to form part of the binding site for PP2A inhibitors [32]. A detailed mass spectrometry-based study of the labeling patterns and effects on catalysis of different classes of thiol alkylating agents [13] revealed that the thiols of C266 and C269 were highly and uniquely reactive but selective modification of these thiols did not affect PP2A activity. Peptide fragments containing the C50-C55 thiol pair were not detected in this study so any effects on catalysis due to modification of these thiols remains unknown.
We addressed the question of the possible formation in PP2Ac of disulfide bonds in vivo by applying very stringent conditions described herein that serve to both promote a high rate of alkylation of protein thiols during brain homogenization and reduce competing thiol oxidation reactions. The restrictions on protein thiol oxidation imparted by these modifications generally meet or exceed the current standards in the field of redox biology for assessment of reversible thiol oxidations in vivo [27]. Upon applying these conditions, we found that a very small fraction of PP2Ac, estimated to be up to 10% of the total PP2Ac, contained disulfide bonds. While we remain cautious in our interpretation, these results suggest that a small fraction of PP2Ac may form intrachain disulfide bonds in vivo. In this context, it is noteworthy that PP2Ac from rabbit skeletal muscle has been found associated with nucleoredoxin [33], a member of the thioredoxin family of oxidoreductases that reduce protein disulfide bonds.
The functional consequences of intrachain disulfide bond formation in PP2Ac cannot be determined at the present time. Our findings that disulfide reducing agents increase the PP2A activity in soluble extracts from rat brain suggest strongly that oxidative inhibition of enzyme catalysis results from disulfide bond formation [17]. However, we cannot at present distinguish the importance for oxidative inhibition of PP2A activity of the intraprotein disulfides shown here from that of interprotein disulfides involving PP2Ac that also appear to form in vitro [17]. Elucidation of the significance of intrachain disulfide bond formation in PP2Ac s for PP2A activity and stability should be facilitated by identification in future studies of the redox-active PAO-binding vicinal thiols of the enzyme.
In summary, the findings reported here demonstrate that PP2Ac contains potential regulatory and redox-active PAO-binding vicinal thiols. PP2Ac vicinal thiols were readily oxidized to form disulfide bonds in vitro and were found to form disulfides, albeit to a much lesser extent, under conditions aimed at preventing protein thiol oxidation during experimental procedures. The suggestion that both oxidation-sensitive and oxidation-resistant vicinal thiol pairs exist on PP2Ac are in keeping with the presence in PP2Ac of two pairs of sequentially-vicinal thiols. The specific cysteine residues comprising the demonstrated PAO binding sites on PP2Ac and possible increases in PP2Ac disulfide bond formation associated with aging and neurodegenerative disease will be examined in future studies.
Acknowledgments
This work was supported in part by NIH Grant AG022357 from the National Institute on Aging.
Abbreviations
- DTT
Dithiothreitol
- EtOH-AS65
The ethanol and ammonium sulfate-precipitated fraction containing dissociated PP2Ac
- PAO
Phenylarsine oxide
- PP2A
Protein phosphatase 2A
- PP2Ac
Protein phosphatase 2A catalytic subunit
- IAM
Iodoacetamide
- S100
The 100,000g supernatant
- TCEP
Tris(2-carboxyethyl)-phosphine
Footnotes
Vicinal is used by us and others in the field to describe thiols that are closely spaced but not necessarily adjacent.
References
- 1.Khew-Goodall Y, Hemmings BA. Tissue-specific expression of mRNAs encoding alpha and beta-catalytic subunits of protein phosphatase 2A. FEBS Lett. 1998;238:265–268. doi: 10.1016/0014-5793(88)80493-9. [DOI] [PubMed] [Google Scholar]
- 2.Millward TA, Zolnierowicz S, Hemmings BA. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem Sci. 1999;24:186–191. doi: 10.1016/s0968-0004(99)01375-4. [DOI] [PubMed] [Google Scholar]
- 3.Chiang CW, Kanies C, Kim KW, et al. Protein phosphatase 2A dephosphorylation of phosphoserine 112 plays the gatekeeper role for BAD-mediated apoptosis. Mol Cell Biol. 2003;23:6350–6362. doi: 10.1128/MCB.23.18.6350-6362.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan SF, Sucher NJ. An NMDA receptor signaling complex with protein phosphatase 2A. J Neurosci. 2001;21:7985–7992. doi: 10.1523/JNEUROSCI.21-20-07985.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sontag E, Nunbhakdi-Craig V, Bloom GS, et al. A novel pool of protein phosphatase 2A is associated with microtubules and is regulated during the cell cycle. J Cell Biol. 1995;128:1131–1144. doi: 10.1083/jcb.128.6.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sontag E, Hladik C, Montgomery L, et al. Downregulation of protein phosphatase 2A carboxyl methylation and methyltransferase may contribute to Alzheimer disease pathogenesis. J Neuropathol Exp Neurol. 2004;63:1080–1091. doi: 10.1093/jnen/63.10.1080. [DOI] [PubMed] [Google Scholar]
- 7.Mumby M. The 3D structure of protein phosphatase 2A: new insights into a ubiquitous regulator of cell signaling. ACS Chem Biol. 2007;2:99–103. doi: 10.1021/cb700021z. [DOI] [PubMed] [Google Scholar]
- 8.de curz e Silva OB, Alemany S, Campbell DG, et al. Isolation and sequence analysis of a cDNA clone encoding the entire catalytic subunit of a type-2A protein phosphatase. FEBS Lett. 1987;221:415–422. doi: 10.1016/0014-5793(87)80966-3. [DOI] [PubMed] [Google Scholar]
- 9.Green DD, Yang SI, Mumby M. Molecular cloning and sequence analysis of the catalytic subunit of bovine type 2A protein phosphatase. Proc Natl Acad Sci. 1987;84:4880–4884. doi: 10.1073/pnas.84.14.4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhuo S, Clemens JC, Stone RL, et al. Mutational analysis of a Ser/Thr phosphatase. Identification of residues important in phosphoesterase substrate binding and catalysis. J Biol Chem. 1994;269:26234–26238. [PubMed] [Google Scholar]
- 11.Nemani R, Lee EY. Reactivity of sulfhydryl groups of the catalytic subunits of rabbit skeletal muscle protein phosphatases 1 and 2A. Arch Biochem Biophys. 1993;300:24–29. doi: 10.1006/abbi.1993.1004. [DOI] [PubMed] [Google Scholar]
- 12.Foley TD, Kintner ME. Brain PP2A is modified by thiol-disulfide exchange and intermolecular disulfide formation. Biochem Biophys Res Commun. 1995;330:1224–1229. doi: 10.1016/j.bbrc.2005.03.108. [DOI] [PubMed] [Google Scholar]
- 13.Codreanu SG, Adams DG, Dawson ES, et al. Inhibition of protein phosphatase 2A activity by selective electrophile alkylation damage. Biochemistry. 2006;45:10020–10029. doi: 10.1021/bi060551n. [DOI] [PubMed] [Google Scholar]
- 14.Whisler RL, Goyette MA, Grants IS, et al. Sublethal levels of oxidant stress stimulate multiple serine/threonine kinases and suppress protein phosphatases in Jurkat T cells. Arch Biochem Biophys. 1995;319:23–35. doi: 10.1006/abbi.1995.1263. [DOI] [PubMed] [Google Scholar]
- 15.Rao RK, Clayton LW. Regulation of protein phosphatase 2A by hydrogen peroxide and glutathionylation. Biochem Biophys Res Commun. 2002;293:610–616. doi: 10.1016/S0006-291X(02)00268-1. [DOI] [PubMed] [Google Scholar]
- 16.Foley TD, Armstrong JJ, Kupchak BR. Identification and H2O2 sensitivity of the major constitutive MAPK phosphatase from rat brain. Biochem Biophys Res Commun. 2004;315:568–574. doi: 10.1016/j.bbrc.2004.01.096. [DOI] [PubMed] [Google Scholar]
- 17.Foley TD, Petro LA, Stredny CM, et al. Oxidative inhibition of brain PP2A activity: role of catalytic subunit disulfides. Neurochem Res. 2007;32:1957–1964. doi: 10.1007/s11064-007-9394-x. [DOI] [PubMed] [Google Scholar]
- 18.Wouters MA, Fan SW, Haworth NL. Disulfides as redox switches: from molecular mechanisms to functional significance. Antioxid Redox Signal. 2010;12:53–91. doi: 10.1089/ars.2009.2510. [DOI] [PubMed] [Google Scholar]
- 19.Rippa M, Bellini T, Signorini M, et al. Evidence for multiple pairs of vicinal thiols in some proteins. J Biol Chem. 1981;256:451–455. [PubMed] [Google Scholar]
- 20.Berg JM. Zinc fingers and other metal-binding domains. Elements for interactions between macromolecules. J Biol Chem. 1990;265:6513–6516. [PubMed] [Google Scholar]
- 21.Foley TD, Stredny CM, Coppa TM, et al. An improved phenylarsine oxide-affinity method identifies triose phosphate isomerase as a candidate redox receptor protein. Neurochem Res. 2010;35:306–314. doi: 10.1007/s11064-009-0056-z. [DOI] [PubMed] [Google Scholar]
- 22.Adams E, Jeter D, Cordes AW, et al. Chemistry of organometalloid complexes with potential antidotes: structure of an organoarsenic(III) dithiolate ring. Inorg Chem. 1990;29:1500–1503. [Google Scholar]
- 23.Hoffman RD, Lane MD. Iodophenylarsine oxide and arsenical affinity chromatography: new probes for dithiol proteins. J Biol Chem. 1992;267:14005–14011. [PubMed] [Google Scholar]
- 24.Getz EB, Xiao M, Chakrabarty T, et al. A comparison between the sulfhydryl reductants Tris(2-carboxyethyl)phosphine and dithiothreitol for use in protein biochemistry. Anal Biochem. 1999;273:73–80. doi: 10.1006/abio.1999.4203. [DOI] [PubMed] [Google Scholar]
- 25.Gitler C, Zarmi B, Kalef E. General method to identify and enrich vicinal thiol proteins present in intact cells in the oxidized, disulfide state. Anal Biochem. 1997;252:48–55. doi: 10.1006/abio.1997.2294. [DOI] [PubMed] [Google Scholar]
- 26.Tran HT, Ferrar TS, Ulke-Lemée A, et al. Purification of PP2Ac from bovine heart. Methods Mol Biol. 2007;365:127–132. doi: 10.1385/1-59745-267-X:127. [DOI] [PubMed] [Google Scholar]
- 27.Hansen RE, Winther JR. An introduction to methods for analyzing thiols and disulfides: Reactions, reagents, and practical considerations. Anal Biochem. 2009;394:147–158. doi: 10.1016/j.ab.2009.07.051. [DOI] [PubMed] [Google Scholar]
- 28.Peters T. All about albumin: biochemistry, genetics, and medical applications. Academic Press; New York: 1996. pp. 9–75. [Google Scholar]
- 29.Erickson AK, Killilea SD. Effects of fructose 2, 6-bisphosphate and glucose 1, 6-bisphosphate on porcine heart protein phosphatase 2A. Biochem Int. 1992;27:353–359. [PubMed] [Google Scholar]
- 30.Ho Y, Samarasinghe R, Knoch ME, et al. Selective inhibition of mitogen-activated protein kinase phosphatases by zinc accounts for extracellular signal-regulated kinase 1/2-dependent oxidative neuronal cell death. Mol Pharmacol. 2008;74:1141–1151. doi: 10.1124/mol.108.049064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Morales P, Minami Y, Luong E, et al. Tyrosine phosphorylation in T cells is regulated by phosphatase activity: studies with phenylarsine oxide. Proc Natl Acad Sci USA. 1990;87:9255–9259. doi: 10.1073/pnas.87.23.9255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xing Y, Xu Y, Chen Y, et al. Structure of protein phosphatase 2A core enzyme bound to tumor-inducing toxins. Cell. 2006;127:341–353. doi: 10.1016/j.cell.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 33.Lechward K, Sugajska E, de Baere I, et al. Interaction of nucleoredoxin with protein phosphatase 2A. FEBS Lett. 2006;580:3631–3637. doi: 10.1016/j.febslet.2006.04.101. [DOI] [PubMed] [Google Scholar]