Stenmark studies how FYVE-finger proteins control cellular membrane dynamics.
Stenmark studies how FYVE-finger proteins control cellular membrane dynamics.
For some time, the mechanisms that drive membrane trafficking events like vesicle fusion in the endocytic pathway were poorly understood. Slowly, a clearer picture has started to emerge, thanks to the work of people like Harald Stenmark (1–5).
Harald Stenmark
PHOTO COURTESY OF ØYSTEIN HORGMO
As a child, Stenmark says he loved taking long treks on cross-country skis, leaving his mark across snowy fields. As an adult, he still loves to ski, but he's leaving most of his marks in a different field: from his lab at the University of Oslo, Norway, Stenmark has blazed new trails, exploring the function of FYVE-finger domain–containing proteins (2,3), which bind to the membrane lipid phosphatidylinositol 3-phosphate (PI3P). His work has helped uncover the essential role of FYVE-finger proteins and PI3P in vesicular fusion (4, 5) and other membrane trafficking events (6). We called him to talk about how he hit his stride in his research.
DIAGONAL STRIDE
Have you lived most of your life in Norway?
Yes, I grew up in a couple of small fishing villages in northern Norway, where the winters are quite long but the skiing is good. My parents were teachers, but my first exposure to science was through an excellent high school teacher named Bjørn Reppen. He was very interested in the natural sciences and mathematics, and he got me interested, too.
“We got these giant endosomes in the cell—it was really striking.”
I wasn't sure what I wanted to study when I reached university, but I was advised that I could find a good career in pharmaceutical research, so I pursued that. At the end of my studies, I heard a seminar by Sjur Olsnes about immunotoxins, which are conjugates of protein toxins and antibodies, and their ability to selectively kill cancer cells. I was intrigued and asked to join his lab at the Norwegian Radium Hospital in Oslo for my PhD.
My first project with Olsnes was to identify the receptor for diphtheria toxin, which at that point was not known. I tried different strategies to identify this receptor, and they were all unsuccessful. I think I worked on this for two years before realizing that it was not going anywhere. Finally, a new postdoc, Stephen McGill, joined the lab. He had expertise in molecular biology, and he taught me how to make plasmid constructs. We made some mutants of diphtheria toxin to try to find out which part of the toxin binds to the receptor and which part is required for its translocation across the membrane. This project was what finally gave me my PhD. I identified the region within the so-called B-fragment of diphtheria toxin that mediates its receptor binding and membrane translocation, and I also showed that a non-toxic mutant of diphtheria toxin can be used to shuttle proteins into cells.
Was your postdoctoral work a large departure from your graduate work?
Not really. I had visited the EMBL at Heidelberg during my PhD and was very impressed with the place. I decided I wanted to do my postdoctoral work there, and a postdoc in Sjur's lab suggested Marino Zerial, who was just setting up his lab there. Marino was working on Rab GTPases, which I thought was quite an interesting subject. Since Rabs are involved in membrane trafficking, it was related to some of the work I had done with diphtheria toxin, and I could learn new skills in molecular biology and cloning there, as well.
When I joined his lab, Marino was excited about functional studies of Rab5, which he had found was targeted to early endosomes. And again, my first project was to identify a receptor: we wanted to find what proteins recruit Rab5 to early endosomes. So again, I tried different approaches to identify a receptor for Rab5, and again I failed. I'm really not good at receptor identification! [laughs] But in parallel, as a kind of side project, I mutated Rab5 to make versions locked in either their GDP- or GTP-bound form to investigate the importance of the GTPase cycle for Rab5 function. And with the GTP-bound form we got these giant endosomes in the cell—it was really striking. That got us thinking that Rab5-GTP might recruit proteins that mediate vesicle fusion, and then of course we went looking for proteins that could bind to Rab5-GTP.
What did you find?
Using a yeast two-hybrid screen, we pulled out a protein that we called Rabaptin5, which we then showed was required for Rab5-dependent homotypic fusion of endosomes in vitro. I must say, I was a little bit naïve at that point—I thought that now that we had identified the effector of Rab5, it would be quite easy to understand how Rab5 works in endosome fusion. Of course, as we know now, that was far from the truth, because I think Marino alone has identified something like 30 Rab5-binding proteins.
TIP-TURN
Harald and his wife Yan hiked off-piste at Svalbard, in Norway, for a nice view.
PHOTO COURTESY OF HARALD STENMARK
Where did that leave you?
Well, then I returned to Oslo for a short-term research associate position at Norwegian Radium Hospital. Toward the end of my time in Marino's lab, I had been working with an immunopathologist, Ban-Hock Toh from Melbourne, to characterize a protein his group had cloned called early endosomal antigen-1 (EEA1). This protein was a more specific marker for early endosomes than even Rab5. I decided to focus my efforts on EEA1 rather than to continue to work on Rab5, in part because I got some good advice from Marino that at this point in my career it would be important to develop my own research interests.
“If [science is] not fun, then it's not worth doing.”
So the first thing we did in my lab was to chop EEA1 into pieces and see what part binds to endosomes, and by this we found that it was the C-terminal part that bound to endosomes. This region contains a zinc-finger motif that is found in many other proteins. We named it a FYVE-finger domain, after the zinc-finger and the initial letters of the first four proteins in which we found this conserved region. It's now been found in more than 30 human proteins.
How does the FYVE-finger domain mediate protein binding to endosomes?
At first we really didn't understand this. We tried to do a screen to find endosomal proteins that bind EEA1, and we didn't succeed. But later, Silvia Corvera's lab showed that EEA1 binding to endosomes was disrupted by the PI3-kinase inhibitor wortmannin, and we realized that we should be looking for a lipid, not a protein. EEA1’s FYVE-finger domain binds to the lipid PI3P, which we later found to be enriched on endosomes. We also realized that the EEA1 FYVE-finger domain alone was not sufficient for binding endosomes; there's another region upstream of the FYVE-finger that's also required… and that turned out to be a Rab5 binding domain! That was a bit ironic, of course, because my idea was to stay away from Rab5-interacting proteins, and the first protein I started studying turned out to bind Rab5.
We've also studied other FYVE-finger proteins. One of these, Hrs, is a protein that helps in sorting of ubiquitinated growth factor receptors to the lysosome.
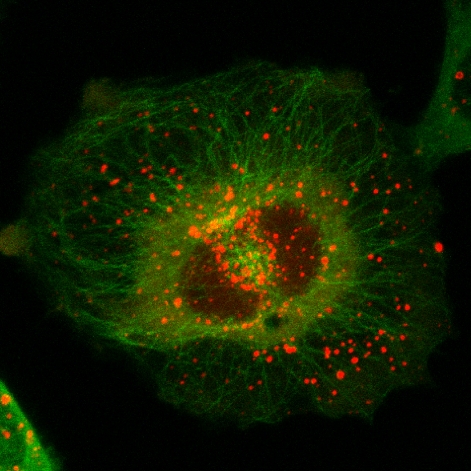
FREE SKATE
PI3P on endosomes is visualized with Stenmark's 2xFYVE construct (red). Microtubules are shown in green.
IMAGE COURTESY OF KAY SCHINK
Are FYVE-finger proteins still a major focus of your lab?
We're still working on characterizing other FYVE-finger proteins. But one thing I am very excited about right now is that we're going in an unexpected direction: cell division. Several years ago we developed a probe for PI3P called 2xFYVE, which is composed of two tandem FYVE-finger domains. When we used it in live cells, in addition to the endosomal staining that we observed in our first studies, we also saw this striking, consistent staining of a structure in between two dividing cells. This turned out to be the mid-body, the stuff that you have between two daughter cells during late cytokinesis.
We then asked if formation of PI3P is important for cell division, and, indeed, when we knocked down subunits of the class III PI3 kinase complex that forms PI3P, we could arrest cells in cytokinesis. So now we're excited to investigate the involvement of membrane trafficking in cell division, not only during cytokinesis but also at earlier stages. That's a field that has not been studied that much so far.
What's your philosophical approach to your work?
My philosophy of science is that it should be fun. If it's not fun, then it's not worth doing, because it's a lot of hard work, and success in science takes persistence. I started out being quite unsuccessful, but I didn't lose my enthusiasm. I feel that it's a little bit of a hobby, not simply an occupation.
References
- 1.Stenmark H., et al. 1995. Cell. 83:423–432. [DOI] [PubMed] [Google Scholar]
- 2.Stenmark H., et al. 1996. J. Biol. Chem. 271:24048–24054. [DOI] [PubMed] [Google Scholar]
- 3.Raiborg C., et al. 2002. Nat. Cell Biol. 4:394–398. [DOI] [PubMed] [Google Scholar]
- 4.Simonsen A., et al. 1998. Nature. 394:494–498. [DOI] [PubMed] [Google Scholar]
- 5.Gillooly D.J., et al. 2000. EMBO J. 19:4577–4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sagona A.P., et al. 2010. Nat. Cell Biol. 12:362–371. [DOI] [PubMed] [Google Scholar]