Abstract
Lineage specific chimerism studies are commonly obtained at several time points after nonmyeloablative haematopoietic cell transplantation to assess the tempo and degree of engraftment, and to monitor graft rejection. For patients who receive myeloablative transplants, the value of frequent chimerism analyses using sensitive molecular techniques is less certain. In this study, a retrospective analysis was performed to assess the transplant outcome of 89 adult patients with acute lymphocytic leukaemia who had chimerism studies of unfractionated bone marrow cells or peripheral blood subsets performed approximately 80 days after transplantation. These patients received unmanipulated, myeloablative transplants using either HLA-identical or HLA-mismatched, related or unrelated donor stem cells. Incomplete donor engraftment was present only in the CD3+ peripheral blood T-cells in a small percentage of patients. There was no correlation of mixed chimerism with transplant outcome. Routine “Day 80” chimerism studies in this group of patients who receive intensive, myeloablative conditioning regimens are not recommended.
Keywords: ALL, myeloablative transplant, chimerism analysis
Introduction
In nonmyeloablative haematopoietic cell transplantation (HCT), chimerism studies are routinely recommended to assess donor engraftment and to detect early signs of graft rejection.1,2 The role of serial chimerism studies, especially leukocyte subset studies, using sensitive molecular markers to monitor recipients of myeloablative HCT is less well defined. A retrospective analysis of adult patients with acute lymphocytic leukaemia (ALL) who received myeloablative transplants at a single centre, using either related or unrelated donors, was performed to determine if chimerism studies done approximately 80 days after transplantation were predictive of transplant outcome.
Materials and Methods
Eighty-nine patients with ALL who were transplanted between January 1998 and December 2004 and had chimerism studies performed two to three months after transplantation were included in this study. Informed consent was obtained from all patients, using forms approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center. Seventy-eight patients had B-cell ALL, 8 had T-cell ALL, and 3 had no phenotyping data. At diagnosis, 30 patients had Philadelphia chromosome positive ALL, 19 had normal cytogenetics, 27 had other clonal cytogenetic abnormalities, and 13 had no cytogenetic analyses available. Median patient age was 33.5 years (range, 18.1–55.9 years). Median duration of disease (time from date of diagnosis to date of transplant) was 8.4 months (range, 2.4–216.3 months). Disease status at the time of transplant was based on marrow morphology: 39 patients were in 1st remission, 29 were in ≥2nd remission and 21 were in relapse. These patient characteristics are summarized in Table 1.
Table 1.
Patient Characteristics
| Parameter | Number1 |
|---|---|
| No. of patients | 89 |
| ALL phenotype | |
| B cell | 78 |
| T cell | 8 |
| Unknown | 3 |
| Cytogenetics | |
| Normal | 19 |
| Ph+2 | 30 |
| Other clonal abnormalities3 | 27 |
| No data | 13 |
| Patient age, yrs | |
| Median (range) | 33.5 (18.1–55.9) |
| Disease duration, mos4 | |
| Median (range) | 8.4 (2.4–216.3) |
| Pretransplant disease status5 | |
| 1st remission | 39 |
| ≥2nd remission | 29 |
| Relapse | 21 |
Number of patients, unless otherwise specified
Ph+ includes patients with a t(9;22) only or t(9;22) plus additional clonal abnormalities
Includes unfavourable risk groups t(4;11), n=4; monosomy 7, n=1; trisomy 8, n=2. There were no patients with t(8;14) or t(1;19).
Hyperdiploidy was present in 9 of these 27 patients.
Time from diagnosis of ALL to date of transplant
Defined by marrow morphology
All patients received a myeloablative conditioning regimen, which included 12.0–13.5 Gy total body irradiation (TBI). The source of stem cells was bone marrow (n=34), G-CSF stimulated peripheral blood (n=54), or umbilical cord blood (n=1). None of the stem cell products were T-cell depleted. Of 34 related donors, 32 were HLA-identical with the recipient and 2 were HLA-mismatched. Of 55 unrelated donors, 37 were HLA-identical with the recipient and 18 were not. HLA typing of patients and related donors included intermediate resolution typing for HLA-A, B, C and DQB1 genes and high-resolution HLA-DRB1 typing. HLA typing for patients undergoing an alternative donor search included high resolution typing for HLA-A, B, C and DRB1, and intermediate resolution typing for HLA-DQB1.3 Of the 19 patients who received adult, HLA-mismatched stem cells the degree of mismatching was as follows: a single class I or class II antigen (n=12), a single class II allele (n=4) and a single class I plus a single class II allele (n=3). The only cord blood recipient was mismatched with the donor for both HLA-A antigens based on HLA-A, B, and DRB1 typing. Fifty-two of the 89 recipients had donors of the same sex and 37 had opposite-sex donors. Eighty-one of the 89 patients received cyclosporine and methotrexate for graft-vs.-host disease (GVHD) prophylaxis.4 Details of the conditioning regimens and GVHD prophylaxis are summarized in Table 2.
Table 2.
Transplant regimens and outcome data
| Parameter | Number1 |
|---|---|
| Preparative regimen | |
| CY, 12.0 Gy TBI | 51 |
| CY, 13.2 Gy TBI | 33 |
| VP-16, 12–13.2 Gy TBI | 4 |
| Other | 1 |
| Donor/recipient HLA compatibility | |
| Related/matched | 32 |
| Related/mismatched | 2 |
| Unrelated/matched | 37 |
| Unrelated/mismatched | 18 |
| Donor/recipient gender | |
| M/M | 34 |
| M/F | 15 |
| F/F | 18 |
| F/M | 22 |
| Stem cell source | |
| Bone marrow | 34 |
| Peripheral blood | 54 |
| Umbilical cord blood | 1 |
| GVHD prophylaxis | |
| CSP + MTX | 81 |
| CSP + MMF | 3 |
| Sirolimus + tacrolimus + MTX | 2 |
| Tacrolimus, MMF | 2 |
| Other | 1 |
| Acute GVHD | |
| Grades 0 – I | 12 |
| Grades II – IV | 77 |
| Day onset acute GVHD (grades I–IV) | |
| Median (range) | 19 (6–97) |
| Chronic extensive GVHD | 58 |
| Day onset chronic GVHD | |
| Median (range) | 139 (85–874) |
| Graft rejection | 0 |
| Day of relapse (n=41) | |
| Median (range) | 231 (75–1397) |
| Survival | |
| Patients alive | 42 |
| Patients dead | 47 |
| Causes of death | |
| Relapse | 35 |
| Infection | 2 |
| DAD/ARDS | 1 |
| Chronic GVHD +/− infection | 7 |
| Acute GVHD +/− infection | 1 |
| Other | 1 |
Number of patients unless otherwise specified
Abbreviations: CY = cyclophosphamide 60 mg/kg once daily i.v. for 2 consecutive days (total dose 120 mg/kg); TBI = total body irradiation; VP-16 = etoposide; GVHD = graft-vs.-host disease; CSP = cyclosporine; MTX = methotrexate; MMF = mycophenolate mofetil; DAD = diffuse alveolar damage; ARDS = acute respiratory distress syndrome
Donor engraftment was initially assessed, per our standard practice, approximately 28 days after transplantation. Morphologic, conventional and molecular cytogenetic, and flow cytometric studies were performed on unfractionated bone marrow specimens at this time point.
At a median of 77 days (range, 65 – 113 days) after transplantation, the “Day 28” studies were repeated and chimerism analysis was also performed. Unfractionated bone marrow cells (BM) or peripheral blood (PB) sorted for CD3+ T-cells and CD33+ neutrophils were studied at this later time point. Patients who had sex-mismatched donors had chimerism studies done by conventional cytogenetic analysis of metaphase chromosomes or by molecular cytogenetic techniques, i.e., fluorescence in situ hybridization (FISH) using X and Y chromosome-specific probes.5 Patients who had donors of the same sex had chimerism studies done by amplification and analysis of variable number tandem repeat (VNTR) or short tandem repeat (STR) polymorphisms by DNA amplification.6 VNTR studies, performed routinely between 1998 and April, 2004, involved amplification of individual VNTR loci with analysis on precast polyacrylamide gels with silver staining.7 As of May 2004, same sex transplant pairs had chimerism studies performed by multiplex amplification of STR loci (PowerPlex 16, Promega, Madison, WI) with analysis by capillary electrophoresis (ABI 3130xl, Applied Biosystems, Foster City, CA). Sensitivity by both methods achieved at least 5% sensitivity as documented by external proficiency challenges.7,8 Depending on the relative size of the informative VNTR markers, sensitivity was as low as 0.1%, as assessed by internal mixing studies. Sensitivity of STR analysis routinely achieved 0.5% sensitivity as assessed by mixing studies.
Disease-free survival probabilities were estimated using the Kaplan-Meier method.9
Results
The incidence of acute and chronic GVHD, survival, relapse, and causes of death are summarized in Table 2.
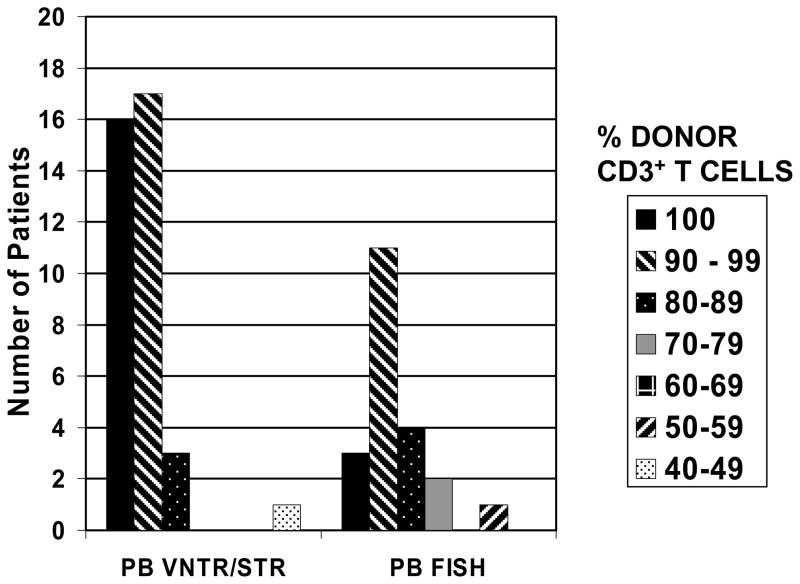
Forty-seven patients had chimerism studies performed on unfractionated BM and 58 patients had studies of fractionated PB samples. (Twenty of the 89 patients had both BM and PB studies.) Only two of the 47 patients who had chimerism studies of BM had <90% donor-derived cells (82.6% and 45%, respectively) at the time of analysis. The patient with the lowest percent donor chimerism was known to be in relapse at the time the chimerism test was done. Both of these patients died <4 months after transplant from relapsed ALL. None of the 58 patients who had PB chimerism studies had <90% donor cells in the CD33+ neutrophil fraction. There was a wider range in donor CD3+ T-cell percentages as shown in Figure 1. Eleven of the 58 patients had <90% donor CD3+ T-cells, but none had <20% donor CD3+ T-cells, a level previously reported to be associated with graft loss.1 None of the patients in this analysis experienced subsequent graft rejection. The actuarial probability of disease-free survival was not significantly different between the group of patients with <90% donor CD3+ T-cells and those with ≥90% donor CD3+ T-cells (62% vs. 48% at three years, respectively, p = 0.49). Similarly, there was no difference in the probability of relapse at three years between these two groups (38% vs. 40%, respectively).
Figure 1.
Variation in percentage of donor-derived CD3+ peripheral blood T-cells, based on VNTR or STR polymorphisms, or sex chromosome analysis by FISH.
Of the 58 patients who had PB chimerism studies performed, the percentage of donor CD3+ T-cells did not correlate with the severity of acute GVHD, but only six of the 58 patients developed mild (grade I) acute GVHD. Similarly, stem cell source (BM versus PB), donor type (related versus unrelated), HLA-matching, and relapse after transplantation did not correlate with the degree of mixed chimerism in PB at the time of evaluation. These observations, however, are based on a small number of patients in each subgroup who had mixed chimerism when tested.
During the time period covered in this analysis, 23 additional adult ALL patients received myeloablative transplants, but did not have chimerism studies performed. Twenty-one patients died early after transplantation (median day of death = day 29, range = 5–66 days). One patient had persistent disease after a syngeneic transplant and died on day 204 after receiving a second transplant from an unrelated donor. Two of the 23 patients relapsed, on days 19 and 47, with the relapse diagnosed by morphology of the peripheral smear or marrow, respectively. None of the 22 patients had evidence of graft failure prior to death. Only one of these 23 patients survived at least 80 days; this patient is alive in remission >7 years after HCT.
Discussion
In patients undergoing nonmyeloablative or reduced-intensity HCT, chimerism analyses provide useful information regarding graft rejection and minimal residual disease.2,8 Serial, quantitative chimerism studies have been recommended for children with ALL after allogeneic HCT to identify those patients at high risk of relapse.10 In general, analysis of PB cells is more useful than BM cells because lineage-specific chimerism can be determined.1 Before the advent of FISH and PCR-based studies, the degree of donor engraftment was assessed by conventional karyotype analyses for sex-mismatched donor-recipient pairs, red blood cell phenotyping, or immunoglobulin isotypes.11 All of these techniques were associated with low sensitivity. FISH analysis of sex chromosomes is more quantitative than conventional cytogenetics, while PCR-based methodology (with VNTR or STR analyses) is not only a more sensitive technique, but also permits analysis of very small numbers of cells. The sensitivity of VNTR and STR analyses by PCR methods is estimated at 1–5 %, but may be as low as 0.1% depending on the informative markers and the strength of amplification.6,7 The laboratory fees at this Center for one chimerism study using FISH or PCR-based technology range from $600 (without cell subset isolation) to $850 (with cell subset isolation by flow cytometry).
The patient population in this retrospective analysis was fairly homogeneous, with all patients having a diagnosis of ALL, being ≥18 years of age and receiving myeloablative, TBI-based conditioning regimens followed by infusion of allogeneic, unmanipulated stem cells. GVHD prophylaxis was standardized with 91% of patients receiving cyclosporine and methotrexate. Twenty-four percent of patients were in relapse at the time of transplantation, a factor which previously has been shown to be associated with a significantly increased risk of post-transplant relapse.12 Two to three months after transplant a low frequency of incomplete donor chimerism was found. In addition, lineage-specific (CD3+ and CD33+) peripheral blood chimerism studies were not predictive of transplant outcome, leading to the recommendation that these studies not be routinely obtained in this patient population. This conclusion is in agreement with the recommendations for assessment of chimerism following myeloablative HCT for haematologic malignancies by the IBMTR/ABMTR.1
In contrast, frequent PB chimerism studies have been recommended for children with ALL undergoing allogeneic transplantation to detect increasing mixed chimerism, allowing for early intervention with immunomodulatory therapy.10 The patient group in this study was more heterogeneous, with results grouped from several transplant centres. Fifteen percent (25/163) of patients received chemotherapy-only preparative regimens and 41% (67/163) received T-cell depleted grafts. Only one of the 163 patients was in relapse at the time of transplant.
The disparate conclusions about the role and timing of chimerism studies in the pediatric ALL study compared to our study illustrate the importance of defining the specific patient populations for whom chimerism studies are useful in monitoring transplant outcome. The value of chimerism studies for other subgroups of patients, including those who undergo myeloablative HCT using chemotherapy-only preparative regimens, patients who receive T-cell depleted grafts, and patients who have greater degrees of HLA disparity with their donors, e.g., haplo-identical transplant recipients, remains to be determined.
References
- 1.Antin JH, Childs R, Filipovich AH, Giralt S, Mackinnon S, Spitzer T, et al. Establishment of complete and mixed donor chimerism after allogeneic lymphohematopoietic transplantation: recommendations from a workshop at the 2001 Tandem Meetings (Review) Biol Blood Marrow Transplant. 2001;7:473–485. doi: 10.1053/bbmt.2001.v7.pm11669214. [DOI] [PubMed] [Google Scholar]
- 2.Baron F, Sandmaier BM. Chimerism and outcomes after allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning (Review) Leukemia. 2006;20:1690–1700. doi: 10.1038/sj.leu.2404335. [DOI] [PubMed] [Google Scholar]
- 3.Petersdorf EW, Gooley TA, Anasetti C, Martin PJ, Smith AG, Mickelson EM, et al. Optimizing outcome after unrelated marrow transplantation by comprehensive matching of HLA class I and II alleles in the donor and recipient. Blood. 1998;92:3515–3520. [PubMed] [Google Scholar]
- 4.Storb R, Deeg HJ, Pepe M, Appelbaum FR, Anasetti C, Beatty P, et al. Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft-versus-host disease in patients given HLA-identical marrow grafts for leukemia: Long-term follow-up of a controlled trial. Blood. 1989;73:1729–1734. [PubMed] [Google Scholar]
- 5.Dewald GW, Schad CR, Christensen ER, Law ME, Zinsmeister AR, Stalboerger PG, et al. Fluorescence in situ hybridization with X and Y chromosome probes for cytogenetic studies on bone marrow cells after opposite sex transplantation. Bone Marrow Transplant. 1993;12:149–154. [PubMed] [Google Scholar]
- 6.Scharf SJ, Smith AG, Hansen JA, McFarland C, Erlich HA. Quantitative determination of bone marrow transplant engraftment using fluorescent polymerase chain reaction primers for human identity markers. Blood. 1995;85:1954–1963. [PubMed] [Google Scholar]
- 7.Smith AG, Martin PJ. Analysis of amplified variable number tandem repeat loci for evaluation of engraftment after hematopoietic stem cell transplantation. Rev Immunogenet. 1999;1:255–264. [PubMed] [Google Scholar]
- 8.Thiede C, Bornhauser M, Oelschlagel U, Brendel C, Leo R, Daxberger H, et al. Sequential monitoring of chimerism and detection of minimal residual disease after allogeneic blood stem cell transplantation (BSCT) using multiplex PCR amplification of short tandem repeat-markers. Leukemia. 2001;15:293–302. doi: 10.1038/sj.leu.2401953. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 10.Bader P, Kreyenberg H, Hoelle W, Dueckers G, Handgretinger R, Lang P, et al. Increasing mixed chimerism is an important prognostic factor for unfavorable outcome in children with acute lymphoblastic leukemia after allogeneic stem-cell transplantation: possible role for pre-emptive immunotherapy? J Clin Oncol. 2004;22:1696–1705. doi: 10.1200/JCO.2004.05.198. [DOI] [PubMed] [Google Scholar]
- 11.Bryant E, Martin PJ. Documentation of engraftment and characterization of chimerism following hematopoietic cell transplantation. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas’ Hematopoietic Cell Transplantation. Blackwell Publishing Ltd; Oxford, UK: 2004. pp. 234–243. [Google Scholar]
- 12.Doney K, Hägglund H, Leisenring W, Chauncey T, Appelbaum FR, Storb R. Predictive factors for outcome of allogeneic hematopoietic cell transplantation for adult acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2003;9:472–481. doi: 10.1016/s1083-8791(03)00149-6. [DOI] [PubMed] [Google Scholar]