Abstract
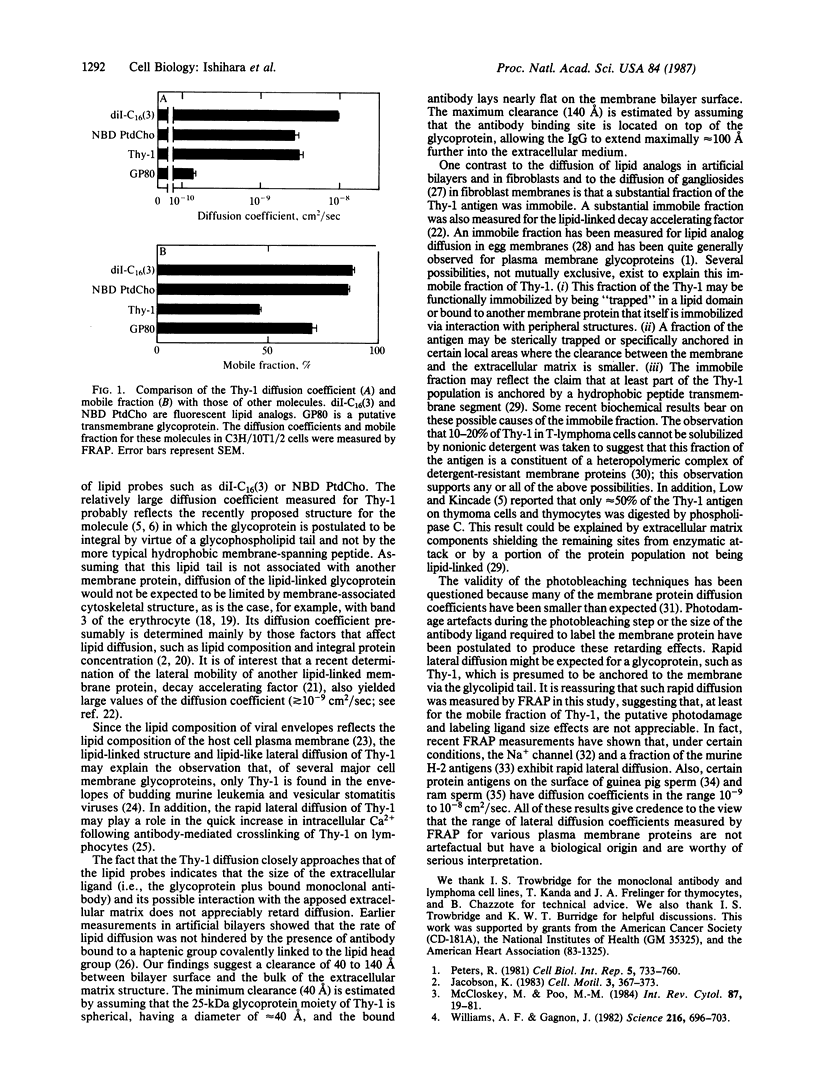
Thy-1 is a plasma membrane protein, but its primary structure lacks the typical membrane-spanning sequence. Recent studies revealed that a glycophospholipid is covalently bound to the carboxyl terminus, suggesting that the protein is integrated into the plasma membrane by this lipid moiety. Lateral diffusion of Thy-1 was measured in mouse thymocytes, lymphoma cells, and fibroblasts by the fluorescence recovery after photobleaching technique. Thy-1 was labeled with rhodamine-conjugated anti-Thy-1 monoclonal antibodies. Diffusion coefficients of 2-4 X 10(-9) cm2/sec were obtained for the antigen-antibody complex in all the cell types. About 50% of the Thy-1 was mobile. The diffusion coefficient for the mobile fraction of Thy-1 is considerably larger than the diffusion coefficients of many other plasma membrane proteins. Rather, the diffusion coefficient of Thy-1 is similar to those of lipid analogs embedded in the same membrane, providing strong support for the suggested lipid anchoring of this antigen.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat J., Janssen H., Démant P., Hilgers J., Závada J. Specific selection of host cell glycoproteins during assembly of murine leukaemia virus and vesicular stomatitis virus: presence of Thy-1 glycoprotein and absence of H-2, Pgp-1 and T-200 glycoproteins on the envelopes of these virus particles. J Gen Virol. 1983 Jun;64(Pt 6):1241–1253. doi: 10.1099/0022-1317-64-6-1241. [DOI] [PubMed] [Google Scholar]
- Davitz M. A., Low M. G., Nussenzweig V. Release of decay-accelerating factor (DAF) from the cell membrane by phosphatidylinositol-specific phospholipase C (PIPLC). Selective modification of a complement regulatory protein. J Exp Med. 1986 May 1;163(5):1150–1161. doi: 10.1084/jem.163.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derzko Z., Jacobson K. Comparative lateral diffusion of fluorescent lipid analogues in phospholipid multibilayers. Biochemistry. 1980 Dec 23;19(26):6050–6057. doi: 10.1021/bi00567a016. [DOI] [PubMed] [Google Scholar]
- Dragsten P., Henkart P., Blumenthal R., Weinstein J., Schlessinger J. Lateral diffusion of surface immunoglobulin, Thy-1 antigen, and a lipid probe in lymphocyte plasma membranes. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5163–5167. doi: 10.1073/pnas.76.10.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin M., Wei T. Lateral diffusion of H-2 antigens on mouse fibroblasts. J Cell Biol. 1982 Nov;95(2 Pt 1):458–462. doi: 10.1083/jcb.95.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge C. A., Elson E. L., Webb W. W. Fluorescence photobleaching recovery measurements of surface lateral mobilities on normal and SV40-transformed mouse fibroblasts. Biochemistry. 1980 May 13;19(10):2075–2079. doi: 10.1021/bi00551a011. [DOI] [PubMed] [Google Scholar]
- Golan D. E., Veatch W. Lateral mobility of band 3 in the human erythrocyte membrane studied by fluorescence photobleaching recovery: evidence for control by cytoskeletal interactions. Proc Natl Acad Sci U S A. 1980 May;77(5):2537–2541. doi: 10.1073/pnas.77.5.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henis Y. I., Elson E. L. Inhibition of the mobility of mouse lymphocyte surface immunoglobulins by locally bound concanavalin A. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1072–1076. doi: 10.1073/pnas.78.2.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoessli D., Rungger-Brändle E. Association of specific cell-surface glycoproteins with a triton X-100-resistant complex of plasma membrane proteins isolated from T-lymphoma cells (P1798). Exp Cell Res. 1985 Jan;156(1):239–250. doi: 10.1016/0014-4827(85)90278-2. [DOI] [PubMed] [Google Scholar]
- Hughes E. N., August J. T. Characterization of plasma membrane proteins identified by monoclonal antibodies. J Biol Chem. 1981 Jan 25;256(2):664–671. [PubMed] [Google Scholar]
- Jacobson K. Lateral diffusion in membranes. Cell Motil. 1983;3(5-6):367–373. doi: 10.1002/cm.970030504. [DOI] [PubMed] [Google Scholar]
- Jacobson K., O'Dell D., August J. T. Lateral diffusion of an 80,000-dalton glycoprotein in the plasma membrane of murine fibroblasts: relationships to cell structure and function. J Cell Biol. 1984 Nov;99(5):1624–1633. doi: 10.1083/jcb.99.5.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroczek R. A., Gunter K. C., Germain R. N., Shevach E. M. Thy-1 functions as a signal transduction molecule in T lymphocytes and transfected B lymphocytes. Nature. 1986 Jul 10;322(6075):181–184. doi: 10.1038/322181a0. [DOI] [PubMed] [Google Scholar]
- Low M. G., Kincade P. W. Phosphatidylinositol is the membrane-anchoring domain of the Thy-1 glycoprotein. Nature. 1985 Nov 7;318(6041):62–64. doi: 10.1038/318062a0. [DOI] [PubMed] [Google Scholar]
- Mason D. W., Williams A. F. The kinetics of antibody binding to membrane antigens in solution and at the cell surface. Biochem J. 1980 Apr 1;187(1):1–20. doi: 10.1042/bj1870001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey M., Poo M. M. Protein diffusion in cell membranes: some biological implications. Int Rev Cytol. 1984;87:19–81. doi: 10.1016/s0074-7696(08)62439-0. [DOI] [PubMed] [Google Scholar]
- Myles D. G., Primakoff P., Koppel D. E. A localized surface protein of guinea pig sperm exhibits free diffusion in its domain. J Cell Biol. 1984 May;98(5):1905–1909. doi: 10.1083/jcb.98.5.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzer E. J., Wagner R. R., Dubovi E. J. Viral membranes: model systems for studying biological membranes. CRC Crit Rev Biochem. 1979;6(2):165–217. doi: 10.3109/10409237909102563. [DOI] [PubMed] [Google Scholar]
- Peacock J. S., Barisas B. G. Photobleaching recovery studies of antigen-specific mouse lymphocyte stimulation by DNP-conjugated polymerized flagellin. J Immunol. 1981 Sep;127(3):900–906. [PubMed] [Google Scholar]
- Peters R. Translational diffusion in the plasma membrane of single cells as studied by fluorescence microphotolysis. Cell Biol Int Rep. 1981 Aug;5(8):733–760. doi: 10.1016/0309-1651(81)90231-9. [DOI] [PubMed] [Google Scholar]
- Seki T., Chang H. C., Moriuchi T., Denome R., Ploegh H., Silver J. A hydrophobic transmembrane segment at the carboxyl terminus of thy-1. Science. 1985 Feb 8;227(4687):649–651. doi: 10.1126/science.2857501. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Schindler M., Koppel D. E. Lateral mobility of integral membrane proteins is increased in spherocytic erythrocytes. Nature. 1980 Jun 12;285(5765):510–511. doi: 10.1038/285510a0. [DOI] [PubMed] [Google Scholar]
- Smith L. M., Parce J. W., Smith B. A., McConnell H. M. Antibodies bound to lipid haptens in model membranes diffuse as rapidly as the lipids themselves. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4177–4179. doi: 10.1073/pnas.76.9.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck D. K., Pagano R. E. Insertion of fluorescent phospholipids into the plasma membrane of a mammalian cell. J Biol Chem. 1980 Jun 10;255(11):5404–5410. [PubMed] [Google Scholar]
- Tse A. G., Barclay A. N., Watts A., Williams A. F. A glycophospholipid tail at the carboxyl terminus of the Thy-1 glycoprotein of neurons and thymocytes. Science. 1985 Nov 29;230(4729):1003–1008. doi: 10.1126/science.2865810. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Gagnon J. Neuronal cell Thy-1 glycoprotein: homology with immunoglobulin. Science. 1982 May 14;216(4547):696–703. doi: 10.1126/science.6177036. [DOI] [PubMed] [Google Scholar]
- Woda B. A., Gilman S. C. Lateral mobility and capping of rat lymphocyte membrane proteins. Cell Biol Int Rep. 1983 Mar;7(3):203–209. doi: 10.1016/0309-1651(83)90227-8. [DOI] [PubMed] [Google Scholar]
- Wolf D. E., Hagopian S. S., Lewis R. G., Voglmayr J. K., Fairbanks G. Lateral regionalization and diffusion of a maturation-dependent antigen in the ram sperm plasma membrane. J Cell Biol. 1986 May;102(5):1826–1831. doi: 10.1083/jcb.102.5.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D. E., Kinsey W., Lennarz W., Edidin M. Changes in the organization of the sea urchin egg plasma membrane upon fertilization: indications from the lateral diffusion rates of lipid-soluble fluorescent dyes. Dev Biol. 1981 Jan 15;81(1):133–138. doi: 10.1016/0012-1606(81)90355-9. [DOI] [PubMed] [Google Scholar]



