Abstract
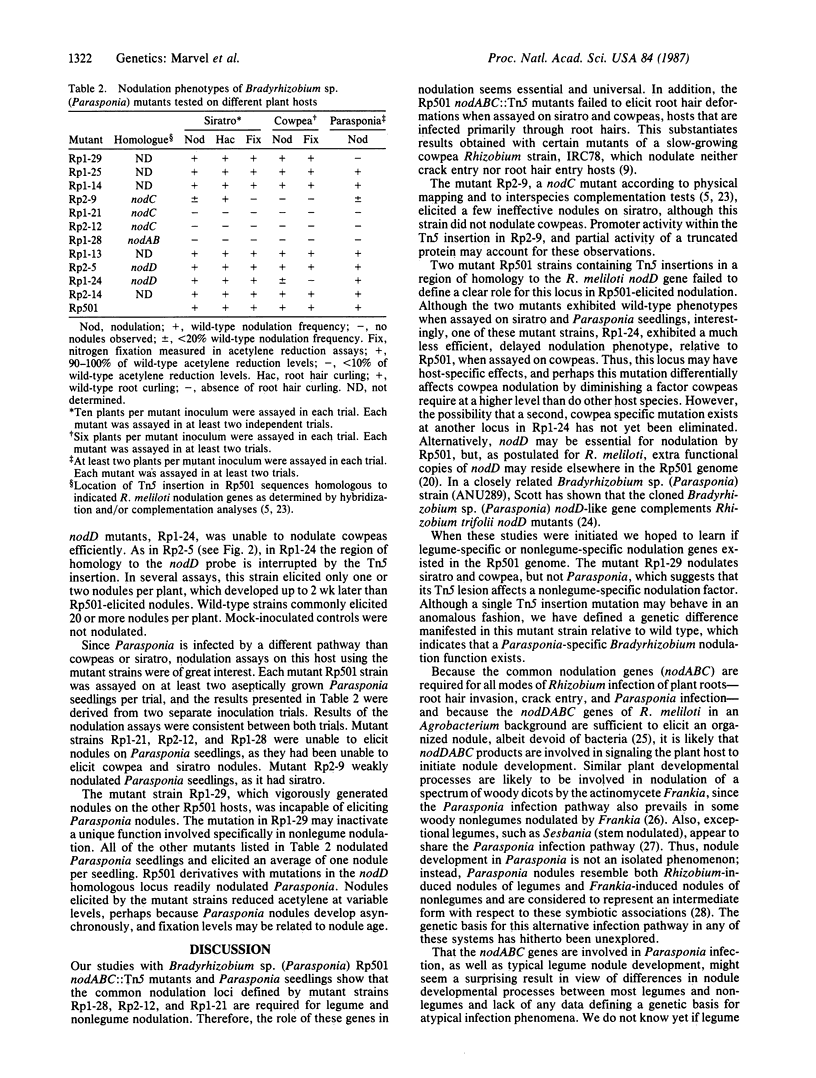
Parasponia, a woody member of the elm family, is the only nonlegume genus whose members are known to form an effective nitrogen-fixing symbiosis with Bradyrhizobium or Rhizobium species. The Bradyrhizobium strain Rp501, isolated from Parasponia nodules, also nodulates the legumes siratro (Macroptilium atropurpureum) and cowpea (Vigna unguiculata). To test whether some of the same genes are involved in the early stages of legume and nonlegume nodulation, we generated transposon Tn5 insertions in the region of three evolutionarily conserved genes (nodA, nodB, and nodC) required for legume nodulation in several Rhizobium and Bradyrhizobium species. Assays of these mutant Rp501 strains on legume hosts and Parasponia seedlings established that nodABC are required for nodulation of legume and nonlegume hosts, indicating that nonlegumes and legumes can respond to the same bacterial signal(s). In addition, a strain carrying a Tn5 insertion adjacent to the nodABC genes vigorously nodulated Rp501 legume hosts but was incapable of nodulating Parasponia, possibly identifying a nonlegume-specific nodulation function.
Keywords: Parasponia Rhizobium, symbiosis, gene replacement
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buikema W. J., Long S. R., Brown S. E., van den Bos R. C., Earl C., Ausubel F. M. Physical and genetic characterization of Rhizobium meliloti symbiotic mutants. J Mol Appl Genet. 1983;2(3):249–260. [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelhoff T. T., Fisher R. F., Jacobs T. W., Mulligan J. T., Long S. R. Nucleotide sequence of Rhizobium meliloti 1021 nodulation genes: nodD is read divergently from nodABC. DNA. 1985 Jun;4(3):241–248. doi: 10.1089/dna.1985.4.241. [DOI] [PubMed] [Google Scholar]
- Hirsch A. M., Drake D., Jacobs T. W., Long S. R. Nodules are induced on alfalfa roots by Agrobacterium tumefaciens and Rhizobium trifolii containing small segments of the Rhizobium meliloti nodulation region. J Bacteriol. 1985 Jan;161(1):223–230. doi: 10.1128/jb.161.1.223-230.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvel D. J., Kuldau G., Hirsch A., Richards E., Torrey J. G., Ausubel F. M. Conservation of nodulation genes between Rhizobium meliloti and a slow-growing Rhizobium strain that nodulates a nonlegume host. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5841–5845. doi: 10.1073/pnas.82.17.5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade H. M., Long S. R., Ruvkun G. B., Brown S. E., Ausubel F. M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982 Jan;149(1):114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan J. T., Long S. R. Induction of Rhizobium meliloti nodC expression by plant exudate requires nodD. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6609–6613. doi: 10.1073/pnas.82.19.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noti J. D., Dudas B., Szalay A. A. Isolation and characterization of nodulation genes from Bradyrhizobium sp. (Vigna) strain IRc 78. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7379–7383. doi: 10.1073/pnas.82.21.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters N. K., Frost J. W., Long S. R. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science. 1986 Aug 29;233(4767):977–980. doi: 10.1126/science.3738520. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Sundaresan V., Ausubel F. M. Directed transposon Tn5 mutagenesis and complementation analysis of Rhizobium meliloti symbiotic nitrogen fixation genes. Cell. 1982 Jun;29(2):551–559. doi: 10.1016/0092-8674(82)90171-4. [DOI] [PubMed] [Google Scholar]
- Scott K. F. Conserved nodulation genes from the non-legume symbiont Bradyrhizobium sp. (Parasponia). Nucleic Acids Res. 1986 Apr 11;14(7):2905–2919. doi: 10.1093/nar/14.7.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien H. C., Dreyfus B. L., Schmidt E. L. Initial stages in the morphogenesis of nitrogen-fixing stem nodules of Sesbania rostrata. J Bacteriol. 1983 Nov;156(2):888–897. doi: 10.1128/jb.156.2.888-897.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]