Abstract
There is an expanding field of research investigating the benefits of alternatives to current pharmacological therapies in psychiatry. N-acetylcysteine (NAC) is emerging as a useful agent in the treatment of psychiatric disorders. Like many therapies, the clinical origins of NAC are far removed from its current use in psychiatry. Whereas the mechanisms of NAC are only beginning to be understood, it is likely that NAC is exerting benefits beyond being a precursor to the antioxidant, glutathione, modulating glutamatergic, neurotropic and inflammatory pathways. This review outlines the current literature regarding the use of NAC in disorders including addiction, compulsive and grooming disorders, schizophrenia and bipolar disorder. N-acetylcysteine has shown promising results in populations with these disorders, including those in whom treatment efficacy has previously been limited. The therapeutic potential of this acetylated amino acid is beginning to emerge in the field of psychiatric research.
Historical use of N-acetylcysteine
N-acetylcysteine (NAC) has been used as an antioxidant precursor to glutathione (γ-glutamylcysteinylglycine; GSH) in the treatment of paracetamol overdose for more than 30 years.1 As more is understood about the actions of NAC, the clinical applications have also broadened. N-acetylcysteine is now widely used as a mucolytic and in the treatment of HIV, and it has reported efficacy in chronic obstructive pulmonary disease and contrast-induced nephropathy.2 Specific to brain disorders, NAC has been trialled with some efficacy in patients with Alzheimer disease.3 The present review will explore the role of NAC in the treatment of psychiatric conditions and the possible mechanisms of benefit for these disorders.
Role in oxidative homeostasis
The use of NAC in restoring GSH levels is well established (Fig. 1). Glutathione is the primary endogenous antioxidant. Glutathione neutralizes reactive oxygen and nitrogen species from the cell through both direct and indirect scavenging. As the most abundant and ubiquitous antioxidant, it is responsible for maintaining the oxidative balance in the cell. This occurs through both direct removal of reactive species through the formation and breakdown of adducts and is also catalyzed by glutathione peroxidase (GPx) in a nicotinamide adenine dinucleotide phosphate (NADPH)–dependent reaction. The resulting oxidized glutathione is then reduced by glutathione reductase to begin the cycle again.4 Glial cells contain much higher levels of GSH than neuronal cells and support neuronal GSH production. Astrocytes release GSH into the extracellular space and γ-glutamyltranspeptidase breaks down GSH to a cysteine–glycine dipeptide and glutamate. The dipeptide is hydrolyzed to glycine and cysteine, and all 3 amino acids are then available for neuronal GSH synthesis. Neuronal GSH production is believed to be primarily mediated by astrocytic GSH release, and astrocytic GSH production is rate-limited by cysteine and the enzyme glutamate–cysteine ligase.4,5
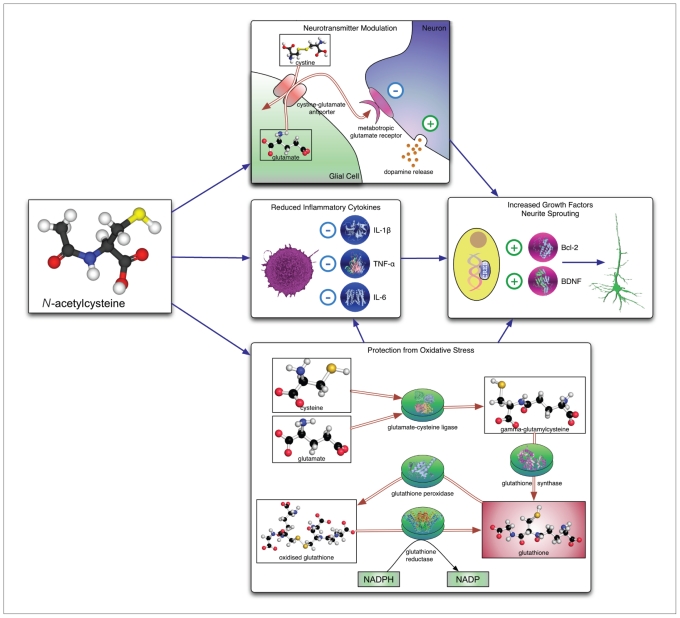
Fig. 1.
Mechanisms of action of N-acetylcysteine (NAC). Top to bottom: increased activity of cystine–glutamate antiporter results in increased activation of metabotropic glutamate receptors on inhibitory neurons and facilitates vesicular dopamine release; NAC is associated with reduced levels of inflammatory cytokines and acts as a substrate for glutathione synthesis. These actions are believed to converge upon mechanisms promoting cell survival and growth factor synthesis, leading to increased neurite sprouting. BDNF = brain-derived neurotrophic factor; IL = interleukin; NADP = nicotinamide adenine dinucleotide phosphate; NADPH = reduced form of NADP; TNF = tumour necrosis factor.
In addition to providing cysteine for GSH production, NAC has been shown to scavenge oxidants directly, particularly the reduction of the hydroxyl radical, ·OH and hypochlorous acid.6
Oral administration of GSH alone does not adequately restore GSH levels. It is rapidly hydrolyzed by the liver and intestines,7 and penetration through the blood–brain barrier is poor. Similarly, oral administration of L-cysteine has also been shown to have little effect on brain GSH levels owing to first-pass metabolism.8–10 Oral NAC administration results in increased plasma cysteine levels, ultimately leading to increases in plasma GSH.11,12 N-acetylcysteine has been shown to successfully penetrate the blood–brain barrier and raise brain GSH levels in animal models,13–15 which may be relevant to psychiatry, where alterations in brain GSH and other redox pathways have been shown.
Interaction with inflammatory mediators
Alterations in pro- and anti-inflammatory cytokines, including interleukin (IL)-6, IL-1β and tumour necrosis factor (TNF)–α, have been reported in populations with depression, and to a lesser extent, bipolar disorder and schizophrenia.16,17 These inflammatory cytokines are potential contributors to the underlying pathophysiology of these disorders. N-acetylcysteine has been shown to have anti-inflammatory properties (Fig. 1) that are linked to oxidative pathways, which may provide another potential mechanism of action in the benefits of NAC in psychiatry.
N-acetylcysteine has been shown to reduce IL-6 levels in hemodialysis patients,18 although no change in these levels were reported following NAC treatment in a rat model of traumatic brain injury.19 Conversely, increased TNF-α and IL-1β levels were reduced following NAC treatment in rat models of both traumatic brain injury and focal cerebral ischemia.19,20 N-acetylcysteine has also been shown to improve outcomes in lipopolysaccharide models of inflammation. Pretreatment with NAC prevented oxidative stress and loss of long-term potentiation following exposure to prenatal inflammation.21 Furthermore, lipopolysaccharide treatment results in inhibited oligodendroglial cell development and myelination that is attenuated by NAC administration in rat mixed glial cultures.22
The reductions in inflammatory cytokines by NAC treatment may be a potential mechanism by which NAC modulates the symptoms of psychiatric disorders. This may be directly associated with the inflammatory pathway, or working through oxidative processes associated with inflammation. Further research is required to elucidate these mechanisms.
Effects on neurotransmission
Glutamate
In addition to the effects on oxidative balance, alterations in cysteine levels have also been shown to modulate neuro-transmitter pathways, including glutamate and dopamine (DA; Fig. 1).23,24 Cysteine assists in the regulation of neuronal intra- and extracellular exchange of glutamate through the cystine–glutamate antiporter. Whereas this antiporter is ubiquitous throughout all cell types, in the brain it is preferentially located on glial cells.25 The dimer, cystine, is taken up by astrocytes and exchanged for glutamate, which is released into the extracellular space. This free glutamate appears to stimulate inhibitory metabotropic glutamate receptors on glutamatergic nerve terminals and thereby reduce the synaptic release of glutamate.26 Given that relation, the amount of cysteine in the system as well as the feedback via GSH production by neurons may directly regulate the amount of glutamate present in the extracellular space. Furthermore, GSH itself has been shown to potentiate brain N-methyl-d-aspartate receptor response to glutamate in rats.27,28 Changes in the levels of neuronal GSH may not only alter available glutamate levels, but also have direct consequences on glutamatergic function.
Dopamine
In addition to modulating glutamate levels through the cystine–glutamate antiporter, NAC has also been shown to alter DA release. Following amphetamine treatment to rat striatal slices, NAC has been shown to facilitate vesicular DA release at low doses in striatal neurons and inhibit release at millimolar concentrations.29 In monkeys, NAC has been shown to protect against reductions in DA transporter levels following repeated methamphetamine administration,30 suggesting one mechanism whereby increased DA release was facilitated in the previous study. Glutathione has also been shown to increase glutamate agonist–evoked DA release in mouse striatal neurons.23
Use in psychiatry
There is a growing body of literature exploring the use of NAC in the treatment of psychiatric illness. There is provisional evidence of the potential benefit of NAC in a wide range of disorders. Many of these disorders have limited treatment options or suboptimal outcomes with current treatments. The present review outlines the clinical use of NAC in psychiatry (summary in Table 1).
Table 1.
Summary of clinical findings of N-acetylcysteine (NAC) treatment in psychiatric illness
| Study | Disorder | No. study participants | Total daily dose, mg | Study design | Outcome measures | Findings |
|---|---|---|---|---|---|---|
| Gray et al.31 | Marijuana addiction | 24 | 2400 | 4-wk open-label trial | Marijuana Craving Questionnaire | Improvement in 3 of the 4 domains of the scale |
| Knackstedt et al.32 | Reduction in nicotine use | 29 | 2400 | 4-wk double-blind placebo-controlled trial | Questionnaire for Smoking Urges- Brief, Minnesota Nicotine Withdrawal Scale | Trend for improvement after covarying for alcohol use but overall a negative trial |
| Van Schooten et al.33 | Chemo-prevention trial in healthy smokers (unable to quit) | 41 | 1200 | 6-mo double-blind placebo-controlled trial | Cotinine (plasma and BAL fluid), urine mutagenicity, 4-ABP-Hb adducts, lipophilic-DNA adducts (PBL and BAL cells), 8-OH-dG adducts (BAL cells), PAH-DNA adducts (MFC and BMC), micronuclei (MFC and SPC), TEAC (plasma and BAL fluid) | Decreases in lipophilic DNA adducts, 8-OH-dG levels and number of micronuclei |
| LaRowe et al.34 | Cocaine addiction | 13 | 2400 | Crossover design, treatment for 2 d in each arm (NAC and placebo) | CSSA, self-reported cocaine use, reported cravings, routine blood tests | Significant decrease in craving, withdrawal and self-reported use in NAC but not placebo group (no between- group differences) |
| LaRowe et al.35 | Cocaine addiction | 15 | 2400 | Crossover design, treatment for 2 d in each arm (NAC and placebo) | Cue-reactivity (general and motivational measures) | Decreased desire and interest in cocaine and reduced time spent looking at cocaine-related slides |
| Mardikian et al.36 | Cocaine addiction | 23 | 1200, 2400 and 3600 | 4-wk, open-label trial | Days and amount of money spent on cocaine, CSSA and urine drug screen | Nonsignificant trends in reductions of amount spent and number of days of use on cocaine and improvements based on CSSA |
| Grant et al.37 | Pathological gambling | 29 O/L 16 random | 1800† | 8-wk O/L study followed by a 6-wk double-blind, placebo- controlled trial (in responders only) | Y-BOCS adapted for Pathological Gambling (PGYBOCS), G-SAS, CGI Improvement and Severity scales, Sheehan Disability Scale, HAM-D, HAM-A, Quality of Life Inventory | Decreased PG-YBOCS scores during O/L phase. Sixteen of original 27 were classified as responders, 13 of whom continued to double-blind phase. There was an increased number of continued response in the NAC group and trends toward significance in the PG-YBOCS and G-SAS scales. |
| Lafleur et al.38 | OCD | 1 | 3000† | 13 wk | Y-BOCS and HAM-D | Improvements between baseline and end point on Y-BOCS and HAM-D |
| Odlaug et al.39 | TTM | 2 | 1800 | 10 and 13 wk | Self-reported behaviour | Complete abstinence from hair pulling |
| Grant et al.40 | TTM | 50 | 1200–2400 | 12-wk double-blind, placebo-controlled trial | MGH-HPS, CGI, PITS, Sheehan Disability Scale, Quality of Life Scale, HAM-A and HAM-D | Significant improvements in MGH-HPS, PITS, CGI severity scale scores in the NAC group compared with placebo |
| Odlaug et al.39 | Nail biting | 1* | 1800† | 13 wk | Self-reported behaviour | Complete abstinence from nail biting after 9 weeks of treatment. A 2-week hiatus caused reinstatement of symptoms, but subsequent NAC treatment again ameliorated this. |
| Berk et al.41 | Nail biting | 3 | 2000 | 6 mo | Self-reported behaviour | Complete abstinence of symptoms that continued after 1-month washout period |
| Odlaug et al.39 | Skin picking | 1 | 1800† | 13 wk | Self-reported behaviour | Decreased urge and act of skin picking |
| Berk et al.42 | Schizophrenia | 140 | 2000 | 6-mo double-blind placebo-controlled trial | PANSS, CGI, GAF, SOFAS, BAS, Simpson–Angus Scale and the Abnormal Involuntary Movements Scale | Improvements seen in negative symptoms based on PANSS; improvements also seen on CGI and BAS. Improvements were lost at the 1-month follow-up visit. |
| Lavoie et al.12 | Schizophrenia | 11 | 2000 | 8-wk double-blind crossover design | Mismatched negativity and plasma glutathione concentration | Significant improvements in mismatch negativity in NAC group. Plasma glutathione levels were increased following NAC treatment. |
| Bulut et al.43 | Schizophrenia | 1 | 600 | 30 d | PANSS, CGI and Calgary depression scale | Reduction in PANSS and CGI scores |
| Berk et al.44 | Bipolar disorder | 76 | 2000 | 6-mo double-blind placebo-controlled trial | MADRS, Bipolar Depression Rating Scale, Young Mania Rating Scale, CGI-bipolar version, GAF, SOFAS, SLICE/LIFE, LIFE-RIFT, and the Quality of Life Enjoyment and Satisfaction Questionnaire | Positive results showed improvements on most rating scales (including primary outcome measure MADRS) in NAC group compared with placebo group |
BAL = bronchoalveolar lavage; BAS = Barnes Akathisia Scale; BMC = buccal mucosa cell; CGI = Clinical Global Impression; CSSA = Cocaine Selective Severity Assessment; GAF = Global Assessment of Functioning; G-SAS = Gambling Symptom Assessment Scale; HAM-A = Hamilton Rating Scale for Anxiety; HAM-D = Hamilton Rating Scale for Depression; LIFE-RIFT = Longitudinal Interval Follow-up Evaluation Range of Impaired Functioning Tool; MADRS = Montgomery–Åsberg Depression Rating Scale; MFC = mouth floor cell; MGH-HPS = Massachusetts General Hospital Hair Pulling Scale; OCD = obsessive–compulsive disorder; O/L = open label; PAH = polycyclic aromatic hydrocarbon; PANSS = Positive and Negative Symptoms Scale; PBL = peripheral blood lymphocyte; PITS = Psychiatric Institute Trichotillomania Scale; random. = randomized; SLICE/LIFE = Streamlined Longitudinal Interview Clinical Evaluation from the Longitudinal Interval Follow-up Evaluation; SOFAS = Social and Occupational Functioning Scale; SPC = soft palate cell; TEAC = trolox equivalent antioxidant capacity; TTM = trichotillomania; Y-BOCS = Yale–Brown Obsessive Compulsive Scale.
This participant also had TTM.
Titrated dose.
Addiction
There is an abundance of literature implicating glutamatergic abnormalities in addiction.47,48 More recently, data are emerging suggesting a role of oxidative stress in the pathophysiology of addiction to drugs of abuse.32,49–51 Research has explored the modulation of glutamatergic pathways by NAC in pre-clinical models.52,53 N-acetylcysteine has been shown to reverse the decline in cystine–glutamate exchange through the cystine–glutamate antiporter and thereby assist in the restoration of glutamatergic pathways in addiction.32,52 These properties have made it a potential prospect for the treatment of addiction. Much of the following literature is based on small clinical trials, nonrandomized cohorts or case reports, but is sufficiently promising to suggest the need for larger well designed studies.
Marijuana dependence
A recent study by Gray and colleagues31 investigated the use of NAC (2400 mg/d) in an open-label study of 24 dependent marijuana users who reported an interest in reducing their use. Following treatment, users reported reductions in days/week of use and “number of hits.” Conversely, urine cannabinoid measures did not significantly change over the treatment period, although the authors state that urine cannabinoid levels in 13 users remained higher than the detection range of the test, thus providing ambiguous results regarding decreases in use. In addition to overall use, reductions in reported compulsivity, emotionality and purposeful-ness regarding marijuana use (measured with the Marijuana Craving Questionnaire) were reported, reflecting an improvement in 3 of the 4 domains of the scale.31
Nicotine addiction
N-acetylcysteine has also been investigated as a treatment for nicotine addiction. In addition to the modulation of glutamate to reduce cravings and reward behaviours, NAC may have a role as an antioxidant in a disorder where oxidative stress is marked. There has been 1 placebo-controlled study (n = 29) investigating 2400 mg/day of NAC as a treatment for tobacco cessation.32 This study recorded participant ratings of use and cravings as well as biochemical measures to confirm reported use. There was no significant difference in the number of cigarettes smoked or carbon monoxide levels between NAC and placebo groups. Treatment adherence and side effects were not reported. The authors noted that alcohol was a significant covariate, and after the removal of 2 outliers based on alcohol consumption and resulting nicotine use, there was only a post hoc trend toward decreased number of cigarettes smoked in the NAC group, and this did not correspond with decreased carbon monoxide levels. Owing to the exclusion of participants from the analysis and the variability of the sample in terms of extraneous factors such as alcohol use, the sample size of this study was too small to make definitive conclusions.
There is another small-scale study that specifically included smokers who were not planning on quitting that investigated biomarkers in smokers after NAC treatment.33 The outcome of the study was to assess the effects of NAC on the detrimental biophysical aspects of smoking. Participants were randomly assigned to placebo or NAC (1200 mg/d) groups and treated for 6 months. The study found that in the NAC group, there were decreases in lipophilic DNA adducts between baseline and end point. Also, 8-OH-dG levels were decreased both between baseline and end point, and compared to the placebo group. These data indicate a decrease in DNA damage over the course of the study. Additionally, there was a decreased number of micronuclei present in oral mucosa in the NAC group after treatment when compared with baseline.
Cocaine addiction
In a small crossover study (n = 13), designed to determine tolerability and safety, participants (currently abstaining from cocaine use) were given 2400 mg of NAC or placebo over 2 days.34 Four days later, participants were crossed over to the alternative arm. Whereas there was no between-group change in reduction of cravings compared with placebo, the within-group analysis showed that the NAC group had a significant reduction in cravings, withdrawals and self-reported use compared with baseline, which was not seen in the placebo group. Whereas this study did not aim to investigate efficacy, a signal was found that provided some evidence to justify further research.
In a follow-up study, a similar sample was treated with 2400 mg of NAC.35 Results of this study showed that, based on cue-reactivity slides, NAC reduced the desire for and interest in cocaine, and also reduced the amount of time spent looking at the cocaine-related slides.
After these studies, this research group went on to conduct a larger open-label trial of NAC using 3 doses over 4 weeks.36 Initially, 8 participants received 1200 mg/day of NAC. After the establishment of tolerability at this dose, a further 9 participants received 1800 mg/day of NAC, and finally 6 participants received 3600 mg/day of NAC. Although not statistically significant, this study found reductions in the amount spent on cocaine, the number of days of use and improvements based on the Cocaine Selective Severity Assessment. The researchers noted that this study was underpowered and required a placebo-controlled design to make concrete assertions regarding the efficacy of NAC in the treatment of cocaine addiction. Considering these results, larger well designed trials are required.
Pathological gambling
In an open-label study involving 29 participants with a confirmed pathologic addiction to gambling, Grant and colleagues37 administered 1800 mg (titrated dose) of NAC over 8 weeks. A randomized trial of 13 responders was then conducted over the following 6 weeks (constant dose of 1800 mg/kg of NAC compared with placebo). During the open-label study, 16 participants experienced significant reductions in gambling behaviour. Of those, 13 agreed to take part in the randomized study. Following a further 6 weeks of NAC treatment, 83% of the NAC group was still considered responders, with only 28% in the placebo group.
Obsessive–compulsive disorder
Similarities exist among brain regions implicated in addiction and obsessive–compulsive disorder (OCD), including the nucleus accumbens and anterior cingulate cortex.54,55 There have been reports of oxidative stress in populations with OCD, including increased lipid peroxidation;56–59 decreased vitamin E,58 catalase, GPx and selenium;59 increased superoxide dismutase;59 and changes in overall oxidative status.60 Some of these alterations have been linked to symptom severity.57,59
Standard first-line therapies for OCD generally include a combination of serotonin reuptake inhibitors (SRIs) and psychotherapy. Whereas there is some efficacy with this treatment regimen, up to 20% of individuals with OCD are treatment-resistant and derive little benefit.61 There is some evidence to suggest glutamatergic abnormalities in individuals with OCD; however, further characterization is required to determine if this is a primary, causal effect or a by-product of hypermetabolism and altered neurotransmission in other pathways.62
At present, there is only 1 case report regarding the use of NAC in patients with OCD.38 This report showed notable benefits in an individual who was treatment-refractory. The participant experienced partial benefit from treatment with fluvoxamine, and continued fluvoxamine during a 13-week trial of 3 g of NAC (including dose titration to 3 g). During the course of the trial, the participant improved on both Yale–Brown Obsessive Compulsive Scale and Hamilton Rating Scale for Depression scores. Continued treatment with fluvoxamine and NAC led to dramatic improvements in control of compulsive washing and obsessional triggers.
Trichotillomania and grooming disorders
A spectral relation between OCD and trichotillomania (TTM) is described, and there is reported efficacy of SRIs in TTM, as with OCD.63 However, the response to treatment with SRIs in individuals with TTM is inconsistent.64 Comparisons between TTM and addictive disorders have also been made, given that impulsivity and dysfunctional reward pathways may be operative in both types of disorder, and there has been some benefit in treating TTM with opioid antagonists.65 Trichotillomania may have a heterogeneous nature, with one subgroup more similar to OCD and another subgroup more similar to addiction.66 Two case studies suggested benefits of NAC treatment in individuals with TTM.39 The first involved a 28-year-old man and the second a 40-year-old woman. These authors reported that 1800 mg of NAC (titrated over a period of several weeks) ameliorated hair pulling.
There has been 1 double-blind, placebo-controlled trial of NAC for the treatment of TTM.40 In this study, 50 individuals (45 women and 5 men) were given 1200 mg of NAC or placebo for 6 weeks, followed by a further 6 weeks of 2400 mg of NAC or placebo. Half of the sample was concurrently taking medication, including SRIs, serotonin-noradrenaline reuptake inhibitors and stimulants. Four participants were undergoing psychotherapy. N-acetylcysteine was administered in combination with these treatments. Over the course of the study, NAC treatment was found to decrease symptoms of TTM compared with placebo. Most (88%) of the participants completed the 12-week study. Effects of treatment were seen at week 9 and continued throughout the remainder of the study. Overall, NAC seemed to be efficacious in the treatment of TTM.
In addition to TTM, promising preliminary results suggest the need for controlled studies in other grooming disorders, including nail biting and skin picking.39,41 A case report was published regarding an individual with both TTM and nail biting behaviours, in whom nail biting ceased following 9 weeks of NAC treatment.39 The participant relapsed after a hiatus in treatment, but recommencement of NAC resulted in a remission of symptoms.39 A serendipitous finding of the benefit of NAC treatment in the reduction of nail biting in a study primarily investigating NAC (2000 mg/d) in the treatment of mood disorders has been reported.41 Three participants taking NAC reported significant reductions in nail biting during the 6-month course of treatment. All 3 participants were still abstinent from nail biting 1 month after the discontinuation of NAC.
Finally, there is a case report regarding skin picking and NAC treatment.39 In a woman who was not receiving pharmacological interventions, 600 mg/day of NAC was administered. Over the subsequent 4 weeks, the dose was increased to 1800 mg/day, after which both urge and actual behaviours regarding skin picking had completely remitted.
Schizophrenia
Dopaminergic abnormalities have historically been in the foreground as research targets for schizophrenia, although all other major neurotransmitter systems, including γ-aminobutyric acid, serotonin, acetylcholine, glutamate and noradrenaline have also been implicated.65 Increased dopaminergic metabolism in the striatum has been reported. This hyperdopaminergic state has been shown to inversely correlate with hypodopaminergia in the prefrontal cortex. These changes are believed to mediate alterations in executive function and many of the positive symptoms of the disorder.
In populations with schizophrenia, dysfunction in glutamate metabolism and decreased glutamate levels in the pre-frontal cortex have been reported.68 The addition of cysteine has been shown to modulate glutamate levels through glutamate–cystine exchange, and GSH has been shown to modulate the binding of glutamate to N-methyl-d-aspartate receptors.69 N-acetylcysteine may be beneficial in the treatment of schizophrenia by targeting both oxidative stress and glutamatergic dysfunction, suggesting that the phenotype is a result of interactions of multiple neurotransmitter pathways70 that interact with oxidative and inflammatory systems, which are additionally implicated in the disorder.
There is an expanding body of evidence suggesting oxidative stress occurs in individuals with schizophrenia, and there are links between oxidative stress symptom severity and diagnostic subtype.45,71–74 Whether the effects are synchronous with altered neurotransmission or the result of these abnormalities requires further research. Evidence for a role of oxidative stress in populations with schizophrenia includes polymorphisms in key GSH pathway genes and altered levels of antioxidants (with correlations between levels and severity of symptoms).75 Oxidative stress may lead to changes in lipid membranes, mitochondrial dysfunction and alterations to DNA and proteins. In individuals with schizophrenia, it is believed that whereas there are few changes to neuronal cell bodies, connections and dendritic sprouting may be affected. This is one potential mechanism by which oxidative stress is involved in this disorder. Similarly, changes in mitochondrial function have been reported, and the link to energy generation may provide a clue to the underlying pathology of schizophrenia. Moreover, links between oxidative stress and neurotransmission in psychiatric illnesses are beginning to be identified.
A large-scale study investigating NAC as an adjunctive therapy for schizophrenia has been conducted,42 which employed a 1000 mg, bi-daily regimen (compared with placebo) in addition to existing medication over 6 months. In all, 140 participants took part in this double-blind, placebo-controlled, randomized trial. Of these, 60% completed the 6-month treatment trial. Improvements were seen in the negative symptoms, measured on the Positive and Negative Symptoms Scale. Furthermore, improvements in global function and improved abnormal movements, particularly akathisia, were also reported. These effect sizes were moderate, and improvements were lost 1 month after the discontinuation of treatment. This sample was considered treatment-refractory, with the average duration of illness being 12 years and more than 60% of participants medicated with clozapine. Given this, the outcomes of the addition of NAC are noteworthy. Gastrointestinal side effects were most commonly reported; however, the NAC and placebo groups did not differ statistically.
These findings were further supported by qualitative analysis of participants’ data. In this report, using a novel methodology, qualitative analysis of patient reports and clinician observations was performed in a blinded manner, and the NAC and placebo groups were compared. Emerging themes showed that participants treated with NAC demonstrated improvements in insight, self-care, social interaction, motivation, volition, psychomotor stability and stabilization of mood.76 In a subset of the primary study, NAC appeared to modulate auditory sensory processing, measured using mismatch negativity, a marker of glutamatergic function and an endophenotype of psychosis. Compared with healthy controls, individuals with schizophrenia were shown to have reduced mismatch negativity at baseline. Following 8 weeks of NAC treatment (2000 mg/d), mismatch negativity was shown to improve significantly.12 A recent case report has also shown significant improvements in symptoms following 600 mg/day of NAC in a young woman with treatment-resistant schizophrenia. However, details of total length of treatment are not provided.43
Bipolar disorder
Alterations in oxidative metabolism have also been described in populations with bipolar disorder.61,77 Similar to schizophrenia, changes in antioxidant levels, increased markers of lipid peroxidation and protein carbonylation have all been reported. These changes appear to be related to state, particularly in mania, where increased oxidative stress seems to be apparent. This is congruent with reports of hyperdopaminergic states during manic episodes.46 Furthermore, links between oxidative status and duration of illness have also been found.78
A double-blind, randomized, placebo-controlled trial of NAC in 75 participants with bipolar disorder was conducted.44 This 6-month trial involved the addition of 2000 mg/d of NAC or placebo to treatment as usual. Over the 6-month period there was no difference between groups in drop-out rates, with 64% of the total sample completing the trial. Rating scores on the Montgomery–Åsberg Depression Rating Scale (MADRS) and the Bipolar Depression Rating Scale showed large decreases in depressive symptoms (about 9 points on the MADRS between NAC and placebo groups at the end point). Akin to the schizophrenia trial, improvements were seen on global improvement, severity and function scales; however, these effects were proportionally larger, with large effect sizes on most measures. Again, after discontinuation of NAC treatment, there was a convergence with scores between the NAC and placebo groups, showing a loss of benefit following washout.
Discussion
N-acetylcysteine appears to be promising in the treatment of several psychiatric disorders. Many of the psychiatric disorders discussed have shown only preliminary data regarding the efficacy of NAC in their treatment, and further research is required. However, NAC appears to be a promising therapeutic target and provides a window of treatment opportunity in a field where current treatments are limited or have remained suboptimal.
The apparent lack of specificity of NAC in initial studies is intriguing and suggests that it may be targeting pathways that are common across disorders; oxidative stress appears to be a fairly nonspecific finding in a range of psychopathologies, and dysregulation of glutamate, inflammatory pathways and DA are similarly widely reported. Given that the current diagnostic systems are phenomenologically based, and that in no other branch of medicine are phenomenology and pathophysiology linearly linked, this may reflect an intrinsic limitation of our classification system. This is high-lighted by the fact that there is extensive overlap of other treatments and biomarkers across disorders. As the body of evidence is currently provisional for many disorders, as the evidence base expands, it is possible that the efficacy will appear to be greater in some areas than others. Additionally, the precise dose of NAC remains to be definitively established. Dose-finding studies may reveal greater efficacy at higher doses or equal efficacy at lower doses. Whereas the tolerability profile of NAC appears benign, it needs to be stressed that there is no extensive evidence base with longer-term use. Some adverse events, such as pulmonary hypertension are reported in very high-dose animal studies, but have not been seen in human studies.79 Whereas NAC appears to be antiepileptic at low doses,80 seizures are reported with overdose.81 Vigilance is necessary.
Given that many of these disorders have many interacting potential pathophysiological pathways, further research is required to determine how NAC is exerting benefits. Bio-marker and neuroimaging platforms have the capacity to illuminate these issues. In disorders such as addiction, glutamate has been the primary candidate for the mechanism of action, whereas in schizophrenia and mood disorders, the GSH hypothesis has been the one postulated as explaining the mechanism of action of NAC. However, given the interaction between glutamate, the most abundant neurotransmitter, and other neurotransmitter pathways, including DA and serotonin, individuals with disorders such as depression and schizophrenia may benefit by indirect modulation of these pathways through changes in glutamatergic function. A common link in treatment efficacy may be oxidative stress, which has been shown to be altered in most of these disorders. However, in cocaine addiction, most of the research focusing on mechanisms of action has implicated the modulation of the cystine–glutamate antiporter by NAC as the most likely cause of benefit.26,82,83 Whereas there are similarities across these disorders with alterations to oxidative biology and neurotransmission, and changes in glutamate-dependent long-term potentiation and neuronal plasticity,84 perhaps the heterogeneity of the underlying pathologies, especially in brain regions implicated, may lead to the revelation of different actions of NAC depending on the disorder.
Similarly, the modulation of inflammatory pathways may also play a role in the benefits seen following NAC treatment. The role of inflammation in depression has received the greatest attention; however, inflammatory pathways are implicated in the etiology of other disorders, such as schizophrenia. As with the atypical antipsychotics, which have new data showing a diversity of mechanisms of action, including on inflammation,85 brain-derived neurotrophic factor86 and oxidative stress,87 efficacy may turn out to be a summative interaction of effects on various pathways.
Overall this unlikely therapeutic tool is implicating novel pathways as viable therapeutic targets. This opens the way for the development of other rational, hypothesis-based therapies. That NAC appears safe, tolerable and affordable and is readily available adds to its interest.
Footnotes
Competing interests: This work was supported in part by a grant from the Australian National Health and Medical Research Council (O.D. and M.B., NHMRC No. 509109) and a Melbourne Research Scholarship (F.G.) to the University of Melbourne. Dr. Berk declares having been a consultant for AstraZeneca, Eli Lilly, GlaxoSmithKline, Janssen Cilag and Servier; his institution has received grants from the Stanley Medical Research Institute, MBF, the National Health and Medical Research Council, Beyond Blue, the Geelong Medical Research Foundation, Bristol Myers Squibb, Eli Lilly, GlaxoSmithKline, Organon, Novartis, Mayne Pharma and Servier; he has received honoraria from Astra Zeneca, Eli Lilly, Janssen Cilag, Lundbeck, Pfizer, Sanofi Synthelabo, Servier, Solvay and Wyeth; and he has travel funding from Janssen Cilag, Astra Zeneca, Wyeth and Pfizer.
Contributors: Drs. Dean and Berk designed the study. Dr. Dean acquired the data and analyzed it with Drs. Giorlando and Berk. All authors wrote and reviewed the article and approved its publication.
References
- 1.Scalley RD, Conner CS. Acetaminophen poisoning: a case report of the use of acetylcysteine. Am J Hosp Pharm. 1978;35:964–7. [PubMed] [Google Scholar]
- 2.Dodd S, Dean O, Copolov DL, et al. N-acetylcysteine for antioxidant therapy: pharmacology and clinical utility. Expert Opin Biol Ther. 2008;8:1955–62. doi: 10.1517/14728220802517901. [DOI] [PubMed] [Google Scholar]
- 3.Adair JC, Knoefel JE, Morgan N. Controlled trial of N-acetylcysteine for patients with probable Alzheimer’s disease. Neurology. 2001;57:1515–7. doi: 10.1212/wnl.57.8.1515. [DOI] [PubMed] [Google Scholar]
- 4.Dringen R, Hirrlinger J. Glutathione pathways in the brain. Biol Chem. 2003;384:505–16. doi: 10.1515/BC.2003.059. [DOI] [PubMed] [Google Scholar]
- 5.Meister A. Glutathione, ascorbate, and cellular protection. Cancer Res. 1994;54(Suppl):1969s–75s. [PubMed] [Google Scholar]
- 6.Aruoma OI, Halliwell B, Hoey BM, et al. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6:593–7. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 7.Witschi A, Reddy S, Stofer B, et al. The systemic availability of oral glutathione. Eur J Clin Pharmacol. 1992;43:667–9. doi: 10.1007/BF02284971. [DOI] [PubMed] [Google Scholar]
- 8.Vina J, Reginald H, Krebs HA. Maintenance of glutathione content in isolated hepatocytes. Biochem J. 1978;170:627–30. doi: 10.1042/bj1700627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sjodin K, Nilsson E, Hallberg A, et al. Metabolism of N-acetyl-L-cysteine. Some structural requirements for the deacetylation and consequences for the oral bioavailability. Biochem Pharmacol. 1989;38:3981–5. doi: 10.1016/0006-2952(89)90677-1. [DOI] [PubMed] [Google Scholar]
- 10.Borgström L, Kågedal B. Dose dependent pharmacokinetics of N-acetylcysteine after oral dosing to man. Biopharm Drug Dispos. 1990;11:131–6. doi: 10.1002/bdd.2510110205. [DOI] [PubMed] [Google Scholar]
- 11.Holdiness MR. Clinical pharmokinetics of N-acetylcysteine. Clin Pharmacokinet. 1991;20:123–34. doi: 10.2165/00003088-199120020-00004. [DOI] [PubMed] [Google Scholar]
- 12.Lavoie S, Murray MM, Deppen P, et al. Glutathione precursor, N-acetylcysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology. 2008;33:2187–99. doi: 10.1038/sj.npp.1301624. [DOI] [PubMed] [Google Scholar]
- 13.Neuwelt EA, Pagel MA, Hasler BP, et al. Therapeutic efficacy of aortic administration of N-acetylcysteine as a chemoprotectant against bone marrow toxicity after intracarotid administration of alkylators, with or without glutathione depletion in a rat model. Cancer Res. 2001;61:7868–74. [PubMed] [Google Scholar]
- 14.Dean O, van den Buuse M, Copolov D, et al. N-acetylcysteine inhibits depletion of brain glutathione levels in rats: implications for schizophrenia [abstract] Int J Neuropsychopharmacol. 2004;7(S1):262. [Google Scholar]
- 15.Farr SA, Poon HF, Dogrukol-Ak D, et al. The antioxidants alphalipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem. 2003;84:1173–83. doi: 10.1046/j.1471-4159.2003.01580.x. [DOI] [PubMed] [Google Scholar]
- 16.Drexhage RC, Knijff EM, Padmos RC, et al. The mononuclear phagocyte system and its cytokine inflammatory networks in schizophrenia and bipolar disorder. Expert Rev Neurother. 2010;10:59–76. doi: 10.1586/ern.09.144. [DOI] [PubMed] [Google Scholar]
- 17.Dinan TG. Inflammatory markers in depression. Curr Opin Psychiatry. 2009;22:32–6. doi: 10.1097/YCO.0b013e328315a561. [DOI] [PubMed] [Google Scholar]
- 18.Nascimento MM, Suliman ME, Silva M, et al. Effect of oral N-acetylcysteine treatment on plasma inflammatory and oxidative stress markers in peritoneal dialysis patients: a placebo-controlled study. Perit Dial Int. 2010;30:336–42. doi: 10.3747/pdi.2009.00073. [DOI] [PubMed] [Google Scholar]
- 19.Chen G, Shi J, Hu Z, et al. Inhibitory effect on cerebral inflammatory response following traumatic brain injury in rats: a potential neuroprotective mechanism of N-acetylcysteine. Mediators Inflamm. 2008;2008:716458. doi: 10.1155/2008/716458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan M, Sekhon B, Jatana M, et al. Administration of N-acetylcysteine after focal cerebral ischemia protects brain and reduces inflammation in a rat model of experimental stroke. J Neurosci Res. 2004;76:519–27. doi: 10.1002/jnr.20087. [DOI] [PubMed] [Google Scholar]
- 21.Lante F, Meunier J, Guiramand J, et al. Late N-acetylcysteine treatment prevents the deficits induced in the offspring of dams exposed to an immune stress during gestation. Hippocampus. 2008;18:602–9. doi: 10.1002/hipo.20421. [DOI] [PubMed] [Google Scholar]
- 22.Paintlia MK, Paintlia AS, Khan M, et al. Modulation of peroxisome proliferator-activated receptor-alpha activity by N-acetyl cysteine attenuates inhibition of oligodendrocyte development in lipo-polysaccharide stimulated mixed glial cultures. J Neurochem. 2008;105:956–70. doi: 10.1111/j.1471-4159.2007.05199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janaky R, Dohovics R, Saransaari P, et al. Modulation of [3H]dopamine release by glutathione in mouse striatal slices. Neurochem Res. 2007;32:1357–64. doi: 10.1007/s11064-007-9315-z. [DOI] [PubMed] [Google Scholar]
- 24.Himi T, Ikeda M, Yasuhara T, et al. Oxidative neuronal death caused by glutamate uptake inhibition in cultured hippocampal neurons. J Neurosci Res. 2003;71:679–88. doi: 10.1002/jnr.10510. [DOI] [PubMed] [Google Scholar]
- 25.Baker DA, Xi ZX, Shen H, et al. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–41. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moran MM, McFarland K, Melendez RI, et al. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci. 2005;25:6389–93. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogita K, Kitago T, Nakamuta H, et al. Glutathione-induced inhibition of Na+-independent and -dependent bindings of L-[3H]glutamate in rat brain. Life Sci. 1986;39:2411–8. doi: 10.1016/0024-3205(86)90482-0. [DOI] [PubMed] [Google Scholar]
- 28.Varga V, Jenei Z, Janaky R, et al. Glutathione is an endogenous ligand of rat brain N-methyl-D-aspartate (NMDA) and 2-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptors. Neurochem Res. 1997;22:1165–71. doi: 10.1023/a:1027377605054. [DOI] [PubMed] [Google Scholar]
- 29.Gere-Paszti E, Jakus J. The effect of N-acetylcysteine on amphetamine-mediated dopamine release in rat brain striatal slices by ion-pair reversed-phase high performance liquid chromatography. Biomed Chromatogr. 2009;23:658–64. doi: 10.1002/bmc.1171. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto K, Tsukada H, Nishiyama S, et al. Effects of N-acetyl-L-cysteine on the reduction of brain dopamine transporters in monkey treated with methamphetamine. Ann N Y Acad Sci. 2004;1025:231–5. doi: 10.1196/annals.1316.028. [DOI] [PubMed] [Google Scholar]
- 31.Gray KM, Watson NL, Carpenter MJ, et al. N-acetylcysteine (NAC) in young marijuana users: an open-label pilot study. Am J Addict. 2010;19:187–9. doi: 10.1111/j.1521-0391.2009.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knackstedt LA, LaRowe S, Mardikian P, et al. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65:841–5. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Schooten FJ, Besaratinia A, De Flora S, et al. Effects of oral administration of N-acetyl-L-cysteine: a multi-biomarker study in smokers. Cancer Epidemiol Biomarkers Prev. 2002;11:167–75. [PubMed] [Google Scholar]
- 34.LaRowe SD, Mardikian P, Malcolm R, et al. Safety and tolerability of N-acetylcysteine in cocaine-dependent individuals. Am J Addict. 2006;15:105–10. doi: 10.1080/10550490500419169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LaRowe SD, Myrick H, Hedden S, et al. Is cocaine desire reduced by N-acetylcysteine? Am J Psychiatry. 2007;164:1115–7. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- 36.Mardikian PN, LaRowe SD, Hedden S, et al. An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:389–94. doi: 10.1016/j.pnpbp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Grant JE, Kim SW, Odlaug BL. N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biol Psychiatry. 2007;62:652–7. doi: 10.1016/j.biopsych.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 38.Lafleur DL, Pittenger C, Kelmendi B, et al. N-acetylcysteine augmentation in serotonin reuptake inhibitor refractory obsessive-compulsive disorder. Psychopharmacology (Berl) 2006;184:254–6. doi: 10.1007/s00213-005-0246-6. [DOI] [PubMed] [Google Scholar]
- 39.Odlaug BL, Grant JE. N-acetyl cysteine in the treatment of grooming disorders. J Clin Psychopharmacol. 2007;27:227–9. doi: 10.1097/01.jcp.0000264976.86990.00. [DOI] [PubMed] [Google Scholar]
- 40.Grant JE, Odlaug BL, Kim SW. N-acetylcysteine, a glutamate modulator, in the treatment of trichotillomania: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2009;66:756–63. doi: 10.1001/archgenpsychiatry.2009.60. [DOI] [PubMed] [Google Scholar]
- 41.Berk M, Jeavons S, Dean O, et al. Nail-biting stuff? The effect of N-acetyl cysteine on nail-biting. CNS Spectr. 2009;14:357–60. doi: 10.1017/s1092852900023002. [DOI] [PubMed] [Google Scholar]
- 42.Berk M, Copolov D, Dean O, et al. N-acetyl cysteine as a glutathione precursor for schizophrenia–a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64:361–8. doi: 10.1016/j.biopsych.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Bulut M, Savas HA, Altindag A, et al. Beneficial effects of N-acetylcysteine in treatment resistant schizophrenia. World J Biol Psychiatry. 2009;10:626–8. doi: 10.1080/15622970903144004. [DOI] [PubMed] [Google Scholar]
- 44.Berk M, Copolov DL, Dean O, et al. N-acetyl cysteine for depressive symptoms in bipolar disorder–a double-blind randomized placebo-controlled trial. Biol Psychiatry. 2008;64:468–75. doi: 10.1016/j.biopsych.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 45.Berk M, Ng F, Dean O, et al. Glutathione: a novel treatment target in psychiatry. Trends Pharmacol Sci. 2008;29:346–51. doi: 10.1016/j.tips.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Kunz M, Gama CS, Andreazza AC, et al. Elevated serum super-oxide dismutase and thiobarbituric acid reactive substances in different phases of bipolar disorder and in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1677–81. doi: 10.1016/j.pnpbp.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Kalivas PW, Lalumiere RT, Knackstedt L, et al. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–73. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baker DA, McFarland K, Lake RW, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–9. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 49.Cunha-Oliveira T, Rego AC, Oliveira CR. Cellular and molecular mechanisms involved in the neurotoxicity of opioid and psycho-stimulant drugs. Brain Res Rev. 2008;58:192–208. doi: 10.1016/j.brainresrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Huang MC, Chen CC, Peng FC, et al. The correlation between early alcohol withdrawal severity and oxidative stress in patients with alcohol dependence. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:66–9. doi: 10.1016/j.pnpbp.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 51.Pereska Z, Dejanova B, Bozinovska C, et al. Prooxidative/antioxidative homeostasis in heroin addiction and detoxification. Bratisl Lek Listy. 2007;108:393–8. [PubMed] [Google Scholar]
- 52.Madayag A, Lobner D, Kau KS, et al. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci. 2007;27:13968–76. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen HH, Stoker A, Markou A. The glutamatergic compounds sarcosine and N-acetylcysteine ameliorate prepulse inhibition deficits in metabotropic glutamate 5 receptor knockout mice. Psychopharmacology (Berl) 2010;209:343–50. doi: 10.1007/s00213-010-1802-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huey ED, Zahn R, Krueger F, et al. A psychological and neuroanatomical model of obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci. 2008;20:390–408. doi: 10.1176/appi.neuropsych.20.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsycho-pharmacology. 2010;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuloglu M, Atmaca M, Tezcan E, et al. Antioxidant enzyme activities and malondialdehyde levels in patients with obsessive-compulsive disorder. Neuropsychobiology. 2002;46:27–32. doi: 10.1159/000063573. [DOI] [PubMed] [Google Scholar]
- 57.Chakraborty S, Singh OP, Dasgupta A, et al. Correlation between lipid peroxidation-induced TBARS level and disease severity in obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:363–6. doi: 10.1016/j.pnpbp.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 58.Ersan S, Bakir S, Erdal Ersan E, et al. Examination of free radical metabolism and antioxidant defence system elements in patients with obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1039–42. doi: 10.1016/j.pnpbp.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 59.Ozdemir E, Cetinkaya S, Ersan S, et al. Serum selenium and plasma malondialdehyde levels and antioxidant enzyme activities in patients with obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:62–5. doi: 10.1016/j.pnpbp.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 60.Selek S, Herken H, Bulut M, et al. Oxidative imbalance in obsessive compulsive disorder patients: a total evaluation of oxidant-antioxidant status. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:487–91. doi: 10.1016/j.pnpbp.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 61.Bloch MH, McGuire J, Landeros-Weisenberger A, et al. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010;15:850–5. doi: 10.1038/mp.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carlsson ML. On the role of prefrontal cortex glutamate for the antithetical phenomenology of obsessive compulsive disorder and attention deficit hyperactivity disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:5–26. doi: 10.1016/s0278-5846(00)00146-9. [DOI] [PubMed] [Google Scholar]
- 63.Dell’Osso B, Altamura AC, Allen A, et al. Epidemiologic and clinical updates on impulse control disorders: a critical review. Eur Arch Psychiatry Clin Neurosci. 2006;256:464–75. doi: 10.1007/s00406-006-0668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hautmann G, Hercogova J, Lotti T. Trichotillomania. J Am Acad Dermatol. 2002;46:807–21. doi: 10.1067/mjd.2002.122749. quiz 22–6. [DOI] [PubMed] [Google Scholar]
- 65.Bloch MH. Trichotillomania across the life span. J Am Acad Child Adolesc Psychiatry. 2009;48:879–83. doi: 10.1097/CHI.0b013e3181ae09f3. [DOI] [PubMed] [Google Scholar]
- 66.Grant JE, Odlaug BL, Potenza MN. Addicted to hair pulling? How an alternate model of trichotillomania may improve treatment out-come. Harv Rev Psychiatry. 2007;15:80–5. doi: 10.1080/10673220701298407. [DOI] [PubMed] [Google Scholar]
- 67.Carlsson A, Waters N, Holm-Waters S, et al. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol. 2001;41:237–60. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- 68.Marek GJ, Behl B, Bespalov AY, et al. Glutamatergic (N-methyl-D-aspartate receptor) hypofrontality in schizophrenia: Too little juice or a miswired brain? Mol Pharmacol. 2010;77:317–26. doi: 10.1124/mol.109.059865. [DOI] [PubMed] [Google Scholar]
- 69.Oja SS, Janaky R, Varga V, et al. Modulation of glutamate receptor functions by glutathione. Neurochem Int. 2000;37:299–306. doi: 10.1016/s0197-0186(00)00031-0. [DOI] [PubMed] [Google Scholar]
- 70.Carlsson A. The neurochemical circuitry of schizophrenia. Pharmacopsychiatry. 2006;39(Suppl 1):S10–4. doi: 10.1055/s-2006-931483. [DOI] [PubMed] [Google Scholar]
- 71.Dean OM, van den Buuse M, Bush AI, et al. A role for glutathione in the pathophysiology of bipolar disorder and schizophrenia? Animal models and relevance to clinical practice. Curr Med Chem. 2009;16:2965–76. doi: 10.2174/092986709788803060. [DOI] [PubMed] [Google Scholar]
- 72.Raffa M, Mechri A, Othman LB, et al. Decreased glutathione levels and antioxidant enzyme activities in untreated and treated schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1178–83. doi: 10.1016/j.pnpbp.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 73.Pazvantoglu O, Selek S, Okay IT, et al. Oxidative mechanisms in schizophrenia and their relationship with illness subtype and symptom profile. Psychiatry Clin Neurosci. 2009;63:693–700. doi: 10.1111/j.1440-1819.2009.02015.x. [DOI] [PubMed] [Google Scholar]
- 74.Ng F, Berk M, Dean O, et al. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11:851–76. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- 75.Carter CJ. Schizophrenia susceptibility genes converge on interlinked pathways related to glutamatergic transmission and long-term potentiation, oxidative stress and oligodendrocyte viability. Schizophr Res. 2006;86:1–14. doi: 10.1016/j.schres.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 76.Berk M, Munib A, Dean O, et al. Qualitative methods in early-phase drug trials: data and methods from a trial of N-acetyl cysteine in schizophrenia. J Clin Psychiatry. 2010 Sep 1; doi: 10.4088/JCP.09m05741yel. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 77.Andreazza AC, Kauer-Sant’anna M, Frey BN, et al. Oxidative stress markers in bipolar disorder: a meta-analysis. J Affect Disord. 2008;111:135–44. doi: 10.1016/j.jad.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 78.Andreazza AC, Kapczinski F, Kauer-Sant’Anna M, et al. 3-Nitrotyrosine and glutathione antioxidant system in patients in the early and late stages of bipolar disorder. J Psychiatry Neurosci. 2009;34:263–71. [PMC free article] [PubMed] [Google Scholar]
- 79.Palmer LA, Doctor A, Chhabra P, et al. S-Nitrosothiols signal hypoxia-mimetic vascular pathology. J Clin Invest. 2007;117:2592–601. doi: 10.1172/JCI29444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Devi PU, Pillai KK, Vohora D. Facilitation action of N-acetylcysteine on the anticonvulsant effect of sodium valproate in mice. Basic Clin Pharmacol Toxicol. 2006;98:521–2. doi: 10.1111/j.1742-7843.2006.pto_377.x. [DOI] [PubMed] [Google Scholar]
- 81.Bailey B, Blais R, Letarte A. Status epilepticus after a massive intravenous N-acetylcysteine overdose leading to intracranial hypertension and death. Ann Emerg Med. 2004;44:401–6. doi: 10.1016/j.annemergmed.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 82.Kau KS, Madayag A, Mantsch JR, et al. Blunted cystine-glutamate an-tiporter function in the nucleus accumbens promotes cocaine-induced drug seeking. Neuroscience. 2008;155:530–7. doi: 10.1016/j.neuroscience.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baker DA, McFarland K, Lake RW, et al. N-acetyl cysteine-induced blockade of cocaine-induced reinstatement. Ann N Y Acad Sci. 2003;1003:349–51. doi: 10.1196/annals.1300.023. [DOI] [PubMed] [Google Scholar]
- 84.Moussawi K, Pacchioni A, Moran M, et al. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–9. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bian Q, Kato T, Monji A, et al. The effect of atypical antipsychotics, perospirone, ziprasidone and quetiapine on microglial activation induced by interferon-gamma. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:42–8. doi: 10.1016/j.pnpbp.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 86.Bai O, Chlan-Fourney J, Bowen R, et al. Expression of brain-derived neurotrophic factor mRNA in rat hippocampus after treatment with antipsychotic drugs. J Neurosci Res. 2003;71:127–31. doi: 10.1002/jnr.10440. [DOI] [PubMed] [Google Scholar]
- 87.Pillai A, Parikh V, Terry AV, Jr, et al. Long-term antipsychotic treatments and crossover studies in rats: differential effects of typical and atypical agents on the expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J Psychiatr Res. 2007;41:372–86. doi: 10.1016/j.jpsychires.2006.01.011. [DOI] [PubMed] [Google Scholar]