Abstract
Background
Abnormalities in the corpus callosum have long been implicated in schizophrenia. Previous diffusion tensor imaging (DTI) studies in patients with different durations of schizophrenia yielded inconsistent results. By comparing patients with different durations of schizophrenia, we investigated if white matter abnormalities of the corpus callosum emerge at an early stage in the illness or result from pathological progression.
Methods
We recruited patients with first-episode schizophrenia, patients with chronic schizophrenia and age-, sex-and handedness-matched healthy controls. We used 2 DTI techniques (voxel-based and fibre-tracking DTI) to investigate differences in corpus callosum integrity among the 3 groups.
Results
With both DTI techniques, significantly decreased fractional anisotropy values were identified in the genu of corpus callosum in patients with chronic schizophrenia, but not first-episode schizophrenia, compared with healthy controls.
Limitations
This study was cross-sectional, and the sample size was relatively small.
Conclusion
Abnormalities in the genu of the corpus callosum might be a progressive process in schizophrenia, perhaps related to disease severity and prognosis.
Introduction
The corpus callosum is the largest white matter tract in the brain. It plays an important role in transmitting information between the 2 hemispheres, especially concerning the integration of high-level cognitive, linguistic and perceptual processing, such as attention, language and memory.1 Abnormalities in these functions have been considered a core symptom of schizophrenia.2 Diffusion tensor imaging (DTI) is a unique magnetic resonance imaging (MRI) technique for probing the directional organization of white matter microstructure in vivo, and this technique has been used in several studies to investigate corpus callosum abnormalities in patients with schizophrenia. Most of the DTI studies have found decreased fractional anisotropy (an important quantitative indicator for DTI) in different corpus callosum subregions, such as the genu,3,4 the splenium5,6 and other portions of the corpus callosum,7,8 in patients with schizophrenia. However, some studies have not detected altered corpus callosum integrity in such patients.9 Inconsistent findings may reflect methodological differences in DTI techniques or in samples studied, including differences in age at onset, duration of illness, number of acute episodes and medication exposures.
In this study, we used complementary DTI techniques (voxel-based and fibre-tracking) to study the structural integrity of the corpus callosum and assess the regional localization of changes in patients with schizophrenia compared with healthy controls. We recruited 2 patient groups with different illness durations but otherwise matched for clinical features to study corpus callosum structural integrity in relation to illness progression.
Methods
Participants
We recrutied patients with first-episode schizophrenia and patients with chronic schizophrenia from inpatient and out-patient units of the Department of Psychiatry, Second Xiangya Hospital. We defined first-episode and chronic schizophrenia according to the course of illness: first-episode schizophrenia with illness duration less than 1.5 years10 and chronic schizophrenia with illness duration of more than 2 years.11 All the patients eligible for the study satisfied the following criteria:
a diagnosis of schizophrenia according to the DSM-IV, determined by administration of the Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-I/P);12
age 17–45 years;
right-handedness;
a total Positive and Negative Syndrome Scale (PANSS)13 score greater than 60; and
more than 9 years of completed education.
Patients were excluded if they had any organic brain disease, substance-related disorders, a history of electroconvulsive therapy or any contraindications to MRI scanning.
We also recruited age- and sex-matched healthy controls, who were screened using the SCID, Non-Patient Edition.14 The inclusion and exclusion criteria were the same as those for the schizophrenia groups, except that the healthy controls did not meet the DSM-IV diagnostic criteria for any psychiatric disorders.
The ethics committee of the Second Xiangya Hospital of Central South University approved the study, and signed informed consent was obtained from all participants after we fully explained the purpose of the program.
We assessed clinical symptoms and social function for the schizophrenia patients using the PANSS13 and the Global Assessment Scale (GAS)15 at the time of scanning.
Image acquisition
Magnetic resonance imaging was performed on a 1.5-T GE Signa Twinspeed MR scanner. The standard head coil was used for radio frequency signal transmission and reception. We minimized head motion with restraining foam pads provided by the manufacturer.
Diffusion tensor imaging was performed with a single-shot echo planar imaging sequence aligned to the straight axial plane. Diffusion sensitizing gradients were applied along 13 noncolinear directions (b value = 1000 s/mm2), together with an acquisition without diffusion weighting (b value = 0). Thirty contiguous axial slices were acquired with slice thickness of 4 mm and no gap. The acquisition parameters were as follows: repetition time [TR] 12000 ms; echo time [TE] 107 ms, matrix 128 × 128, field of view [FOV] 240 × 240 mm, number of excitations 5.
Voxel-based DTI processing and statistical analysis
Voxel-based DTI processing has been described previously.16 Briefly, we calculated fractional anisotropy with DTI studio software version 2.40 (www.mristudio.org). For each participant, the b0 image was first normalized to the standard Montreal Neurological Institute (MNI) space using Statistical Parameters Maps (SPM2; Wellcome Department of Cognitive Neurology), and then the transformation matrix was applied to the fractional anisotropy map and b1–b13 images to normalize them to standard MNI space. The b1–b13 images were also used in the fibre-tracking DTI processing, as described in the fibre-tracking section. All images were resampled with a final voxel size of 2 × 2 × 2 mm3 during the normalization procedure. Each normalized fractional anisotropy map was spatially smoothed with an 8-mm full-width at half-maximum Gaussian kernel.
We performed voxel-based analysis of variance (ANOVA) with SPM2 to detect white matter integrity differences among the 3 groups using fractional anisotropy values as the dependent variables. Findings in the whole brain were considered significant at p < 0.005 (uncorrected) and clusters of more than 30 voxels.
The fractional anisotropy value for each cluster showing significant differences in the ANOVA analysis was obtained from each participant using self-developed software. Post hoc analyses were performed by pair-wise comparison between the healthy control and chronic schizophrenia groups, the healthy control and first-episode schizophrenia groups and chronic schizophrenia and first-episode schizophrenia groups. We report all significant main effects (p < 0.05) in the results section.
Fibre-tracking DTI processing and statistical analysis
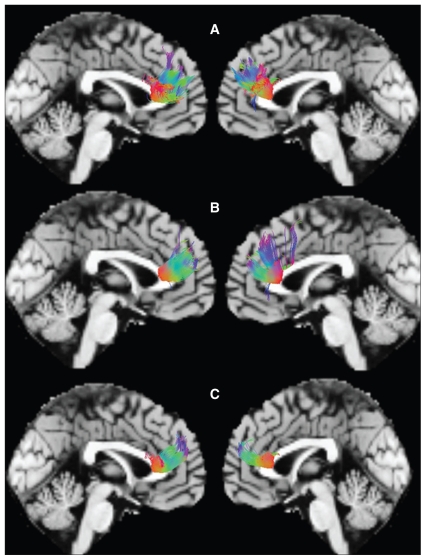
For each participant, the normalized b0–b13 images were loaded into the MedINRIA1.8 software (www-sop.inria.fr/asclepios/software/MedINRIA), and the diffusion tensor matrices in each voxel were estimated. After median smoothing, fractional anisotropy maps were computed for all participants. The brain regions with significantly different fractional anisotropy in the voxel-based analysis (VBA) were saved as regions of interest (ROIs). The tracking parameters used were fractional anisotropy threshold 0.2; minimum length of fibres 10 mm; maximal length 100 mm; maximal angle 45°. The 3-dimensional structure was reconstructed for fibre tracts (Fig. 1). The mean fractional anisotropy values were calculated from each identified fibre tract of every participant for further analysis. Then, we used the same models as in the voxel-based DTI processing to detect differences among the 3 groups and pair-wise comparisons in the mean fractional anisotropy.
Fig. 1.
The sagittal images display the reconstructed fibres traversing the genu of the corpus callosum in (A) healthy controls, (B) patients with a first episode of schizophrenia and (C) patients with chronic schizophrenia.
Statistical analysis for clinical features
We performed 1-way ANOVA to compare age and years of completed education, and we used the χ2 test to compare sex distribution among the 3 groups. Two-sample t tests were used to compare the age at onset of illness, the duration of illness, positive and negative PANSS scores and GAS scores between the 2 patient groups. We performed a linear correlation analysis to detect associations between fractional anisotropy values in all patients with schizophrenia that we obtained from the significant clusters in voxel-based DTI analysis and the global PANSS scores, GAS scores and duration of illness, as well as dosage of antipsychotic medications.
Results
Participants
We included 15 patients with a first-episode of schizophrenia and 15 patients with chronic schizophrenia (10 men and 5 women in each group) in the study. At the time of scanning, 11 patients were on a single antipsychotic medication, 6 patients were on 2 or more antipsychotic medications and only 1 was naive for medication. The medication information could not be determined for 12 participants. We also included 15 age- and sex-matched healthy controls (10 men and 5 women).
The demographic and clinical data for the 3 groups are shown in Table 1. There was no significant difference among the 3 groups regarding age, years of completed education and distribution of sex (all p > 0.05). The PANSS scores, age at onset of illness, dosage of antipsychotic medication equivalent to 100 mg/day of chlorpromazine were not significantly different between the 2 patient groups (all p > 0.05). However, patients with chronic schizophrenia had significantly lower GAS scores (p = 0.045) than patients with first-episode schizophrenia.
Table 1.
Demographic and clinical characteristics for all study participants
| Group; mean (SD)* |
|||
|---|---|---|---|
| Characteristic | Chronic schizophrenia, n = 15 | First-episode schizophrenia, n = 15 | Healthy controls, n = 15 |
| Age, yr | 24.3 (6.4) | 24.3 (6.4) | 24.2 (6.2) |
| Education, yr | 12.7 (2.5) | 12.1 (2.8) | 12.7 (3.2) |
| Sex, male:female | 10:5 | 10:5 | 10:5 |
| Handedness, left:right | 0:15 | 0:15 | 0:15 |
| Age of onset, yr | 20.5 (6.7) | 23.3 (6.5) | |
| Duration of illness, mo† | 45.4 (19.5) | 8.3 (5.3) | |
| PANSS score | |||
| Total | 88.5 (27.2) | 81.1 (25.1) | |
| Positive subscale | 19.7 (7.6) | 18.3 (5.8) | |
| Negative subscale | 19.1 (6.3) | 18.1 (6.1) | |
| Antipsychotic equivalent dosage of CPZ, mg | 544 (323) | 657 (444) | |
| GAS score† | 59.1 (16.3) | 70.3 (12.7) | |
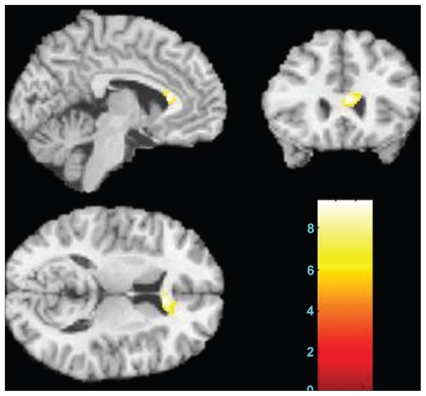
The results from voxel-based DTI analysis showed significant differences in fractional anisotropy values in the genu of the corpus callosum (p < 0.001, uncorrected; Fig. 2). There were no other regions showing significant differences among the 3 groups. Post-hoc analyses showed significantly lower fractional anisotropy values in the chronic group compared with the first-episode group (p = 0.020) and healthy participants (p < 0.001; Table 2). However, there was no significant difference between the first-episode group and healthy controls (p = 0.12; Table 2).
Fig. 2.
The sagittal, coronal and axial images display the genu of corpus callosum where fractional anisotropy values were significantly different among healthy controls, patients with a first episode of schizophrenia and patients with chronic schizophrenia (Montreal Neurological Institute coordinate for the maximal point of difference: x, y, z = 4, 26, 12 mm, 168 voxels, t = 3.33, p < 0.001 uncorrected). The colour bar represents the range of T values. The findings are displayed on a T1 template.
Table 2.
Fractional anisotropy and mean fractional anisotropy values in the genu of the corpus callosum
| Group; mean (SD) |
p value |
|||||
|---|---|---|---|---|---|---|
| Measure | Chronic schizoprenia, n = 15 | First-episode schizophrenia, n = 15 | Healthy controls, n = 15 | Chronic schizoprenia v. first-episode schizophrenia | First-episode schizophrenia v. healthy controls | Chronic schizoprenia v. healthy controls |
| Fractional anisotropy | 0.292 (0.043) | 0.335 (0.044) | 0.364 (0.060) | 0.020 | 0.12 | < 0.001 |
| Mean fractional anisotropy* | 0.335 (0.020) | 0.345 (0.015) | 0.355 (0.029) | 0.24 | 0.21 | 0.017 |
SD = standard deviation.
The mean fractional anisotropy of region-of-interest fibre.
Furthermore, we compared mean fractional anisotropy values of the white matter fibre tracts traversing the genu of the corpus callosum to verify our voxel-based DTI findings. We found that mean fractional anisotropy values showed significant differences among the 3 groups (F = 4.535, p = 0.039). Post-hoc tests demonstrated that the mean fractional anisotropy values were significantly lower in the genu of the corpus callosum in the patients with chronic schizophrenia than in healthy controls (p = 0.017); the patients with first-episode schizophrenia had fractional anisotropy values between those of the patients with chronic schizophrenia (p = 0.24) and the healthy controls (p = 0.21), but did not show significant difference (Table 2).
Linear correlation analyses showed that fractional anisotropy values in the genu of the corpus callosum in all patients with schizophrenia were positively correlated with GAS scores (r = 0.567, p = 0.001) and negatively correlated with global PANSS scores (r = −0.468, p = 0.009).
Discussion
Converging evidence from neuropathological,17–20 postmortem,21,22 neurogenetic18,23,24 and neuroimaging25–27 studies has increasingly implicated the corpus callosum in schizophrenia. The present study, combining VBA and fibre-tracking DTI techniques, found that fractional anisotropy was significantly reduced in the genu of the corpus callosum in patients with chronic schizophrenia, but not in those with first-episode schizophrenia. This finding suggests that the corpus callosum integrity abnormalities may not emerge or are too subtle to detect by DTI techniques at the early stage of the illness and that these deficits are progressive.
Consistent with our findings, recent DTI studies have reported decreased fractional anisotropy values in patients with chronic schizophrenia and less pronounced differences in patients with first-episode schizophrenia. For example, Pomarol-Clotet and colleagues28 reported a positive finding in patients with chronic schizophrenia, but Peters and colleagues9 and Price and colleagues29 could not detect corpus callosum abnormalities in patients with first-episode schizophrenia, nor in participants at ultra-high risk for psychosis. Most interestingly, Friedman and colleagues30 conducted a cross-sectional DTI study including patients with first-episode and chronic schizophrenia and obtained similar findings to those of our current study: less prominent changes in white matter at illness onset that progress in more chronic states of illness. In the study by Friedman and colleagues, the authors set 2 control groups to match the first-episode and chronic patient groups, respectively, for age and sex. Since aging has been found to be related to white matter integrity in healthy populations31 and in populations with schizophrenia,32–34 we enrolled age- and sex-matched groups to minimize the effect of aging. Of note, the findings from a study by Mitelman and colleagues35 contrasted those of the above studies. The discrepancy may be related to differences in participant characteristics and DTI methodologies.
Corpus callosum abnormalities in patients with schizophrenia have been increasingly reported, and the genu region is the most commonly reported. Fasciculi crossing the genu of the corpus callosum terminate in the bilateral pre-frontal cortex,36 perhaps mediating cognitive functions. These functions have been related with symptom severity and outcome in schizophrenia.37–39 In this study, we found that fractional anisotropy in the genu of the corpus callosum was negatively correlated with total PANSS score and positively correlated with GAS scores in patients with schizophrenia; these results were consistent with those of a prior study.40 A study of patients with chronic schizophrenia also reported similar results: specifically, that fractional anisotropy decreased in the areas of the corpus callosum interconnecting with frontal regions and that the integrity of the anterior corpus callosum was significantly correlated with negative and positive symptoms.41 Mitelman and colleagues35,42,43 found that patients with schizophrenia and poor outcomes showed greater decline in fractional anisotropy and smaller corpus callosum size compared with patients with good outcomes. All these studies suggest that fractional anisotropy values may be an indicator of illness severity and outcome in patients with schizophrenia.
Interestingly, some studies reported significant negative correlations between clinical symptoms and fractional anisotropy values in the right anterior cingulum, left uncinate and superior longitudinal fasciculus and inferior frontal white matter,44–46 suggesting that impaired white matter integrity in patients with schizophrenia is associated with clinical symptoms. Additionally, Karlsgodt and colleagues47 found that lower baseline fractional anisotropy values in the medial temporal lobe and inferior longitudinal fasciculus predicted deterioration in social and role functioning in participants at ultra-high risk for schizophrenia.
In addition to the corpus callosum, other white matter bundles and regions have shown altered white matter integrity in schizophrenia. For example, Konrad and Winterer48 reviewed 26 DTI studies of schizophrenia and identified the most frequently reported white matter brain regions with decreased fractional anisotropy values located in the prefrontal and temporal lobes and fiber tracts between these areas (cingulum, arcuate and uncinate fasciculi), which all play distinct roles in cognitive function; dysfunction in these brain regions is associated with schizophrenia.26,49
Limitations
There are some limitations to this study. The study was cross-sectional. In addition, most patients were medicated during the study. The current study only identified differences in the genu of the corpus callosum among the 3 groups. The discrepancies might be related to sample heterogeneity, such as differences in age, illness duration, sex distributions and medication history, all of which could affect white matter integrity.30 In our study, a comparatively large smoothing kernel was used, therefore small fibre or minor impairment may not have been detected.
Conclusion
To our knowledge, ours is the first study to directly compare patients with first-episode or chronic schizophrenia with a healthy control group using 2 complementary DTI techniques (voxel-based and fibre-tracking DTI) simultaneously. Both DTI techniques identified reduced fractional anisotropy values in the genu of the corpus callosum in patients with chronic schizophrenia and less robust abnormalities in patients with first-episode schizophrenia compared with healthy controls.
A longitudinal study for patients with first-episode schizophrenia is warranted to draw a more definite conclusion. Future studies will also be needed to systematically assess potential influences of treatment on white matter integrity. Additionally, owing to our modest sample size, replication in larger samples will be important.
Acknowledgements
The authors gratefully acknowledge Zhong He from the Department of Radiology of Second Xiangya Hospital, Central South University, for his assistance in image acquisition.
Footnotes
Competing interests: The authors were supported by research grants from the National Natural Science Foundation of China (30670752, Zhening Liu), the National Basic Research Program of China (2006CB500808 and 2007CB512300), the 11th Five Year Key Program for Science and Technology Development of China (2007BAI17B05) and National Institution of Health (K01MH086621, Fei Wang).
Contributors: Drs. Kong, Zhao, Xue, Jiang and Z. Liu designed the study. Drs. Kong, Ouyang, Tao, H. Liu and Li acquired the data, which Drs. Kong, Ouyang, Tao, H. Liu, Li, Wang and Shan analyzed. Drs. Kong, Ouyang, Wang and Z. Liu wrote the article, which Drs. Tao, H. Liu, Li, Zhao, Xue, Wang, Jiang, Shan and Z. Liu critically reviewed. All authors approved publication of the article.
References
- 1.Gazzaniga MS. Cerebral specialization and interhemispheric communication: Does the corpus callosum enable the human condition? Brain. 2000;123:1293–326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- 2.Brewer WJ, Wood SJ, Phillips LJ, et al. Generalized and specific cognitive performance in clinical high-risk cohorts: a review highlighting potential vulnerability markers for psychosis. Schizophr Bull. 2006;32:538–55. doi: 10.1093/schbul/sbj077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price G, Cercignani M, Parker GJ, et al. Abnormal brain connectivity in first-episode psychosis: a diffusion MRI tractography study of the corpus callosum. Neuroimage. 2007;35:458–66. doi: 10.1016/j.neuroimage.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caan MW, Vermeer KA, van Vliet LJ, et al. Shaving diffusion tensor images in discriminant analysis: a study into schizophrenia. Med Image Anal. 2006;10:841–9. doi: 10.1016/j.media.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Gasparotti R, Valsecchi P, Carletti F, et al. Reduced fractional anisotropy of corpus callosum in first-contact, antipsychotic drug-naive patients with schizophrenia. Schizophr Res. 2009;108:41–8. doi: 10.1016/j.schres.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Cheung V, Cheung C, McAlonan GM, et al. A diffusion tensor imaging study of structural dysconnectivity in never-medicated, first-episode schizophrenia. Psychol Med. 2008;38:877–85. doi: 10.1017/S0033291707001808. [DOI] [PubMed] [Google Scholar]
- 7.Koch K, Wagner G, Dahnke R, et al. Disrupted white matter integrity of corticopontine-cerebellar circuitry in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2010;260:419–26. doi: 10.1007/s00406-009-0087-0. [DOI] [PubMed] [Google Scholar]
- 8.Rotarska-Jagiela A, Schoenmeyer R, Oertel-Knoechel V, et al. Anatomical brain connectivity and positive symptoms of schizophrenia: a diffusion tensor imaging study. Psychiatry Res. 2009;174:9–16. doi: 10.1016/j.pscychresns.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Peters BD, de Haan L, Dekker N, et al. White matter fibertracking in first-episode schizophrenia, schizoaffective patients and subjects at ultra-high risk of psychosis. Neuropsychobiology. 2008;58:19–28. doi: 10.1159/000154476. [DOI] [PubMed] [Google Scholar]
- 10.Ellison-Wright I, Glahn DC, Laird AR, et al. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–23. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YF. Chinese classification of mental disorders (CCMD-3): towards integration in international classification. Psychopathology. 2002;35:171–5. doi: 10.1159/000065140. [DOI] [PubMed] [Google Scholar]
- 12.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-I/P) 2nd ed. New York (NY): Biometrics Research, New York State Psychiatric Institute; 1996. [Google Scholar]
- 13.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 14.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York (NY): Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 15.Endicott J, Spitzer RL, Fleiss JL, et al. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33:766–71. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 16.Hao Y, Liu Z, Jiang T, et al. White matter integrity of the whole brain is disrupted in first-episode schizophrenia. Neuroreport. 2006;17:23–6. doi: 10.1097/01.wnr.0000195664.15090.46. [DOI] [PubMed] [Google Scholar]
- 17.Coger RW, Serafetinides EA. Schizophrenia, corpus callosum, and interhemispheric communication: a review. Psychiatry Res. 1990;34:163–84. doi: 10.1016/0165-1781(90)90017-y. [DOI] [PubMed] [Google Scholar]
- 18.Walterfang M, Wood SJ, Velakoulis D, et al. Neuropathological, neurogenetic and neuroimaging evidence for white matter pathology in schizophrenia. Neurosci Biobehav Rev. 2006;30:918–48. doi: 10.1016/j.neubiorev.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Foong J, Symms MR, Barker GJ, et al. Neuropathological abnormalities in schizophrenia: evidence from magnetization transfer imaging. Brain. 2001;124:882–92. doi: 10.1093/brain/124.5.882. [DOI] [PubMed] [Google Scholar]
- 20.Davis KL, Stewart DG, Friedman JI, et al. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60:443–56. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- 21.Highley JR, Esiri MM, McDonald B, et al. The size and fibre composition of the corpus callosum with respect to gender and schizophrenia: a post-mortem study. Brain. 1999;122:99–110. doi: 10.1093/brain/122.1.99. [DOI] [PubMed] [Google Scholar]
- 22.Rosenthal R, Bigelow LB. Quantitative brain measurements in chronic schizophrenia. Br J Psychiatry. 1972;121:259–64. doi: 10.1192/bjp.121.3.259. [DOI] [PubMed] [Google Scholar]
- 23.Narr KL, Cannon TD, Woods RP, et al. Genetic contributions to altered callosal morphology in schizophrenia. J Neurosci. 2002;22:3720–9. doi: 10.1523/JNEUROSCI.22-09-03720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camchong J, Lim KO, Sponheim SR, et al. Frontal white matter integrity as an endophenotype for schizophrenia: diffusion tensor imaging in monozygotic twins and patients’ nonpsychotic relatives. Front Hum Neurosci. 2009;3:35. doi: 10.3389/neuro.09.035.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanaan RA, Kim JS, Kaufmann WE, et al. Diffusion tensor imaging in schizophrenia. Biol Psychiatry. 2005;58:921–9. doi: 10.1016/j.biopsych.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 26.Kubicki M, McCarley R, Westin CF, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White T, Nelson M, Lim KO. Diffusion tensor imaging in psychiatric disorders. Top Magn Reson Imaging. 2008;19:97–109. doi: 10.1097/RMR.0b013e3181809f1e. [DOI] [PubMed] [Google Scholar]
- 28.Pomarol-Clotet E, Canales-Rodriguez EJ, Salvador R, et al. Medial prefrontal cortex pathology in schizophrenia as revealed by convergent findings from multimodal imaging. Mol Psychiatry. 2010;15:823–30. doi: 10.1038/mp.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price G, Bagary MS, Cercignani M, et al. The corpus callosum in first episode schizophrenia: a diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2005;76:585–7. doi: 10.1136/jnnp.2004.042952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedman JI, Tang C, Carpenter D, et al. Diffusion tensor imaging findings in first-episode and chronic schizophrenia patients. Am J Psychiatry. 2008;165:1024–32. doi: 10.1176/appi.ajp.2008.07101640. [DOI] [PubMed] [Google Scholar]
- 31.Yang J, Guo Y, Gao Y, et al. A MRI quantitative study of corpus callosum in normal adults. NeuroImage. 2009;47(Suppl 1):S175. [Google Scholar]
- 32.Pfefferbaum A, Sullivan EV, Hedehus M, et al. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med. 2000;44:259–68. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 33.Jones DK, Catani M, Pierpaoli C, et al. Age effects on diffusion tensor magnetic resonance imaging tractography measures of frontal cortex connections in schizophrenia. Hum Brain Mapp. 2006;27:230–8. doi: 10.1002/hbm.20179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenberger G, Kubicki M, Nestor PG, et al. Age-related deficits in fronto-temporal connections in schizophrenia: a diffusion tensor imaging study. Schizophr Res. 2008;102:181–8. doi: 10.1016/j.schres.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitelman SA, Torosjan Y, Newmark RE, et al. Internal capsule, corpus callosum and long associative fibers in good and poor outcome schizophrenia: a diffusion tensor imaging survey. Schizophr Res. 2007;92:211–24. doi: 10.1016/j.schres.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 36.Pandya DN, Seltzer B. The topography of commissural fibers. In: Leporé F, Prito M, Jasper HH, editors. Two hemispheres, one brain Functions of the corpus callosum. New York (NY): Alan R. Liss, Inc; 1986. pp. 47–73. [Google Scholar]
- 37.Sanfilipo M, Lafargue T, Rusinek H, et al. Cognitive performance in schizophrenia: relationship to regional brain volumes and psychiatric symptoms. Psychiatry Res. 2002;116:1–23. doi: 10.1016/s0925-4927(02)00046-x. [DOI] [PubMed] [Google Scholar]
- 38.Shergill SS, Kanaan RA, Chitnis XA, et al. A diffusion tensor imaging study of fasciculi in schizophrenia. Am J Psychiatry. 2007;164:467–73. doi: 10.1176/ajp.2007.164.3.467. [DOI] [PubMed] [Google Scholar]
- 39.Prasad KMR, Sahni SD, Rohm BR, et al. Dorsolateral prefrontal cortex morphology and short-term outcome in first-episode schizophrenia. Psychiatry Res. 2005;140:147–55. doi: 10.1016/j.pscychresns.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Skelly LR, Calhoun V, Meda SA, et al. Diffusion tensor imaging in schizophrenia: relationship to symptoms. Schizophr Res. 2008;98:157–62. doi: 10.1016/j.schres.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kubicki M, Styner M, Bouix S, et al. Reduced interhemispheric connectivity in schizophrenia-tractography based segmentation of the corpus callosum. Schizophr Res. 2008;106:125–31. doi: 10.1016/j.schres.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitelman SA, Newmark RE, Torosjan Y, et al. White matter fractional anisotropy and outcome in schizophrenia. Schizophr Res. 2006;87:138–59. doi: 10.1016/j.schres.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 43.Mitelman SA, Nikiforova YK, Canfield EL, et al. A longitudinal study of the corpus callosum in chronic schizophrenia. Schizophr Res. 2009;114:144–53. doi: 10.1016/j.schres.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang J, Liao Y, Zhou B, et al. Abnormal anterior cingulum integrity in first episode, early onset schizophrenia: a diffusion tensor imaging study. Brain Res. 2010;1343:199–205. doi: 10.1016/j.brainres.2010.04.083. [DOI] [PubMed] [Google Scholar]
- 45.Skelly LR, Calhoun V, Meda SA, et al. Diffusion tensor imaging in schizophrenia: relationship to symptoms. Schizophr Res. 2008;98:157–62. doi: 10.1016/j.schres.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolkin A, Choi SJ, Szilagyi S, et al. Inferior frontal white matter anisotropy and negative symptoms of schizophrenia: a diffusion tensor imaging study. Am J Psychiatry. 2003;160:572–4. doi: 10.1176/appi.ajp.160.3.572. [DOI] [PubMed] [Google Scholar]
- 47.Karlsgodt KH, Niendam TA, Bearden CE, et al. White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biol Psychiatry. 2009;66:562–9. doi: 10.1016/j.biopsych.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Konrad A, Winterer G. Disturbed structural connectivity in schizophrenia primary factor in pathology or epiphenomenon? Schizophr Bull. 2008;34:72–92. doi: 10.1093/schbul/sbm034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barch DM. The cognitive neuroscience of schizophrenia. Annu Rev Clin Psychol. 2005;1:321–53. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]