Abstract
Background
Reduced prepulse inhibition (PPI) of the auditory startle reflex is a hallmark feature of attention-processing deficits in patients with schizophrenia and other psychotic disorders. Recent evidence suggests that these deficits may also be present before the onset of psychosis in individuals at ultra-high risk (UHR) and become progressively worse as psychosis develops. We conducted a longitudinal follow-up study to observe the development of PPI over time in UHR adolescents and healthy controls.
Methods
Two-year follow-up data of PPI measures were compared between UHR adolescents and a matched control group of typically developing individuals.
Results
We included 42 UHR adolescents and 32 matched controls in our study. Compared with controls, UHR individuals showed reduced PPI at both assessments. Clinical improvement in UHR individuals was associated with an increase in PPI parameters.
Limitations
A developmental increase in startle magnitude partially confined the interpretation of the association between clinical status and PPI. Furthermore, post hoc analyses for UHR individuals who became psychotic between assessments had limited power owing to a low transition rate (14%).
Conclusion
Deficits in PPI are present before the onset of psychosis and represent a stable vulnerability marker over time in UHR individuals. The magnitude of this marker may partially depend on the severity of clinical symptoms.
Introduction
Disrupted filtering of preattentive, irrelevant sensory information, as measured by prepulse inhibition (PPI) of the auditory startle blink response, is thought to represent a core feature of schizophrenia.1 Prepulse inhibition is the automatic suppression of startle magnitude that occurs when the startling stimulus is preceded for 30–500 ms by a weak prepulse.2,3 It has consistently been shown that PPI is diminished in individuals with schizophrenia and other psychotic disorders, individuals in remission of psychosis and unaffected relatives.4–6 These findings suggest that reduced PPI may serve as a vulnerability marker for the onset of overt psychosis and thereby potentially aid in the early detection and prevention of imminent psychosis. However, the timing of manifestation and predictive validity of this marker are still unclear.
Clinical or ultra-high risk (UHR) designs have helped identify individuals at increased risk for psychosis on the basis of subsyndromal symptoms.7 Although transition rates vary between UHR study sites, the incidence of psychosis is generally high and occurs in relatively close proximity to initial intake.8,9 A previous cross-sectional study investigating acoustic PPI in UHR adults demonstrated a reduction in PPI for prodromal individuals and a negative association between startle reactivity and severity of clinical symptoms.10 Additionally, a recent study from our group also found a reduction of PPI in UHR adolescents who were not taking antipsychotic medication (unpublished data, 2009), suggesting an early presence of this particular vulnerability marker. Owing to an absence of longitudinal reports in UHR individuals, it is unknown whether PPI represents a stable vulnerability marker of the prodromal state or whether it is associated with change in clinical status over time.
To investigate change over time, we collected longitudinal PPI data in a 2-year follow-up of UHR adolescents and typically developing controls (aged 12–18 yr at baseline). The first aim of this study was to determine whether suppressed PPI is a stable vulnerability marker for UHR individuals over time. We hypothesized that UHR individuals would show lower startle reactivity and %PPI over time than controls and that the magnitude of this group difference would depend on the general clinical outcome. The second aim was to explore post hoc whether onset of psychosis between assessments was associated with increased reduction of PPI parameters. We hypothesized that UHR individuals with a psychotic transition (UHR-P) would show more pronounced reduction in startle reactivity (which has also been reported in people with schizophrenia11,12) and %PPI than UHR individuals without psychosis (UHR-NP). Finally, we hypothesized that a change in PPI over time would show a correlation with a change in clinical symptom scores over time.
Methods
Participants
This study is part of the Dutch Prediction of Psychosis Study, a longitudinal research project at the University Medical Center Utrecht in Utrecht, the Netherlands,13 and was approved by the Dutch Central Committee on Research Involving Human Subjects. Adolescents meeting at least 1 criterion of 4 for UHR were referred by general practitioners or other psychiatric clinics at baseline (T1). We recruited the control group of typically developing adolescents from secondary schools in the region of Utrecht. Both groups participated in a follow-up assessment 2 years after the initial assessment (T2). All individuals were between 12 and 18 years of age at baseline recruitment and were included after informed consent was given. Consent could be withdrawn at any time during the follow-up period or after a clinical expert opinion that an individual was incapable of adequately judging the nature of participation in the research project. Individuals younger than 16 years of age signed for assent, and their parents signed for consent. Individuals aged 16 years or older provided informed consent themselves.
Ultra-high risk status was assigned to individuals who met at least 1 criterion of 4 for UHR at T1. The UHR criteria employed in this study have previously been published14 and resemble other frequently used criteria for UHR.8 Briefly, the first 3 inclusion criteria were 1) attenuated positive symptoms; 2) brief, limited or intermittent psychotic symptoms; and 3) a 30% reduction in overall level of social, occupational/school and psychologic functioning (i.e., global assessment of functioning [GAF]) in the past year, combined with a genetic risk of psychosis. We assessed these criteria using the Structured Interview for Prodromal Symptoms (SIPS).15 The fourth inclusion criterion was 2 or more of a selection of 9 basic symptoms, such as subjective deficits in cognitive functioning; we assessed this criterion using the Bonn Scale for the Assessment of Basic Symptoms-Prediction List(BSABS-P).16
Presence of a psychotic transition during follow-up was determined by use of the SIPS. A SIPS diagnosis of a psychotic syndrome refers to psychotic symptoms of particular intensity (e.g., delusional conviction) and frequency or duration (≥ 1 h/d for ≥ 4 d/wk during the past month) or of particular impact (seriously disorganized or dangerous), designed to operationalize the threshold for a DSM-IV17 Axis I psychotic disorder diagnosis.9 We performed chart reviews to retrospectively confirm psychotic transition by clinical consensus (H.vE., P.S.), and diagnoses were subsequently derived according to DSM-IV guidelines.
We excluded controls if they met 1 of the UHR criteria, if they or any first-degree relative had a history of any psychiatric illness, or if there was a second-degree relative with a psychotic disorder. Both controls and UHR individuals were excluded if there was evidence of any past or present neurologic disorder (e.g., epilepsy). Drug and alcohol abuse were additional exclusion criteria, although UHR individuals were permitted a history of drug use if symptoms had also been present in the absence of drugs. We assessed verbal intellectual functioning with the Wechsler Intelligence Scales.18,19 For participants who smoked, smoking was allowed ad libitum before assessment.
Prepulse inhibition paradigm
Participants were seated upright in a comfortable chair in an acoustically shielded room. Auditory stimuli were presented binaurally through stereo insert earphones (Eartone ABR). Software settings were calibrated by an artificial ear (Brüel and Kjær, type 4152) to ensure adequate stimulus intensities.
Recordings were obtained by using a BioSemi Active Two system. Two extra system electrodes called CMS/DRL provided the mechanism for internally referencing the signals: the Common Mode Sense (CMS) electrode is the online reference, and the Driven Right Leg (DRL) electrode provides the active ground for the system. Electromyographic (EMG) activity of the right orbicularis oculi muscle was recorded from bipolar electrodes. One was placed over the medial aspect of the muscle and one displaced 0.5 cm laterally. All data were analyzed using the software package Brain Vision Analyzer (Brain Products GmbH).
The PPI paradigm was assessed at a fixed order in a neurophysiologic test battery (6 out of a total of 8 tasks) that lasted about 2 hours in total, including preparation time. Before testing, 4 startle stimuli of rising intensity were presented, 2 of which were preceded by a prepulse, to accustom the participants to the loud noise. The actual experiment consisted of 24 randomized trials: 12 startle stimuli preceded by a pre-pulse and 12 startle stimuli with no prepulse. The prepulse and startle stimuli consisted of bursts of white noise (duration 22.5 and 32.5 ms, intensity 87 and 106.5 dB(A), respectively, rise/fall 0.1 ms) over a 30 dB(A) background noise, with a fixed interstimulus interval of 120 ms. The intertrial interval varied between 12 and 23 seconds.
Data were filtered offline with a high-pass filter of 30 Hz and a low-pass filter of 200 Hz. Epochs from 50 ms prestimulus until 200 ms poststimulus were extracted from the continuous data, and the baseline was corrected using the data for 50 ms before stimulus onset. Thereafter, the data were rectified. Finally, assessment of the maximal peak amplitude and PPI quantification took place within a window of 20–90 ms after stimulus onset using a computerized algorithm for peak detection.
Startle amplitude was defined as the amplitude of the response to the pulse alone trials. We defined %PPI as the percentage of reduction of the startle amplitude over prepulse–pulse trials, compared with the pulse alone trials (PPI = 100 × [1 – pp/p]), where pp indicates amplitude over prepulse trials and p indicates amplitude over pulse alone trials.
Statistical analysis
We compared group characteristics using analysis of variance (ANOVA) for age, IQ and clinical scores. We performed χ2 tests for sex distribution and handedness. Repeated-measures ANOVAs were conducted for clinical scores to determine outcome over time. Nonparametric analyses yielded similar results and are therefore not reported. Startle reactivity amplitudes were log-transformed to diminish the effect of a skewed distribution. For the same reason, 3 extreme outliers (2 controls, 1 UHR individual), falling 3 standard deviations from the mean of absolute difference scores between T1 and T2, were removed for %PPI analyses. Repeated-measures ANOVAs were used, with startle parameters as the within-subject variables and group (UHR v. controls) as a between-subject factor. Using ANOVA, we checked data at T1 for potential baseline differences between UHR individuals and controls. We repeated all startle analyses for comparisons between control, UHR-P and UHR-NP groups using Tukey honestly significant difference post hoc tests to identify potential subgroup differences. Cohen’s d was used to report effect sizes for startle parameters. For all analyses, we considered results to be statistically significant at α = 0.05, 2-tailed. To examine potential influence of moderating effects of sex, medication and smoking on startle parameters, we conducted separate analyses for these variables. Spearman correlation coefficients were used to explore the relation between PPI and clinical scores in UHR individuals.
Results
Participants
We included 42 adolescents meeting at least 1 of 4 criteria for UHR and 32 matched controls aged 12–18 years at baseline in this study. All individuals had a level of verbal intellectual functioning of 75 or greater, as assessed with the Wechsler Intelligence Scales.18,19 The DSM-IV Axis I diagnoses were as follows: 295.10 schizophrenia, disorganized type (n = 1); 295.30 schizophrenia, paranoid subtype (n = 3); 295.70 schizo-affective disorder (n = 1); and 296.04 bipolar I disorder (n = 1). None of these individuals was considered to have overt psychotic symptoms at the time of T2 assessment. Demographic and clinical characteristics, including smoking status and medication use, are reported in Table 1.
Table 1.
Demographic characteristics and symptom scores for controls and individuals at ultra-high risk for psychosis, separated into subgroups with and without transition to psychosis
| Group; mean (SD)* |
Controls v. UHR |
Controls v. UHR-NP v. UHR-P |
||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Controls, n = 32 | UHR, n = 42 | UHR-NP, n = 36 | UHR-P, n = 6 | χ2/F | p value | χ2/F | p value |
| Sex, male:female (% male) | 15:17 (47) | 25:17 (60) | 20:16 (56) | 5:1 (83) | χ2 = 1.17 | 0.27 | χ2 = 2.77 | 0.25 |
| Handedness, right/left/mixed (% right) | 27/3/2 (84) | 38/3/1 (90) | 33/2/1 (92) | 5/1/0 (83) | χ2 = 0.86 | 0.65 | χ2 = 1.78 | 0.78 |
| Premorbid IQ† | 106.0 (9.6) | 100.6 (13.5) | 102.1 (13.4) | 91.1 (10.5) | F = 3.76 | 0.06 | F = 4.22 | 0.018 |
| Age, yr | ||||||||
| At baseline | 15.5 (1.7) | 15.6 (2.1) | 15.4 (2.0) | 17.0 (2.5) | F = 0.15 | 0.70 | F = 1.79 | 0.17 |
| At 2-yr follow-up | 17.5 (1.8) | 17.6 (2.1) | 17.3 (2.0) | 19.1 (2.5) | F = 0.02 | 0.89 | F = 2.28 | 0.11 |
| SIPS total score | ||||||||
| At baseline‡ | 1.5 (2.1) | 24.7 (11.4) | 22.9 (10.8) | 35.2 (9.8) | F = 129.56 | < 0.001 | F = 80.21 | < 0.001 |
| At 2-yr follow-up‡ | 3.4 (3.3) | 16.6 (11.4) | 15.2 (10.7) | 25.0 (12.6) | F = 40.79 | < 0.001 | F = 25.44 | < 0.001 |
| BSABS-P, total score | ||||||||
| At baseline§ | 1.0 (1.5) | 21.4 (15.2) | 20.5 (14.0) | 20.8 (15.0) | F = 56.92 | < 0.001 | F = 29.86 | < 0.001 |
| At 2-yr follow-up‡ | 0.9 (1.2) | 6.4 (1.2) | 4.9 (7.1) | 15.5 (16.3) | F = 10.63 | < 0.001 | F = 12.75 | < 0.001 |
| GAF score | ||||||||
| At baseline§ | 94.0 (6.6) | 58.4 (15.2) | 58.5 (15.3) | 57.7 (16.4) | F = 151.95 | < 0.001 | F = 74.96 | < 0.001 |
| At 2-yr follow-up§ | 88.8 (7.7) | 60.8 (12.8) | 61.9 (12.9) | 54.0 (11.3) | F = 118.78 | < 0.001 | F = 62.25 | < 0.001 |
| Days from baseline to transition | NA | NA | NA | 299 (159) | NA | NA | ||
| Days from transition to baseline | NA | NA | NA | 484 (187) | NA | NA | ||
| Smoking, no. (%) | ||||||||
| At baseline§ | 0 (0) | 9 (21) | 8 (22) | 1 (17) | χ2 = 7.54 | 0.005 | χ2 = 7.56 | 0.019 |
| At 2-yr follow-up§ | 1 (3) | 13 (31) | 12 (33) | 1 (17) | χ2 = 9.87 | 0.001 | χ2 = 10.55 | 0.003 |
| Medication at baseline, no. (%)¶ | NA | NA | ||||||
| Any | 15 (36) | 12 (33) | 3 (50) | χ2 = 0.62 | 0.43 | |||
| Antipsychotic** | 7 (17) | 7 (19) | 1 (17) | χ2 = 0.26 | 0.87 | |||
| Mood stabilizer | 6 (14) | 4 (11) | 2 (33) | χ2 = 2.07 | 0.15 | |||
| Psychostimulant | 3 (7) | 3 (13) | 0 (0) | χ2 = 0.54 | 0.46 | |||
| Other | 1 (2) | 1 (3) | 0 (0) | χ2 = 0.17 | 0.68 | |||
| Medication at 2-yr follow-up, no. (%)§ | NA | NA | ||||||
| Any | 16 (38) | 13 (36) | 3 (50) | χ2 = 0.42 | 0.52 | |||
| Antipsychotic†† | 7 (5) | 5 (14) | 2 (33) | χ2 = 1.40 | 0.24 | |||
| Mood stabilizer | 6 (14) | 5 (14) | 1 (17) | χ2 = 0.03 | 0.86 | |||
| Psychostimulant | 5 (12) | 5 (14) | 0 (0) | χ2 = 0.95 | 0.33 | |||
| Anxiolytic | 0 (0) | 0 (0) | 0 (0) | NA | ||||
| Other | 2 (4) | 1 (3) | 1 (17) | χ2 = 5.34 | 0.07 | |||
BSABS-P = Bonn Scale for the Assessment of Basic Symptoms-Prediction List;17 GAF = Global Assessment of Functioning; NA = not applicable; SD = standard deviation; SIPS = Structured Interview for Prodromal Symptoms;16 UHR = total group at ultra-high risk for psychosis; UHR-NP = subgroup of people at ultra-high risk for psychosis who do not transition to psychosis; UHR-P = subgroup of people at ultra-high risk for psychosis who transition to psychosis.
Unless otherwise indicated.
Post hoc comparisons significant (p < 0.05) for controls versus UHR-P.
Post hoc comparisons significant (p < 0.05) for controls versus UHR-NP, controls versus UHR-P and UHR-NP versus UHR-P.
Post hoc comparisons significant (p < 0.05) for controls versus UHR-NP and controls versus UHR-P.
Comparisons between UHR-NP versus UHR-P only.
All UHR-NP individuals used atypical antipsychotic medication; the UHR-P individual used typical antipsychotic medication.
All UHR individuals used atypical antipsychotic medication.
Groups were matched for sex, handedness, IQ and age. The UHR individuals reported more clinical symptoms than controls at T1 and T2 (all p < 0.001), and there was a significant time × group within-subject interaction for all clinical scales (all p < 0.001). The UHR individuals showed a decrease in symptoms (total SIPS and total BSABS-P) and an increase in daily functioning (GAF) over time compared with controls (all p < 0.001).
Post hoc tests showed an IQ difference between groups with lower total IQ scores for the UHR-P group than controls (p = 0.017). Both the UHR-NP and UHR-P groups reported more clinical symptoms than controls at T1 and T2 (all p < 0.001). Additionally, the UHR-P group reported more clinical symptoms than the UHR-NP group for total SIPS scores at T1 and T2 and total BSABS-P scores at T2 (all p < 0.05).
Startle reactivity
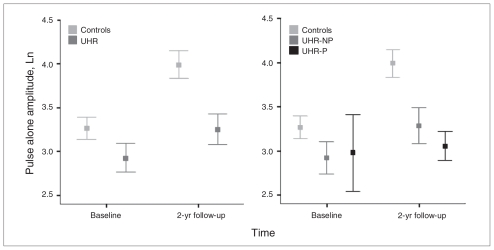
There was a main effect for pulse alone amplitude, caused by an increase in amplitude over time (F1.1, 72 = 27.27, p < 0.001). Additionally, there was a group difference, with UHR individuals showing lower amplitude than controls (F1.1,72 = 6.60, p = 0.010; Fig. 1). The time × group within-subject interaction indicated a trend (F1.1,72 = 3.93, p = 0.05), and there was no difference in amplitude between groups at T1 (t71.15 = 1.62, p = 0.11). A post hoc group difference was found only between controls and the UHR-NP group (p = 0.048), but the effect size of the group differences was larger for the UHR-P than the UHR-NP group compared with controls (d = 0.53 and 0.74, respectively. Because the UHR-P group was not ideally matched for age, sex and total IQ, the analysis was repeated with age at T1, sex and total IQ added as covariates, which did not affect the outcome.
Fig. 1.
Means of the log-transformed pulse alone amplitude for the control and ultra-high risk (UHR) groups at baseline (T1) and 2-year follow-up (T2) assessment (left), separated for UHR individuals who did not experience a psychotic transition (UHR-NP) and those who did (UHR-P; right).
Prepulse inhibition
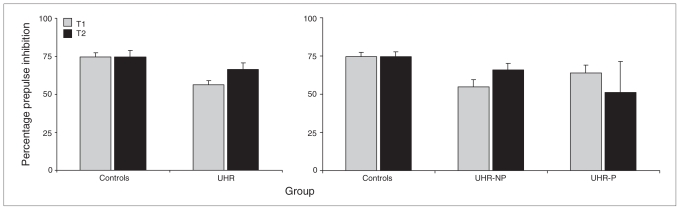
The UHR individuals exhibited a lower %PPI than controls over time (F1.1,69 = 7.20, p = 0.009), and there was a significant time × group interaction (F1.1,69 = 4.17, p = 0.045), potentially due to a relative increase in %PPI over time for UHR individuals and a stable %PPI for controls (Fig. 2). This group difference was already apparent at T1 (F1,72 = 12.49, p = 0.001). Post hoc %PPI differed only between controls and the UHR-NP group (p = 0.028). As with startle reactivity, the effect size of the group differences was larger for the UHR-P than the UHR-NP group compared with controls (d = 0.67 and 1.05, respectively). Including age at T1, sex or total IQ as covariates for this analysis did not affect the outcome.
Fig. 2.
Mean percentage prepulse inhibition (+ 1 standard error) for the control and ultra-high risk (UHR) groups at baseline (T1) and 2-year follow-up (T2) assessment (left), separated for UHR individuals who did not experience a psychotic transition (UHR-NP) and those who did (UHR-P; right).
Sex
No differences were found on startle parameters when boys were compared with girls for the entire sample, nor when the control and UHR groups were separated. Adding sex as a covariate to the initial group analyses also failed to reveal an influence of sex on startle parameters.
Medication
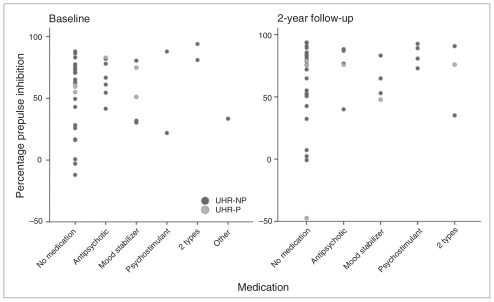
For pulse alone amplitude and %PPI change over time, there were no differences between UHR individuals who never used medication (n = 22), UHR individuals who used medication at either T1 or T2 (n = 9) and UHR individuals who used medication at both assessments (n = 11). The %PPI for different medication types are displayed in Figure 3. Removing all individuals who received medication at T1 or T2 (n = 20) from the analyses did not change the outcome of the initial comparisons between UHR individuals and controls.
Fig. 3.
Percentage prepulse inhibition per ultra-high risk (UHR) individual, categorized for each medication type at baseline (T1) assessment (left) and 2-year follow-up (T2) assessment (right). Individuals who did not experience a psychotic transition during follow-up (UHR-NP) are indicated with dark circles and those with a psychotic transition between assessments (UHR-P) are indicated with light circles.
Smoking
Only 1 individual in the control group smoked on a regular basis at T2 (Table 1), therefore a minimum influence of smoking status on PPI was assumed for the control group. For pulse alone amplitude and %PPI change over time, there were no differences between UHR individuals who never smoked (n = 27), UHR individuals who smoked regularly at either T1 or T2 (n = 8) and UHR individuals who smoked at both assessments (n = 7). Removing all individuals who smoked at T1 or T2 from the analyses (n = 15) did not change the outcome of the initial comparisons between UHR individuals and controls, except the interaction effect between %PPI and group was no longer significant (F1.1,54 = 3.82, p = 0.06).
Correlation between PPI and clinical variables
Correlations between PPI and clinical variables are displayed in Table 2. There were significant correlations between startle reactivity and GAF scores at T1 (p = 0.039) and between %PPI and total SIPS scores at T2 (p = 0.048). Change over time in both PPI parameters was associated with a change over time in total BSABS-P scores (p = 0.031 and p = 0.034, respectively).
Table 2.
Correlations between prepulse inhibition variables and clinical variables in the group of individuals at ultra-high risk for psychosis
| Correlation | Startle reactivity | %PPI |
|---|---|---|
| At baseline | ||
| Total SIPS | R = 0.078 | R = −0.046 |
| Total BSABS-P | R = −0.003 | R = −0.207 |
| GAF | R = −0.320* | R = −0.010 |
| At 2-yr follow-up | ||
| Total SIPS | R = 0.182 | R = 0.307* |
| Total BSABS-P | R = 0.000 | R = 0.115 |
| GAF score | R = 0.166 | R = 0.057 |
| Changes in PPI variables and clinical variables | ||
| Total SIPS change | R = 0.042 | R = 0.239 |
| Total BSABS-P change | R = −0.337* | R = −0.331* |
| GAF score change | R = 0.050 | R = −0.104 |
Discussion
To our knowledge, we present the first longitudinal, 2-year follow-up study of PPI of the auditory startle blink response in a large sample of UHR adolescents and typically developing controls. We hypothesized that UHR individuals would show deficient PPI over time compared with controls and that the magnitude of this deficit would depend on the clinical outcome. The results confirmed that, compared with controls, PPI parameters (startle reactivity and %PPI) were reduced in UHR individuals over an extended period of time, irrespective of sex, psychotropic medication use and smoking status. Additionally, clinical improvement over time was associated with an increase in %PPI for UHR individuals. Owing to limited statistical power, no evidence was found for progressive worsening of PPI in a small group of UHR individuals (n = 6) who experienced a psychotic transition between assessments (UHR-P). A novel finding of this study was a developmental effect of startle magnitude, which in-creased with age during adolescence for both controls and UHR individuals.
One cross-sectional study10 has previously reported on PPI deficits in UHR cohorts, and the findings were replicated in a study from our own group (unpublished data, 2009). Both studies suggest that PPI deficits present a stable vulnerability marker or trait and potentially predispose UHR individuals to oncoming psychosis. The presented longitudinal results showed that this deficit is stable over time in UHR adolescents. Additionally, this study showed that PPI may be state-sensitive, as the magnitude of reduced PPI was associated with severity of clinical status in UHR individuals. This was illustrated by improvement of both clinical variables and %PPI for UHR individuals over time, whereas controls were proven to have stable and higher levels of %PPI and clinical scores. Furthermore, the most pronounced clinical improvement over time for UHR individuals was reflected by a decline in total BSABS-P scores and was significantly correlated with the enhancement of PPI parameters. This suggests that PPI is an early vulnerability marker that is potentially mediated by alterations of the psychopathologic state, which has been proposed and supported by others11,20,21 based on longitudinal PPI observations in patients with chronic schizophrenia.
In line with the study by Quednow and colleagues,10 no evidence was found for greater PPI deficits in UHR-P individuals before psychosis onset. Additionally, UHR-P individuals showed no subsequent reduction in PPI over time. Importantly, none of the UHR-P individuals was psychotic at the time of assessment, and their level of global functioning (GAF score) was similar to that of UHR-NP individuals. However, the results for the UHR-P subgroup have to be interpreted with reservation, as they are obscured by lack of statistical power owing to a low transition rate (14%). Although transition rates are generally higher in UHR cohorts,8 a 2-year follow-up study of a large UHR cohort in Mel-bourne, Australia, recently reported a similar transition rate of 16%.22 Considering the relatively young age of UHR individuals in our study and the long-term conversion rate based on similar inclusion criteria (70%),23 higher transition rates are expected as we continue to monitor these individuals as they progress into adulthood.
An unanticipated outcome of this study was the developmental increase of startle reactivity with age, demonstrated by larger magnitudes in pulse alone trials. Whereas PPI reportedly reaches adult levels in children around 8 years of age,24 and startle magnitude is known to decrease with age in adulthood,25,26 very few data have been published on the typical progression of startle magnitude during childhood and adolescence. One study found that adolescents aged 12–13 years (n = 94) who were in a mid-/late-pubertal stage showed increased startle magnitude compared with those who were in a pre-/early-pubertal stage.27 Another study with a smaller number of participants (n = 27) but a broader age range (8–17 yr) failed to find evidence for age effects of startle magnitude during adolescence.28 However, comparison between these studies is hindered by several methodologic differences, including the definition of startle magnitude (peak amplitude v. area-under-the-curve approach) and the cross-sectional study design. Nevertheless, maturational changes may play a role in the ability to pick up potential vulnerability markers during adolescence. For example, in the current study, startle magnitude only differed between groups over time and not at baseline. Additionally, the time × group interaction nearly reached significance (p = 0.05), indicating a slower increase of startle magnitude for (a portion of) UHR individuals over time. However, these results await confirmation from longitudinal PPI reports in similar adolescent cohorts.
A number of factors are known to influence PPI and may partially be reflected in our results. Sex has been shown to affect PPI parameters with men showing higher levels of PPI than women,29–31 although some studies have also reported an absence of sex differences.26,30 Given that our study groups were matched for sex, we further controlled for potential influence of this factor by directly comparing boys to girls, and no sex differences were detected. However, we could not correct for the potential influence of phase of the menstrual cycle in girls, as these data were unavailable. Numerous studies have also examined the effects of psychotropic medication on PPI.32,33 Because UHR individuals in our study were receiving multiple types of medication, which in some cases changed in type or dosage over time, statistical correction for this factor was restricted. Despite this limitation, our results demonstrated that unmedicated individuals did not differ from medicated individuals and that group results did not change when medicated individuals were excluded from the analysis.
Limitations
A possible limitation of the current study is that smoking status was based on self report, which cannot exclude the possibility that unreliable data were submitted.34 Nicotine intake can increase PPI levels in controls and patients with schizophrenia35–37 and consequently may have interfered with our outcome. Furthermore, smoking withdrawal effects can decrease PPI levels;38 hence, smokers were allowed to smoke ad libitum before the assessment. The reported amount of regular smoking behaviour was quite low in our study and, except for 1 control participant, restricted to the UHR group. Therefore, if smoking did have an effect in the expected direction, a superior correction for smoking status would most likely have resulted in larger group differences and a strengthening of our results.
Conclusion
In this paper, we report on a 2-year follow-up study of PPI measures in a sample of UHR adolescents and typically developing controls. Results indicated that both startle magnitude and %PPI represent stable vulnerability markers of the psychosis prodrome. Based on a small group of UHR individuals (n = 6) with a psychotic episode between assessments, we did not find evidence for increased PPI deficits associated with transition to psychosis. However, we were able to show an association between PPI, symptom severity and subsequent improvement over time, suggesting that the magnitude of PPI deficits may partially depend on clinical status. This is in line with the idea put forth by others that reduced PPI can be studied as both a trait and a state marker in schizophrenia research.11,12,39 Future longitudinal studies would benefit from larger groups of UHR individuals converting to psychosis and multiple repeated measures over time to establish the nature of the relation between clinical progression and PPI deficits. Direct comparisons between UHR individuals with and without a genetic vulnerability for schizophrenia-spectrum disorders and unaffected family members of patients with schizophrenia-spectrum disorders could also provide additional insight into the relative contribution of state and trait aspects of these types of vulnerability markers.
Acknowledgements
The authors thank Mirjam Simons-Sprong, Petra Klaassen, Anneke Schouten, Nieke Kobussen and Emmie van Schaffelaar for their contributions in collecting the data for this study.
Footnotes
Competing interests: Drs. Ziermans, Schothorst, Magnée and van Engeland’s work was supported in part by a ZonMw grant (the Netherlands organisation for health research and development, project 2630.0001) to their institution. Dr. Kemner’s work was supported in part by NWO-VIDI and NCU grants from the Dutch Science Foundation to his institutions.
Contributors: Drs. Ziermans, Schothorst, van Engeland and Kemner designed the study. Dr. Ziermans acquired the data, which Drs. Ziermans, Magnée and Kemner analyzed. Drs. Ziermans and Kemner wrote the article. All authors critically reviewed the article and approved its publication.
References
- 1.Braff D, Stone C, Callaway E, et al. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–43. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- 2.Graham FK. Presidential Address, 1974. The more or less startling effects of weak prestimulation. Psychophysiology. 1975;12:238–48. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 3.Swerdlow NR, Light GA, Cadenhead KS, et al. Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function. Arch Gen Psychiatry. 2006;63:1325–35. doi: 10.1001/archpsyc.63.12.1325. [DOI] [PubMed] [Google Scholar]
- 4.Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–58. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- 5.Thaker GK. Neurophysiological endophenotypes across bipolar and schizophrenia psychosis. Schizophr Bull. 2008;34:760–73. doi: 10.1093/schbul/sbn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turetsky BI, Calkins ME, Light GA, et al. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophr Bull. 2007;33:69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGorry PD, Yung AR, Phillips LJ. The “close-in” or ultra high-risk model: a safe and effective strategy for research and clinical intervention in prepsychotic mental disorder. Schizophr Bull. 2003;29:771–90. doi: 10.1093/oxfordjournals.schbul.a007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsen KA, Rosenbaum B. Prospective investigations of the prodromal state of schizophrenia: review of studies. Acta Psychiatr Scand. 2006;113:247–72. doi: 10.1111/j.1600-0447.2005.00697.x. [DOI] [PubMed] [Google Scholar]
- 9.Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quednow BB, Frommann I, Berning J, et al. Impaired sensorimotor gating of the acoustic startle response in the prodrome of schizophrenia. Biol Psychiatry. 2008;64:766–73. doi: 10.1016/j.biopsych.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Meincke U, Morth D, Voss T, et al. Prepulse inhibition of the acoustically evoked startle reflex in patients with an acute schizophrenic psychosis — a longitudinal study. Eur Arch Psychiatry Clin Neurosci. 2004;254:415–21. doi: 10.1007/s00406-004-0523-0. [DOI] [PubMed] [Google Scholar]
- 12.Quednow BB, Wagner M, Westheide J, et al. Sensorimotor gating and habituation of the startle response in schizophrenic patients randomly treated with amisulpride or olanzapine. Biol Psychiatry. 2006;59:536–45. doi: 10.1016/j.biopsych.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Sprong M, Becker HE, Schothorst PF, et al. Pathways to psychosis: a comparison of the pervasive developmental disorder subtype multiple complex developmental disorder and the “at risk mental state”. Schizophr Res. 2008;99:38–47. doi: 10.1016/j.schres.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 14.Ziermans TB, Durston S, Sprong M, et al. No evidence for structural brain changes in young adolescents at ultra high risk for psychosis. Schizophr Res. 2009;112:1–6. doi: 10.1016/j.schres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 15.McGlashan TH, Miller TJ, Woods SW. Pre-onset detection and intervention research in schizophrenia psychoses: current estimates of benefit and risk. Schizophr Bull. 2001;27:563–70. doi: 10.1093/oxfordjournals.schbul.a006896. [DOI] [PubMed] [Google Scholar]
- 16.Schultze-Lutter F, Klosterkötter J. Bonn Scale for the Assessment of Basic Symptoms-Prediction list (BSABS-P) Cologne (Germany): University of Cologne; 2002. [Google Scholar]
- 17.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 1994. [Google Scholar]
- 18.Wechsler D. Wechsler Adult Intelligence Scale-III NL: Afname en scoringshandleiding [Translated by Uterwijk JM] Lisse (the Netherlands): Swets & Zeitlinger; 2000. [Google Scholar]
- 19.Wechsler D. In: Wechsler Intelligence Scale for Children-III NL: Handleiding en verantwoording. Kort W, Compaan EL, Bleichrodt N, et al., editors. Amsterdam: Harcourt Test Publishers; 2002. [Google Scholar]
- 20.Martinez-Gras I, Rubio G, del Manzano BA, et al. The relationship between prepulse inhibition and general psychopathology in patients with schizophrenia treated with long-acting risperidone. Schizophr Res. 2009;115:215–21. doi: 10.1016/j.schres.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 21.Ludewig K, Geyer MA, Etzensberger M, et al. Stability of the acoustic startle reflex, prepulse inhibition, and habituation in schizophrenia. Schizophr Res. 2002;55:129–37. doi: 10.1016/s0920-9964(01)00198-0. [DOI] [PubMed] [Google Scholar]
- 22.Yung AR, Nelson B, Stanford C, et al. Validation of “prodromal” criteria to detect individuals at ultra high risk of psychosis: 2 year follow-up. Schizophr Res. 2008;105:10–7. doi: 10.1016/j.schres.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Klosterkotter J, Hellmich M, Steinmeyer EM, et al. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58:158–64. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- 24.Ornitz EM, Guthrie D, Sadeghpour M, et al. Maturation of prestimulation-induced startle modulation in girls. Psychophysiology. 1991;28:11–20. doi: 10.1111/j.1469-8986.1991.tb03381.x. [DOI] [PubMed] [Google Scholar]
- 25.Ellwanger J, Geyer MA, Braff DL. The relationship of age to pre-pulse inhibition and habituation of the acoustic startle response. Biol Psychol. 2003;62:175–95. doi: 10.1016/s0301-0511(02)00126-6. [DOI] [PubMed] [Google Scholar]
- 26.Ludewig K, Ludewig S, Seitz A, et al. The acoustic startle reflex and its modulation: effects of age and gender in humans. Biol Psychol. 2003;63:311–23. doi: 10.1016/s0301-0511(03)00074-7. [DOI] [PubMed] [Google Scholar]
- 27.Quevedo KM, Benning SD, Gunnar MR, et al. The onset of puberty: effects on the psychophysiology of defensive and appetitive motivation. Dev Psychopathol. 2009;21:27–45. doi: 10.1017/S0954579409000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bakker MJ, Boer F, van der Meer JN, et al. Quantification of the auditory startle reflex in children. Clin Neurophysiol. 2009;120:424–30. doi: 10.1016/j.clinph.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 29.Kumari V, Aasen I, Sharma T. Sex differences in prepulse inhibition deficits in chronic schizophrenia. Schizophr Res. 2004;69:219–35. doi: 10.1016/j.schres.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Swerdlow NR, Auerbach P, Monroe SM, et al. Men are more inhibited than women by weak prepulses. Biol Psychiatry. 1993;34:253–60. doi: 10.1016/0006-3223(93)90079-s. [DOI] [PubMed] [Google Scholar]
- 31.Swerdlow NR, Hartman PL, Auerbach PP. Changes in sensorimotor inhibition across the menstrual cycle: implications for neuropsychiatric disorders. Biol Psychiatry. 1997;41:452–60. doi: 10.1016/S0006-3223(96)00065-0. [DOI] [PubMed] [Google Scholar]
- 32.Geyer MA, Krebs-Thomson K, Braff DL, et al. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–54. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- 33.Swerdlow NR, Weber M, Qu Y, et al. Realistic expectations of pre-pulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl) 2008;199:331–88. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mair M, Barlow A, Woods SE, et al. Lies, damned lies and statistics? Reliability and personal accounts of smoking among young people. Soc Sci Med. 2006;62:1009–21. doi: 10.1016/j.socscimed.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Duncan E, Madonick S, Chakravorty S, et al. Effects of smoking on acoustic startle and prepulse inhibition in humans. Psychopharmacology (Berl) 2001;156:266–72. doi: 10.1007/s002130100719. [DOI] [PubMed] [Google Scholar]
- 36.Evans LH, Gray NS, Snowden RJ. Prepulse inhibition of startle and its moderation by schizotypy and smoking. Psychophysiology. 2005;42:223–31. doi: 10.1111/j.1469-8986.2005.00280.x. [DOI] [PubMed] [Google Scholar]
- 37.Postma P, Gray JA, Sharma T, et al. A behavioural and functional neuroimaging investigation into the effects of nicotine on sensorimotor gating in healthy subjects and persons with schizophrenia. Psychopharmacology (Berl) 2006;184:589–99. doi: 10.1007/s00213-006-0307-5. [DOI] [PubMed] [Google Scholar]
- 38.Kumari V, Gray JA. Smoking withdrawal, nicotine dependence and prepulse inhibition of the acoustic startle reflex. Psychopharmacology (Berl) 1999;141:11–5. doi: 10.1007/s002130050800. [DOI] [PubMed] [Google Scholar]
- 39.Saccuzzo DP, Braff DL. Information-processing abnormalities: trait-and state-dependent components. Schizophr Bull. 1986;12:447–59. doi: 10.1093/schbul/12.3.447. [DOI] [PubMed] [Google Scholar]