Abstract
Crohn's disease results from a combination of genetic and environmental factors that trigger an inappropriate immune response to commensal gut bacteria. The aryl hydrocarbon receptor (AhR) is well known for its involvement in the toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), an environmental contaminant that affects people primarily through the diet. Recently, TCDD was shown to suppress immune responses by generating regulatory T cells (Tregs). We hypothesized that AhR activation dampens inflammation associated with Crohn's disease. To test this hypothesis, we utilized the 2,4,6-trinitrobenzenesulfonic acid (TNBS) murine model of colitis. Mice were gavaged with TCDD prior to colitis induction with TNBS. Several parameters were examined including colonic inflammation via histological and flow cytometric analyses. TCDD-treated mice recovered body weight faster and experienced significantly less colonic damage. Reduced levels of interleukin (IL) 6, IL-12, interferon-gamma, and tumor necrosis factor-α demonstrated suppression of inflammation in the gut following TCDD exposure. Forkhead box P3 (Foxp3)egfp mice revealed that TCDD increased the Foxp3+ Treg population in gut immune tissue following TNBS exposure. Collectively, these results suggest that activation of the AhR by TCDD decreases colonic inflammation in a murine model of colitis in part by generating regulatory immune cells. Ultimately, this work may lead to the development of more effective therapeutics for the treatment of Crohn's disease.
Keywords: aryl hydrocarbon receptor; 2,3,7,8-tetrachlorodibenzo-p-dioxin; Crohn's disease; inflammation; regulatory T cells
Crohn's disease, one of the conditions encompassed by inflammatory bowel disease (IBD), is a chronic inflammatory disease of the gastrointestinal tract that primarily affects younger European Americans (Head and Jurenka, 2004). The exact cause of Crohn's disease remains unknown; however, it is suspected that both genetics and environmental factors contribute to an inappropriate immune response to commensal bacteria. Normally, there exists a state of tolerance in the gut such that effector and regulatory cell differentiation is balanced and immune responses are not mounted against commensal bacteria or food substances. In Crohn's disease, however, imbalanced differentiation of T effector cells (Teffs) and T regulatory cells (Tregs) results in T helper type 1 (Th1)- and Th17-dominated inflammatory responses (Sanchez-Munoz et al., 2008). Since current medical treatment can be associated with severe side effects and life-threatening complications, it is essential to better understand this immune dysfunction so that novel and more effective therapeutics can be developed.
The aryl hydrocarbon receptor (AhR) is a member of the basic helix-loop-helix/Per-Arnt-Sim protein family and acts as a cytosolic, ligand-activated transcription factor that binds a variety of synthetic and natural compounds. The AhR is perhaps most recognized in the immune system for its role in mediating the immunotoxic effects of the prototypical ligand 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Canonical signaling involves ligand-activated AhR interaction with dioxin response elements to modulate gene transcription. Many immune cells express the AhR, and several cytokine genes possess dioxin response elements so AhR activation can significantly impact immune responses. Recently, it has been shown that the generation of CD4+CD25+ Tregs is the likely mechanism responsible for the immunosuppressive effects following TCDD exposure (Funatake et al., 2005; Marshall et al., 2008). Moreover, AhR-deficient mice do not experience immunosuppression following exposure to TCDD and TCDD-like compounds, thus demonstrating that the effects of these chemicals are AhR mediated.
To examine the potential of new therapeutic agents to treat Crohn's disease, the 2,4,6-trinitrobenzenesulfonic acid (TNBS)–induced mouse model resembles inflammatory responses in human Crohn's disease so it is commonly used for this purpose (Neurath et al., 1995; Pizarro et al., 2003). In this model, TNBS binds colonic proteins to trigger a Th1-mediated response primarily driven by the proinflammatory cytokine interleukin (IL)-12 as well as a Th17-mediated response driven by IL-17. Excessive amounts of tumor necrosis factor-α (TNF-α) and interferon-gamma (IFN-γ) along with deficiencies in the regulatory cytokines IL-10 and transforming growth factor-β (TGF-β) have also been observed (Kawada et al., 2007). Based on the potential for TCDD and other AhR ligands to modulate inflammatory and immune responses by altering the differentiation of T cells, the effects of AhR activation may be quite pronounced in this model of colitis involving Th1 and Th17 cells.
Since Crohn's disease affects millions of people worldwide, it is essential to investigate the underlying mechanisms of pathogenesis so that new and more effective therapeutics can be developed. Additionally, activation of the AhR modulates immune and inflammatory responses, so studying the effects of AhR activation on the pathogenesis of Crohn's disease is warranted. Therefore, we explored the effects of TCDD on the generation of TNBS-induced colitis in mice. We hypothesized that AhR activation reduces the inflammation generated in a murine model of Crohn's disease by promoting an immunosuppressive environment in the gut. Collectively, our data demonstrate for the first time that AhR activation by TCDD in the gut suppresses inflammation and generates regulatory cells in the TNBS model of colitis in mice. These results also provide novel information regarding gut mucosal immunity and suggest that the AhR may be a potential therapeutic target for Crohn's disease.
MATERIALS AND METHODS
Laboratory animals.
Six- to 8-week-old male and female AhR+/+, AhR−/−, and forkhead box P3 (Foxp3)egfp mice (all on the C57Bl/6 background) were bred and maintained in the animal research facility at the University of Montana. C57Bl/6 AhR+/+ were originally obtained from the Jackson Laboratories (Bar Harbor, ME) and bred in-house. C57Bl/6 AhR−/− (B6.AhRtm1Bra) mice were obtained from Dr Paige Lawrence (University of Rochester Medical College, Rochester, NY) and bred as previously described (Schmidt and Bradfield, 1996). Foxp3egfp mice were kindly provided by Dr R. Noelle (Dartmouth Medical School, Lebanon, NH) who originally obtained these mice from Dr A. Rudensky (University of Washington School of Medicine, Seattle, WA). All mice were housed under specific pathogen-free conditions and maintained on 12-h dark/light cycles. Throughout each experiment, animals were individually caged and standard laboratory food and water were provided ad libitum. All protocols for the use of animals were approved by the University of Montana Institutional Animal Care and Use Committee and adhered to the current National Institutes of Health (NIH) guidelines for animal usage.
Chemicals.
TCDD was obtained from Cambridge Isotope Laboratories Inc., (Woburn, MA), initially dissolved in anisole (Sigma-Aldrich, St Louis, MO), and further diluted in peanut oil, which was used as a vehicle control for gavages. TNBS was also obtained from Sigma-Aldrich and dissolved in acetone/olive oil for presensitization or 40% ethanol for enema administration.
Colitis induction.
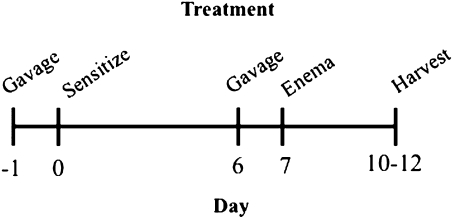
As described in Figure 1, mice were gavaged on day 1 and day 6 with 15 μg TCDD per kilogram body weight (∼200 to 250 μl total volume) or a comparable volume of peanut oil vehicle control prior to presensitization and colitis induction. Presensitization on day 0 involved an application of 150 μl 5% TNBS in a 4:1 acetone:olive oil solution between the shoulder blades such that mice were not orally exposed to TNBS. Colitis was then induced via an intrarectal injection with TNBS on day 7, as previously described with modifications (Wirtz et al., 2007). Briefly, mice were anesthetized with isoflurane and received an enema of 2.5 mg TNBS in 40% ethanol (50 μl total volume) via a 3.5 F catheter inserted 3 cm into the colon. To ensure distribution of TNBS in the colon, mice were held in a vertical position for 90 s before being returned to their cages. Body weight loss and the severity of clinical symptoms (overall body condition, stool consistency, and dehydration state via a skin pinch test) were monitored daily, as fully described in Supplementary table 1A. Briefly, the severity of each clinical sign was scored on a scale 0–3 and combined to determine the total severity score. On days 10–12, mice were euthanized via CO2 asphyxiation followed by cervical dislocation.
FIG. 1.
This schematic represents the experimental design utilized for the studies in which animals were gavaged with 15 μg/kg TCDD, peripherally sensitized with 5% TNBS, intrarectally injected with 2.5 mg TNBS, and subsequently harvested for evaluation.
Colon processing and histology.
Colons were excised from each mouse and washed with PBS to remove debris. For histological analysis, a 1-cm section from the distal colon was fixed in 2% paraformaldehyde, processed in the Shandon Citadel 2000 Automated Tissue Processor (Thermo Fisher Scientific, Waltham, MA) with vacuum unit, embedded using the Shandon Histocentre 2 Embedding unit, sectioned at 7 μm using the Thermo Shandon Finesse 325 microtome, and stained with hematoxylin and eosin (H&E) in the Shandon Veristain 24-4 Slide Stainer. Colons were assessed microscopically by a blinded individual to determine the severity of inflammation based on the modified scale shown in Supplementary table 1B (Hartmann et al., 2000). Briefly, the extent of inflammatory cell infiltration and tissue lesions was scored on a scale 0–3 and combined to determine a total damage score. Slides were imaged using the Nikon E800 Microscope with Cambridge Research Instrumentation, Inc. and Nuance camera software version 1.62 at ×4 magnification. The remaining colon was divided into two sections for cell isolation, as described below, and homogenization in lysis buffer from which the supernatants were analyzed for cytokine production using commercially available ELISA kits.
Cytokine assays.
Supernatants from homogenized colon tissue were examined for the levels of IL-6, IL-10, IL-12, IL-17, IFN-γ, and TNF-α via ELISA. Samples were analyzed according to the manufacturer's instructions using mouse cytokine-specific BD OptEIA ELISA kits (BD Biosciences, San Diego, CA) or R&D ELISA kits (Minneapolis, MN).
Quantitative real-time-PCR.
RNA was isolated from colon tissue with Trizol reagent (Invitrogen, Carlsbad, CA) followed by RNA cleanup using the Total RNA Kit with optional DNase treatment (Omega Bio-Tek, Norcross, GA) according to the manufacturer's instructions. First-strand complementary DNA (cDNA) synthesis was performed using qScript cDNA Supermix (Quanta Biosciences, Gaithersburg, MD). Resulting cDNA was subjected to quantitative real-time (qRT)-PCR using commercially obtained primers (SABiosciences, Frederick, MD) including aryl hydrocarbon receptor repressor (AhRR), aldehyde dehydrogenase family 1, subfamily A2 (Aldh1a2), a proliferation-inducing ligand (APRIL), B cell–activating factor (BAFF), cytochrome P450, family 1, subfamily a, polypeptide 1 (Cyp1a1), Foxp3, indoleamine-2,3-dioxygenase 1 (IDO1), IDO2, IL17A, IL17F, RAR-related orphan receptor gamma (Rorc), Tgfβ1, Tgfβ2, and Tgfβ3. Reactions were performed with PerfeCTa SYBR Green Supermix for iQ (Quanta Biosciences) on a Bio-Rad iQ5 Multicolor RT-PCR Detection System. Resulting data were normalized to β-actin, and fold changes were calculated using the ΔΔCt method, which compares threshold values of the samples of interest for a particular gene relative to a housekeeping gene.
Preparation of cells from MLNs and colon.
Mesenteric lymph nodes (MLNs) were removed, washed in media, homogenized using the end of a syringe plunger, and subsequently filtered through a 40-μm nylon cell strainer, as previously described (Bankoti et al., 2010a). Lamina propria mononuclear cells were also isolated from the colon tissue, as previously described (Weigmann et al., 2007). Briefly, colons were excised and washed with PBS, digested with collagenase/DNase (Sigma-Aldrich/Invitrogen), filtered, and subjected to Percoll (GE Healthcare, Pittsburg, PA) density centrifugation. Cells from both tissues were counted via Trypan blue exclusion on a hemacytometer, and 2 × 106 cells were subsequently washed with buffer prior to cell staining.
Flow cytometry.
Expression of accessory molecules on isolated cells was determined by flow cytometry, as previously described (Shepherd et al., 2001). Briefly, cells were washed with phosphate azide buffer (1% bovine serum albumin and 0.1% sodum azide in PBS). To eliminate nonspecific staining, cells were blocked with 30 μg purified rat and/or hamster immunoglobulin G (IgG) (Jackson ImmunoResearch, West Grove, PA). Optimal concentrations of fluorochrome-conjugated monoclonal antibodies were used to stain cells for an additional 10 min. Antibodies used in these experiments included CD4-AlexaFluor 700 (GK1.5), CD25-PerCP/Cy5.5 (PC61), CTLA4-APC (UC10-4B9), GITR-PECy7 (YGITR), α4β7-PE (DATK32), CD103-Pacific Blue (2E7), CD11c-PE (HL3), MHC2-PECy7 (M5/114.15.2), CD86-APC (GL-1), and their corresponding isotype controls, all of which were purchased from BioLegend (San Diego, CA) or BD Biosciences (San Jose, CA). One to five hundred thousand events were collected using a BD FACSAria flow cytometer and analyzed using FACSDiva (Version 6.1.2, BD Biosciences) and FlowJo (Version 8.7.1, TreeStar Inc., Ashland, OR) software programs.
Immunohistochemistry.
Using the embedded colon tissue prepared as described above, 5-μm sections were mounted on slides and allowed to air-dry prior to being deparaffinized and coverslipped using the ProLong Gold anti-fade reagent with 4′,6–diamidino–2–phenylindole mounting media (Invitrogen Molecular Probes). Slides were imaged at ×40 magnification using the Olympus FluoView 1000 LSC on an inverted 1 × 81 microscope with spectral detection and total internal reflection fluorescence module (Version 2.1b software with 405, 458, 488, 515, 559, and 635 laser lines available).
Statistics.
All statistical analyses were performed using GraphPad Prism 4.0a for Macintosh (GraphPad Software, San Diego, CA). Student's t-test was used to compare vehicle-treated groups and TCDD-treated groups, whereas two-way ANOVA with Bonferroni's post hoc test was used to compare multiple groups. Values of p ≤ 0.05 were determined to be significant.
RESULTS
AhR Activation by TCDD Decreases the Severity of Clinical Symptoms in TNBS-Treated Mice
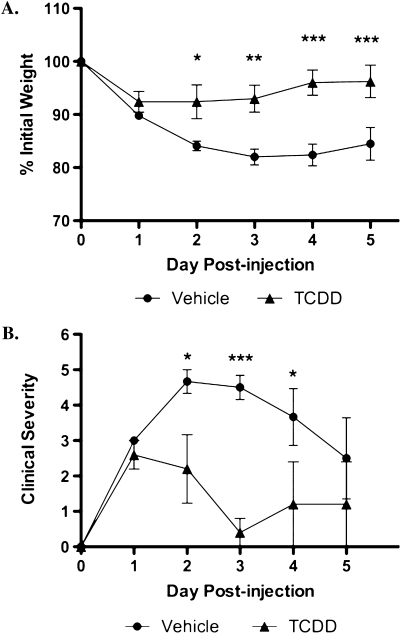
In order to characterize the effects of TCDD on the progression of TNBS-induced colitis in C57Bl/6 AhR+/+ mice, clinical symptoms were monitored throughout the course of the experiment. Crohn's disease is a progressive wasting disease so body weight loss is a hallmark indicator of clinical severity. As shown in Figure 2A, vehicle-treated mice rapidly lost approximately 17% of their initial body weights following TNBS exposure and did not recover by the end of the study. Conversely, TCDD-treated mice lost only 8% of their initial weights and recovered weight rapidly. The protective effect of TCDD was also observed in the clinical severity of colitis, as measured by stool consistency, dehydration, and body condition (Supplementary table 1A). Vehicle-treated mice typically had loose stools, mild dehydration, and slower body movements, whereas the TCDD-treated mice had semiformed stools initially but recovered quite rapidly (Fig. 2B). Thus, TCDD decreases the severity of clinical symptoms of mice in the TNBS-induced model of Crohn's disease.
FIG. 2.
TCDD reduces wasting disease and clinical symptoms associated with TNBS-induced colitis. Animals were monitored daily after disease induction for body weight loss (A) and other clinical symptoms including body condition, dehydration state, and stool consistency (B). Clinical severity was determined by combining the symptom scores for a total possible severity score of nine, as described in Supplementary table 1A. Results are shown as mean ± SEM (n = 6) and representative of three separate experiments. “*” Indicates significances of p ≤ 0.05, “**” indicates significance of p ≤ 0.01, and “***” indicates significance of p ≤ 0.0001.
Inflammation and Tissue Damage in the Colon Decreases following Treatment with TCDD
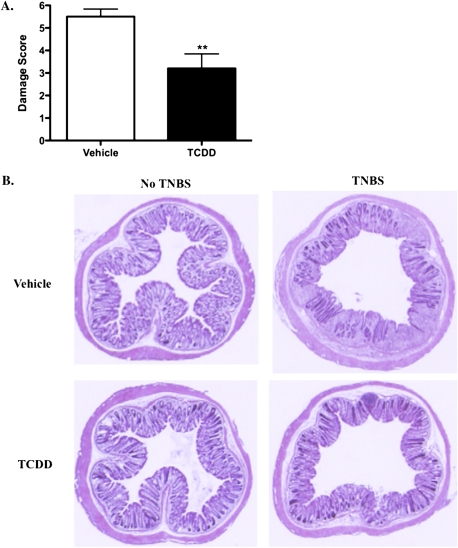
Crohn's disease results in transmural inflammation and ulceration of the colon tissue. To examine the degree of inflammatory cell infiltration and tissue lesions, cross sections of the distal colon were stained with H&E and assessed microscopically to determine damage (Supplementary table 1B and Fig. 3A). Transmural infiltration of inflammatory cells and extensive mucosal damage were frequently observed in vehicle-treated mice following TNBS exposure, which resulted in an average damage score of 5.5. However, the extent of inflammatory cell infiltration and lesions was significantly less in TCDD-treated mice, as represented by an average damage score of 3.2. Representative images of each treatment group are shown in Figure 3B. These results demonstrate that TCDD exposure prior to colitis induction significantly suppresses colonic inflammation following TNBS insult.
FIG. 3.
Colonic inflammation is reduced following exposure to TCDD. Animals were harvested 5 days after disease induction and sections of the distal colon were assessed microscopically. Damage score (A) was determined by combining the scores for inflammatory cell infiltration (0–3) and tissue damage (0–3), as described in Supplementary table 1B. Representative images of H&E-stained tissue sections of vehicle-treated and TCDD-treated animals are shown in (B). Results are shown as mean ± SEM (n = 6) and are representative of three separate experiments. “**” Indicates significance of p ≤ 0.01.
AhR Activation Decreases the Production of Inflammatory Mediators in Colitis
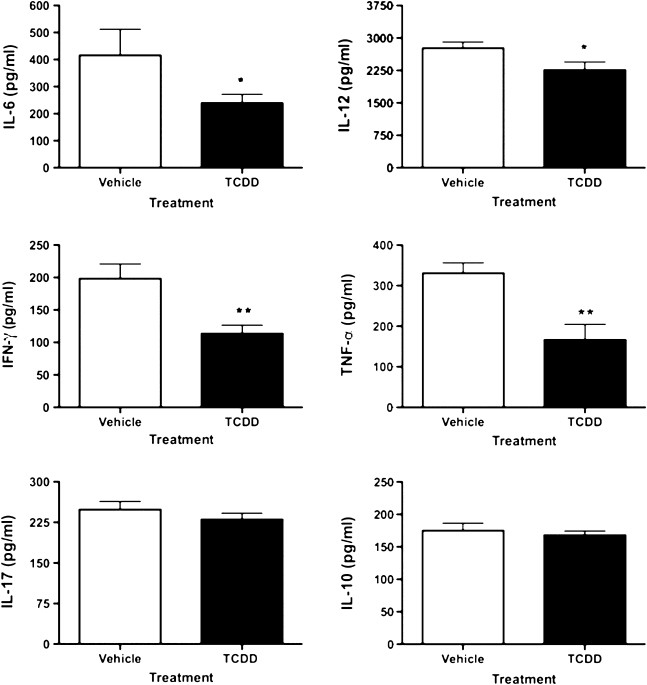
TNBS-induced colitis is driven by proinflammatory cytokines including IL-6, IL-12, and IL-17 (Kawada et al., 2007). Accordingly, cytokine production in supernatants from homogenized colon tissue was evaluated. As shown in Figure 4, TCDD decreased the production of IL-6, IFN-γ, and TNF-α by approximately 45% each as well as the production of IL-12 by 18%. In a separate preliminary experiment, neither IL-6 (TCDD = 363 ± 18 pg/ml; vehicle = 382 ± 22 pg/ml) nor IL-12 (TCDD = 2763 ± 141 pg/ml; vehicle = 2633 ± 190 pg/ml) colonic levels were significantly reduced in TCDD-treated AhR-null mice when compared with the vehicle-treated wild-type controls. Furthermore, production of IL-10 and IL-17 in the colon was unaffected by TCDD in TNBS-treated animals. Together, these results suggest that TCDD can alter the gut immune environment by suppressing proinflammatory cytokines in an AhR-dependent manner and decrease inflammation associated with TNBS-induced colitis.
FIG. 4.
Modulation of cytokine production by TCDD in colon tissue. On day 5 following disease induction, colons were excised, washed, and homogenized. The supernatants were assessed for cytokine production, as described in the “Materials and Methods” section. Results are shown as mean ± SEM (n = 6) and representative of three separate experiments. “*” Indicates significance of p ≤ 0.05 and “**” indicates significance of p ≤ 0.01.
The effects of TCDD on antibody production were also assessed because IgA plays an important role in regulating immune responses in the gut. Following TCDD treatment, IgA levels in the supernatants from homogenized colon tissue and feces increased from 38.89 to 54.99 μg/ml and from 5.85 to 15.46 μg/ml, respectively (Supplementary table 2), an effect that was not observed in AhR-null mice treated with TCDD (data not shown). Although a trend was observed for a TCDD-induced increase in IgA in the serum of TNBS-exposed mice, this effect was not statistically significant. TCDD also increased total Ig production in the colon from 48.67 to 113.1 μg/ml but did not alter total Ig levels in the feces or serum. Interestingly, without the TNBS-triggered inflammatory response, TCDD did not induce significant increases in IgA production in the gut (data not shown). Thus, TCDD modulates humoral immune responses in the gut of TNBS-treated mice.
We also examined the potential for intestinal epithelial cells (IECs) to contribute to the suppression of inflammation following TCDD treatment because these cells are important in maintaining the mucosal barrier against gut flora and mounting immune responses by acting as antigen presenting cells. CMT-93 cells, a murine intestinal epithelial cell line, were stimulated with 1 μg/ml lipopolysaccharide (LPS) and concurrently treated with TCDD. The LPS-induced production of IL-6, as determined by ELISA, was suppressed by TCDD treatment (Supplementary table 3A). Similar effects were observed when cells were stimulated with cytosine-phosphate-guanine and concomitantly treated with TCDD (data not shown). IL-10, IL-12, and IFN-γ were not detected in either of these experiments (data not shown).
TCDD Alters Transcription of Immunoregulatory Genes in Colonic Tissue of TNBS-Treated Mice
To gain further insight into factors that may be responsible for the immunomodulatory effects of TCDD in colitis, intestinal tissue was evaluated by qRT-PCR to assess changes in gene transcription. Three days after colitis induction, which was the peak of clinical disease severity, TCDD enhanced the expression of AhRR, Cyp1a1, and IDO1 by 6.74-, 216.40-, and 2.91-fold, respectively (Table 1). Conversely, messenger RNA (mRNA) levels of APRIL, IDO2, and IL17A were decreased. Five days after enema administration, a significant upregulation of AhRR, Cyp1a1, and IDO was observed; however, mRNA levels of IL17A and IL17F were unaffected by TCDD, which was consistent with the results obtained from the ELISA. Although not significant, a modest trend for increased TGFβ 3 mRNA following AhR activation by TCDD was observed in wild-type animals. These effects, with the exception of Cyp1a1 and AhRR induction, were specific to the inflamed colonic tissue and did not affect tissue from the small intestine.
TABLE 1.
TCDD Alters Gene Transcription in the Intestinal Tissue of TNBS-Exposed Mice
| Small intestine day 3 |
Colon day 3 |
Colon day 5 |
||||
| Gene | Fold change | p Value | Fold change | p Value | Fold change | p Value |
| AhRR | 2.17 | 0.026 | 6.74 | 0.009 | 25.49 | < 0.0001 |
| Aldh1a2 | 1.01 | 0.494 | 1.42 | 0.088 | 1.43 | 0.179 |
| APRIL | −1.01 | 0.475 | −2.05 | 0.024 | −1.29 | 0.179 |
| BAFF | −1.06 | 0.413 | −1.62 | 0.115 | −1.22 | 0.191 |
| Cyp1a1 | 50.56 | 0.0009 | 216.40 | 0.0002 | 1807 | < 0.0001 |
| Foxp3 | 1.26 | 0.331 | −1.07 | 0.450 | −1.17 | 0.366 |
| IDO1 | 1.65 | 0.109 | 2.91 | 0.006 | 2.15 | 0.026 |
| IDO2 | −1.27 | 0.271 | −1.67 | 0.067 | −1.05 | 0.440 |
| IL17A | 2.89 | 0.255 | −9.40 | 0.009 | −1.47 | 0.265 |
| IL17F | 1.64 | 0.165 | 1.46 | 0.220 | 1.25 | 0.337 |
| Rorc | 1.24 | 0.143 | 1.23 | 0.198 | −1.38 | 0.236 |
| Tgfβ1 | 1.08 | 0.328 | 1.36 | 0.116 | −1.19 | 0.299 |
| Tgfβ2 | −1.25 | 0.270 | 1.00 | 0.495 | 1.16 | 0.335 |
| Tgfβ3 | −1.05 | 0.400 | 1.16 | 0.274 | 1.71 | 0.051 |
Three or 5 days following colitis induction, colon tissue from wild-type mice treated with TCDD was homogenized in Trizol and subjected to qRT-PCR to assess alterations in gene transcription. Fold change is relevant to vehicle-treated mice and normalized to β-actin. Bolded values indicate significance of p ≤ 0.05. Data are representative of a single experiment with n = 6 mice per treatment group.
The ability of TCDD to also alter inflammatory responses by IECs was assessed via qRT-PCR. TCDD induced Cyp1a1 mRNA and downregulated the LPS-induced expression of IL-6 (Supplementary table 3B). Moreover, TCDD treatment of LPS-stimulated IECs resulted in a 6.85- and 2.45-fold upregulation in APRIL and BAFF, respectively. Thus, IECs may be directly affected by TCDD to help mount immunosuppressive responses in the inflamed gut by decreasing the production of proinflammatory cytokines and enhancing the activity of B cells and their subsequent IgA responses.
Immunoregulatory Cells in the MLNs and Colon Are Induced following AhR Activation
AhR activation by TCDD promotes the generation of Tregs in several disease models, so we hypothesized that TCDD dampened inflammation in TNBS-treated mice by increasing regulatory cell populations in the gut. Therefore, Foxp3egfp mice that contain a knock-in allele encoding a green fluorescent protein (GFP)-Foxp3 fusion protein were utilized to specifically assess the presence of Foxp3+ Tregs. Initial experiments with these mice revealed no significant changes in regulatory cell populations when mice were harvested 5 days after TNBS enema administration (data not shown). Since the peak of clinical disease severity occurred 3 days after disease induction, Foxp3egfp mice were evaluated 3 days following the TNBS enema. Populations of regulatory cells, both Tregs and dendritic cells (DCs), following TCDD treatment were analyzed in the colon tissue and MLNs (Table 2). Several markers were used to define regulatory cell populations: Tregs were characterized by the expression of CD4, CD25, Foxp3, CTLA4, GITR, CD103, and α4β7, whereas DCs were characterized by the expression of CD11c, major histocompatibility complex (MHC) class II, CD86, and CD103.
TABLE 2.
Effects of TCDD on Immune Cells and Their Regulatory Cell Surface Marker Expression during TNBS-Induced Colitis in Foxp3egfp Mice
| MLNs |
Colon |
|||||
| Vehicle | TCDD | p Value | Vehicle | TCDD | p Value | |
| A. Tregs in the gut | ||||||
| CD4+CD25+Foxp3+ | 6.34 | 7.74 | 0.036 | 26.8 | 15.98 | 0.017 |
| CTLA4 | 11.78 | 12.30 | 0.411 | 4.05 | 13.80 | 0.001 |
| GITR | 83.86 | 85.52 | 0.274 | 386 | 710 | < 0.0001 |
| CD103 | 45.36 | 51.90 | 0.008 | 1945 | 2072 | 0.104 |
| α4β7 | 18.12 | 20.04 | 0.263 | 235 | 263 | 0.029 |
| CD4+CD25−Foxp3+ | 3.90 | 3.98 | 0.444 | 16.78 | 17.02 | 0.445 |
| CTLA4 | 2.20 | 2.94 | 0.151 | 0.62 | 1.42 | 0.033 |
| GITR | 78.66 | 78.88 | 0.472 | 325 | 458 | 0.005 |
| CD103 | 32.90 | 42.74 | < 0.0001 | 1639 | 1915 | 0.007 |
| α4β7 | 5.60 | 8.36 | 0.009 | 155 | 185 | 0.013 |
| B. DCs in the gut | ||||||
| CD11c+ | 3.43 | 2.74 | 0.036 | 9.56 | 11.65 | 0.030 |
| MHC2 | 81.73 | 82.4 | 0.369 | 20.56 | 11.07 | 0.001 |
| CD86 | 652 | 592 | 0.228 | 338 | 364 | 0.288 |
| CD103 | 24.43 | 33.14 | 0.005 | 88.54 | 92.43 | 0.160 |
| MHC2+CD103+ | 8.673 | 11.88 | 0.024 | 6.595 | 4.910 | 0.089 |
Leukocytes from colon tissue and MLNs were isolated 3 days after disease induction and immunophenotypically evaluated by flow cytometry to determine the presence of Tregs and DCs. Values are listed as the percentage of the bolded parent population indicated in the table, except for GITR, CD103, and α4β7 expression on colon T cells and CD86 expression on DCs, which indicate the median fluorescence intensity values. Bolded p values indicate significance of p ≤ 0.05. Data are representative of a single experiment (n = 6). Representative histograms are shown in Supplementary figures 1–3.
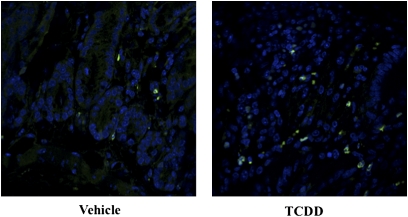
In the MLNs, TCDD increased the percentage of CD4+CD25+Foxp3+ cells from 6.34 to 7.74%, and the percentage of these Tregs that expressed CD103 increased by 6.5% (Table 2A), an effect that did not occur in AhR-null mice treated with TCDD (data not shown). Additionally, the CD4+CD25−Foxp3+ Tregs were also evaluated because it has recently been shown that TCDD-induced CD25− Tregs can effectively suppress immune responses (Funatake et al., 2009). The percentage of CD103+ and α4β7+ cells increased in the CD4+CD25−Foxp3+ Treg population by approximately 10 and 3%, respectively. In the colon, TCDD increased the number of cells expressing CTLA4 from 4.05 to 13.80% as well as the relative expression (median fluorescence intensity [MFI]) of GITR from 386 to 710 in the CD4+CD25+Foxp3+ Tregs, even though this population was significantly decreased following TCDD exposure. Additionally, this population of Tregs also exhibited a 12% increase in cells expressing α4β7. AhR activation by TCDD also increased the percentage of CD4+CD25−Foxp3+ cells expressing CTLA4 as well as the relative expression of GITR from 325 to 458, CD103 from 1639 to 1915, and α4β7 from 155 to 185. Immunohistochemistry performed on the colon tissue revealed an increased Treg population in TNBS-treated mice, as more Foxp3+ expression was observed in mice treated with TCDD (Fig. 5). It should be noted that TCDD treatment in the absence of the TNBS enema did not induce an increased frequency of Foxp3+ cells in the gut (data not shown).
FIG. 5.
TCDD increases Foxp3 expression in colon tissue. Colons were harvested from Foxp3egfp mice 3 days after disease induction. Tissues were sectioned, mounted on slides, and stained with 4′,6–diamidino–2–phenylindole to visualize the nucleus. Slides were imaged at ×40 magnification using the Olympus FluoView 1000 LSC on an inverted 1 × 81 microscope with spectral detection and total internal reflection fluorescence module. Images are representative of a single experiment (n = 6).
Regulatory DCs were also examined in these experiments because these cells can promote the generation of Tregs. TCDD increased the percentage of CD11c+MHC2+CD103+ DCs in the MLNs from 8.673 to 11.88% (Table 2B), an effect that was also not observed in AhR-null mice (data not shown). However, a greater frequency of CD11c+ DCs isolated from the colon expressed decreased levels of MHC class II. Collectively, these results suggest that AhR activation by TCDD induces regulatory immune cells in the gut that may reduce the inflammation in mice experiencing TNBS-induced colitis.
DISCUSSION
Crohn's disease arises from a combination of genetic and environmental factors that trigger an inappropriate immune response against commensal bacteria. This disease is characterized by progressive weight loss, transmural inflammation in the intestines, and infiltration of inflammatory cells that produce inflammatory mediators. More specifically, many proinflammatory cytokines, such as IL-12, are upregulated in the inflamed intestinal mucosa of Crohn's disease patients (Abraham and Cho, 2009; Eastaff-Leung et al., 2010; Liu et al., 1999; Monteleone et al., 1997; Parronchi et al., 1997). Thus, the first goal of this study was to characterize the effects of TCDD treatment in gut mucosal inflammation associated with TNBS-induced colitis. When compared with vehicle-treated mice, TCDD treatment significantly reduced disease severity, as indicated by decreased body weight loss, recovery periods, and colonic inflammation. TCDD also altered the cytokine environment by decreasing production of the inflammatory mediators IL-6, IL-12, IFN-γ, and TNF-α. Notably, 5 days after colitis induction, TCDD did not appear to affect IL-10 or IL-17 protein levels, which have both been implicated in human IBD. Although the TNBS-induced colitic response in mice is mediated primarily by Th1 cells and may not correlate exactly with the human condition that involves both Th17 and Th1 cells, no animal model perfectly represents human IBD. However, TNBS-induced colitis in mice does induce many of the inflammatory sequelae that contribute to human IBD, and thus, this model is very useful for assessment of these early events.
Recently, Takamura et al. (2010) utilized the dextran sodium sulfate (DSS) model of ulcerative colitis, another type of inflammatory bowel disease, to study the effects of TCDD on gut inflammation. In their model, pretreatment with TCDD ameliorated disease severity, inflammation, and TNF-α production in colonic tissue of DSS-treated mice. Although these results were obtained in a model that elicits a slightly different immune response, our results are consistent with those obtained in this independent study. Thus, TCDD treatment suppresses proinflammatory cytokine production that may be responsible for propagating inappropriate Teff responses in the gut. Interestingly, the TCDD dose (15 μg/kg) that was used in our studies was slightly higher than the TCDD dose (5 μg/kg) used by Takamura et al. (2010). Selection of the dose of TCDD in our experiments was based on the reproducible, but not overtly toxic, effects of 15 μg/kg in many models of immunity performed in C57Bl/6 mice. To date, we have not performed dose-response studies; however, it would be of interest to evaluate the potential for both higher and lower doses to affect inflammation associated with the colitic response in TNBS-treated mice.
The intestinal immune system is comprised of organized lymph tissue, various innate and adaptive immune cells, and IECs. In addition to the cells located in the lamina propria, the MLNs are essential sites for mounting successful adaptive immune responses against pathogens in the gut. In the healthy gut, there is a balance between effector and regulatory cells such that no response is elicited against commensal organisms. In Crohn's disease, this balance is disrupted and many cellular components contribute to the exaggerated immune and inflammatory responses. In the inflamed mucosa of Crohn's disease patients, there is a significant decrease in Foxp3 expression in CD4+CD25+ cells along with decreased numbers of CD4+CD25+Foxp3+ cells, which may contribute to enhanced Th1- and Th17-mediated immune responses (Eastaff-Leung et al., 2010). Moreover, TCDD has been reported to induce CD4+CD25+ T cells that express elevated levels of GITR and CTLA4 and suppress proliferation of effector T cells (Funatake et al., 2005). Thus, we investigated potential alterations in several key immune cell populations in the context of gut mucosal immunity. Pretreatment of Foxp3egfp mice with TCDD increased Foxp3+ cells in the gut of TNBS-treated mice. These cells displayed increased levels of GITR, CTLA4, and CD103, which indicate a regulatory potential for these cells. It must be acknowledged that our flow cytometry data revealed a decreased frequency of Foxp3+ cells, whereas the immunohistochemistry images revealed increased Foxp3+ expression in the colonic tissue. This discrepancy is potentially because of the fact that the colon section removed for histology was at the site of TNBS administration and therefore possessed the most severe inflammation, whereas surrounding tissue was used to isolate leukocytes for flow cytometric analyses. Even though the GFP fluorescence was not quantified in these images, the increase of Foxp3+ Tregs at the site of inflammation in TCDD-treated mice most likely contributes to decreased inflammation. Furthermore, Treg induction by TCDD may primarily occur in the context of an inflammatory environment because we did not observe increased Foxp3 expression with TCDD treatment alone. Regulatory DCs were also present in the MLNs, as indicated by increased frequencies of CD103+ DCs. Although not directly assessed by flow cytometry, downregulation of IL-17A mRNA in the colon suggests that TCDD may decrease Th17 cells; however, no corresponding changes in IL-17 protein levels were observed. We expect that performing these studies in Rorγt reporter mice will fully define the contribution of Th17 cells in this model and how TCDD might affect this population of inflammatory T cells. Taken together, our results demonstrate that TCDD generates regulatory cells that may contribute to decreased inflammation in the gut of TNBS-treated mice.
Successful antibody responses in the gut are essential to limiting inappropriate responses against commensal bacteria. IgA is the primary antibody responsible for limiting bacterial penetration in the gut (Macpherson and Uhr, 2004), and its production is promoted by TGF-β. In the normal intestinal immune environment, DCs maintain a tolerogenic environment, as they are conditioned by TGF-β and prostaglandin E-2 to secrete IL-10 and/or TGF-β (Chirdo et al., 2005; Iwasaki and Kelsall, 1999). In addition, IECs play an important role in the innate and adaptive immune responses in the gut. The epithelium is not only the first line of defense against bacteria but IECs can also act as antigen-presenting cells capable of influencing intestinal immune function. Defects in the epithelial barrier have been associated with Crohn's disease pathogenesis (Baumgart and Dignass, 2002). In our experiments, IgA production was increased in the colon tissue and feces, suggesting that TCDD may be acting on DCs and/or IECs to produce switch factors necessary to induce IgA production by B cells in the gut. Additionally, BAFF and APRIL are expressed in many immune cells and play important roles in lymphocyte homeostasis (Ng et al., 2005). Although BAFF transcript levels were reduced in the colon, TGF-β upregulation was observed, which may be responsible in part for the increased IgA production in the gut following TCDD treatment in TNBS-exposed mice. Moreover, stimulated IECs upregulated BAFF and APRIL mRNA levels following TCDD exposure. Thus, IECs may contribute to the amelioration of disease severity in the TNBS-induced colitis model by contributing to increased production of IgA. Additional studies focused on further defining the effects of TCDD on IgA production in the gut, and the mechanisms responsible for these effects are warranted. It must be emphasized that TCDD may be acting on T cells, B cells, and other immune and nonimmune cells to reduce inflammation in the gut, and we believe that these events need not be mutually exclusive. Indeed, it is very likely that the effects of TCDD on all these cells collectively may be necessary to generate a regulatory environment during TNBS-induced colonic inflammation. Thus, to definitively determine if TCDD is acting directly on T cells or indirectly on B cells, DCs, or IECs to mount protective immune responses during the generation of colitis, it would be necessary to perform adoptive transfer experiments or generate TNBS-induced colitis in AhR conditional knockout mice. Our laboratory is currently pursuing these experimental possibilities.
The effects of TCDD notably occur via activation of the AhR, which is a ligand-activated transcription factor present in many immune cells including T cells, B cells, DCs, and macrophages (Marshall and Kerkvliet, 2010). As such, activation of the AhR by TCDD results in the suppression of immune and inflammatory responses. Since it has been demonstrated in AhR-deficient mice that the immunosuppressive effects of TCDD are AhR- dependent, we anticipate that TCDD is also acting through the AhR to suppress colitis. Although our preliminary study using AhR-null mice suggested that many of our observed TCDD-induced effects are dependent on the AhR, additional studies are currently underway in our laboratory to fully examine the role of the AhR in mediating the effects of TCDD as well as endogenous AhR ligands in TNBS-induced colitis.
IDO has been implicated in promoting the generation of Tregs. Tryptophan, an essential amino acid, is primarily catabolized via induction of IDO, an enzyme that has been linked to immune tolerance. Furthermore, tryptophan metabolites can activate the AhR (Belladonna et al., 2006; Denison and Nagy, 2003; Fallarino et al., 2002, 2006; Frumento et al., 2002; Terness et al., 2002). It has been suggested that by binding to the AhR in activated CD4+ T cells, tryptophan metabolites can elicit their immunosuppressive effects by inducing a Treg phenotype. Moreover, Bankoti et al. (2010b) recently demonstrated that TCDD upregulated IDO1, IDO2, TGFβ-1, and TGFβ-2 in LPS-stimulated inflammatory bone marrow–derived DCs. In our study, TCDD treatment increased IDO1 transcript levels in the colon of TNBS-treated mice. It is therefore possible that TCDD induces IDO in the intestinal DCs and/or CD4+ T cells subsequently leading to a significant induction of Tregs in mice exposed to TNBS. Furthermore, tolerogenic DCs capable of promoting Treg differentiation can be generated following the interaction of ligand-activated AhR with RelB via the noncanonical nuclear factor-kappa β signaling pathway, an effect that is mediated via IDO induction in DCs and subsequent Foxp3 expression in T cells (Bankoti et al., 2010b; Belladonna et al., 2007; Curti et al., 2007; Mellor et al., 2004; Tas et al., 2007; Vogel et al., 2008.
Current therapeutic strategies for Crohn's disease involve a combination of corticosteroids and immunomodulatory drugs to rapidly reduce inflammation and induce remission. These therapeutics, however, often possess severe adverse effects. Although TCDD suppressed colonic inflammation in this model, it is recognized that TCDD would not serve as a useful therapeutic agent for Crohn's disease patients because of its potential toxic side effects. There are numerous other AhR ligands present in the diet, such as indole-3-carbinol in cruciferous vegetables and resveratrol in grapes that have documented anti-inflammatory properties and have little or no associated toxicity (Aggarwal and Shishodia, 2006; Cottart et al., 2010; Leibelt et al., 2003). Thus, it is possible that AhR agonists found naturally in the diet can activate the AhR to reduce disease severity similar to TCDD. Recent studies have suggested a potential protective effect in the colitic response for 3,3′-diindolylmethane, the acid condensation product of indole-3-carbinol (Kim et al., 2009) and resveratrol (Cui et al., 2010; Larrosa et al., 2009; Martin et al., 2006). Although these studies were not performed in TNBS-treated mice, they demonstrated decreased disease severity and gut inflammation during a colitic response; however, the role of the AhR in mediating the effects in each of these studies was not evaluated. Conversely, AhR activation by 6-formylindolo[3,2-b]carbazole (FICZ), a typtophan metabolite, exacerbated inflammatory responses by promoting Th17 differentiation in some murine disease models, such as experimental autoimmune encephalitis (Ho and Steinman, 2008; Martin et al., 2006; Quintana et al., 2008; Veldhoen et al., 2008). Although ligand-specific effects may be because of the timing and duration of AhR activation, it must be emphasized that, to date, it is not well understood why some AhR ligands, such as FICZ, have the potential to promote the development of Th17-mediated inflammatory responses, whereas others, such as TCDD, promote the development of Treg-mediated suppressive responses. Therefore, it is critical to continue to evaluate the effects of AhR activation by multiple ligands in the context of gut inflammation.
Collectively, our results demonstrate for the first time that the potent AhR ligand TCDD suppresses gut inflammation and disease severity in the murine model of TNBS-induced colitis, an effect that correlates with an induction of Tregs. Further investigations are needed to elucidate the signaling events responsible for Treg generation in the gut, as TCDD may induce Tregs directly or alternatively via the generation of regulatory DCs. It would also be interesting to evaluate other AhR ligands, as these compounds may prove to represent a new class of therapeutics to treat Crohn's disease in humans.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health (ES013784 to D.M.S., RR017670, RR015583) and National Center for Complementary and Alternative Medicine (F31AT005557 to J.M.B.).
Acknowledgments
In addition to our NIH funding sources, we acknowledge Dr Don Gardner, a veterinary pathologist at the Rocky Mountain Laboratories branch of the National Institute of Allergy and Infections Diseases, who provided assistance in the histological scoring of tissues. The authors also thank Drs Andrij Holian, Kevan Roberts, and Jerry Smith for their critical reviews of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or NCCAM. The authors wish to thank the Center for Environmental Health Sciences Molecular Histology and Fluorescent Imagery Core and the Fluorescence Cytometry Core at the University of Montana for their support.
References
- Abraham C, Cho JH. IL-23 and autoimmunity: new insights into the pathogenesis of inflammatory bowel disease. Annu. Rev. Med. 2009;60:97–110. doi: 10.1146/annurev.med.60.051407.123757. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Bankoti J, Burnett A, Navarro S, Miller AK, Rase B, Shepherd DM. Effects of TCDD on the fate of naive dendritic cells. Toxicol. Sci. 2010a;115:422–434. doi: 10.1093/toxsci/kfq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankoti J, Rase B, Simones T, Shepherd DM. Functional and phenotypic effects of AhR activation in inflammatory dendritic cells. Toxicol. Appl. Pharmacol. 2010b;246:18–28. doi: 10.1016/j.taap.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart DC, Dignass AU. Intestinal barrier function. Curr. Opin. Clin. Nutr. Metab. Care. 2002;5:685–694. doi: 10.1097/00075197-200211000-00012. [DOI] [PubMed] [Google Scholar]
- Belladonna ML, Grohmann U, Guidetti P, Volpi C, Bianchi R, Fioretti MC, Schwarcz R, Fallarino F, Puccetti P. Kynurenine pathway enzymes in dendritic cells initiate tolerogenesis in the absence of functional IDO. J. Immunol. 2006;177:130–137. doi: 10.4049/jimmunol.177.1.130. [DOI] [PubMed] [Google Scholar]
- Belladonna ML, Puccetti P, Orabona C, Fallarino F, Vacca C, Volpi C, Gizzi S, Pallotta MT, Fioretti MC, Grohmann U. Immunosuppression via tryptophan catabolism: the role of kynurenine pathway enzymes. Transplantation. 2007;84:S17–S20. doi: 10.1097/01.tp.0000269199.16209.22. [DOI] [PubMed] [Google Scholar]
- Chirdo FG, Millington OR, Beacock-Sharp H, Mowat AM. Immunomodulatory dendritic cells in intestinal lamina propria. Eur. J. Immunol. 2005;35:1831–1840. doi: 10.1002/eji.200425882. [DOI] [PubMed] [Google Scholar]
- Cottart CH, Nivet-Antoine V, Laguillier-Morizot C, Beaudeux JL. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010;54:7–16. doi: 10.1002/mnfr.200900437. [DOI] [PubMed] [Google Scholar]
- Cui X, Jin Y, Hofseth AB, Pena E, Habiger J, Chumanevich A, Poudyal D, Nagarkatti M, Nagarkatti PS, Singh UP, et al. Resveratrol suppresses colitis and colon cancer associated with colitis. Cancer Prev. Res. (Phila) 2010;3:549–559. doi: 10.1158/1940-6207.CAPR-09-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curti A, Pandolfi S, Valzasina B, Aluigi M, Isidori A, Ferri E, Salvestrini V, Bonanno G, Rutella S, Durelli I, et al. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25- into CD25+ T regulatory cells. Blood. 2007;109:2871–2877. doi: 10.1182/blood-2006-07-036863. [DOI] [PubMed] [Google Scholar]
- Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- Eastaff-Leung N, Mabarrack N, Barbour A, Cummins A, Barry S. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J. Clin. Immunol. 2010;30:80–89. doi: 10.1007/s10875-009-9345-1. [DOI] [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–1077. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J. Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J. Exp. Med. 2002;196:459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funatake CJ, Ao K, Suzuki T, Murai H, Yamamoto M, Fujii-Kuriyama Y, Kerkvliet NI, Nohara K. Expression of constitutively-active aryl hydrocarbon receptor in T-cells enhances the down-regulation of CD62L, but does not alter expression of CD25 or suppress the allogeneic CTL response. J. Immunotoxicol. 2009;6:194–203. doi: 10.1080/15476910903124454. [DOI] [PubMed] [Google Scholar]
- Funatake CJ, Marshall NB, Steppan LB, Mourich DV, Kerkvliet NI. Cutting edge: activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells. J. Immunol. 2005;175:4184–4188. doi: 10.4049/jimmunol.175.7.4184. [DOI] [PubMed] [Google Scholar]
- Hartmann G, Bidlingmaier C, Siegmund B, Albrich S, Schulze J, Tschoep K, Eigler A, Lehr HA, Endres S. Specific type IV phosphodiesterase inhibitor rolipram mitigates experimental colitis in mice. J. Pharmacol. Exp. Ther. 2000;292:22–30. [PubMed] [Google Scholar]
- Head K, Jurenka JS. Inflammatory bowel disease. Part II: Crohn's disease—pathophysiology and conventional and alternative treatment options. Altern. Med. Rev. 2004;9:360–401. [PubMed] [Google Scholar]
- Ho PP, Steinman L. The aryl hydrocarbon receptor: a regulator of Th17 and Treg cell development in disease. Cell Res. 2008;18:605–608. doi: 10.1038/cr.2008.63. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Kelsall BL. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J. Exp. Med. 1999;190:229–239. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada M, Arihiro A, Mizoguchi E. Insights from advances in research of chemically induced experimental models of human inflammatory bowel disease. World J. Gastroenterol. 2007;13:5581–5593. doi: 10.3748/wjg.v13.i42.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Kwon HS, Kim DH, Shin EK, Kang YH, Park JH, Shin HK, Kim JK. 3,3'-Diindolylmethane attenuates colonic inflammation and tumorigenesis in mice. Inflamm. Bowel Dis. 2009;15:1164–1173. doi: 10.1002/ibd.20917. [DOI] [PubMed] [Google Scholar]
- Larrosa M, Yanez-Gascon MJ, Selma MV, Gonzalez-Sarrias A, Toti S, Ceron JJ, Tomas-Barberan F, Dolara P, Espin JC. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J. Agric. Food Chem. 2009;57:2211–2220. doi: 10.1021/jf803638d. [DOI] [PubMed] [Google Scholar]
- Leibelt DA, Hedstrom OR, Fischer KA, Pereira CB, Williams DE. Evaluation of chronic dietary exposure to indole-3-carbinol and absorption-enhanced 3,3'-diindolylmethane in sprague-dawley rats. Toxicol. Sci. 2003;74:10–21. doi: 10.1093/toxsci/kfg103. [DOI] [PubMed] [Google Scholar]
- Liu Z, Colpaert S, D'Haens GR, Kasran A, de Boer M, Rutgeerts P, Geboes K, Ceuppens JL. Hyperexpression of CD40 ligand (CD154) in inflammatory bowel disease and its contribution to pathogenic cytokine production. J. Immunol. 1999;163:4049–4057. [PubMed] [Google Scholar]
- Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- Marshall NB, Kerkvliet NI. Dioxin and immune regulation: emerging role of aryl hydrocarbon receptor in the generation of regulatory T cells. Ann. N. Y. Acad. Sci. 2010;1183:25–37. doi: 10.1111/j.1749-6632.2009.05125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall NB, Vorachek WR, Steppan LB, Mourich DV, Kerkvliet NI. Functional characterization and gene expression analysis of CD4+ CD25+ regulatory T cells generated in mice treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. J. Immunol. 2008;181:2382–2391. doi: 10.4049/jimmunol.181.4.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AR, Villegas I, Sanchez-Hidalgo M, de la Lastra CA. The effects of resveratrol, a phytoalexin derived from red wines, on chronic inflammation induced in an experimentally induced colitis model. Br. J. Pharmacol. 2006;147:873–885. doi: 10.1038/sj.bjp.0706469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor AL, Chandler P, Baban B, Hansen AM, Marshall B, Pihkala J, Waldmann H, Cobbold S, Adams E, Munn DH. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int. Immunol. 2004;16:1391–1401. doi: 10.1093/intimm/dxh140. [DOI] [PubMed] [Google Scholar]
- Monteleone G, Biancone L, Marasco R, Morrone G, Marasco O, Luzza F, Pallone F. Interleukin 12 is expressed and actively released by Crohn's disease intestinal lamina propria mononuclear cells. Gastroenterology. 1997;112:1169–1178. doi: 10.1016/s0016-5085(97)70128-8. [DOI] [PubMed] [Google Scholar]
- Neurath MF, Fuss I, Kelsall BL, Stuber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J. Exp. Med. 1995;182:1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng LG, Mackay CR, Mackay F. The BAFF/APRIL system: life beyond B lymphocytes. Mol. Immunol. 2005;42:763–772. doi: 10.1016/j.molimm.2004.06.041. [DOI] [PubMed] [Google Scholar]
- Parronchi P, Romagnani P, Annunziato F, Sampognaro S, Becchio A, Giannarini L, Maggi E, Pupilli C, Tonelli F, Romagnani S. Type 1 T-helper cell predominance and interleukin-12 expression in the gut of patients with Crohn's disease. Am. J. Pathol. 1997;150:823–832. [PMC free article] [PubMed] [Google Scholar]
- Pizarro TT, Arseneau KO, Bamias G, Cominelli F. Mouse models for the study of Crohn's disease. Trends Mol. Med. 2003;9:218–222. doi: 10.1016/s1471-4914(03)00052-2. [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J. Gastroenterol. 2008;14:4280–4288. doi: 10.3748/wjg.14.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JV, Bradfield CA. Ah receptor signaling pathways. Annu. Rev. Cell Dev. Biol. 1996;12:55–89. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- Shepherd DM, Steppan LB, Hedstrom OR, Kerkvliet NI. Anti-CD40 treatment of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-exposed C57Bl/6 mice induces activation of antigen presenting cells yet fails to overcome TCDD-induced suppression of allograft immunity. Toxicol. Appl. Pharmacol. 2001;170:10–22. doi: 10.1006/taap.2000.9080. [DOI] [PubMed] [Google Scholar]
- Takamura T, Harama D, Matsuoka S, Shimokawa N, Nakamura Y, Okumura K, Ogawa H, Kitamura M, Nakao A. Activation of the aryl hydrocarbon receptor pathway may ameliorate dextran sodium sulfate-induced colitis in mice. Immunol. Cell Biol. 2010;88:685–689. doi: 10.1038/icb.2010.35. [DOI] [PubMed] [Google Scholar]
- Tas SW, Vervoordeldonk MJ, Hajji N, Schuitemaker JH, van der Sluijs KF, May MJ, Ghosh S, Kapsenberg ML, Tak PP, de Jong EC. Noncanonical NF-kappaB signaling in dendritic cells is required for indoleamine 2,3-dioxygenase (IDO) induction and immune regulation. Blood. 2007;110:1540–1549. doi: 10.1182/blood-2006-11-056010. [DOI] [PubMed] [Google Scholar]
- Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, Opelz G. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J. Exp. Med. 2002;196:447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem. Biophys. Res. Commun. 2008;375:331–335. doi: 10.1016/j.bbrc.2008.07.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigmann B, Tubbe I, Seidel D, Nicolaev A, Becker C, Neurath MF. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat. Protoc. 2007;2:2307–2311. doi: 10.1038/nprot.2007.315. [DOI] [PubMed] [Google Scholar]
- Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.