Abstract
The objectives of this study were to determine the structural characteristics of perfluoroalkyl acids (PFAAs) that confer estrogen-like activity in vivo using juvenile rainbow trout (Oncorhynchus mykiss) as an animal model and to determine whether these chemicals interact directly with the estrogen receptor (ER) using in vitro and in silico species comparison approaches. Perfluorooctanoic (PFOA), perfluorononanoic (PFNA), perfluorodecanoic (PFDA), and perfluoroundecanoic (PFUnDA) acids were all potent inducers of the estrogen-responsive biomarker protein vitellogenin (Vtg) in vivo, although at fairly high dietary exposures. A structure-activity relationship for PFAAs was observed, where eight to ten fluorinated carbons and a carboxylic acid end group were optimal for maximal Vtg induction. These in vivo findings were corroborated by in vitro mechanistic assays for trout and human ER. All PFAAs tested weakly bound to trout liver ER with half maximal inhibitory concentration (IC50) values of 15.2–289μM. Additionally, PFOA, PFNA, PFDA, PFUnDA, and perlfuorooctane sulfonate (PFOS) significantly enhanced human ERα-dependent transcriptional activation at concentrations ranging from 10–1000nM. Finally, we employed an in silico computational model based upon the crystal structure for the human ERα ligand-binding domain complexed with E2 to structurally investigate binding of these putative ligands to human, mouse, and trout ERα. PFOA, PFNA, PFDA, and PFOS all efficiently docked with ERα from different species and formed a hydrogen bond at residue Arg394/398/407 (human/mouse/trout) in a manner similar to the environmental estrogens bisphenol A and nonylphenol. Overall, these data support the contention that several PFAAs are weak environmental xenoestrogens of potential concern.
Keywords: perfluoroalkyl acid, estrogen, perfluorooctanoic acid, perfluorooctane sulfonate, vitellogenin, molecular docking
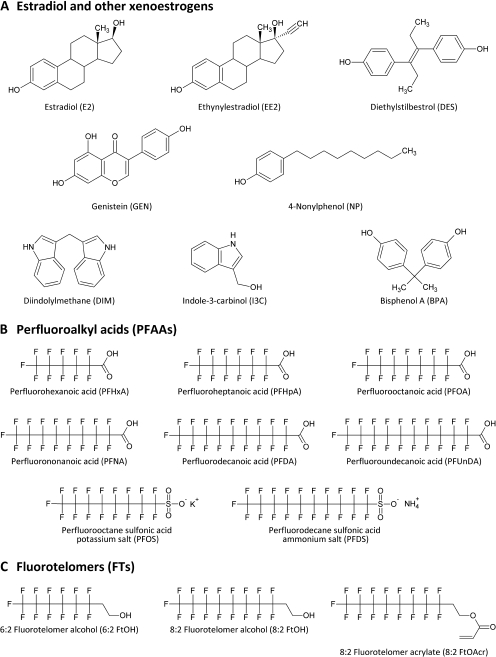
The widespread industrial and commercial use of polyfluorinated chemicals (PFCs) as surfactants and surface protectors for paper and textile coatings, polishes, food packaging, and fire-retardant foams has led to the pervasive presence of these chemicals in the environment, wildlife, and humans (see reviews by Calafat et al., 2007; Houde et al., 2006). The general structure of PFCs resembles that of fatty acids in that each compound has a hydrophobic polyfluorinated carbon tail of varying length and a functional end group, which provides the basis for classification (Fig. 1). Perfluoroalkyl acids (PFAAs) and fluorotelomers comprise the two major structural groups of PFCs. Human blood levels of perfluorooctanoic acid (PFOA) and perlfuorooctane sulfonate (PFOS), the two most commonly studied PFCs, are about 4 and 20 ppb, respectively, although national survey data suggest that these levels have decreased in recent years (Calafat et al., 2007). Levels of PFOS and PFOA in wildlife tend to be higher, in the range of tens to thousands ppb (Kannan et al., 2002). Other PFCs commonly detected in biological samples include perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUnDA), and perfluorododecanoic acid (PFDoDA).
FIG. 1.
Structures for known xenoestrogens (A), select PFAAs (B), and fluorotelomer (C) compounds are shown.
The toxicology and toxicokinetics of PFOA, as an example PFC, have been thoroughly reviewed by Kennedy et al. (2004). PFOA does not accumulate in fatty tissues because of its dual lipophobic and hydrophobic chemical properties but instead binds to blood proteins and is distributed primarily to liver, plasma, and kidney. The measured biological half-life of PFOA varies among species ranging from hours in female rat to days in dogs or rainbow trout (Hanhijarvi et al., 1988; Martin et al., 2003). The estimated half-life of PFOA in humans is nearly 4 years, pointing to a lower capacity for elimination of the compound compared with other species (Olsen et al., 2007). Finally, PFOA is not metabolized or defluorinated in vivo, although some fluorotelomer compounds may be metabolized to PFAAs in rodents (Martin et al., 2005; Nabb et al., 2007).
PFOA and other PFCs are members of a large group of chemicals called peroxisome proliferators (PPs), which increase the abundance of hepatic peroxisomes and induce peroxisomal and mitochondrial enzymes involved with β-oxidation, cytochrome P450 fatty acid ω-oxidation, and cholesterol homeostasis (reviewed in Bosgra et al., 2005) via ligand-dependent activation of the hepatic PP-activated receptor α (PPARα) (Holden and Tugwood, 1999). Prolonged exposure to PPs, including PFOA, can result in hepatocarcinogenesis (Abdellatif et al., 1991), although marked species differences in susceptibility to PPs have been observed. Whereas rodents are highly susceptible, humans and nonhuman primates are insensitive or nonresponsive (Lai, 2004), possibly due low expression of PPARα in liver (Palmer et al., 1998). Recent evidence points to PPARα-independent mechanisms of action for increased risk of liver cancer by PPs, suggesting that the assumption that PPARα agonists pose little risk for human hepatocarcinogenesis may be unsound (reviewed in Guyton et al., 2009). For example, chronic exposure to the environmental PPARα agonist di(2-ethylhexyl) phthalate increased liver cancer incidence in PPARα knockout mice (Ito et al., 2007).
Estrogens have long been known to be associated with cancer in estrogen-responsive tissues including the breast, ovary, and uterus as complete carcinogens, promoters, or possible chemoprotective agents (Cavalieri et al., 2000; Eliassen and Hankinson, 2008; Ho, 2003; Persson, 2000). Moreover, estrogens also influence carcinogenesis in other tissues not usually considered to be estrogen responsive. Experimental studies investigating hepatic cancer in mammalian animal models have implicated estrogens as tumor promoters and in some cases complete carcinogens (reviewed in De Maria et al., 2002; Yager et al., 1991), although the higher rate of hepatic cancer in males suggests the potential for a protective role of estrogens against liver cancer (Villa, 2008; Yeh and Chen, 2010). Several studies in our laboratory using the rainbow trout cancer model have also demonstrated that estrogen exposure promotes hepatocellular carcinogenesis in rainbow trout (Núñez et al., 1989; Orner et al., 1995).
Previously, we reported that PFOA may act via an alternative mechanism of action, not involving peroxisome proliferation, to increase risk of liver cancer in rainbow trout, an animal model that mimics human insensitivity to PPs (Tilton et al., 2008). Dietary exposure to PFOA significantly increased expression of the sensitive estrogen biomarker protein vitellogenin (Vtg) and induced an overall hepatic gene expression profile highly similar to that of 17β-estradiol (E2). We concluded that the cancer-enhancing effects of PFOA in trout were due to novel mechanisms related to estrogen signaling rather than the typical PP response observed for this chemical in rodent models (Tilton et al., 2008). Studies in other model organisms support the concept that certain PFCs may have estrogen-like activity. Liu et al. (2007) reported that PFOA, PFOS, and 6:2 fluorotelomer alcohol (6:2FtOH) increased Vtg expression in cultured tilapia hepatocytes, and Wei et al. (2007) showed that PFOA increased Vtg expression and caused disruption of gonad development in rare minnows (Gobiocypris rarus). Evidence for estrogen-like activity of some fluorotelomers has also been shown in vitro (Ishibashi et al., 2007; Maras et al., 2006).
Though several laboratories have shown estrogenic effects of certain PFCs in vitro and in vivo, it is yet unknown whether any of these chemicals interact directly with the estrogen receptor (ER). This receptor is generally considered to be quite promiscuous because dozens of structurally diverse exogenous chemicals, including phytochemicals, hydroxylated polychlorinated biphenyls, pesticides, and other industrial compounds, have been shown to bind to the receptor with varying degrees of affinity (Blair et al., 2000; Matthews et al., 2000; Olsen et al., 2005). The optimal chemical structure for ligand binding to the ER is a four-ring arrangement with opposing hydroxyl end groups, although long-chain compounds, such as nonylphenol, have been shown to effectively bind to the protein as well (Matthews et al., 2000; Sivanesan et al., 2005).
In this present study, we determined the relationship between PFC structure and estrogen-like activity using the Vtg induction bioassay in rainbow trout to test the hypotheses that PFCs structurally similar to PFOA are estrogenic and that the Vtg response depends largely on chemical structure. We also assessed the interaction of PFAAs with the ER using direct steroid receptor-binding assays for trout liver ER and a gene reporter assay for human ERα. Finally, we employed computational modeling to determine the molecular interaction of these compounds with the ligand-binding domains (LBDs) of human, mouse, and trout ERα proteins.
MATERIALS AND METHODS
Chemicals
A complete list of PFCs investigated in this study, including their common names, abbreviations, and registry numbers for reference, is provided in Supplementary table 1. Perfluoroalkyl carboxylic acid and sulfonate compounds were all purchased from Sigma-Aldrich (St Louis, MO), except for perfluorodecane sulfonate (PFDS), which was graciously donated by Dr Jennifer Field (Oregon State University). Fluorotelomer alcohol and acrylate compounds were obtained from SynQuest Labs (Alachua, FL). E2, genistein (GEN), diethylstilbestrol (DES), ethynylestradiol (EE2), indole-3-carbinol (I3C), 3,3′-diindolylmethane (DIM), 4-nonylphenol (NP), and all other reagents were purchased from Sigma-Aldrich or other general laboratory suppliers and were of the highest purity available. [2,4,6,7-3H]-17β-estradiol (70 Ci/mmol) was purchased from Perkin Elmer (Waltham, MA).
Animal Care
Mt Shasta strain rainbow trout were hatched and reared at the Sinnhuber Aquatic Research Laboratory (SARL) at Oregon State University in Corvallis, OR. Fish were maintained in flow-through 375-l tanks at 12°C with activated carbon water filtration on a 12:12 h light:dark cycle. Two weeks prior to experimental treatments, fish were fed a maintenance ration (2% of body weight [bw]) of Oregon Test Diet (OTD), a semipurified casein-based diet with menhaden oil as the lipid. All experimental diets described below were administered for 14 days, and feeding occurred 5 days per week. On day 15, trout were euthanized with an overdose (250 ppm) of tricane methanesulfonate and necropsied. Body and liver weights were recorded to calculate the liver somatic index (LSI = liver weight/bw × 100), and blood samples from each donor animal were obtained. The plasma fraction of each blood sample was obtained by centrifugation at 850 × g and then frozen at −80°C. All procedures for treatment, handling, maintenance, and euthanasia of trout used in this study were approved by the Oregon State University Institutional Animal Care and Use Committee.
In Vivo Experimental Procedures
Experiment 1—subchronic dietary exposure to PFCs.
A variety of structurally diverse PFCs were chosen to test in vivo in juvenile rainbow trout, including perfluoroalkyl carboxylic acids with 6–14 fluorinated carbons, two perfluoroalkyl sulfonates, and three fluorotelomers. Eleven-month-old juvenile trout (initial bw approximately 70 g) were distributed to holding tanks 1 week prior to the start of feeding experimental diets. Test chemicals were dissolved in dimethylsulfoxide (DMSO) vehicle (≤ 0.5 ppm) and added directly to the oil portion of the custom trout OTD diet. PFCs were administered at a diet concentration of 250 ppm calculated with respect to diet wet weight (approximately 5 mg/kg bw/day) to six fish per treatment group. Additionally, PFOA, PFNA, PFDA, and PFUnDA were selected for testing in a simple mixture study. Two additional dietary groups at 5 and 50 ppm (0.1 and 1 mg/kg bw/day) were added to the experiment for these four compounds so that three concentrations in total were tested for each individual chemical (5, 50, and 250 ppm). Mixture treatments were prepared by combining equal mass amounts of each test compound at each concentration level. Thus, mixture A consisted of 5 ppm each PFOA, PFNA, PFDA, and PFUnDA for a total perfluoroalkyl carboxylic acid concentration of 20 ppm. Similarly, mixture B was made by combining 50 ppm each chemical for a total diet concentration of 200 ppm; finally, mixture C was prepared by combining 250 ppm each compound for total concentration of 1000 ppm. The control group (N = 24, six fish in four replicate tanks) was fed OTD diet with no modifications; the vehicle control group was fed diet with 0.5 ppm DMSO (N = 6), and 5 ppm E2 was included as a positive control (N = 12, six fish in two replicate tanks). The additional fish were included in the E2 positive and the control groups to account for multiple sampling days.
Experiment 2—dose-dependent induction of Vtg by PFOA and PFDA.
The goal of this experiment was to determine the dose-dependent impact of dietary exposure to PFOA and PFDA on trout blood Vtg levels. PFOA and PFDA were selected as test compounds for this experiment because of their common detection in wildlife and humans and the observed robust induction of Vtg by both chemicals in experiment 1. Each experimental group consisted of a single tank of 5-month-old individual juvenile trout with an initial approximate bw of 30 g. The control group (N = 16) was fed OTD diet with no modifications, and 5 ppm E2 diet was used as a positive control (N = 16). Eight trout per treatment group were fed experimental diets containing either PFOA or PFDA at concentrations of 0.026, 0.128, 0.64, 3.2, 16, 80, 400, or 2000 ppm calculated with respect to diet wet weight (range of 0.52 μg/kg bw/day up to 40 mg/kg bw/day). In this study, the test compounds were added directly to the oil portion of the diet, which was gently heated (< 50°C) to aid in chemical dissolution.
Vtg ELISA
Blood plasma Vtg was measured following an ELISA method previously described (Shilling and Williams, 2000) with modifications as outlined by Benninghoff and Williams (2008). For this study, the interassay coefficient of variance for the Vtg ELISA was 7.1% with an assay detection limit of 6.25 ng/ml.
Measurement of Palmitoyl Coenzyme A Oxidation and Catalase Activity
Peroxisomal fractions of liver tissues were prepared by differential centrifugation, and catalase activity was assessed following the methods described by Orner et al. (1995). Beta-oxidation of palmitoyl coenzyme A (CoA) was measured using a spectrophotometric assay as previously described (Mitchell et al., 1985).
PFOA and PFDA Blood Content by Liquid Chromatography-Tandem Mass Spectrometry
Complete methods for the determination of PFOA and PFDA content in trout blood plasma samples are provided in the Supplementary materials including details on the instrumentation, quality control, sample preparation, liquid chromatography-tandem mass spectrometry/(LC-MS/MS) conditions, and quantitation. Briefly, measurement of PFOA and PFDA was performed at the 3M Medical Department, and this laboratory was blinded to any information on the effects of PFOA or PFDA on Vtg expression in trout. A Shimadzu High Performance Liquid Chromatography (HPLC) system free of contaminating polytetrafluoroethylene-based materials was used in conjunction with an API 5000 triple stage quadrupole mass spectrometer. Samples were prepared by solid phase extraction with Waters Oasis HLB cartridges. PFOA and PFDA standard materials were the free acid linear isoforms, and internal dual 13C-labeled PFOA and PFDA standards were used for quantitation.
Trout Liver ER-Binding Studies with PFCs
Preparation of trout liver cytosol.
Juvenile trout were fed control OTD diet (N = 24) or supplemented with 5 ppm E2 (N = 24). Livers were pooled in groups of six, homogenized in two volumes of ice-cold TEMS buffer (10mM Tris-HCl, 1mM EDTA, 12mM monothioglycerol, 20mM sodium molybdate, 10% vol/vol glycerol, and 0.1mM phenylmethanesulfonylfluoride), and centrifuged at 100,000 × g for 15 min at 4°C. Two volumes of ice-cold TEMS buffer containing 5% wt/vol activated charcoal and 0.5% wt/vol dextran were added to the supernatant, which was mixed, incubated on ice for 15 min, and then centrifuged at 100,000 × g for 90 min at 4°C. The upper lipid layer was removed, and the remaining supernatant was spun at 100,000 × g for an additional 30 min. Again, the lipid layer was aspirated, and the resulting supernatant (the cytosol fraction) was collected, aliquoted, and stored at −80°C until use.
ER saturation binding assays.
Saturation analyses were performed by incubating 200μl of charcoal-stripped cytosol (5 mg protein per milliliter) with 25 μl of [3H]-E2 (final concentration 0.1–25nM) in TEMS buffer for 24 h at 12°C. ER saturation binding assays were performed with separate cytosol preparations from control and E2-exposed trout. Nonspecific binding was determined by incubating cytosol with [3H]-E2 and DES (10–2.5μM) and was defined as the fraction of total binding displaced by 100-fold excess DES. Free steroid was separated from bound by incubation with 500μl of TEMS with 2.5% wt/vol activated charcoal and 0.25% wt/vol dextran for 30 min on ice followed by centrifugation at 3000 × g for 10 min. Bound [3H]-E2 was measured in the supernatant by liquid scintillation counting (Beckman Instruments Inc., Fullerton, CA).
ER competitive binding assays.
Competitive binding assays were performed by incubating 200μl charcoal-stripped cytosol (5 mg protein per milliliter, 200 microliters per assay tube) extracted from livers of E2-exposed trout, 25μl of [3H]-E2 in TEMS buffer at a final concentration of 4nM (near saturating concentration), and 25μl of cold competitor for 24 h at 12°C. Competitor stock solutions were prepared in TEMS buffer with DMSO (5% final assay concentration). Bound [3H]-E2 was measured as described above. Competitors were assayed according to the anticipated affinity of the putative ligand (1pM–10μM for strong binders and 10μM–100mM for weak binders). Maximum nonspecific binding was determined using 100μM E2 and was subtracted from all total count values to determine specific binding.
Human ERα Gene Reporter Assay
Human embryonic kidney (HEK-293T) cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with L-glutamine (Mediatech, Manassas, VA) supplemented with 10% fetal bovine serum (FBS, Tissue Culture Biologicals, Seal Beach, CA) and 10,000 U/ml penicillin-streptomycin (Mediatech) in a humidified, 5% CO2 atmosphere. HEK-293T cells were seeded in 96-well microplates at 10,000 cells per well in 100 μl phenol red-free DMEM with 10% charcoal-stripped FBS. Cells were cotransfected 24 h after seeding with the following: 70 ng of the XTEL luciferase reporter plasmid containing a consensus estrogen-responsive element (ERE) sequence from the Xenopus Vtg promoter (graciously donated by Dr Michael Garabedian, New York University School of Medicine) and 10 ng of the human ERα expression vector (Kolluri et al., 2003), an independent β-galactosidase reporter driven by a cytomegalovirus (CMV) promoter and pcDNA for DNA input normalization (Invitrogen, Carlsbad, CA). Each well received 0.2 μl of PLUS reagent with 0.2 μl of lipofectamine (Invitrogen). Twenty-four hours after transfection, cells were treated with E2, PFOA, PFNA, PFDA, PFUnDA, PFOS, or 8:2FtOH at concentrations ranging from 1 to 1000nM for 24 h. Cells were then lysed and measured for reporter gene activity as previously described (Bisson et al., 2009). Raw luminescence data were normalized by β-galactosidase activity to account for any variability in transfection efficiency; then, values for ERα gene reporter activity were expressed as fold induction with respect to vehicle-only cells.
In Silico Modeling of PFC Interaction with Human, Mouse, and Trout ERα Proteins
Molecular modeling.
The 3D coordinates of the human ERα (hERα) LBD complexed with the natural agonist E2 were taken from the crystal structures available in the Protein Data Bank (accession 1ERE) (Brzozowski et al., 1997). Following protein sequence alignment of the LBD regions for the mouse Esr1 and trout era1 proteins (UniProt accessions P19785 and P16058, respectively) using ClustalW2 (EMBL-EBI), homology models for mouse ERα (mERα) and rainbow trout ERα (rtERα) proteins were built based upon 1ERE as the 3D-template with Molsoft ICM v3.5-1p (Molsoft, L.L.C., La Jolla, CA). Rainbow trout have two isoforms of ERα, era1 and era2 (Nagler et al., 2007); because era1 is the ERα isoform abundantly expressed in the liver, we selected this sequence for constructing the homology model. The percent identities of the mouse Esr1 and trout era1 protein sequences to that of the human ERα were 89 and 46%, respectively. All models were energetically refined in the internal coordinate space with Molsoft ICM v3.5-1p (Cardozo et al., 1995).
Molecular docking.
The receptor is represented by five types of interaction potentials: (1) van der Waals potential for a hydrogen atom probe, (2) van der Waals potential for a heavy-atom probe (generic carbon of 1.7 Å radius), (3) optimized electrostatic term, (4) hydrophobic terms, and (5) loan-pair–based potential, which reflects directional preferences in hydrogen bonding. The energy terms are based on the Merck Molecular Force Field to account for solvation free energy and entropic contribution (Totrov and Abagyan, 2001). Modified intermolecular terms such as soft van der Waals and hydrogen bonding, as well as a hydrophobic term, are added. Conformational sampling is based on the biased probability Monte Carlo procedure, which randomly selects a conformation in the internal coordinate space and then makes a step to a new random position independent of the previous one, but according to a predefined continuous probability distribution. It has also been shown that after each random step, full local minimization greatly improves the efficiency of the procedure. In the ICM-virtual ligand screening (VLS) procedure (Molsoft ICM v3.5-1p), the ligand scoring is optimized to obtain maximal separation between the binders and nonbinders. Each compound is assigned a score according to its fit within the receptor; this ICM score accounts for continuum and discreet electrostatics, hydrophobicity, and entropy parameters (Abagyan et al., 1994; Totrov and Abagyan, 1997, 2001).
Data Analysis
For in vivo experiments, an individual animal consisted of a single biological replicate, and the sample size is indicated in each figure legend or table. For most experiments, a one-way ANOVA test with Dunnett's multiple comparisons post hoc test was employed for statistical analyses of the data (Prism 5, GraphPad Software, LaJolla, CA). For analyses of Vtg data, all values were log10 transformed to equalize variance prior to statistical analyses. A significant effect of an experimental treatment was inferred when p < 0.05.
RESULTS
Experiment 1—Subchronic Dietary Exposure to PFCs
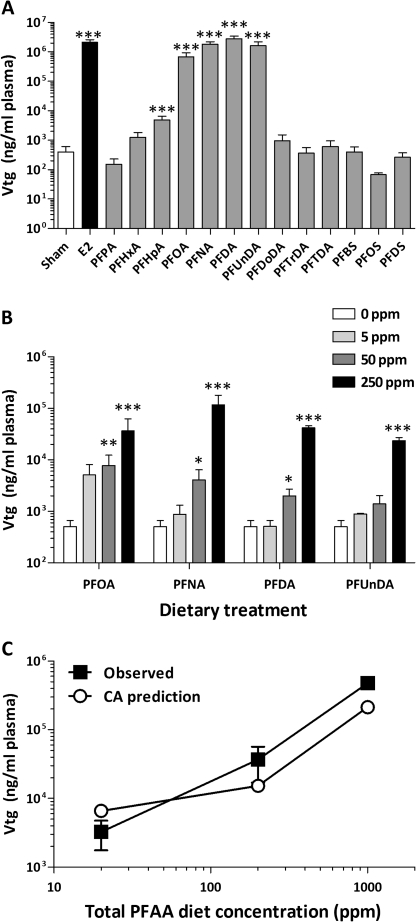
The objective of this experiment was to determine the impact of subchronic dietary exposure to structurally diverse PFCs, including PFAAs and fluorotelomers, on Vtg expression and to determine whether a structure-activity relationship was evident. As expected, short-term dietary exposure to 5 ppm E2 caused a large > 10,000-fold increase in plasma Vtg levels (Fig. 2A) compared with control fish. Exposure to small perfluoroalkyl carboxylic acids with six or seven fluorinated carbons did not significantly alter plasma Vtg levels, whereas medium and long-chain perfluoroalkyl carboxylic acids with 8–14 fluorinated carbons significantly induced Vtg expression by 7- to 190-fold. PFOA, PFNA, PFDA, and PFUnDA were the most potent inducers of Vtg, although the level of response was substantially lower than that of E2. The perfluoroalkyl sulfonates PFOS and PFDS induced a minor three- to fourfold increase in blood Vtg levels. Overall, the fluorotelomers tested had little effect on plasma Vtg levels, with only one compound, 6:2FtOH, causing a modest eightfold increase.
FIG. 2.
Effects of subchronic dietary exposure to select PFAAs and fluorotelomers on plasma Vtg concentration. (A) Mean blood plasma Vtg values are shown on a logarithmic scale + SEM (N = 18 for control, N = 12 for E2, and N = 6 for all other treatments). The diet concentration was 250 ppm (approximately 5 mg/kg bw/day) for all perfluoroalkyl compounds and 5 ppm for E2, the positive control. (B) For individual chemical treatments, fish were fed diets containing 0, 5, 50, or 250 ppm of PFOA, PFNA, PFDA, and PFUnDA. Mean blood plasma Vtg values are shown on a logarithmic scale + SEM (N = 18 for control and N = 6 for all other treatments). (C) The mixture treatments were prepared by adding equal amounts of each constituent chemical to achieve total concentrations of 20, 200, and 1000 ppm as described in the “Materials and Methods” section. Closed squares represent the measured mean blood plasma Vtg values ± SEM for the mixture treatments (N = 6). Open circles represent the predicted level of Vtg response at each concentration calculated using a simple CA model. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with control (for B, within a diet group) as determined by one-way ANOVA with Dunnett's multiple comparisons post hoc test.
The perfluoroalkyl carboxylic acids PFOA, PFNA, PFDA, PFUnDA, and PFDoDA all caused a significant increase in relative liver size, though the response to PFDoDA was unexpectedly high (Supplementary table 2). Although only the fluorotelomer compound 6:2FtOH was effective in increasing Vtg expression, 8:2FtOH and 8:2FtOAcr caused a significant increase in relative liver weight. Overall, for this dietary screening study, there was a significant correlation between Vtg response and LSI with a Spearman's correlation r value of 0.62 (p = 0.022).
Four perfluoroalkyl carboxylic acids, PFOA, PFNA, PFDA, and PFUnDA, were tested at diet concentrations ranging from 5 to 250 ppm and also in a quaternary mixture (20–1000 ppm). For each individual chemical, a significant concentration-dependent induction of Vtg expression was observed (p < 0.0001 by one-way ANOVA) (Fig. 2B). Bonferroni post hoc tests revealed that PFOA, PFNA, and PFDA exposures at 50 and 250 ppm caused significant increases in plasma Vtg levels, whereas the response to PFUnDA was only significant at 250 ppm. To calculate the predicted response to the PFOA/PFNA/PFDA/PFUnDA mixture, a simple concentration addition (CA) model was employed, where the response for each individual compound was summed at each concentration level (Fig. 2C). For example, at the middle exposure, Vtg levels of 7726 ng/ml for PFOA + 4083 ng/ml for PFNA + 2008 ng/ml for PFNA + 1410 ng/ml for PFUnDA yielded a CA prediction model value of 15,227 ng/ml for the quaternary mixture. At the lowest concentration level, the observed response to the carboxylic acid mixture was about half of that predicted by the CA model. Alternatively, at the medium and higher diet concentrations, the mixture treatment induced Vtg at levels 1.8- and 2.2-fold higher, respectively, than the predicted response. As these in vivo data sets were limited in the number of exposure levels examined, a nonlinear regression analysis for curve comparisons was not performed.
Finally, palmitoyl CoA oxidation and catalase activity were measured as markers of peroxisome proliferation; a subset of the compounds tested in experiment 1 were selected for enzyme activity analyses, including those chemicals that strongly induced Vtg as well as representative compounds from each structural group. None of the test agents significantly increased peroxisomal palmitoyl CoA oxidation or catalase activity in trout liver, whereas 6:2FtOH and 8:2FtOH apparently decreased catalase activity (Supplementary fig. 1).
Experiment 2—Dose-Dependent Induction of Vtg by PFOA and PFDA
PFOA and PFDA levels in trout blood.
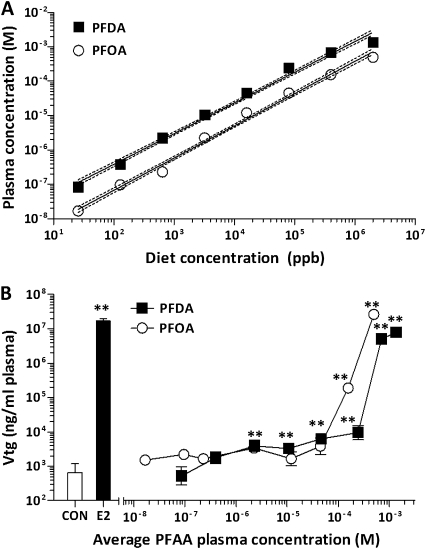
A strong linear dose-response relationship between the concentration of PFOA or PFDA administered in the diet and the amount of compound measured in trout blood plasma was observed (Fig. 3A) with linear regression R2 values of 0.86 and 0.95, respectively. However, at the very highest concentration of PFDA tested (2000 ppm), the measured blood concentration of PFDA was slightly lower than indicated by the linear model, suggesting that this or higher dietary concentrations may exceed the limits for PFDA absorption in trout. The lowest dietary concentration tested (25.6 ppb or 0.5 μg/kg bw/day) resulted in blood levels of these compounds in the low nanomolar range: 17.3 ± 1.82nM for PFOA and 85.8 ± 4.79nM for PFDA, whereas the highest dietary concentrations (2000 ppm or 40 mg/kg bw/day) resulted in blood levels of 508 ± 35μM for PFOA and 1.36 ± 0.11mM PFDA.
FIG. 3.
Dose-dependent effects of subchronic dietary exposure to PFOA and PFDA on chemical concentrations in blood plasma and Vtg expression. (A) Blood plasma concentrations of PFOA and PFDA following 2-week exposure to a broad range of diet concentrations (0.0256–2000 ppm) are shown (N = 8) in a log-log plot. Results of linear regression analyses of nontransformed data are represented by the solid lines, whereas the 95% confidence intervals are represented by the dashed lines. (B) Mean blood plasma Vtg values are shown ± SEM (N = 8) for PFOA- and PFDA-exposed fish plotted according to the measured blood concentrations of PFOA and PFDA, respectively. *p < 0.05 and **p < 0.01 compared with the 0 ppm treatment group (CON) as determined by one-way ANOVA with Dunnett's multiple comparisons post hoc test.
Dose-dependent induction of Vtg by PFOA and PFDA.
PFOA and PFDA caused a significant dose-dependent induction of Vtg expression in trout with a maximal response similar to that of E2 (p < 0.0001 by separate one-way ANOVA for PFOA and PFDA) (Fig. 3B). Bonferroni post hoc tests indicated that the lowest observed effect level (LOEL) for PFOA exposure was at the 80 ppm diet (1.6 mg/kg bw/day) level or 35.4μM PFOA in blood plasma (Table 1). Alternatively, the LOEL for PFDA was much lower at 0.64 ppm (12.8 μg/kg bw/day) or 2.0μM PFDA in blood plasma. Although PFOA did not induce a significant increase in Vtg below the relatively high diet concentration of 80 ppm, the dose-response curve for this compound was substantially left shifted compared with that of PFDA, resulting in an substantially lower estimated effective concentration at 50% response (EC50) value, 182μM for PFOA compared with 360μM for PFDA. However, this lower EC50 value did not correlate with a lower PFOA dietary exposure, likely a result of the higher half-life and lower elimination rate of PFDA in trout compared with PFOA (Martin et al., 2003). The corresponding amount of dietary PFOA to achieve the EC50 value of 182μM is 458 ppm, whereas a dietary PFDA concentration of 214 ppm corresponds with its EC50 value of 360μM (Table 1). A dose-dependent effect of increasing diet concentrations of PFOA and PFDA on relative liver size was less evident in this experiment, as only the highest diet concentrations significantly increased LSI values, 2000 ppm for PFOA and > 400 ppm for PFDA (Supplementary table 3).
TABLE 1.
Dose-Response Curve Parameters for PFOA and PFDA Induction of Vtg
| PFOA |
PFDA |
|||
| Parameter | Diet (ppm) | Blood level (μM) | Diet (ppm) | Blood level (μM) |
| LOEL | 80 | 35.4 | 0.64 | 2.00 |
| NOEL | 16 | 7.83 | 0.128 | 0.48 |
| EC50 | 458 | 182 | 214 | 360 |
| EC10 | 141 | 60.1 | 118 | 212 |
Note. Experimentally determined values (diet concentrations) are shown in bold for the LOEL and NOEL estimates, whereas the corresponding blood levels were extrapolated from the standard curve shown in Figure 3A (diet concentration vs. blood level). Experimentally calculated values (blood levels) are shown in bold for EC50 and EC10 estimates, whereas the corresponding dietary exposure level was extrapolated from the dose-response curve shown in Figure 3B (Vtg induction vs. blood level). NOEL, no observed effect level; EC10, effective concentration at 10% response.
Trout Hepatic ER-Binding Studies
Radiolabeled E2 binding in liver cytosol extract from E2-exposed rainbow trout showed a single class of high-affinity binding sites that saturated at 5–10nM [3H]-E2 with an equilibrium dissociation constant (Kd) value of 4.4 ± 0.7nM and measured binding capacity (Bmax) value of 152 ± 4.9pM (38 pmol/g protein) (Supplementary fig. 2). At the near saturating concentration of 4nM [3H]-E2, a larger fraction of total binding was specific binding (about 75%) for the E2-exposed liver cytosol compared with only about 50% for the control cytosol. Therefore, all competitive binding experiments were conducted using the cytosol protein from E2-exposed fish because of the greater dynamic range in which to perform these assays.
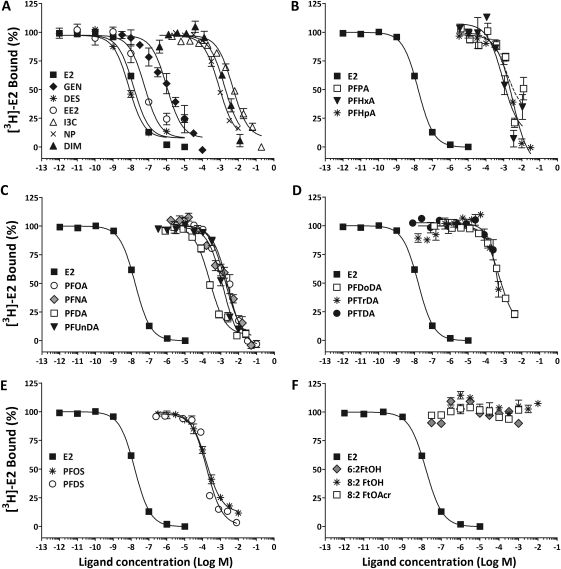
In the radioligand competitive binding assays, the natural hormone E2 bounds to the trout hepatic ER with an IC50 value of 13.9nM (Fig. 4A, Table 2). The synthetic estrogen DES was also a strong binder with a relative binding affinity (RBA) value nearly twice that of E2, whereas EE2 binding was about fivefold weaker. The dietary phytoestrogen GEN had moderate binding affinity for the trout liver ER, with an RBA value of about 1%. Though NP, DIM, and I3C all effectively displaced [3H]-E2 from the ER, these compounds were classified as weak-affinity ligands with IC50 values in the high micromolar range and RBA values well below 0.01%.
FIG. 4.
Binding of known xenoestrogens and various PFCs to the trout hepatic ER. Competition assays were performed with 1 mg cytosolic protein in the presence of 4nM [3H]-estradiol and increasing concentrations of test ligands, including known xenoestrogens (A), perfluoroalkyl carboxylic acids of increasing carbon chain length (B–D), perfluoroalkyl sulfonic acids (E), and fluorotelomers (F). Symbols represent mean ± SEM specific binding for each test chemical.
TABLE 2.
IC50 and RBA Values for Competitive Ligands for the Trout Liver ER
| Treatment | Abbreviation | IC50 (M)a | RBA (%)b | Classificationc |
| Xenoestrogens | ||||
| Estradiol | E2 | 1.39 × 10−8 | 100 | S |
| Diethylstilbestrol | DES | 7.59 × 10−9 | 186 | S |
| Ethynylestradiol | EE2 | 5.89 × 10−8 | 23.7 | S |
| Genistein | GEN | 1.01 × 10−6 | 1.38 | M |
| 4-Nonylphenol | NP | 8.42 × 10−4 | 0.0017 | W |
| Indole-3-carbinol | I3C | 4.81 × 10−3 | 0.0003 | W |
| 3,3′-Diindolylmethane | DIM | 1.76 × 10−3 | 0.0008 | W |
| Perfluoroalkyl carboxylic acids | ||||
| Perfluoropentanoic acid | PFPA | 2.89 × 10−3 | 0.0005 | VW |
| Perfluorohexanoic acid | PFHxA | 1.22 × 10−3 | 0.0011 | W |
| Perfluoroheptanoic acid | PFHpA | 1.78 × 10−3 | 0.0008 | VW |
| Perfluorooctanoic acid | PFOA | 1.82 × 10−3 | 0.0008 | VW |
| Perfluorononanoic acid | PFNA | 1.63 × 10−3 | 0.0009 | VW |
| Perfluorodecanoic acid | PFDA | 2.34 × 10−4 | 0.0060 | W |
| Perfluoroundecanoic acid | PFUnDA | 1.01 × 10−3 | 0.0014 | W |
| Perfluorododecanoic acid | PFDoDA | 6.51 × 10−4 | 0.0021 | W |
| Perfluorotridecanoic acid | PFTrDA | 5.22 × 10−4 | 0.0027 | W |
| Perfluorotetradecanoic acid | PFTDA | L | L | |
| Perfluoroalkyl sulfonates | ||||
| Perfluorooctane sulfonic acid potassium salt | PFOS | 2.01 × 10−4 | 0.0069 | W |
| Perfluorodecane sulfonic acid ammonium salt | PFDS | 1.52 × 10−4 | 0.0092 | W |
| Fluorotelomers | ||||
| 6:2 Fluorotelomer alcohol | 6:2FtOH | ND | ND | |
| 8:2 Fluorotelomer alcohol | 8:2FtOH | ND | ND | |
| 8:2 Fluorotelomer acrylate | 8:2FtOAcr | ND | ND | |
Note. ND, no significant displacement; L, less than 50% displacement.
IC50 is the competitor concentration that causes 50% displacement of [3H]-E2.
RBA values were calculated by normalizing IC50 values to that of E2 which was set at 100%.
Letters indicate classification of RBA values into four broad groups indicating calculated affinity for trout liver ER as follows: S, strong binding (RBA > 10%); M, moderate binding (RBA 0.1–10%); W, weak binding (RBA 0.001–0.1%); and VW, very weak binding (RBA < 0.001%).
An apparent relationship between the PFAA carbon chain length and the strength of binding to the trout ER was evident (Fig. 4, Table 2). The shortest (perfluoropentanoic acid) and longest (PFDoDa, PFTrDA, and perfluorotetradecanoic acid) compounds did not completely displace E2 from the ER. Compounds of medium size (7–11 fluorinated carbons), including perfluorohexanoic acid (PFHpA), PFOA, PFNA, PFDA, and PFUnDA, had the highest affinities of the carboxylic acids for the ER and completely displaced E2 from the receptor. Because the calculated RBA values for these compounds were more than 10,000-fold lower than that of E2, they were classified as weak or very weak binders. PFDA had the highest affinity of the carboxylic acids tested for the trout liver ER with IC50 and RBA values comparable to that of the weak environmental estrogen NP. PFOS and PFDS were also weak ligands for the trout liver ER, with RBA values comparable to that of the similarly sized carboxylic acid PFDA. In contrast, none of the fluorotelomers tested displaced E2 from the receptor and were classified as nonbinders in this study (Fig. 4F).
Human ERα Gene Reporter Assay
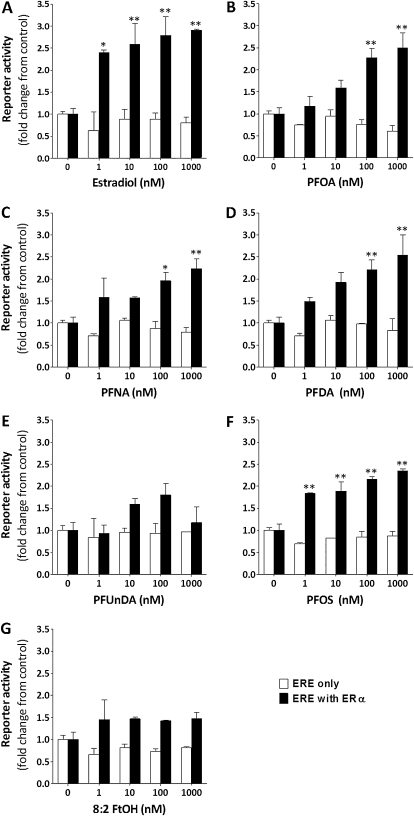
For all test compounds evaluated, the presence of the hERα-expressing plasmid was required to increase gene reporter activity (Fig. 5), although the assay had a somewhat limited dynamic range because of fairly high basal activity of the gene reporter under the culture conditions described above. (For this reason, EC50 and relative activity values were not calculated for this assay.) Treatment of cells with 1–1000nM E2 caused a significant, concentration-dependent induction of hERα gene reporter activity, with a maximal response of nearly threefold observed. PFOA, PFNA, and PFDA significantly induced gene reporter activity up to 2.5-fold at concentrations of 100–1000nM. PFOS appeared to be the most effective of the PFAAs tested, with significant induction of gene reporter activity observed at 1nM. Alternatively, PFUnDA and 8:2FtOH did not significantly alter gene reporter activity.
FIG. 5.
Transactivation of hERα by PFAAs. HEK-293T cells were transfected with (solid bar) or without (open bar) a human ERα-expressing plasmid, the XTEL luciferase reporter plasmid containing a consensus ERE sequence, and a β-galactosidase–containing plasmid for normalization of raw luminescence data. Twenty-four hours after transfection, cells were treated with increasing concentrations of E2 (A), PFOA (B), PFNA (C), PFDA (D), PFUnDA (E), PFOS (F), and 8:2FtOH (G). Values are the mean gene reporter activity expressed as fold change with respect to control (0nM treatment) (N = 3 replicate experiments). Within the ERE + ERα data set, *p < 0.05, **p < 0.01 as determined by one-way ANOVA with Bonferonni post hoc tests compared with 0nM.
Molecular Docking Studies with hERα, mERα, and rtERα1
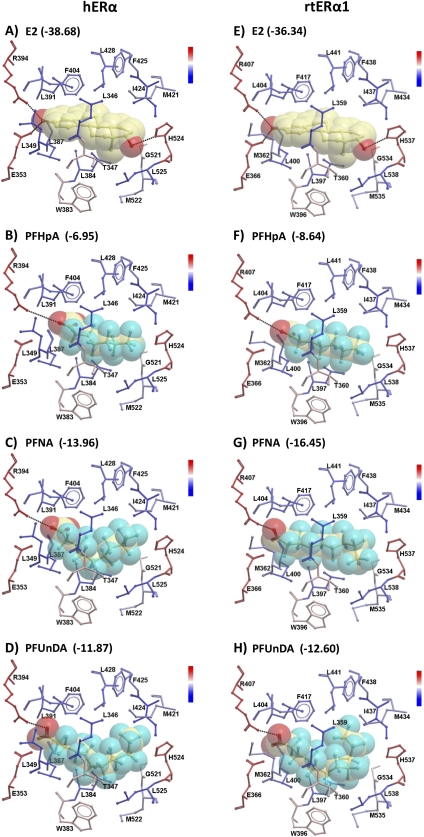
For this comparative molecular docking study, we included human and trout ERα proteins as well as the mouse ERα, as most of the toxicological assessments for this compound class have been performed in mouse models. The LBD regions of the human, mouse, and trout ERα proteins are highly homologous, with perfect identity at amino acid sites previously recognized as key for hydrogen bonding with E2 (Supplementary fig. 3) (Brzozowski et al., 1997; Marchand-Geneste et al., 2006). The molecular models for human, mouse, and trout ERα proteins were constructed using the experimentally resolved crystal structure available for hERα and were energetically minimized in the internal coordinate space as described in “Materials and Methods” section (Supplementary fig. 4). The root-mean-square deviation (RMSD) values for superimposition of the mERα and rtERα1 homology models with the 3D crystal structure of hERα were 0.087 and 0.078 Å, respectively (SuperImpose Algorithm, ICM-Browser, Molsoft).
The natural ligand E2 docked within a 4-Å sphere around its 3D position in the crystal structure with favorable ICM scores of −38.68 (human), −37.75 (mouse), and −36.34 (trout) and a very low all atoms RMSD (< 0.151 Å for all three models) when compared with the crystallographic orientation. The hydrogen bond interactions observed in the crystal structure between the phenolic hydroxyl group of the A-ring and the hydroxyl group of the D-ring of the natural ligand and the side chains of residues Glu353/357/366 (hERα/mERα/rtERα1) of helix 2, Arg394/398/407 of helix 3, and His524/528/537 of helix 8 were confirmed (Fig. 6). Sites of hydrophobic interactions with the natural ligand were similar to those previously identified (Marchand-Geneste et al., 2006).
FIG. 6.
Effect of fluorinated carbon chain length on perfluoroalkyl carboxylic acid docking orientation and efficiency for human or trout ERα proteins. The docked orientation of E2 is represented by the stick/space-filling model in the binding pocket of the receptor (A and E). Dockings of three representative perfluoroalkyl carboxylic acids with different fluorinated carbon chain length are shown, including PFHpA (seven carbons), PFNA (nine carbons), and PFUnDA (11 carbons), with hERα (B–D) or rtERα1 (F–H). Interacting residues of the LBDs are shown as sticks and are colored according to their hydrophobicity (inset scale) with hydrophobic residues colored blue and hydrophilic colored red. Hydrogen bonds are represented by black dotted lines between the donor (D) and the acceptor (A) and are defined as follows: Distance D–A: 2.8–3.2 Å; Angle D–H–A: 140–180°. Residue labels are numbered according to the indicated protein sequences, which are available in Supplementary figure 3. The corresponding ICM docking scores are indicated for each ligand-protein pair; scores for all PFAAs tested are available in Table 3.
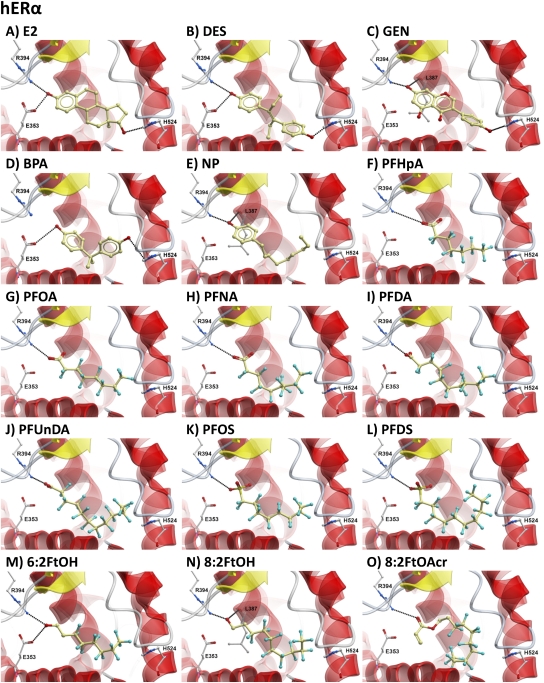
To explain the effects observed in vitro (radioligand-binding assay, cell-based assay) through ERα activation, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFOS, PFDS, 6:2FtOH, 8:2FtOH, and 8:2FtOAcr were docked into the human, mouse, and trout models of the ERα-LBD binding pocket. In addition, DES (a strong ERα binder), GEN (a moderate binder), and NP and bisphenol A (BPA) (weak binders) were included in the docking study as reference compounds with known binding affinities toward the ERα (this study; Matthews et al., 2000; Olsen et al., 2005). The results of the molecular docking for all test ligands into the LBD of hERα are shown in Figure 7, mERα in Supplementary figure 5, and rtERα1 in Supplementary figure 6. When considered by broad groupings (strong, moderate, weak, and very weak), the ranking by ICM score of the docked reference compounds correlated well with the difference in ERα-binding affinity and activity of the compounds (Table 3). The higher score for BPA in the mouse model is likely because of the third hydrogen bond interaction between the compound and the side chain of residue T351 (Supplementary fig. 5D). The moderate binder GEN and the weak binders BPA and NP docked into the human, mouse, and trout ERα-LBD binding pockets with lower ICM scores than E2 and DES because of loss of the classical triple hydrogen-bonding pattern observed with the two strongest binders (e.g., hERα in Figs. 7A–E). In the binding site, GEN establishes a triple hydrogen-bonding pattern like E2 and DES but with the carboxyl backbone of L387/391/400 instead; this bonding pattern is the likely cause for the improved docking score of GEN compared with BPA and NP, which form only two hydrogen bonds.
FIG. 7.
In silico model showing docking of estrogens and select PFCs into the hERα LBD. Docking of test ligands into the hERα LBD was performed using Molsoft ICM, and the lowest ICM-scored poses for each calculated ligand-protein docking are shown. Ligands are colored by atom type (the carbon atoms in tan) and are displayed as ball and sticks. Relevant protein residues, including E353, R394, H524, and L387, are displayed as ball and sticks and colored by atom type: carbon atoms in gray, oxygen in red, sulfur in yellow, and fluorine in cyan. Hydrogen bonds are represented by black dotted lines between the donor (D) and the acceptor (A) and are defined as follows: Distance D–A: 2.8–3.2 Å; Angle D–H–A: 140–180°.
TABLE 3.
Molecular Modeling of Ligand Interaction with Human, Mouse, and Trout ERα LBDs
| ICM docking score |
Hydrogen-bonding interactions |
||||||
| Ligand | hERα | mERα | rtERα1 | HERα | mERα | rtERα | Classificationa |
| E2 | −38.68 | −37.75 | −36.34 | R394, E353, H524 | R398, E357, H528 | R407, E366, H537 | S |
| DES | −38.34 | −37.40 | −37.25 | R394, E353, H524 | R398, E357, H528 | R407, E366, H537 | S |
| GEN | −30.10 | −32.70 | −31.63 | R394, L387, H524 | R398, L391, H528 | R407, L400, H537 | M |
| NP | −23.41 | −24.49 | −23.39 | R394, L387 | R398, L391 | R407, L400 | W |
| BPA | −18.77 | −27.86 | −17.34 | E353, H524 | R398, E357, T351 | E366 | M,W |
| PFHpA | −6.95 | −9.63 | −8.64 | R394 | R398, E357 | R407 | VW |
| PFOA | −11.84 | −17.75 | −11.70 | R394 | R398, L391 | R407 | VW |
| PFNA | −13.96 | −22.54 | −16.45 | R394 | R398 | R407 | VW,W |
| PFDA | −18.77 | −20.05 | −13.41 | R394 | R398 | R407 | VW,W |
| PFUnDA | −11.87 | −17.85 | −12.60 | R394 | R398 | R407 | VW,W |
| PFOS | −15.77 | −19.06 | −10.96 | R394 | R398 | R407 | VW,W |
| PFDS | −11.52 | −14.79 | −11.12 | R394 | R398 | R407 | VW |
| 6:2FtOH | −20.48 | −20.46 | −20.30 | R394, E353 | R398 | R407, E366 | W |
| 8:2FtOH | −18.57 | −25.68 | −18.06 | R394, L387 | R398 | R407, L400 | W |
| 8:2FtOAcr | −15.60 | −14.50 | −17.06 | R394 | R398 | R407 | W |
Note. ICM scores are a measure of fit within the receptor-binding pocket, taking into account continuum and discreet electrostatics, hydrophobicity, and entropy parameters. The predicted amino acid residue locations for hydrogen bonding are indicated for each ligand-protein docking.
Letters indicate classification of ICM docking scores into four broad groups indicating predicted affinity for ERα as follows: S, strong affinity (ICM scores > −35); M, moderate affinity (scores −25 and −35); W, weak affinity (scores −15 to −25); and VW, very weak affinity (scores > −15). Two classifications are given when scores for different receptors fit within multiple ranges.
All PFAAs and fluorotelomer alcohol derivatives docked in all three ERα models with similar orientations. The negative charged oxygen of the carboxylic (PFHpA, PFOA, PFNA, PFDA, and PFUnDA) and the sulfonic (PFOS and PFDS) groups establishes a hydrogen bond interaction with the side chain of R394/398/407 but not with E353/357/366 (e.g., hERα in Figs. 7F–L). The loss of the classical hydrogen-bonding pattern present in E2, DES, and GEN docking poses is caused by the repulsion between the negative charge of the ligand (carboxylic and sulfonic groups) and the side chain of E353/357/366. Alternatively, 8:2FtOH and 6:2FtOH docked with higher scores (with the exception of 6:2FtOH in the mouse model) with the hydroxyl group interacting with both side chains of R394/398/407 and E353/357/366 (e.g., hERα in Figs. 7M and 7N). The fluorotelomer acrylate compound 8:2FtOAcr displayed a similar hydrogen-bonding pattern as the carboxylic acids tested rather than the fluorotelomer alcohols, likely because of the positioning of the acrylate side chain near R394/398/407. Unlike several of the xenoestrogens studied, no hydrogen bonding was possible with H524/528/537 for the PFCs tested because of the orientation of the fluorinated carbon tail near this residue. The PFAA compounds with the best docking scores had fluorinated carbon chains of medium length, PFDA for hERα (−18.77) and PFNA for mERα and rtERα1 (−22.54 and −16.45, respectively), whereas shorter or longer compounds have lower scores (Table 3, Fig. 6). Space-filling models for the PFAAs showed that short carbon chain fluorinated chemicals (e.g., PFHpA) did not adequately fill the LBD pocket (Figs. 6B and 6F), whereas longer fluorinated carbon chain structures (e.g., PFUnDA) were nearly too large and required the fluorinated carbon tail to bend so that the ligand could fit into the binding pocket (Figs. 6D and 6H).
No major differences in the pattern of xenoestrogen and PFC docking to the LBD region of ERα were observed for the species studied, aside from the noted different site of hydrogen bonding for BPA in the mERα model. However, the ICM scores for PFAA docking to the mERα model were generally somewhat higher than those for the hERα or rtERα1 models (Table 3).
DISCUSSION
This study details the first comprehensive screen of structurally diverse PFCs for estrogen-like activity in an animal model that mimics human insensitivity to peroxisome proliferation, the presumed mechanism of action for PFAAs. As has been shown previously by our laboratory for PFOA (Tilton et al., 2008), dietary exposure to PFAAs did not elicit a change in palmitoyl CoA oxidation or catalase activity, indicative of a peroxisome proliferation response. Rather, eight different PFAAs significantly increased expression of the estrogen biomarker protein Vtg, and perfluoroalkyl carboxylic acids were more estrogenic compared with the perfluoroalkyl sulfonates and fluorotelomers tested.
Additionally, we provide the first evidence that PFAAs interact directly with the ER to exert their estrogenic activity. Results from competitive steroid-binding assays show that multiple PFAAs effectively bind to the trout liver ER with a structure-activity relationship similar to that observed for in vivo Vtg induction in trout. Perfluoroalkyl carboxylic acids ranging in size from 7 to 13 carbons were identified as competitive binders for the rtER, with the 10-carbon compound PFDA being the strongest binder of the chemical group. Although not considered strong ER agonists, these PFAAs had sufficient binding affinity for the ER that, in the absence of endogenous estrogen (as is the case in juvenile trout), the compounds are capable of eliciting a weak estrogen-like effect as measured by the Vtg bioassay. The rtER is considered more promiscuous than in other species (Matthews et al., 2000) and has been shown to weakly bind a wide variety of environmental xenobiotics; in this study, PFAA binding was comparable to some of these weak estrogens, such as nonylphenol and I3C. Interestingly, PFOS and PFDS showed the highest affinity of all the PFCs tested for this receptor, contradicting the fairly modest Vtg response observed for these sulfonate compounds in vivo. This discrepancy is not likely due to poor bioavailability of perfluoroalkyl sulfonates because PFOS is reported to have a four-fold greater half-life in trout than PFOA (Martin et al., 2003). Currently, it is unknown why PFOS and PFDS are less estrogenic in vivo than would be predicted by their RBA for the trout ER. Moreover, results of steroid-binding assays for trout liver ER suggest that the observed difference in Vtg response between carboxylic acids and sulfonates is not likely the result of differing affinities for the ER. It should be noted, as well, that the ER-binding assay does not discriminate between the four trout ER isoforms, of which rtERα1 and rtERβ2 are highly expressed in trout liver (Nagler et al., 2007). Finally, we observed that dietary PFOA, PFNA, PFDA, and PFUnDA induced a marked increase in Vtg expression in vivo, even though the binding affinity for these compounds was classified as weak or very weak. This biomarker assay integrates several factors that impact the biological response to PFCs, including chemical-specific patterns of assimilation, distribution, and elimination. The perceived discrepancy in the effectiveness of PFAAs in vivo versus in vitro could be explained by the apparent accumulation of these compounds in trout over time (14 days), which allowed for blood levels to achieve concentrations sufficient for PFAA binding to the trout ER and a robust Vtg response (Fig. 3).
In general, the fluorotelomers tested in this study were not potent estrogens in vivo, save for 6:2FtOH which caused a slight increase in Vtg expression. Similar to our observations in trout, Liu et al. (2007) showed that PFOA, PFOS, and 6:2FtOH significantly increased Vtg expression by tilapia hepatocytes in vitro, whereas 8:2FtOH was ineffective. However, subsequent reports showed that aqueous exposure to 6:2FtOH or 8:2FtOH caused significant induction of Vtg in male zebrafish, though this may be the result of changes in steroid metabolism rather than direct action of the chemicals at the ER (Liu et al., 2009, 2010). On the other hand, Ishibashi et al. (2008) reported that waterborne 6:2FtOH and 8:2FtOH increased hepatic Vtg gene expression in male medaka and that this effect was likely linked to interaction with the medaka ERα. Interestingly, in this same study (Ishibashi et al., 2008) as well as another report using human breast cancer cells (Maras et al., 2006), the authors found no evidence for ER interaction with PFAAs. Overall, these differences suggest that the estrogenic response to PFAAs and fluorotelomers may depend on the route of exposure or the species studied.
This report also describes the first broad-scale dose-response study for estrogen-like activity of PFOA and PFDA, two PFAAs commonly detected in humans and wildlife. Overall, the dose-response data suggest that the compounds tested are weakly estrogenic, as fairly high diet levels were required to induce expression of the estrogen biomarker protein Vtg. Neither chemical caused a significant increase in Vtg expression at blood levels for PFOA or PFDA in trout that correspond to the levels of these compounds observed in the general human population, about 2–7 ppb (reviewed in Fromme et al., 2009; Kannan et al., 2004). However, a significant estrogenic response was observed at levels that may reflect certain human exposures, such as residents living near a contaminated site or occupationally exposed workers (Emmett et al., 2006). It should be noted, however, that the in vivo studies described above are short-term (14 day) experiments that do not precisely reflect the typical chronic exposure to these chemicals observed in humans. Moreover, humans and wildlife have detectable blood levels of multiple PFCs (Calafat et al., 2007), which could have additive or synergistic toxic effects depending on the mechanism of action. In this study, results of a simple in vivo mixture experiment with PFOA, PFNA, PFDA, and PFUnDA suggest that perfluoroalkyl carboxylic acids impact Vtg expression in at least an additive manner at higher exposures.
Subsequent experiments sought to expand the findings in trout to humans using a reporter gene assay for hERα. We determined that certain PFAAs activate hERα-induced transcription, including the perfluoroalkyl carboxylic acids PFOA, PFNA, and PFDA and one sulfonate compound, PFOS, whereas 8:2FtOH was ineffective. Although our observations for PFC activation of hERα largely match those for ER binding and in vivo activity in trout, they follow a pattern opposite to that observed by Ishibashi et al. (2007); results of their studies using a yeast two-hybrid assay suggested that fluorotelomer alcohols, but not PFOA or PFOS, interacted with hERα. The apparent differences in observations of these two research groups might be explained by interassay variation associated with the cellular and molecular responses in the bioassays employed; one may reasonably expect that our reporter gene assay better represents the molecular context for ER activation in human cells. The estrogen-like activity of some fluorotelomers could be the result of metabolic production of polyfluorinated derivatives, such as 8:2 or 7:3 fluorotelomer acids (Nabb et al., 2007), which have chemical structures and lengths similar to the estrogenic perfluoroalkyl carboxylic acids identified in this study. Moreover, substantial differences in the metabolism of fluorotelomers in human, rodent, and trout hepatocytes have been reported (Nabb et al., 2007).
A major focus of the present study was to understand the potential molecular interactions of PFAAs with the LBD region of the ER, particularly because the chemical structures of PFAAs previously identified as estrogenic have little in common with the structure of the native hormone or other environmental xenoestrogens. To this end, we employed an in silico molecular docking model to investigate docking of these putative ligands with hERα, mERα, and rtERα1. From the results obtained and after considering the score ranking of the reference compounds E2, DES, GEN, BPA, and NP, the PFAAs and fluorotelomers examined were predicted to be in vitro weak binders and ERα activators. For the three species investigated, the overall pattern of xenoestrogen and PFC docking to the LBD region of ERα was fairly consistent, as all compounds docked into each model with similar orientations. Hydrogen bonding with the R394/398/407 (human/mouse/rainbow trout) residue was critical for all putative ligands, whereas more the estrogen-like compounds DES and GEN docked with the classic triple hydrogen bond pattern, with additional hydrogen bonds predicted at residues E353/357/366 and H524/528/537. For the PFAAs examined, docking scores for mERα were moderately more favorable than those for the human and trout receptors. However, it is uncertain whether this modest difference in computed affinity for mERα would translate into greater in vivo activity for PFAAs in mice, especially because these chemicals are reported to have quite different pharmacokinetic properties in rodents, humans, and trout (Kudo and Kawashima, 2003; Martin et al., 2003).
The homology model developed for rtERα1 agreed well with a similar computational model described by Marchand-Geneste et al. (2006), where the natural ligand E2 formed hydrogen bonds with active site residues E366, R407, and H537. Our docking model appropriately predicted PFAA interaction with the trout ER and in vivo estrogen activity, as the medium-sized compounds PFNA and PFDA docked to the rtERα1 with the most favorable ICM scores of the carboxylic acids examined. However, the model was not predictive for fluorotelomer alcohols. Despite the higher computed docking scores for all the fluorotelomers examined in silico, none of these compounds showed activity in vitro, and only 6:2FtOH was slightly estrogenic in vivo in trout. Moreover, the computational models presented here do not perfectly align with prior studies predicting positive interaction of fluorotelomers, but not PFOA or PFOS, with human or medaka ERα (Ishibashi et al., 2007, 2008). In silico modeling is a useful tool for predicting potential ligand-receptor interactions or, in the case of this study, a retrospective tool used to gain insight into ligand-receptor interaction. However, these computational models are not perfect predictors of in vivo or in vitro interactions. Other factors, such as metabolism, bioavailability, or other biophysical parameters, could account for prediction errors in translating results from computational modeling to in vivo or in vitro experimental systems.
In this study, we investigated molecular interactions with ERα isoforms only, primarily because of its high expression in the liver (Flouriot et al., 1998), which is generally considered the primary target organ for PFC toxicity. Because the LBDs for ERα and ERβ isoforms are highly similar, it is reasonable to predict that in silico modeling with the ERβ crystal structure would generate similar docking results as described above for ERα (Supplementary fig. 7).
In conclusion, we present here the first comprehensive evaluation of PFAAs for estrogenic activity in vivo using an animal model that mimics human insensitivity to PPs. Multiple estrogenic PFAAs have been identified, and a structure-activity relationship was observed, where a fluorinated carbon chain length of 8–10 carbons and a carboxylic acid end group were optimal for maximal induction of plasma biomarker proteins. Perfluoroalkyl carboxylic acids, in particular PFOA, PFNA, PFDA, and PFUnDA, were all potent inducers of Vtg, although at fairly high dietary exposures. Moreover, this study provides the first evidence for direct interaction of PFAAs with the ER, including the persistent environmental pollutants PFOA and PFOS. As with the in vivo Vtg assay, a structure-activity relationship between ER interaction and fluorinated carbon chain length was evident, with medium-sized PFAAs having the highest binding affinity and effective concentration values and most favorable docking scores for hERα, mERα, and/or rtERα1. Collectively, these findings support the idea that several PFAAs could act as weak environmental xenoestrogens.
SUPPLEMENTARY DATA
Supplementary data are available online at http://www.toxsci.oxfordjournals.org.
Supplementary materials and methods.pdf includes experimental details for measurement of PFOA and PFDA in trout blood plasma by LC-MS/MS. Supplementary tables.pdf contains nine additional tables, including a list of PFCs investigated in this report (Suppl. Table 1) and morphometric data from the in vivo experiments described above (Suppl. Tables 2 and 3). Supplementary Figures.pdf contains seven additional figures, including activity of peroxisome proliferator-responsive enzymes in trout liver (Suppl. Fig. 1), trout ER saturation analyses (Suppl. Fig 2), alignment of human, mouse and fish ERs (Suppl. Figs 3 and 7), the mouse and trout ER homology models (Suppl. Fig 4) and results of in silico docking of PFCs into the mouse and trout ERa LBDs (Suppl. Figs. 5 and 6).
FUNDING
National Institute of Environmental Health Sciences (P30 ES03850, T32 ES07060, P30 ES00210, R01 ES013534); Society of Toxicology Colgate/Palmolive Grants for Alternative Research to A.D.B.
Acknowledgments
The authors wish to acknowledge the assistance of Eric Johnson, Greg Gonnerman, and Cari Buchner at the SARL for care of the animals used in this study. We also thank Dr Jennifer Field for the donation of some PFCs used in these experiments. The technical assistance of Marilyn Henderson and Beth Siddens is also gratefully acknowledged. Parts of the manuscript were presented at the Society of Environmental Toxicology and Chemistry North America 28th Annual Meeting, Milwaukee, Wisconsin, November 11–15, 2007, and the Society of Environmental Toxicology and Chemistry 4th World Congress, Sydney, Australia, August 3–7, 2008.
References
- Abagyan R, Totrov M, Kuznetsov D. ICM—a new method for protein modeling and design: applications to docking and structure prediction from the distorted native conformation. J. Comput. Chem. 1994;15:488–506. [Google Scholar]
- Abdellatif AG, Preat V, Taper HS, Roberfroid M. The modulation of rat liver carcinogenesis by perfluorooctanoic acid, a peroxisome proliferator. Toxicol. Appl. Pharmacol. 1991;111:530–537. doi: 10.1016/0041-008x(91)90257-f. [DOI] [PubMed] [Google Scholar]
- Benninghoff AD, Williams DE. Identification of a transcriptional fingerprint of estrogen exposure in rainbow trout liver. Toxicol. Sci. 2008;101:65–80. doi: 10.1093/toxsci/kfm238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson WH, Koch DC, O'Donnell EF, Khalil SM, Kerkvliet NI, Tanguay RL, Abagyan R, Kolluri SK. Modeling of the aryl hydrocarbon receptor (AhR) ligand binding domain and its utility in virtual ligand screening to predict new AhR ligands. J. Med. Chem. 2009;52:5635–5641. doi: 10.1021/jm900199u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RM, Fang H, Branham WS, Hass BS, Dial SL, Moland CL, Tong W, Shi L, Perkins R, Sheehan DM. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands. Toxicol. Sci. 2000;54:138–153. doi: 10.1093/toxsci/54.1.138. [DOI] [PubMed] [Google Scholar]
- Bosgra S, Mennes W, Seinen W. Proceedings in uncovering the mechanism behind peroxisome proliferator-induced hepatocarcinogenesis. Toxicology. 2005;206:309–323. doi: 10.1016/j.tox.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engstrom O, Ohman L, Greene GL, Gustafsson JA, Carlquist M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–758. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003-2004 and comparisons with NHANES 1999-2000. Environ. Health Perspect. 2007;115:1596–1602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardozo T, Totrov M, Abagyan R. Homology modeling by the ICM method. Proteins. 1995;23:403–414. doi: 10.1002/prot.340230314. [DOI] [PubMed] [Google Scholar]
- Cavalieri E, Frenkel K, Liehr JG, Rogan E, Roy D. Estrogens as endogenous genotoxic agents—DNA adducts and mutations. J. Natl. Cancer Inst. Monogr. 2000;27:75–93. doi: 10.1093/oxfordjournals.jncimonographs.a024247. [DOI] [PubMed] [Google Scholar]
- De Maria N, Manno M, Villa E. Sex hormones and liver cancer. Mol. Cell. Endocrinol. 2002;193:59–63. doi: 10.1016/s0303-7207(02)00096-5. [DOI] [PubMed] [Google Scholar]
- Eliassen AH, Hankinson SE. Endogenous hormone levels and risk of breast, endometrial and ovarian cancers: prospective studies. Adv. Exp. Med. Biol. 2008;630:148–165. [PubMed] [Google Scholar]
- Emmett EA, Shofer FS, Zhang H, Freeman D, Desai C, Shaw LM. Community exposure to perfluorooctanoate: relationships between serum concentrations and exposure sources. J. Occup. Environ. Med. 2006;48:759–770. doi: 10.1097/01.jom.0000232486.07658.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flouriot G, Griffin C, Kenealy M, Sonntag-Buck V, Gannon F. Differentially expressed messenger RNA isoforms of the human estrogen receptor-alpha gene are generated by alternative splicing and promoter usage. Mol. Endocrinol. 1998;12:1939–1954. doi: 10.1210/mend.12.12.0209. [DOI] [PubMed] [Google Scholar]
- Fromme H, Tittlemier SA, Volkel W, Wilhelm M, Twardella D. Perfluorinated compounds—exposure assessment for the general population in Western countries. Int. J. Hyg. Environ. Health. 2009;212:239–270. doi: 10.1016/j.ijheh.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Guyton KZ, Chiu WA, Bateson TF, Jinot J, Scott CS, Brown RC, Caldwell JC. A reexamination of the PPAR-alpha activation mode of action as a basis for assessing human cancer risks of environmental contaminants. Environ. Health Perspect. 2009;117:1664–1672. doi: 10.1289/ehp.0900758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanhijarvi H, Ylinen M, Haaranen T, Nevalainen T. In: A Proposed Species Difference in the Renal Excretion of Perfluorooctanoic Acid in the Beagle Dog and Rat. Beynen AC, Solleveld HA, editors. Dordrecht, The Netherlands: Martinus Nijhoff Publishers; 1988. pp. 409–412. [Google Scholar]
- Ho SM. Estrogen, progesterone and epithelial ovarian cancer. Reprod. Biol. Endocrinol. 2003;1:73. doi: 10.1186/1477-7827-1-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden PR, Tugwood JD. Peroxisome proliferator-activated receptor alpha: role in rodent liver cancer and species differences. J. Mol. Endocrinol. 1999;22:1–8. doi: 10.1677/jme.0.0220001. [DOI] [PubMed] [Google Scholar]
- Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DC. Biological monitoring of polyfluoroalkyl substances: a review. Environ. Sci. Technol. 2006;40:3463–3473. doi: 10.1021/es052580b. [DOI] [PubMed] [Google Scholar]
- Ishibashi H, Ishida H, Matsuoka M, Tominaga N, Arizono K. Estrogenic effects of fluorotelomer alcohols for human estrogen receptor isoforms alpha and beta in vitro. Biol. Pharm. Bull. 2007;30:1358–1359. doi: 10.1248/bpb.30.1358. [DOI] [PubMed] [Google Scholar]
- Ishibashi H, Yamauchi R, Matsuoka M, Kim JW, Hirano M, Yamaguchi A, Tominaga N, Arizono K. Fluorotelomer alcohols induce hepatic vitellogenin through activation of the estrogen receptor in male medaka (Oryzias latipes) Chemosphere. 2008;71:1853–1859. doi: 10.1016/j.chemosphere.2008.01.065. [DOI] [PubMed] [Google Scholar]
- Ito Y, Yamanoshita O, Asaeda N, Tagawa Y, Lee CH, Aoyama T, Ichihara G, Furuhashi K, Kamijima M, Gonzalez FJ, et al. Di(2-ethylhexyl)phthalate induces hepatic tumorigenesis through a peroxisome proliferator-activated receptor alpha-independent pathway. J. Occup. Health. 2007;49:172–182. doi: 10.1539/joh.49.172. [DOI] [PubMed] [Google Scholar]
- Kannan K, Corsolini S, Falandysz J, Fillmann G, Kumar KS, Loganathan BG, Mohd MA, Olivero J, Van Wouwe N, Yang JH, et al. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ. Sci. Technol. 2004;38:4489–4495. doi: 10.1021/es0493446. [DOI] [PubMed] [Google Scholar]
- Kannan K, Corsolini S, Falandysz J, Oehme G, Focardi S, Giesy JP. Perfluorooctanesulfonate and related fluorinated hydrocarbons in marine mammals, fishes, and birds from coasts of the Baltic and the Mediterranean Seas. Environ. Sci. Technol. 2002;36:3210–3216. doi: 10.1021/es020519q. [DOI] [PubMed] [Google Scholar]
- Kennedy GL, Jr, Butenhoff JL, Olsen GW, O'Connor JC, Seacat AM, Perkins RG, Biegel LB, Murphy SR, Farrar DG. The toxicology of perfluorooctanoate. Crit. Rev. Toxicol. 2004;34:351–384. doi: 10.1080/10408440490464705. [DOI] [PubMed] [Google Scholar]
- Kolluri SK, Bruey-Sedano N, Cao X, Lin B, Lin F, Han YH, Dawson MI, Zhang XK. Mitogenic effect of orphan receptor TR3 and its regulation by MEKK1 in lung cancer cells. Mol. Cell. Biol. 2003;23:8651–8667. doi: 10.1128/MCB.23.23.8651-8667.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo N, Kawashima Y. Toxicity and toxicokinetics of perfluorooctanoic acid in humans and animals. J. Toxicol. Sci. 2003;28:49–57. doi: 10.2131/jts.28.49. [DOI] [PubMed] [Google Scholar]
- Lai DY. Rodent carcinogenicity of peroxisome proliferators and issues on human relevance. J. Environ. Sci. Health C. 2004;22:37–55. doi: 10.1081/GNC-120038005. [DOI] [PubMed] [Google Scholar]
- Liu C, Deng J, Yu L, Ramesh M, Zhou B. Endocrine disruption and reproductive impairment in zebrafish by exposure to 8:2 fluorotelomer alcohol. Aquat. Toxicol. 2010;96:70–76. doi: 10.1016/j.aquatox.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Liu C, Du Y, Zhou B. Evaluation of estrogenic activities and mechanism of action of perfluorinated chemicals determined by vitellogenin induction in primary cultured tilapia hepatocytes. Aquat. Toxicol. 2007;85:267–277. doi: 10.1016/j.aquatox.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Liu C, Yu L, Deng J, Lam PK, Wu RS, Zhou B. Waterborne exposure to fluorotelomer alcohol 6:2 FTOH alters plasma sex hormone and gene transcription in the hypothalamic-pituitary-gonadal (HPG) axis of zebrafish. Aquat. Toxicol. 2009;93:131–137. doi: 10.1016/j.aquatox.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Maras M, Vanparys C, Muylle F, Robbens J, Berger U, Barber JL, Blust R, De Coen W. Estrogen-like properties of fluorotelomer alcohols as revealed by mcf-7 breast cancer cell proliferation. Environ. Health Perspect. 2006;114:100–105. doi: 10.1289/ehp.8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand-Geneste N, Cazaunau M, Carpy AJ, Laguerre M, Porcher JM, Devillers J. Homology model of the rainbow trout estrogen receptor (rtERalpha) and docking of endocrine disrupting chemicals (EDCs) SAR QSAR Environ. Res. 2006;17:93–105. doi: 10.1080/10659360600562137. [DOI] [PubMed] [Google Scholar]
- Martin JW, Mabury SA, O'Brien PJ. Metabolic products and pathways of fluorotelomer alcohols in isolated rat hepatocytes. Chem. Biol. Interact. 2005;155:165–180. doi: 10.1016/j.cbi.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Martin JW, Mabury SA, Solomon KR, Muir DC. Dietary accumulation of perfluorinated acids in juvenile rainbow trout (Oncorhynchus mykiss) Environ. Toxicol. Chem. 2003;22:189–195. [PubMed] [Google Scholar]
- Matthews J, Celius T, Halgren R, Zacharewski T. Differential estrogen receptor binding of estrogenic substances: a species comparison. J. Steroid Biochem. Mol. Biol. 2000;74:223–234. doi: 10.1016/s0960-0760(00)00126-6. [DOI] [PubMed] [Google Scholar]
- Mitchell AM, Lhuguenot JC, Bridges JW, Elcombe CR. Identification of the proximate peroxisome proliferator(s) derived from di(2-ethylhexyl) phthalate. Toxicol. Appl. Pharmacol. 1985;80:23–32. doi: 10.1016/0041-008x(85)90097-3. [DOI] [PubMed] [Google Scholar]
- Nabb DL, Szostek B, Himmelstein MW, Mawn MP, Gargas ML, Sweeney LM, Stadler JC, Buck RC, Fasano WJ. In vitro metabolism of 8-2 fluorotelomer alcohol: interspecies comparisons and metabolic pathway refinement. Toxicol. Sci. 2007;100:333–344. doi: 10.1093/toxsci/kfm230. [DOI] [PubMed] [Google Scholar]
- Nagler JJ, Cavileer T, Sullivan J, Cyr DG, Rexroad C., III The complete nuclear estrogen receptor family in the rainbow trout: discovery of the novel ERalpha2 and both ERbeta isoforms. Gene. 2007;392:164–173. doi: 10.1016/j.gene.2006.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez O, Hendricks JD, Arbogast DN, Fong AT, Lee BC, Bailey GS. Promotion of aflatoxin B1 hepatocarcinogenesis in rainbow trout by 17β-estradiol. Aquat. Toxicol. 1989;15:289–302. [Google Scholar]
- Olsen CM, Meussen-Elholm ET, Hongslo JK, Stenersen J, Tollefsen KE. Estrogenic effects of environmental chemicals: an interspecies comparison. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005;141:267–274. doi: 10.1016/j.cca.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 2007;115:1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orner GA, Mathews C, Hendricks JD, Carpenter HM, Bailey GS, Williams DE. Dehydroepiandrosterone is a complete hepatocarcinogen and potent tumor promoter in the absence of peroxisome proliferation in rainbow trout. Carcinogenesis. 1995;16:2893–2898. doi: 10.1093/carcin/16.12.2893. [DOI] [PubMed] [Google Scholar]
- Palmer CN, Hsu MH, Griffin KJ, Raucy JL, Johnson EF. Peroxisome proliferator activated receptor-alpha expression in human liver. Mol. Pharmacol. 1998;53:14–22. [PubMed] [Google Scholar]
- Persson I. Estrogens in the causation of breast, endometrial and ovarian cancers—evidence and hypotheses from epidemiological findings. J. Steroid Biochem. Mol. Biol. 2000;74:357–364. doi: 10.1016/s0960-0760(00)00113-8. [DOI] [PubMed] [Google Scholar]
- Shilling AD, Williams DE. Determining relative estrogenicity by quantifying vitellogenin induction in rainbow trout liver slices. Toxicol. Appl. Pharmacol. 2000;164:330–335. doi: 10.1006/taap.2000.8912. [DOI] [PubMed] [Google Scholar]
- Sivanesan D, Rajnarayanan RV, Doherty J, Pattabiraman N. In-silico screening using flexible ligand binding pockets: a molecular dynamics-based approach. J. Comput. Aided Mol. Des. 2005;19:213–228. doi: 10.1007/s10822-005-4788-9. [DOI] [PubMed] [Google Scholar]
- Tilton SC, Orner GA, Benninghoff AD, Carpenter HM, Hendricks JD, Pereira CB, Williams DE. Genomic profiling reveals an alternate mechanism for hepatic tumor promotion by perfluorooctanoic acid in rainbow trout. Environ. Health Perspect. 2008;116:1047–1055. doi: 10.1289/ehp.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totrov M, Abagyan R. Flexible protein-ligand docking by global energy optimization in internal coordinates. Proteins. 1997;(Suppl 1):215–220. doi: 10.1002/(sici)1097-0134(1997)1+<215::aid-prot29>3.3.co;2-i. [DOI] [PubMed] [Google Scholar]
- Totrov M, Abagyan R. In: Protein-Ligand Docking as an Energy Optimization Problem. Raffa RB, editor. New York, NY: John Wiley & Sons; 2001. pp. 603–624. [Google Scholar]
- Villa E. Role of estrogen in liver cancer. Womens Health (Lond. Engl.) 2008;4:41–50. doi: 10.2217/17455057.4.1.41. [DOI] [PubMed] [Google Scholar]
- Wei Y, Dai J, Liu M, Wang J, Xu M, Zha J, Wang Z. Estrogen-like properties of perfluorooctanoic acid as revealed by expressing hepatic estrogen-responsive genes in rare minnows (Gobiocypris rarus) Environ. Toxicol. Chem. 2007;26:2440–2447. doi: 10.1897/07-008R1.1. [DOI] [PubMed] [Google Scholar]
- Yager JD, Zurlo J, Ni N. Sex hormones and tumor promotion in liver. Proc. Soc. Exp. Biol. Med. 1991;198:667–674. doi: 10.3181/00379727-198-43305. [DOI] [PubMed] [Google Scholar]
- Yeh SH, Chen PJ. Gender disparity of hepatocellular carcinoma: the roles of sex hormones. Oncology. 2010;78(Suppl. 1):172–179. doi: 10.1159/000315247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.