Abstract
Manganese (Mn) is an essential nutrient with potential neurotoxic effects. Mn deposited in the nose is apparently transported to the brain through anterograde axonal transport, bypassing the blood-brain barrier. However, the role of the olfactory epithelial cells in Mn transport from the nasal cavity to the blood and brain is not well understood. We utilized the methyl bromide (MeBr) lesion model wherein the olfactory epithelium fully regenerates in a time-dependent and cell type–specific manner over the course of 6–8 weeks postinjury. We instilled 54MnCl2 intranasally at different recovery periods to study the role of specific olfactory epithelial cell types in Mn transport. 54MnCl2 was instilled at 2, 4, 7, 21, and 56 days post-MeBr treatment. 54Mn concentrations in the blood were measured over the first 4-h period and in the brain and other tissues at 7 days postinstillation. Age-matched control rats were similarly studied at 2 and 56 days. Blood and tissue 54Mn levels were reduced initially but returned to control values by day 7 post-MeBr exposure, coinciding with the reestablishment of sustentacular cells. Brain 54Mn levels also decreased but returned to control levels only by 21 days, the period near the completion of neuronal regeneration/bulbar reinnervation. Our data show that Mn transport to the blood and brain temporally correlated with olfactory epithelial regeneration post-MeBr injury. We conclude that (1) sustentacular cells are necessary for Mn transport to the blood and (2) intact axonal projections are required for Mn transport from the nasal cavity to the olfactory bulb and brain.
Keywords: Mn transport, neurotoxicity, axonal transport, methyl bromide, olfactory epithelium
Manganese (Mn) is an essential trace element that is required for multiple essential metabolic functions, such as a constituent of the antioxidant Mn superoxide dismutase and of many other enzymes involved in hydrolysis, phosphorylation, decarboxylation, and transamination. Humans obtain Mn from many food sources, such as fruits, whole grains, and drinking water. The average daily intake of Mn from food ranges from 1 to 5 mg/day (Environmental Protection Agency [EPA] U.S.EPA, 1992). The reference dose (RfD) for Mn developed by the EPA is 0.14 mg/kg body weight/day based on central nervous system effects in humans. A modified RfD of 0.05 mg/kg/day is recommended for Mn from drinking water or soil (U.S.EPA, 1992). Ingested Mn, later found in the blood, typically does not result in toxicity because of the rapid clearance of Mn from the blood by the liver. In contrast, Mn aerosols deposited in the lungs can be absorbed across the air-blood barrier (Brain et al., 2006; Heilig et al., 2006). Mn deposited in the airways or nose is consequently thought to be a more important source of toxicity. Mn is in many industrial products including building materials, alloys, welding materials, antiknocking agents, and batteries. Some occupations are associated with significant exposures. In ambient urban air, Mn-containing aerosols can be formed from an antiknocking agent methylcyclopentadienyl Mn tricarbonyl, which is sometimes used in automobile fuels. There is debate as to how much airborne Mn is needed to cause injury. Chronic exposure to excessive Mn, however, can lead to neurological problems (Hanzlik et al., 1980). One such condition is manganism—a Parkinson's-like disease in which Mn accumulates in the brain and disturbs motor functions (Bouchard et al., 2008; Cotzias, 1958; Mergler et al., 1994, 1999; Perl and Olanow, 2007).
The presence of neurological deficits stemming from occupational exposures to Mn led to studies involving the nose and olfactory system as a route of direct entry to the central nervous system. Several excellent studies have identified the olfactory pathway as a key contributor to brain Mn accumulation (Dobson et al., 2003; Dorman et al., 2004; Finkelstein et al., 2008; Mergler et al., 1994, 1999) and found evidence of toxicity following intranasal administration (Dobson et al., 2003, 2004; Henriksson and Tjalve, 2000; Villalobos et al., 2009). In this route, the olfactory epithelium (OE) is the gateway for Mn uptake and transport. A number of studies have suggested that Mn present in the olfactory bulb is transported to the brain via olfactory sensory neurons (OSNs) that bypass the blood-brain barrier. These studies established that Mn, once present in the olfactory bulb, is then transported along secondary and tertiary olfactory pathways to other regions of the brain (Brenneman et al., 2000; Dorman et al., 2002; Henriksson et al., 1999; Tjalve et al., 1995, 1996). Mn-enhanced magnetic resonance imaging (MEMRI) is a novel tool that utilizes injected MnCl2 to analyze neuronal activity (Matsuda et al., 2010; Obenaus and Jacobs, 2007). The MEMRI method has been applied to trace olfactory and visual pathways and supports the transport of MnCl2 from the nose to the brain by neuronal pathways (Pautler and Koretsky, 2002; Pautler et al., 1998). Additionally, the transport of Mn from the nose has been shown to increase with anemia. It involves the transporter divalent metal transporter-1 (DMT1; Thompson et al., 2007). Although there is clear evidence for transneuronal transport of Mn, specific reports examining olfactory pathway transport have assumed that Mn is taken up by OE cells and transported through the primary OSNs to the olfactory bulb. To our knowledge, there are no studies specifically examining the role of the OE cells in Mn uptake and transport to the brain. Moreover, which OE cell types are responsible for the initial uptake of Mn and its transport to the olfactory bulb are unknown.
The OE is composed of three major cell types: the bipolar olfactory sensory neurons, supporting cells (sustentacular cells and a smaller population of microvillar cells), and the functionally and structurally heterogeneous population of basal cells (Graziadei and Graziadei, 1979; Schwob et al., 1995; Tjalve and Henriksson, 1999). Because the dendrites and cilia of OSNs project above the apical surface of the OE, they are directly exposed to the external environment and are vulnerable to a variety of airborne insults (Caggiano et al., 1994; Huard et al., 1998; Morrison and Costanzo, 1990). Damage to the epithelium eliminates the olfactory sensory neurons as well as other epithelial cell types (Schwob, 2005). As a consequence, the population of OSNs and the OE as a whole need to be regenerated after injury. Among the basal cells are stem and progenitor cells that are responsible for this regeneration. Although the epithelium as a whole reconstitutes after injury, the regeneration kinetics differ considerably across these different cell populations. This can be used to an advantage when assessing the contribution of each cell type to Mn transport. When the OE is exposed to methyl bromide gas (MeBr; 330 ppm) via inhalation, all the sustentacular cells and neurons are destroyed in 90% or more of the epithelium (Schwob et al., 1995). Subsequently, sustentacular cells begin to differentiate within 1–2 days, whereas it takes about 4 or more days for the first, very immature OSNs to reappear. It takes about 6–8 weeks for the epithelium to reestablish a normal appearing phenotype (Huard et al., 1998; Schultz, 1960; Schwob et al., 1995). During that time, the innervations of the olfactory bulb are also restored; the first nascent olfactory axons contact a limited area of the bulb at 7 days, surround the bulb and reform the olfactory nerve layer by 2 weeks, enter and fill the glomeruli by 3 weeks, and stabilize between 6 and 8 weeks (Schwob et al., 1999). The characteristic kinetics of the regeneration of the OE and the reinnervation of the bulb are utilized in this study to examine mechanisms of 54Mn uptake and transport from the nasal epithelium to the blood and the brain.
MATERIALS AND METHODS
Animals.
Animal protocols used in this study were approved by the Harvard Medical Area Animal Care and Use Committee. The rats were given a standard diet (Purina Laboratories Diet #5053, PharmaServ, Framingham, MA) and water ad libitum and housed at the Harvard School of Public Health (HSPH) animal facility with a 12-h light-dark cycle.
54MnCl2 pharmacokinetics in intranasally instilled and iv-injected normal rats.
Male Hsd:Sprague-Dawley rats (284 ± 6.9 g) were obtained from Harlan Laboratories (Indianapolis, IN). Radioactive Mn was administered by intranasal instillation or iv injection to normal rats anesthetized with vaporized isoflurane (Halocarbons Lab, North Augusta, SC) at a dose of 7.5 μCi/kg. For intranasal instillation, 54MnCl2 (Perkin Elmer, Boston, MA) was diluted in PBS to 75 μCi/ml and a volume of 0.1 ml/kg body weight was administered as described previously (Thompson et al., 2007; Tjalve et al., 1996). For iv injection, 54MnCl2 was diluted in PBS to 15 μCi/ml and a volume of 0.5 ml/kg was injected into the penile vein. The amount of Mn given was 1 ng/kg body weight.
To examine 54Mn transport to the blood and brain by intranasal and iv routes over time, the blood and brain concentration of 54Mn was determined at 4 or 72 h postdosing. Rats were humanely killed by isoflurane anesthesia followed by exsanguination. Rats were dissected, and brain and blood samples were collected, weighed, and placed in tubes. Radioactivity was measured in a gamma counter (Cobra Quantum, Packard Instrument, Downers Grove, IL). All data were expressed as nanocurie per gram tissue standardized (corrected) to a selected date/time.
MeBr model of OE injury.
The MeBr model for olfactory epithelial lesion was used to determine the role of the cells of the OE in Mn transport to the blood, brain, and peripheral tissues. Male Sprague-Dawley Germfree rats, approximately 9 weeks old (287 ± 5.8 g), were obtained from Taconic Farms (Germantown, NY). The MeBr exposure was performed at the Department of Anatomy and Cellular Biology, Tufts University School of Medicine (Boston, MA), as previously described (Schwob et al., 1995, 1999). Briefly, awake 9-week-old Sprague-Dawley rats were exposed to MeBr gas at 330 ppm in purified air at a flow rate of 10 l/min for 6 h. The gas and the air into which the MeBr was diluted were both delivered under the control of separate electronic mass flow controllers (EMFCs: 10 ml/min capacity for the MeBr controller and 10 l/min capacity for the air controller from Teledyne Hastings Instruments). The controllers have a direct readout of flow (based on cooling of a sensor in the flow stream, which alters the resistance) and were calibrated by the manufacturer for the use of the two gases. The flow was monitored with a separate Electronic Mass Flowmeter (Teledyne Hastings Instruments) to insure that the EMFC remained calibrated. After this 6-h exposure to MeBr, the rats were then returned to the HSPH animal facility and allowed to recover for 2, 4, 7, 21, and 56 days post-MeBr exposure. At each time point, rats were used to study the kinetics of intranasally instilled 54Mn. Age-matched control rats (9-week and 17-week old, 436.2 ± 8.6 g) were not exposed to MeBr.
54MnCl2 pharmacokinetics in MeBr-treated rats.
To determine the effects of MeBr exposure on Mn transport from the nasal cavity to the blood, brain, and other tissues, treated rats were administered 54MnCl2 by the intranasal route at specified days after MeBr exposure. Figure 1 shows the experimental design for the MeBr experiment and subsequent 54Mn administration. Four MeBr-treated rats were studied at each time point: 2, 4, 7, 21, and 56 days postexposure. Age-matched control groups at 9 weeks and 17 weeks (n = 4 per group) were evaluated at the same time as their corresponding experimental cohort. For intranasal instillation, 54MnCl2 (Perkin Elmer/NEN) was diluted in PBS to 300 μCi/ml and a volume of 0.1 ml/kg body weight was administered as described previously (Thompson et al., 2007; Tjalve et al., 1996). Absorption into the blood was studied by collecting blood at 1, 2, and 4 h. Additional blood samples were collected at 7 days, the time of euthanasia. All rats were humanely killed by isoflurane anesthesia followed by exsanguination at 7 days after 54MnCl2 administration. Rats were dissected, and samples of blood, brain, lungs, heart, spleen, kidneys, skeletal muscle, liver, and bone marrow were collected, weighed, and placed in tubes. After an initial gamma counting, the brain samples were microdissected into individual regions (olfactory bulb, cortex, brain stem, hippocampus, basal ganglia [without substantia nigra], substantia nigra, and cerebellum) and 54Mn levels were similarly measured. The amount of Mn administered was 35 ng/kg body weight. The increased radiation dose (30 μCi/kg) was necessary for detection of 54Mn in much smaller microdissected brain samples.
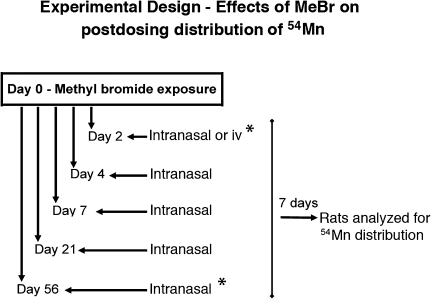
FIG. 1.
Experimental design of MeBr exposure protocol. Male Sprague-Dawley Germfree rats, 9 weeks old (287 ± 5.8 g), were obtained from Taconic Farms. After 3–4 days of acclimatization in the animal facilities, rats were transported to the Department of Anatomy and Cellular Biology, Tufts University School of Medicine, and subjected to MeBr exposure (Day 0). Rats were returned to HSPH animal facility at the end of exposure and were sequentially studied at 2, 4, 7, 21, and 56 days after MeBr exposure when rats were instilled intranasally with 54MnCl2 (Perkin Elmer/NEN) at 30 μCi/kg and a volume of 0.1 ml/kg body weight (four rats per group). All instilled rats were analyzed 7 days later for brain and other tissue concentration of 54Mn. *, At these times, separate cohorts of untreated age-matched control rats were also studied.
The 56-day rats had significant weight gains since the start of the experiment (305.0 ± 6.15 vs. 431.8 ± 7.68 g). Body weight and OE do change with age. The epithelium increases in surface area with increasing age of rats. During this period, there is a corresponding high rate of proliferation of the basal cells in the OE in order to provide for that increase (Hinds and McNelly, 1981; Loo et al., 1996). To address whether age or weight may alter 54Mn absorption kinetics, we studied a separate age- and weight-matched control group corresponding to the 56-day-recovered group (Fig. 1).
We also wanted to determine if MeBr exposure disrupts Mn transport to the brain and peripheral tissues based on the direct effects of MeBr gas on OE or rather via systemic toxicity. To assess whether MeBr exposure in our model could cause systemic toxicity and alter the uptake of 54Mn from the blood to tissues, we compared the 54Mn concentrations of iv-injected 54Mn in control and MeBr-treated rats at 2 days post-MeBr exposure. 54MnCl2 was diluted in PBS to 60 μCi/ml, and a volume of 0.5 ml/kg was injected into the penile vein. The same set of tissue samples was collected at 7 days, the time of euthanasia, and postmortem tissues were processed as above.
Statistical analysis.
Results are shown as the mean ± SE. Data on the blood and brain concentrations of 54Mn in intranasally instilled and iv-injected Hsd:Sprague-Dawley rats were evaluated using Student's unpaired t-test. Pharmacokinetic data on 54Mn from MeBr-exposed Sprague-Dawley Germfree rats were analyzed with multivariate ANOVA using the general linear model procedure (SAS statistical analysis software; SAS Institute Inc., Cary. NC). Statistical significance was considered when p < 0.05.
RESULTS
Brain Uptake of 54Mn from OE
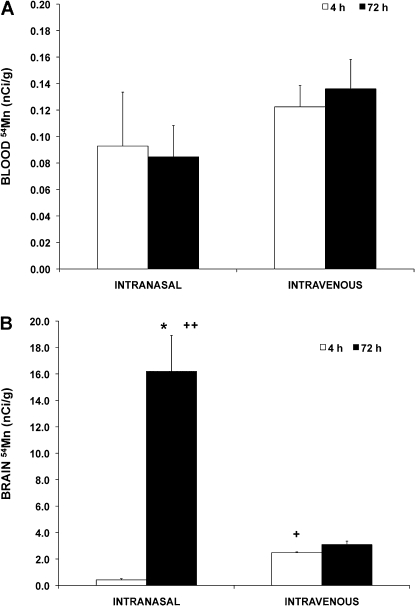
We compared the blood and brain concentrations of intranasally instilled with iv-injected 54MnCl2 in normal Hsd:Sprague-Dawley rats. Blood levels of 54Mn were not different between the intranasal and iv routes of administration, nor were they different at 4 versus 72 h (Fig. 2A). However, 54Mn concentration in the brain was initially higher after iv than after intranasal instillation (Fig. 2B, see 4-h time point). By 72 h, however, the brain levels significantly increased after intranasal but not after iv administration. These data and others from previous reports indicate that intranasal routes of exposure lead to increased concentrations in the brain and suggest the existence of a more direct pathway from the nose to the brain for Mn (Brenneman et al., 2000; Dorman et al., 2002; Henriksson et al., 1999; Tjalve et al., 1995, 1996). In addition, the results of this study were useful in determining the time course for subsequent analyses and point of euthanasia for the MeBr studies.
FIG. 2.
Blood (A) and brain (B) concentrations of 54Mn after a single intranasal instillation or iv injection of 54MnCl2 in normal CD rats. Rats were intranasally instilled or iv injected with 7.5 μCi/kg 54MnCl2 and analyzed at 4 h (open bars) or 72 h (closed bars) postdosing. Each point is a mean ± SE of four rats. The 54Mn brain concentration was initially higher after iv than after intranasal instillation at 4 h (+). However, from 4 to 72 h, the brain levels significantly increased after intranasal instillation but not after iv dosing (*). Moreover, the 54Mn brain levels at 72 h were significantly higher after intranasal instillation than after iv administration (++). (*,+,++p < 0.05, Student's t-test)
Effects of MeBr Exposure on Blood Absorption of Intranasally Instilled 54Mn
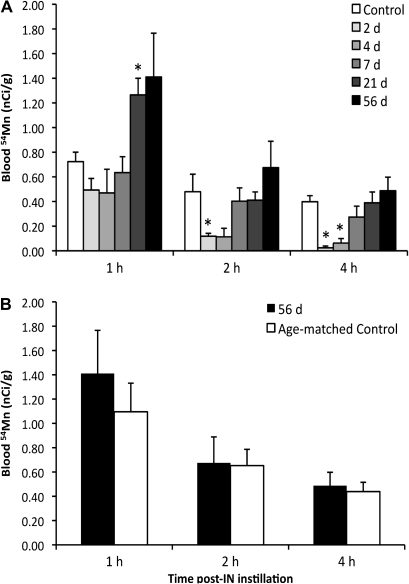
The cells in the nasal epithelium responsible for uptake and transport of Mn to the brain are unknown. Using the MeBr rat model of OE injury and regeneration, we investigated the temporal pattern of accumulation of intranasally instilled 54Mn in the brain (Fig. 1) and correlated it with the cellular regeneration profile of OE previously described (Schwob et al., 1995, 1999). The blood levels following intranasal instillation of 54Mn at different days after MeBr lesion of the OE were obtained to examine the absorption into the blood prior to euthanasia. Blood levels at 1, 2, and 4 h postinstillation of 54MnCl2 are shown in Figure 3A. Two and 4 days after the MeBr lesions, the 2- and 4-h blood levels of 54Mn in intranasally instilled rats were significantly lower compared with untreated control levels. However, at days 7, 21, and 56 after MeBr lesion, the blood levels during the first 4 h were back to control values. Interestingly, rats that had recovered for 21 days had significantly higher blood levels than did control rats at 1 h.
FIG. 3.
54Mn blood concentrations in control and in MeBr-treated rats at 1, 2, and 4 h after 54Mn intranasal instillation. Rats were exposed to MeBr and allowed to recover 2, 4, 7, 21, or 56 days prior to 54MnCl2 instillation. (A) Control rats were not exposed to MeBr. Blood 54Mn levels are nanocurie per gram. Each point is a mean ± SE of four rats. Multivariate ANOVA showed that the different groups of MeBr-exposed rats were significantly different from the 9-week-old controls. Significant decrease over time was also seen in all rat groups (*p < 0.05). 54Mn blood concentrations in 56-day-recovered MeBr-treated rats and age-matched controls (17 weeks old) were not different (B).
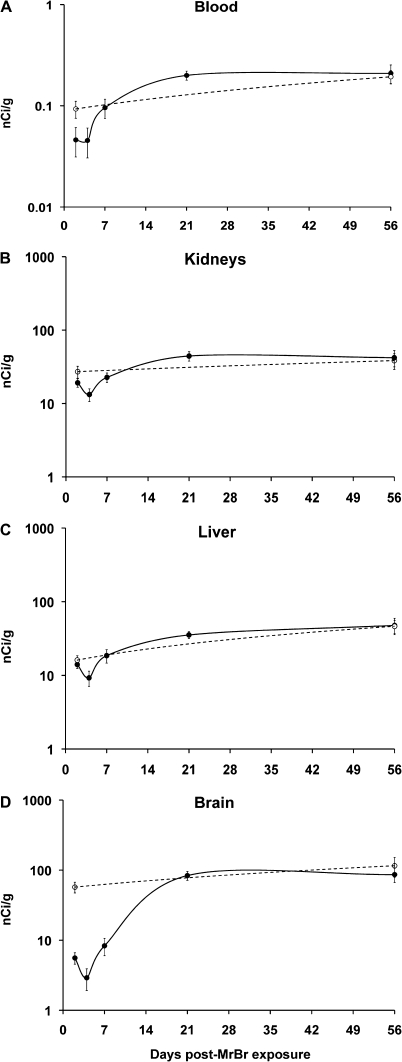
To address whether age or weight may alter 54Mn absorption kinetics, we studied a separate age- and weight-matched control group corresponding to the 56-day-recovered group. The blood levels of intranasally instilled 54Mn in age-matched control rats were not different from MeBr-treated rats recovered for 56 days (Figs. 3B and 4A). Other tissue concentrations, including the brain, were not significantly different indicating normal 54Mn kinetics by the 56-day recovery (Table 1, see 56 days vs. age-matched control).
FIG. 4.
Brain and nonneuronal tissue uptake of 54Mn. Blood (A), kidney (B), and liver (C) concentrations of 54Mn were significantly decreased at 4 days post-MeBr treatment but returned to normal by day 7 of recovery. In contrast, the total brain (D) concentrations were significantly decreased at 2, 4, and 7 days and returned to control values only from day 21 post-MeBr treatment.
TABLE 1.
Tissue Concentration of 54Mn at 7 Days Post-Intranasal Instillation
| Tissue | Untreated control | Days after MeBr exposure |
|||||
| 2 | 4 | 7 | 21 | 56 | Age-matched control-56 days | ||
| Brain | 57.22 ± 9.94 | 5.58 ± 1.03* | 2.91 ± 1.00* | 8.31 ± 2.31* | 83.61 ± 12.13 | 86.38 ± 19.98 | 115.47 ± 36.52 |
| Spleen | 7.15 ± 1.16 | 5.35 ± 0.59 | 4.09 ± 0.93 | 7.23 ± 1.26 | 13.71 ± 1.64* | 13.41 ± 2.97 | 11.68 ± 2.05 |
| Kidneys | 27.14 ± 5.00 | 19.14 ± 2.53 | 13.25 ± 2.59* | 22.68 ± 3.32 | 44.2 ± 6.49 | 42.04 ± 10.52 | 38.39 ± 9.22 |
| Heart | 7.64 ± 0.09 | 5.11 ± 0.67 | 4.18 ± 0.78* | 7.09 ± 1.13 | 12.71 ± 1.84 | 11.69 ± 3.02 | 11.72 ± 2.10 |
| Liver | 16.12 ± 2.36 | 14.02 ± 1.59 | 9.26 ± 2.16 | 18.53 ± 3.85 | 35.37 ± 3.78* | 47.66 ± 11.96* | 46.39 ± 9.46 |
| Skeletal muscle | 1.28 ± 0.16 | 0.71 ± 0.09* | 0.63 ± 0.12* | 1.06 ± 0.17 | 1.61 ± 0.24 | 1.60 ± 0.38 | 1.32 ± 0.31 |
| Lungs | 14.99 ± 6.94 | 4.37 ± 0.93 | 3.92 ± 0.81 | 11.45 ± 5.52 | 10.38 ± 1.35 | 9.57 ± 1.34 | 11.01 ± 2.81 |
| Testes | 5.67 ± 0.89 | 3.53 ± 0.57* | 2.89 ± 0.61 | 4.89 ± 0.65 | 8.64 ± 1.60 | 7.76 ± 2.04 | 7.08 ± 1.35 |
Note. Data are means ± SE concentration of 54Mn in nanocurie per gram (n = 4; *p < 0.05 compared with untreated control, multivariate ANOVA). Data from age-matched untreated and from MeBr-exposed rats at 56 days of recovery were not different.
Effects of MeBr Exposure on Tissue Uptake of Intranasally Instilled 54Mn
The overall tissue 54Mn levels in MeBr-treated rats were lower at 2 and 4 days after injury. Significant differences at 2 and 4 days were found in the brain, kidney, skeletal muscle, and heart (Table 1). Kidney, skeletal muscle, and heart 54Mn concentrations returned to control levels by day 7. Brain levels, however, remained significantly lower at day 7, reaching control levels only at day 21 of recovery (Table 1). This time delay was unique for the brain. Spleen and liver 54Mn concentrations increased at day 21 and days 21 and 56, respectively. The comparison of MeBr-treated animals to age-matched controls over the recovery period is further illustrated in Figure 4. Figure 4 shows blood, two major nonneural tissues (liver and kidney), and whole brain concentrations of 54Mn at various time points post-MeBr recovery periods. The blood (Fig. 4A), kidney (Fig. 4B), and liver (Fig. 4C) 54Mn concentrations were back to control levels by day 7, whereas the whole brain (Fig. 4C) was still significantly lower. Only by day 21 did the brain levels returned to control values.
Comparison of the Effects of MeBr Exposure on Brain Distribution of IV-Injected and Intranasally Instilled 54Mn at an Early Recovery Period
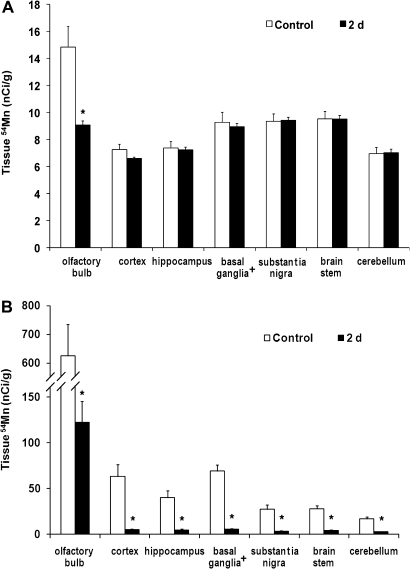
Brain 54Mn distribution was further analyzed in both the intranasally instilled and iv-injected groups by examining the radioactivity levels in microdissected brain samples. Each brain was subdivided into the olfactory bulb, cortex, brain stem, cerebellum, hippocampus, and regions of the substantia nigra and the basal ganglia (without substantia nigra). Because a fraction of 54Mn retained in the brain would be absorbed directly from the blood, we examined whether MeBr lesions would alter the tissue distribution of 54Mn directly injected iv. The brain distribution of iv-injected 54Mn in rats 2 days after MeBr lesion was nearly identical to untreated control rats, except in the olfactory bulb (Fig. 5A). All other peripheral tissues examined were not different from control values (data not shown). Although MeBr exposure decreased the olfactory bulb uptake of 54Mn from the blood, it was unlikely to contribute to the significant reduction in the olfactory bulb uptake of intranasally instilled 54Mn. The concentration of 54Mn in the olfactory bulb after intranasal instillation (Fig. 5B) was an order of magnitude higher than after iv injection (Fig. 5A). The overall brain concentrations of 54Mn were higher in the intranasally instilled rats compared with iv-injected rats. The 54Mn concentrations in different brain regions were also significantly lower in MeBr-treated rats compared with control (Fig. 5B). The olfactory bulb, which was anatomically close to the nose, had the highest concentration of 54Mn (Fig. 5B).
FIG. 5.
Comparison of intranasally instilled versus iv-injected 54MnCl2 in MeBr-exposed rats. Shown are brain tissue concentrations of 54Mn after iv injection (A) or intranasal instillation (B) of 54MnCl2 in MeBr-exposed (closed bars) or age-matched untreated controls (open bars) 2 days after MeBr exposure. The retained 54Mn in all dissected brain regions was significantly decreased in MeBr-exposed rats intranasally instilled with the radioisotope. In contrast, only the olfactory bulb had significantly reduced 54Mn in MeBr-exposed iv-injected rats. *p < 0.05. (Basal ganglia+, these data excluded the substantia nigra).
Distribution of 54Mn in the Brain during the Cellular Reconstitution of the Rat OE Post-MeBr Exposure
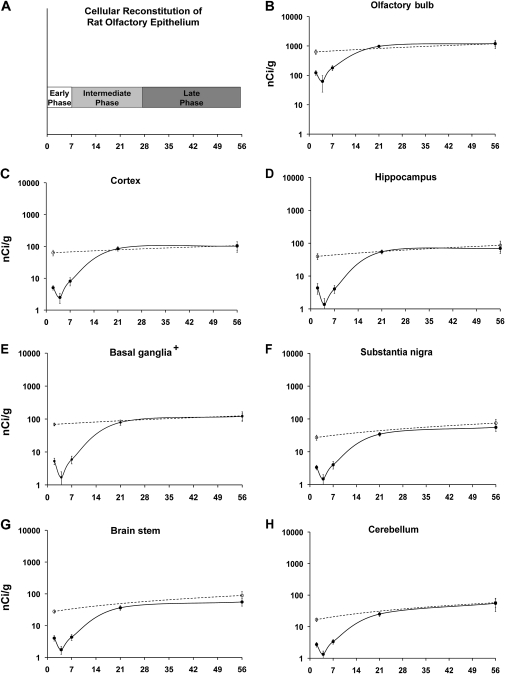
Figure 6A depicts phases of OE regeneration along the time line of our experiment (Schwob et al., 1995). The early phase, corresponding to the first week postinjury, is a period of proliferation of sustentacular and neuronal cells, and it is during this time period where there are significant decreases in brain 54Mn levels between control and MeBr-treated rats. The intermediate phase ranges from 7 to 28 days postinjury and corresponds to the time period when reconstitution of the epithelium as well as neuronal differentiation and maturation are occurring. Near the end of this intermediate phase and throughout the late phase, 54Mn levels in the brain reached and maintained the control levels (Figs. 4D and 6B–H). This is true of all brain regions analyzed. The results are in contrast to the findings in the blood and peripheral tissues.
FIG. 6.
54Mn levels in dissected brain regions of intranasally instilled MeBr-treated rats. Rats were instilled with 54MnCl2 at specific time point post-MeBr treatment (closed circles): 2, 4, 7, 21, or 56 days. Two groups of weight-matched control rats were not exposed to MeBr and were studied at the beginning and at the end of the 56-day MeBr postexposure recovery period (open circles). Seven days after intranasal instillation of 54MnCl2, rats were humanely killed and brains were collected for microdissection. Brain tissue 54Mn levels are expressed as nanocurie per gram. Figure 6A depicts phases of OE regeneration along the time line of our experiment. In all the brain regions, there was a significant reduction in 54Mn concentration at 2, 4, and 7 days post-MeBr exposure (B–H). The values for all brain regions were back to control levels at 21 days postexposure. The return to control levels correlates well with the end of the intermediate phase of OE regeneration (A). Each point is a mean ± SE of four rats. *p < 0.05. (Basal ganglia+, these data excluded the substantia nigra).
DISCUSSION
This study was undertaken to better characterize the transport of Mn via olfactory pathways using a tracer amount of 54MnCl2 that is orders of magnitude below toxic levels (U.S.EPA, 1992). Specifically, we sought to identify which olfactory epithelial cell types are responsible for the initial uptake of Mn in the nasal epithelium. We used the MeBr model of OE injury/regeneration to test our hypothesis that olfactory receptor neurons transport Mn to the olfactory bulb and from there to the posterior regions of the brain. The MeBr model is characterized by a reproducible temporal pattern of OE reconstitution and olfactory bulb reinnervation after the initial injury (Schwob et al., 1995, 1999). It has been shown that supporting cells and neuronal cells have different regeneration rates, with the former achieving structural recovery well before the OSNs (Schwob et al., 1995, 1999).
There are three phases in the olfactory epithelial regeneration process: early (cellular proliferation—up to 1 week), intermediate (numerical reconstitution and neuronal differentiation proceeding toward full maturation—1–3 weeks), and late (stabilization and full neuronal maturation—3–8 weeks) (Schwob et al., 1995). Likewise, there are three phases to the reinnervation of the olfactory bulb following MeBr injury: early (olfactory axons reach the bulb and restore the olfactory nerve layer—1–2 weeks), intermediate (axons penetrate the glomeruli—2–4 weeks), and late (the axonal projection onto the olfactory bulb stabilizes—4–6 weeks) (Schwob et al., 1999). We utilized these temporal characteristics to investigate which cell types are important for the initial uptake and transport of 54Mn from OE into the blood and brain. The same dose of 54MnCl2 per kilogram was administered to the animals following different recovery periods post-MeBr treatment.
Our results show that acute injury to the OE results in decreased absorption of 54Mn into the blood, as shown by lower blood concentrations at 2 and 4 days post-MeBr exposure. Absorption returned to normal by day 7, the time when the foot processes of the sustentacular cells have grown down from the somata at the apex of the epithelium and come into contact with the basal lamina (Drapeau and Nachshen, 1984; Schwob et al., 1995, 1999). The blood kinetic observations indicate that the sustentacular cells play an important role in 54Mn transport from the OE to the blood. In contrast, transport of 54Mn to the brain from the OE returned to normal only by day 21. Previous studies have shown that the reinnervation of the olfactory bulb by OSNs takes 3–8 weeks to recover following MeBr damage (Schwob et al., 1995, 1999). The temporal correlation between neuronal regeneration/bulbar reinnervation and 54Mn uptake in the brain suggests that the regeneration of OSNs in OE and the restoration of their projection onto the olfactory bulb are crucial for Mn uptake and transport to the brain.
Neurons can take up Mn through several transport mechanisms, such as Ca2+ channels (Anderson, 1979; Bouchard et al., 2008; Cotzias, 1958; Takeda et al., 1998), active Ca2+ uniporter (Tjalve et al., 1995), and DMT1 (Thompson et al., 2007). DMT1 plays an important role in Mn transport following intranasal instillation (Thompson et al., 2007). Both olfactory receptor neurons and supporting cells express DMT1 (Thompson et al., 2007). Our results indicate that OSNs are primarily responsible for the uptake of Mn that is destined to reach the olfactory bulb. However, contributions to brain uptake of 54Mn could derive from the trigeminal nerve. The trigeminal pathway enters the brain through the cribiform plate alongside the olfactory pathway. Transport along the trigeminal axons would lead to the brain stem (where the sensory fibers of the trigeminal terminate). Lewis et al. (2005) have demonstrated evidence in support of trigeminal transport of Mn using proton-induced x-ray emission analysis for Mn determination. However, the trigeminal nerve, which innervates the lamina propria deep to the OE, is unlikely to be damaged, as the network of loose axons formed by the trigeminal within the lamina propria remains undamaged by the destruction of the OE by MeBr (Schwob, unpublished observations). These intact deeper fibers far outnumber the rare trigeminal axons that extend apically into the OE. Presumably, those few trigeminal axons are damaged, along with the surrounding cells, by the exposure to MeBr. It is likely that the trigeminal fibers regrow into the epithelium as the epithelial cells are regenerated, which means that they would be capable of transporting material before the olfactory axons have reinnervated the bulb. The fact that the timing of Mn transport is delayed past the regeneration of the epithelium and, instead, closely parallels olfactory reinnervation of the bulb suggests that the trigeminal contribution is relatively insignificant.
Vascular pathways and extracellular pathways are additional routes from the nose to the brain (Dhuria et al., 2010; Mathison et al., 1998; Thorne et al., 2004; Yang et al., 2009). Our data do not exclude these pathways as contributors to transport of 54Mn to the olfactory bulb and brain. The early recovery time points where olfactory receptor neurons are nonexistent but 54Mn levels are still evident in the brain strengthen the evidence for these pathways in our model. Interestingly, iv-injected 54Mn was also significantly reduced in the olfactory bulb of MeBr-treated rats during the acute stage of OE injury. This decrease may be because of activation of the glia of the olfactory nerves in reaction to the degeneration of the axons of the destroyed neurons (Schwob et al., 1999), invasion of microglial cells/macrophages (Williams et al., 2004), distortion of the cellular architecture in the outer nerve layer, disruption of olfactory/trigeminal extracellular pathways, and/or a reaction by the vasculature near the olfactory bulb. All these may impede the perfusion of blood containing 54Mn and thus its delivery to the olfactory bulb. Nonetheless, the increase in brain 54Mn concentration in parallel with the timing of olfactory receptor neuron reinnervation does suggest that intact axonal pathways results in increased 54Mn levels and that other pathways alone are not sufficient for this level of transport.
Thus, based on our MeBr study, we favor the conclusion that the olfactory axonal pathway is a major route for 54Mn accumulation in the brain. Our iv data also support this conclusion. Our data showed that even when injected directly into the circulation, brain 54Mn concentration was more than 10 times lower than when instilled intranasally (Fig. 2), which is also observed when 54Mn concentration is determined in the olfactory bulb and other brain regions as they were also lower for the iv group than the intranasal group (Fig. 5). Together these points, along with the MeBr data, indicate that 54Mn present in the circulation either by direct injection or transport to the vasculature from the nose does not reach the brain at the same efficiency as it does via olfactory pathways directly from the nose.
A few peripheral tissues also show decreased 54Mn concentrations during the early recovery periods. We see a decrease in 54Mn concentration in blood, testes, skeletal muscle, and brain at 2 days and a decrease at 4 days in brain, blood, kidney, skeletal muscle, and heart (Table 1). All tissues except for the brain return to control levels by 7 days. At 2 days recovery, the OE is more permeable. However, at 4 days, there may be an increased barrier because of the process of OE cellular reconstitution that prevents the 54Mn from being absorbed/transported by vascular pathways. These early phase recovery concentrations in the tissues, except for brain, follow the same trend as the blood and reflect blood concentrations. The kidney and heart consist of more connective tissue with blood flow through the tissue accounting for 54Mn concentration rather than actual 54Mn absorption and storage by the tissue and therefore may be why the levels are more significantly decreased in these tissues. The other tissues may accumulate a small amount of the 54Mn tracer and maintain slightly higher levels and variable data. We do not feel the decrease reflects any effect of MeBr on the tissues.
We also found a few differences in 54Mn concentrations in peripheral tissues at the later recovery periods. The tissues involved in Mn clearance (liver) and red blood cell recycling (spleen) increase in 54Mn concentration at day 21 compared with controls (Table 1). The liver is also increased at 56 days, but this is not significant when compared with the age-matched control. Our data are also consistent with the fact that the liver plays a major role in Mn homeostasis by clearing Mn via biliary excretion (Persson et al., 2003).
Understanding the mechanisms involved in Mn transport from the OE is an important step in monitoring and preventing the neurotoxic effects of Mn. Inhaled particles containing Mn that are deposited in the nose may contribute to metal toxicity in the central nervous system. Our study shows that the sustentacular cells in the OE play an important role in Mn transport to the blood. The neuronal cells that begin to regenerate 1 week post-MeBr injury are most likely responsible for the initial uptake of Mn from the OE to the olfactory bulb and subsequent transport to the rest of the brain via secondary axons.
Our data show that an intact OE is necessary for Mn transport to the brain and blood where (1) sustentacular cells are necessary for Mn transport to the blood and (2) intact axonal projections are required for Mn transport from the nasal cavity to the brain. Whereas previous studies have identified transport of Mn from the OE to the olfactory bulb, the results presented here are the first to specifically examine cellular transport of Mn at the entry point to the OE and to identify the critical role of OSNs in Mn transport. It is important to acknowledge that environmental sources of Mn include many forms such as Mn sulfate, Mn phosphates, and Mn oxides. Because of the different chemical and physical properties of these Mn compounds, they may have different bioavailability and uptake kinetics. Additional research incorporating studies on transport of these other forms of Mn and studies on other ways of disrupting this transport pathway will be helpful in developing strategies to prevent Mn neurotoxicity.
FUNDING
National Institute of Environmental Health Sciences (P01 ES012874, ES000002); U.S. Environmental Protection Agency (RD-83172501); Elizabethtown College, Elizabethtown, PA.
Acknowledgments
We thank Melissa Curran for editorial advice. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Environmental Health Sciences, U.S. Environmental Protection Agency, and Elizabethtown College.
References
- Anderson M. Mn2+ ions pass through Ca2+ channels in myoepithelial cells. J. Exp. Biol. 1979;82:227–238. doi: 10.1242/jeb.82.1.227. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Mergler D, Baldwin ME, Panisset M. Manganese cumulative exposure and symptoms: a follow-up study of alloy workers. Neurotoxicology. 2008;29:577–583. doi: 10.1016/j.neuro.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Brain JD, Heilig E, Donaghey TC, Knutson MD, Wessling-Resnick M, Molina RM. Effects of iron status on transpulmonary transport and tissue distribution of Mn and Fe. Am. J. Respir. Cell Mol. Biol. 2006;34:330–337. doi: 10.1165/rcmb.2005-0101OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman KA, Wong BA, Buccellato MA, Costa ER, Gross EA, Dorman DC. Direct olfactory transport of inhaled manganese ((54)MnCl(2)) to the rat brain: toxicokinetic investigations in a unilateral nasal occlusion model. Toxicol. Appl. Pharmacol. 2000;169:238–248. doi: 10.1006/taap.2000.9073. [DOI] [PubMed] [Google Scholar]
- Caggiano M, Kauer JS, Hunter DD. Globose basal cells are neuronal progenitors in the olfactory epithelium: a lineage analysis using a replication-incompetent retrovirus. Neuron. 1994;13:339–352. doi: 10.1016/0896-6273(94)90351-4. [DOI] [PubMed] [Google Scholar]
- Cotzias GC. Manganese in health and disease. Physiol. Rev. 1958;38:503–532. doi: 10.1152/physrev.1958.38.3.503. [DOI] [PubMed] [Google Scholar]
- Dhuria SV, Hanson LR, Frey WH., II Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J. Pharm. Sci. 2010;99:1654–1673. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]
- Dobson AW, Erikson KM, Aschner M. Manganese neurotoxicity. Ann. N. Y. Acad. Sci. 2004;1012:115–128. doi: 10.1196/annals.1306.009. [DOI] [PubMed] [Google Scholar]
- Dobson AW, Weber S, Dorman DC, Lash LK, Erikson KM, Aschner M. Oxidative stress is induced in the rat brain following repeated inhalation exposure to manganese sulfate. Biol. Trace Elem Res. 2003;93:113–126. doi: 10.1385/BTER:93:1-3:113. [DOI] [PubMed] [Google Scholar]
- Dorman DC, Brenneman KA, McElveen AM, Lynch SE, Roberts KC, Wong BA. Olfactory transport: a direct route of delivery of inhaled manganese phosphate to the rat brain. J. Toxicol. Environ. Health A. 2002;65:1493–1511. doi: 10.1080/00984100290071630. [DOI] [PubMed] [Google Scholar]
- Dorman DC, McManus BE, Parkinson CU, Manuel CA, McElveen AM, Everitt JI. Nasal toxicity of manganese sulfate and manganese phosphate in young male rats following subchronic (13-week) inhalation exposure. Inhal. Toxicol. 2004;16:481–488. doi: 10.1080/08958370490439687. [DOI] [PubMed] [Google Scholar]
- Drapeau P, Nachshen DA. Manganese fluxes and manganese-dependent neurotransmitter release in presynaptic nerve endings isolated from rat brain. J. Physiol. 1984;348:493–510. doi: 10.1113/jphysiol.1984.sp015121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein Y, Zhang N, Fitsanakis VA, Avison MJ, Gore JC, Aschner M. Differential deposition of manganese in the rat brain following subchronic exposure to manganese: a T1-weighted magnetic resonance imaging study. Isr. Med. Assoc. J. 2008;10:793–798. [PMC free article] [PubMed] [Google Scholar]
- Graziadei PP, Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J. Neurocytol. 1979;8:1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- Hanzlik RP, Stitt R, Traiger GJ. Toxic effects of methylcyclopentadienyl manganese tricarbonyl (MMT) in rats: role of metabolism. Toxicol. Appl. Pharmacol. 1980;56:353–360. doi: 10.1016/0041-008x(80)90068-x. [DOI] [PubMed] [Google Scholar]
- Heilig EA, Thompson KJ, Molina RM, Ivanov AR, Brain JD, Wessling-Resnick M. Manganese and iron transport across pulmonary epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;290:L1247–L1259. doi: 10.1152/ajplung.00450.2005. [DOI] [PubMed] [Google Scholar]
- Henriksson J, Tallkvist J, Tjalve H. Transport of manganese via the olfactory pathway in rats: dosage dependency of the uptake and subcellular distribution of the metal in the olfactory epithelium and the brain. Toxicol. Appl. Pharmacol. 1999;156:119–128. doi: 10.1006/taap.1999.8639. [DOI] [PubMed] [Google Scholar]
- Henriksson J, Tjalve H. Manganese taken up into the CNS via the olfactory pathway in rats affects astrocytes. Toxicol. Sci. 2000;55:392–398. doi: 10.1093/toxsci/55.2.392. [DOI] [PubMed] [Google Scholar]
- Hinds JW, McNelly NA. Aging in the rat olfactory system: correlation of changes in the olfactory epithelium and olfactory bulb. J. Comp. Neurol. 1981;203:441–453. doi: 10.1002/cne.902030308. [DOI] [PubMed] [Google Scholar]
- Huard JM, Youngentob SL, Goldstein BJ, Luskin MB, Schwob JE. Adult olfactory epithelium contains multipotent progenitors that give rise to neurons and non-neural cells. J. Comp. Neurol. 1998;400:469–486. [PubMed] [Google Scholar]
- Lewis J, Bench G, Myers O, Tinner B, Staines W, Barr E, Divine KK, Barrington W, Karlsson J. Trigeminal uptake and clearance of inhaled manganese chloride in rats and mice. Neurotoxicology. 2005;26:113–123. doi: 10.1016/j.neuro.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Loo AT, Youngentob SL, Kent PF, Schwob JE. The aging olfactory epithelium: neurogenesis, response to damage, and odorant-induced activity. Int. J. Dev. Neurosci. 1996;14:881–900. doi: 10.1016/s0736-5748(96)00046-9. [DOI] [PubMed] [Google Scholar]
- Mathison S, Nagilla R, Kompella UB. Nasal route for direct delivery of solutes to the central nervous system: fact or fiction? J. Drug Target. 1998;5:415–441. doi: 10.3109/10611869808997870. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Wang HX, Suo C, McCombe D, Horne MK, Morrison WA, Egan GF. Retrograde axonal tracing using manganese enhanced magnetic resonance imaging. Neuroimage. 2010;50:366–374. doi: 10.1016/j.neuroimage.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Mergler D, Baldwin M, Belanger S, Larribe F, Beuter A, Bowler R, Panisset M, Edwards R, de Geoffroy A, Sassine MP, et al. Manganese neurotoxicity, a continuum of dysfunction: results from a community based study. Neurotoxicology. 1999;20:327–342. [PubMed] [Google Scholar]
- Mergler D, Huel G, Bowler R, Iregren A, Belanger S, Baldwin M, Tardif R, Smargiassi A, Martin L. Nervous system dysfunction among workers with long-term exposure to manganese. Environ. Res. 1994;64:151–180. doi: 10.1006/enrs.1994.1013. [DOI] [PubMed] [Google Scholar]
- Morrison EE, Costanzo RM. Morphology of the human olfactory epithelium. J. Comp. Neurol. 1990;297:1–13. doi: 10.1002/cne.902970102. [DOI] [PubMed] [Google Scholar]
- Obenaus A, Jacobs RE. Magnetic resonance imaging of functional anatomy: use for small animal epilepsy models. Epilepsia. 2007;48(Suppl. 4):11–17. doi: 10.1111/j.1528-1167.2007.01237.x. [DOI] [PubMed] [Google Scholar]
- Pautler RG, Koretsky AP. Tracing odor-induced activation in the olfactory bulbs of mice using manganese-enhanced magnetic resonance imaging. Neuroimage. 2002;16:441–448. doi: 10.1006/nimg.2002.1075. [DOI] [PubMed] [Google Scholar]
- Pautler RG, Silva AC, Koretsky AP. In vivo neuronal tract tracing using manganese-enhanced magnetic resonance imaging. Magn. Reson. Med. 1998;40:740–748. doi: 10.1002/mrm.1910400515. [DOI] [PubMed] [Google Scholar]
- Perl DP, Olanow CW. The neuropathology of manganese-induced Parkinsonism. J. Neuropathol. Exp. Neurol. 2007;66:675–682. doi: 10.1097/nen.0b013e31812503cf. [DOI] [PubMed] [Google Scholar]
- Persson E, Henriksson J, Tjalve H. Uptake of cobalt from the nasal mucosa into the brain via olfactory pathways in rats. Toxicol. Lett. 2003;145:19–27. doi: 10.1016/s0378-4274(03)00266-2. [DOI] [PubMed] [Google Scholar]
- Schultz EW. Repair of the olfactory mucosa with special reference to regeneration of olfactory cells (sensory neurons) Am. J. Pathol. 1960;37:1–19. [PMC free article] [PubMed] [Google Scholar]
- Schwob JE. Restoring olfaction: a view from the olfactory epithelium. Chem. Senses. 2005;30(Suppl. 1):i131–i132. doi: 10.1093/chemse/bjh149. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Youngentob SL, Mezza RC. Reconstitution of the rat olfactory epithelium after methyl bromide-induced lesion. J. Comp. Neurol. 1995;359:15–37. doi: 10.1002/cne.903590103. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Youngentob SL, Ring G, Iwema CL, Mezza RC. Reinnervation of the rat olfactory bulb after methyl bromide-induced lesion: timing and extent of reinnervation. J. Comp. Neurol. 1999;412:439–457. doi: 10.1002/(sici)1096-9861(19990927)412:3<439::aid-cne5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Takeda A, Sawashita J, Okada S. Manganese concentration in rat brain: manganese transport from the peripheral tissues. Neurosci. Lett. 1998;242:45–48. doi: 10.1016/s0304-3940(98)00006-8. [DOI] [PubMed] [Google Scholar]
- Thompson K, Molina RM, Donaghey T, Schwob JE, Brain JD, Wessling-Resnick M. Olfactory uptake of manganese requires DMT1 and is enhanced by anemia. FASEB J. 2007;21:223–230. doi: 10.1096/fj.06-6710com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne RG, Pronk GJ, Padmanabhan V, Frey WH., II Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127:481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Tjalve H, Henriksson J. Uptake of metals in the brain via olfactory pathways. Neurotoxicology. 1999;20:181–195. [PubMed] [Google Scholar]
- Tjalve H, Henriksson J, Tallkvist J, Larsson BS, Lindquist NG. Uptake of manganese and cadmium from the nasal mucosa into the central nervous system via olfactory pathways in rats. Pharmacol. Toxicol. 1996;79:347–356. doi: 10.1111/j.1600-0773.1996.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Tjalve H, Mejare C, Borg-Neczak K. Uptake and transport of manganese in primary and secondary olfactory neurones in pike. Pharmacol. Toxicol. 1995;77:23–31. doi: 10.1111/j.1600-0773.1995.tb01909.x. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (U.S.EPA) Manganese Compounds. 1992. Available at: http://www.epa.gov/ttn/atw/hlthef/manganes.html. Updated February 16, 2010. Accessed November 30, 2010. [Google Scholar]
- Villalobos V, Bonilla E, Castellano A, Novo E, Caspersen R, Giraldoth D, Medina-Leendertz S. Ultrastructural changes of the olfactory bulb in manganese-treated mice. Biocell. 2009;33:187–197. [PubMed] [Google Scholar]
- Williams SK, Franklin RJ, Barnett SC. Response of olfactory ensheathing cells to the degeneration and regeneration of the peripheral olfactory system and the involvement of the neuregulins. J. Comp. Neurol. 2004;470:50–62. doi: 10.1002/cne.11045. [DOI] [PubMed] [Google Scholar]
- Yang JP, Liu HJ, Cheng SM, Wang ZL, Cheng X, Yu HX, Liu XF. Direct transport of VEGF from the nasal cavity to brain. Neurosci. Lett. 2009;449:108–111. doi: 10.1016/j.neulet.2008.10.090. [DOI] [PubMed] [Google Scholar]