Abstract
TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) is a ubiquitous environmental contaminant and known endocrine disruptor. Since humans and animals are most sensitive to toxicant exposure during development, we previously developed a mouse model of in utero TCDD exposure in order to examine the impact of this toxicant on adult reproductive function. Our initial in utero toxicant-exposure study revealed a dose-dependent reduction in uterine sensitivity to progesterone; however, we did not previously explore establishment or maintenance of pregnancy. Thus, in the current study, we examined pregnancy outcomes in adult C57BL/6 mice with a history of developmental TCDD exposure. Herein we demonstrate reduced fertility and an increased incidence of premature birth (PTB) in F1 mice exposed in utero to TCDD as well as in three subsequent generations. Finally, our studies revealed that mice with a history of developmental TCDD exposure exhibit an increased sensitivity to inflammation which further negatively impacted gestation length in all generations examined.
Keywords: TCDD, fertility, preterm birth, inflammation, progesterone receptor, development
1. Introduction
Over the past decade, a considerable body of evidence has begun to accumulate which suggests that many diseases and conditions affecting adult health may actually be initiated much earlier in life, specifically, within fetal and neonatal periods of development. The resultant theory, known as the developmental origins of human disease (DOHaD), requires that we begin to examine the potential role of fetal/neonatal programming on adult pathology. Although fetal programming is a normal component of development, programming processes can be negatively impacted by various factors, including maternal stress, poor nutrition and exposure to environmental toxicants. In particular, numerous chemicals, both natural and manufactured, may act as endocrine disruptors during critical periods of developmental programming, potentially affecting adult health related to a number of diseases, such as cancer, impaired immune function and obesity [1]. In addition to the development of specific diseases, there is also strong experimental evidence linking early life exposure to environmental toxicants to the disruption of adult reproductive processes [2]. An equally important, but poorly understood, element of reproductive toxicology related to DOHaD is the question of whether toxicant exposure within the in utero environment also poses a risk to the health of future generations. In this regard, researchers have begun to focus on the disruption of epigenetic events as a causative mechanism behind the negative effects of in utero toxicant exposure during developmental programming [3–5].
Endocrine disrupting toxicants, such as halogenated arylhydrocarbons (HAHs), are known to interfere with molecular and cellular aspects of the mature mammalian reproductive axis and are suspected of increasing the incidence of infertility and reproductive tract disease in human populations [6,7]. Unfortunately, both human and animal populations are most sensitive environmental toxicants, including the HAHs, during development. TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin or dioxin) is the most potent member of the polychlorinated dibenzo-p-dioxin family of HAHs, all of which are formed as unwanted by-products of industrial processes [8]. This ubiquitous environmental contaminant is a known endocrine disruptor and acute exposure of women and lower primates to high levels of TCDD acts as an abortofacient and teratogen [9–12]. Additionally, TCDD and other HAHs are highly resistant to degradation, thus they accumulate within our environment contaminating soil and groundwater, eventually entering the food supply (primarily meat and dairy sources) [13]. For these reasons, in human populations, ingestion of contaminated food is the major source of exposure to the dioxin family of HAHs [14–16]. Importantly, TCDD is lipophilic and accumulates within the body in areas of fat storage [17]; thus this toxicant is a significant tissue contaminant in the breast. Consequently, breast milk samples have been found to contain very high levels of this compound [18], making prenatal and neonatal exposure of humans to toxicants such as TCDD the norm rather than the exception.
Since prospective experimental studies of early life toxicant exposures are not possible in humans, we recently developed a mouse model of developmental TCDD exposure to examine this toxicant’s impact on adult reproductive function. In this model, we initially reported that developmental exposure to TCDD leads to a reduced uterine sensitivity to progesterone in the sexually mature female offspring [19]. Perhaps not surprisingly, we demonstrated a frequency dependent effect of developmental TCDD exposure, with the greatest disruption in progesterone response noted in the animals exposed to this toxicant multiple times during development and at puberty. Progesterone action may be an especially critical toxicant target since inadequate response to this steroid has been associated with pregnancy failure and spontaneous abortion in women and mice [20–23]. However, while toxicant-mediated disruption of progesterone action can be linked to numerous reproductive disorders, the potential that early life exposure of a single individual to TCDD or other HAHs may transmit adverse pregnancy outcomes to future generations has not previously been described.
In the current study we explored the impact of TCDD exposure at a single time-point versus multiple time-points during development. Our primary objective was to determine whether or not the reproductive affects we previously identified following exposure to this toxicant [19] could be transmitted to the female descendants of exposed dams. It is important to note that the current animal study was not designed to address the issue of relevant human exposure levels or risk assessment for reproductive age human populations, but rather to determine the impact of TCDD exposure at the same dose level as previously reported [19] on the fertility of successive generations of female mice. As will be discussed below, not only was the establishment and maintenance pregnancy affected in the toxicant-exposed F1 mice, but in several subsequent generations as well. Perhaps equally important, an unexpected outbreak of mouse parvovirus (MPV) within the Vanderbilt Vivarium, led to the finding that pregnant mice with a history of early life TCDD exposure frequently exhibited preterm birth (PTB). The increased occurrence of PTB in animals with both an early life toxicant exposure and a subsequent viral infection suggested that two independent exposures may have affected PTB in our colony. To test this two hit hypothesis, we conducted a second series of studies using lipopolysaccharide as the secondary inflammatory trigger in TCDD exposed mice known to be free of MPV infection. Herein, we describe our findings from these studies which support the concept that early life TCDD exposure affects reproductive tract sensitivity to inflammatory processes impacting both fertility and risk for PTB.
2. Materials and Methods
2.1 Animals
Ten-week old, virgin female C57BL/6 mice and adult breeder males were purchased from Harlan Sprague-Dawley (Indianapolis, IN). Animals were housed in the Animal Care Facility according to National Institutes of Health and institutional guidelines for laboratory animals. All animals received low phytoestrogen rodent chow (Picolab 5VO2, Purina TestDiets, Richmond, IN) and water ad libitum. Animal rooms were maintained at a temperature of 22–24°C and a relative humidity of 40–50% on a 12 hour light:dark schedule. Experiments described herein were approved by Vanderbilt University Institutional Animal Care and Use Committee in accordance with the Animal Welfare Act.
2.2 Chemicals
TCDD (99% purity) in nonane solution (50 ug/mL) was obtained from Cambridge Isotope Laboratories (Andover, MA). Lipopolysaccharide (LPS), obtained from Enzo Life Sciences (Plymouth Meeting, PA), was derived from E. coli (serotype 055:B5 S-form, TLR grade). 17β-estradiol, corn oil and all other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
2.3 Animal TCDD Exposure
Following at least a one week acclimation period, female C57BL/6 mice (15–16 per group) were mated to control breeder males and received vehicle (corn oil) or TCDD (10µg/kg) by gavage at 1100 hours CST on gestation day 15.5 (E15.5), when organogenesis is complete. TCDD is estimated to have a half-life of approximately 11 days in this strain [24]; thus pups were exposed in utero and postnatally via lactation (i.e., perinatal exposure). Note: vaginal plug = day 0.5 of gestation (E0.5). Some in utero exposed offspring additionally received TCDD (10µg/kg) by gavage at 4 weeks of age (n=15) or at both 4 weeks and 9 weeks of age (n=5). Thus, we generated single, double and triple-exposed animals. Our laboratory has previously demonstrated the effect of this dose on the expression of progesterone-responsive proteins in the uterus of exposed animals [19]. Additionally, the dose of TCDD selected reflects the more rapid clearance of this toxicant in mice compared to humans and is well below the LD50 for adult mice of this strain (230µg/kg) [25]. Nevertheless, we recognize that this is a high dose of TCDD as the studies herein were designed to determine whether or not this compound could adversely affect fertility and pregnancy. Studies examining the toxicological profile of TCDD were beyond the scope of this study. Dams were euthanized when offspring were weaned on post natal day 21 (PND 21).
2.4. Monitoring of Pregnancy and Parturition
Adult offspring (10–12 weeks) of exposed (F1) and vehicle-exposed (control offspring) mice were mated to unexposed, control breeder males and monitored for pregnancy. Females were weighed prior to mating and again on E16.5. Females were monitored daily until delivery. Mice which did not achieve pregnancy after 3 positive plugs, or if vaginal plugs were never found (some triply-exposed mice), were placed with a single, proven breeder male for four weeks and observed for pregnancy. Mice which were unable to become pregnant were considered infertile and euthanized as described below (Section 2.5). Mice which became pregnant were allowed to continue until delivery and were similarly euthanized and examined after pups (F2) were weaned. Surviving F2 pups were mated in adulthood as were surviving F3 and a limited number of F4 mice and similarly examined as the F1 animals.
Parturition in C57bl/6 mice normally occurs 19.5 days after identification of a vaginal plug with little variation if the timing of mating is accurate [26]. However, it is important to note that pregnancy nomenclature varies by laboratory; thus, within the literature, identification of the vaginal plug has been denoted E0, E0.5 or E1 with term pregnancy occurring on E19.5, E20 or E20.5, respectively. As stated above, for studies described herein, the day the vaginal plug was identified was considered E0.5 and parturition expected on E20. Preterm parturition in mice has been defined as spontaneous labor 12–24 hrs prior to term [27–28]. For our studies, we used the more stringent definition for PTB (spontaneous delivery more than 24 hrs prior to term). All mice were monitored twice daily for timing of delivery of the first pup.
2.5 Lipopolysaccharide Exposure
LPS, obtained from Enzo Life Sciences, was diluted in sterile phosphate-buffered saline (PBS; to a volume of about 200µL/dose) to achieve 200µg/kg. Pregnant mice were weighed and subjected to isofluorane anesthesia prior to administering of diluted LPS by intraperitoneal injection using a tuberculin syringe (dosage administered between 1400 and 1500 hours CST on E16.5). Control mice were similarly weighed and anesthetized and provided 200µL sterile PBS only by intraperitoneal injection.
2.6 Euthanasia and Collection of Tissues
Mice which were unable to achieve pregnancy were euthanized by cervical dislocation under anesthesia at 16–18 weeks of age. Adult dams were similarly euthanized immediately after pups were weaned, typically at about 18 weeks of age. At the time of euthanasia, necropsy was performed and relevant organs removed, including uteri and ovaries. Tissues were formalin-fixed and paraffin-embedded for microscopic analysis and subjected to standard H and E staining.
2.7 Immunohistochemistry
Immunohistochemical localization of progesterone receptor (PR) was conducted using a commercially available antibody (BD Biosciences, San Jose, CA), a universal biotinylated secondary antibody and streptavidin-HRP (DAKO LSAB®+ Kit, DakoCytomation California Inc, Carpinteria, CA) according to the manufacturer’s instructions. In brief, formalin-fixed paraffin-embedded uterine sections (5µm) were deparaffinized, hydrated then subjected heat-mediated antigen retrieval in 10mM sodium citrate buffer solution (pH 6.0). Endogenous peroxidase activity was quenched by immersing sections in 3% H2O2 for 12 minutes followed by incubation in 3% bovine serum albumin in PBS containing 0.05% Tween-20 (PBST) for 1 hour at room temperature (RT) in order to block nonspecific reaction. Sections were then incubated with primary antibody (2µg/ml) diluted with blocking solution overnight at 4°C. Next, sections were washed using PBST then incubated with biotinylated MultiLink secondary antibody (anti-goat, anti-mouse and anti-rabbit immunoglobulins) for 30 minutes at RT. After rinsing with PBST, the sections were covered with streptavidin-horseradish peroxidase complex for 15 minutes at RT. The reaction was visualized using 3-3′diaminobenzidene (DAB) chromagen solution (Vector Laboratories, Burlingame, CA) resulting in a brown precipitate. The negative control sections were incubated only with the secondary antibody to verify the specificity of immunostaining; no positive staining was observed (data not shown).
2.8 PCR Amplification for Mouse Parvovirus Detection
Tissue from the small intestine was collected at the time of animal sacrifice and frozen at −20°C until use. DNA was extracted from frozen samples using a Qiagen DNeasy Kit (Qiagen, Valenica, CA, USA). The PCR amplification reaction was performed in a 25µl volume in a TECHNE GENUIS thermocycler using the following primer set: Forward primer, 5′-GCAGCAATGATGTAACTGAAGCT-3′, Reverse primer, 5′-CCATCTGCCTGAATCATAGCTTAA-3′. The conditions of the PCR were 30 repetitive cycles of denaturing (94°C for 15 seconds), annealing (60°C for 15 seconds) and extending (72°C for 25 seconds), followed by a final elongation step (72°C for 10 minutes). PCR products amplified from mouse tissues were subjected to electrophoresis on a 2% agarose gel followed by ethidium bromide staining and visualization by UV light. The predicted product size of MPV was 260 base pairs. All PCR assays included positive and negative controls.
3. Results
3.1 Infertility and Pregnancy Outcomes Across Multiple Generations
We had previously identified a near complete loss of progesterone receptor-A (PR-A) and PR-B expression in mice exposed to TCDD at three intervals of reproductive tract development and a less marked reduction in PR protein expression following a single or dual exposure [19]. Since PR is required for establishment and maintenance of pregnancy in women and mice [29–31], in the current study, we examined the ability of similarly exposed animals to achieve pregnancy. As shown in Table 1, 44% of mice exposed to TCDD only once (in utero) and 66% of mice exposed both in utero and at 4 weeks of age demonstrated an ability to establish pregnancy. None of the mice which were exposed to TCDD at three time-points during development (in utero, 4 weeks and 9 weeks) ever exhibited external signs of pregnancy (weight gain, nipple prominence).
Table 1.
Impact of Developmental TCDD Exposure on Reproductive Outcomes in Adult C57bl/6 Mice During a Mouse Parvovirus Outbreak
| Mouse Exposure History generation | Pregnancy Rate | Pregnancy Outcome | |
|---|---|---|---|
| Full-term | Preterm | ||
| Vehicle Control1 | |||
| conF1 | 15/15 (100%) | 15/152 | 0/15 |
| conF2 | 10/10 (100%) | 10/10 | 0/10 |
| conF3 | 8/8 (100%) | 8/8 | 0/8 |
| conF4 | 12/12 (100%) | 12/12 | 0/12 |
| TCDD in utero3 | |||
| F1 | 7/16 (44%) | 1/7 | 6/7 |
| F2 | 3/7 (43%) | 2/3 | 1/3 |
| F3 | 5/9 (55%) | 3/5 | 2/5 |
| TCDD in utero + prepubertal4 | |||
| F1 | 10/15 (66%) | 4/11 | 7/11 |
| F2 | 2/7 (29%) | 1/2 | 1/2 |
| F3 | 3/6 (50%) | 2/3 | 1/3 |
| F4 | 6/8 (75%) | 5/6 | 1/6 |
| TCDD in utero + prepubertal + puberty5 | 0/5 (0%) | N/A | |
Pregnant mice were exposed to corn oil vehicle (control) on E15.5 and control offspring (conF1 mice) mated at 10–12 weeks of age. Offspring of conF1 mice (conF2 mice) were mated at a similar age, as were the conF3 and conF4 generations of mice.
Only a subset of control F1–F3 offspring were used to obtain additional generations of unexposed mice.
Pregnant mice were exposed to 10 ug/kg TCDD in corn oil vehicle on E15.5 and singly exposed offspring (F1 mice) were mated at 10–12 weeks of age. Offspring of F1 mice (F2 mice) were mated at a similar age, as were the F3 generation.
Pregnant mice were exposed to 10 ug/kg TCDD in corn oil vehicle on E15.5 and offspring were additionally exposed to 10 ug/kg TCDD in corn oil vehicle, resulting in dually-exposed F1 mice (F1); mice were mated at 10–12 weeks of age. Offspring of F1 mice (F2 mice) were mated at a similar age, as were the F3 and F4 generations of mice.
Pregnant mice were exposed to 10 ug/kg TCDD in corn oil vehicle on E15.5 and offspring were additionally exposed to 10 ug/kg TCDD in corn oil vehicle at 4 weeks of age and at 9 weeks of age, resulting in triply exposed F1 mice (F1). Adult mice were mated at 10–12 weeks of age; no pregnancies were observed.
All control F1 mice (conF1, exposed only to vehicle) were able to become pregnant and delivered at term on E20. Singly- or dually-exposed F1 mice which were able to achieve pregnancy frequently delivered early, most commonly on E17.5, but also on E17 and E18; resulting in high perinatal mortality. Surviving F2 female mice in each exposure group were mated to unexposed breeder males at 12 weeks of age. As shown in Table 1, rates of infertility in F2 mice descended from singly-exposed F1 mice was similar to that of their mothers (43% compared to 44%). However, mice whose mothers were exposed twice (in utero and at 4 weeks of age) fared worse with regard to fertility (29% fertility in F2 mice compared to 66% in F1 mice which were dually-exposed). The dramatic further reduction in fertility among F2 mice may be a reflection of the impact of TCDD exposure on F1 germ cells (which would ultimately become the F2 mice). The limited number of offspring of singly or dually-exposed F1 mice which were able to become pregnant limits our ability to fully assess the incidence of PTB in the F2 generation. Nevertheless, 33% of pregnant mice descended from the singly exposed group delivered preterm while one of only two pregnant F2 mice in the dual exposure group delivered preterm.
Offspring of singly or dually exposed F2 mice, the F3 generation, also exhibited infertility and PTB compared to F3 mice descended from control mice (Table 1). These data are of particular importance since the F3 mice are the first generation of animals which did not have a direct exposure to TCDD and thus suggest that heritable epigenetic alterations have been induced by ancestral exposure to this toxicant. Similarly, offspring of F3 mice (F4), descended from dually exposed F1 mice, continued to exhibit some degree of both infertility and incidence of PTB, further supporting an epigenetic consequence of developmental TCDD exposure (Table 1).
3.2 PR Expression in adult mice subsequent to developmental TCDD exposure
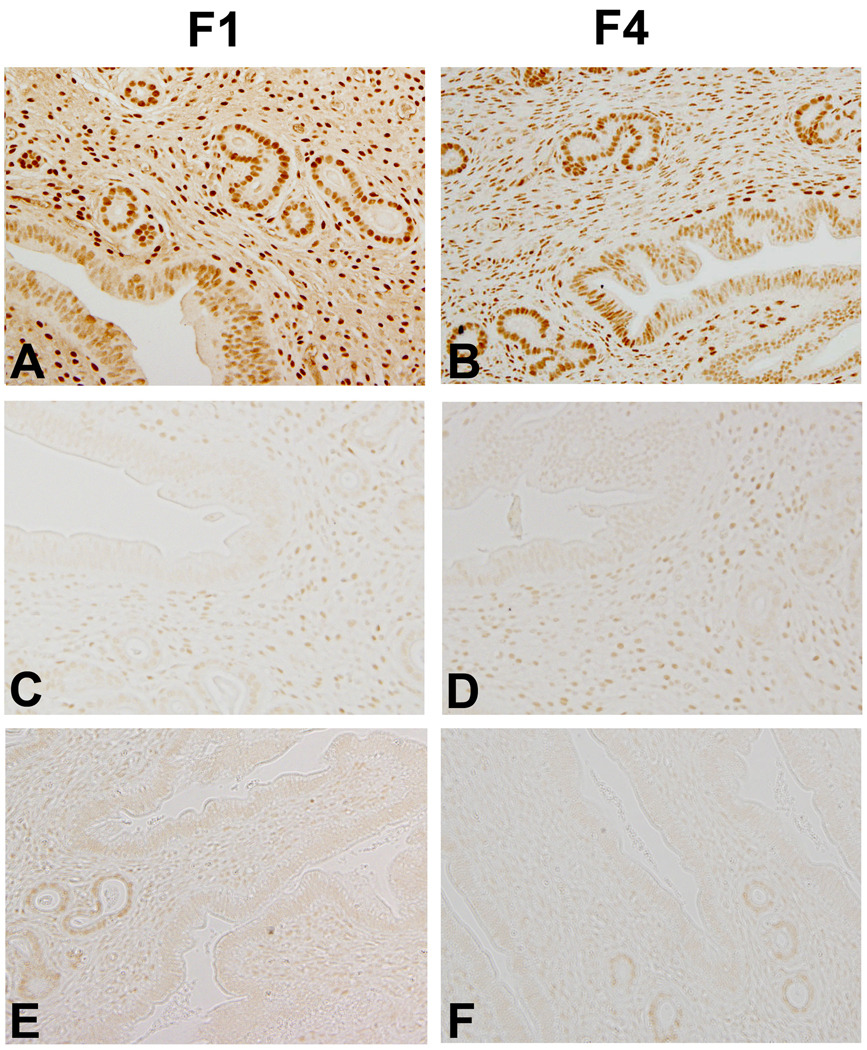
We previously described a progressive loss of both PR-A and PR-B proteins within the uteri of TCDD-exposed F1 mice as the number of exposures increased [19]. For the current study, uteri were analyzed by immunohistochemistry using an antibody which detects both PR-A and PR-B isoforms. As previously reported, we found that first generation control mice, which were exposed only to corn oil during development, exhibit abundant PR expression within both the uterine glands and stroma (Figure 1A). Importantly, F4 control mice which were descended from corn oil exposed control F1 mice, also exhibit strong immunostaining for PR (Figure 1B). In contrast, uteri from infertile F1 mice which were exposed to TCDD in utero exhibit a greatly reduced PR expression (Figure 1C) which was also apparent in the F4 generation (Figure 1D). Similarly, uteri from F1 mice, which were exposed to TCDD both in utero and before puberty, exhibit minimal PR immunostaining (Figure 1E) which also persisted in F4 infertile mice (Figure 1E). In contrast to infertile mice, fertile littermates euthanized during estrous after weaning of pups exhibited a variable, but generally robust, pattern PR of expression, regardless of exposure status or generation (data not shown).
Figure 1. Immunolocalization of PR in Uteri of Adult Mice.
(A) Vehicle-exposed control F1 (conF1) mouse euthanized during estrous exhibits abundant PR immunolocalization; (B) PR immunolocalization of a conF4 mouse descended from a conF1 mouse also reveals abundant PR immunostaining. (C) PR immunolocalization in an infertile F1 female exposed to TCDD in utero only demonstrates reduced stromal and epithelial cell PR expression. (D) PR immunostaining of a uterus from an infertile F4 mouse, descended from a fertile F1 mouse which was exposed to TCDD in utero. (E) PR immunolocalization in the uteri of an infertile F1 female exposed to TCDD in utero and just prior to puberty reveals minimal PR expression in the stromal and epithelial compartments. (F) PR immunolocalization in the uterus infertile F4 female descended from a dually-exposed F1 female also exhibits a greatly reduced expression of PR protein in all compartments. Original magnification, 20×. Images are representative of results from at least four mice per group.
3.3 Impact of Mouse Parvovirus
Late in the studies described above, we were informed by Vanderbilt’s Department of Animal Care that our colony was contaminated with mouse parvovirus (MPV). MPV occurs commonly in mouse colonies and does not cause overt disease in these animals [32–33]. Although this latent infection was not expected to significantly impact our reproductive outcome study, we established a new mouse colony in an MPV-free facility and repeated a subset of our studies. Furthermore, we began conducting routine screenings of our mice for MPV infection (data not shown) in order to eliminate this confounder.
As shown in Table 2, F1 mice which were exposed in utero to TCDD in the absence of MPV contamination exhibited a similar rate of infertility as F1 mice presumed to be infected with this virus (Table 1). In contrast, the rate of PTB among the mice known to be free of MPV infection was greatly reduced compared to those mice exposed to MPV (36% versus 86% PTB rate among singly-exposed F1 mice, respectively). Moreover, mice which delivered early in the absence of MPV exposure did not deliver more than 36 hours early compared to mice in the MPV-infected group, which delivered up to 72 hours early.
Table 2.
Impact of Developmental TCDD Exposure on Reproductive Outcome in MPV-free C57bl/6Mice over Multiple Generations
| Exposure | Pregnancy Rate | Pregnancy Outcome | |
|---|---|---|---|
| Full-term | Preterm | ||
| Vehicle Control1 | |||
| conF1 | 10/10 (100%) | 10/102 | 0/10 |
| conF3 | 12/12 (100%) | 12/12 | 0/12 |
| TCDD in utero3 | |||
| F1 | 11/28 (39%) | 7/11 | 4/11 |
| F3 | 8/14 (57%) | 6/8 | 2/8 |
Pregnant mice were exposed to corn oil vehicle (control) on E15.5 and control offspring (cF1 mice) mated at 10–12 weeks of age. Offspring of conF1 mice (conF2 mice) were mated at a similar age, as were the conF3 mice.
Only a subset of conF1–F3 offspring were used to obtain additional generations of unexposed mice.
Pregnant mice were exposed to 10 ug/kg TCDD in corn oil vehicle on E15.5 and singly exposed offspring (F1 mice) were mated at 10–12 weeks of age. Offspring of singly exposed F1 mice (F2 mice) were mated at a similar age, as were the F3 mice.
F3 mice, descended from F1 mice which were exposed in utero to TCDD were also examined for pregnancy outcomes in the absence of MPV infection. Similar to the data in the F1 mice described above, the infertility rates of F3 mice descended from singly exposed F1 mice in the absence of MPV were markedly similar to the pregnancy rate of F3 mice during the MPV outbreak (57% vs 55%, respectively) while the PTB rate was reduced in the MPV-free mice (25%) compared to the mice presumed infected with MPV (40%) (Tables 1 and 2).
Importantly, control mice (conF1–conF3) never delivered prior to E20, regardless of the presence or absence of MPV exposure, indicating that the presence of this virus alone did not affect gestational length. These data demonstrate an important relationship between developmental TCDD exposure and a weakened resistance to an inflammatory challenge mediated by a chronic viral infection. Furthermore, our data suggest that an increased sensitivity to inflammation, resulting from a single in utero exposure to TCDD, can also be transmitted through multiple generations.
3.4 Impact of TCDD Exposure on LPS-Induced PTB
In order to further examine a potential relationship between developmental TCDD exposure and an increased sensitivity to inflammation, we exposed MPV-free mice to an alternate inflammatory stressor. LPS, the major component of the outer membrane of gram negative bacteria, has been used for many years for experimental induction of inflammation-associated PTB [34,35]. We previously determined the lowest intraperitoneal dose of LPS which induces PTB in control C57BL/6 mice is 250µg/kg and that doses below this amount have no apparent adverse affect on length of pregnancy or health of pups [36]. In the current study, in order to determine whether or F1 mice with a history of developmental TCDD exposure exhibited a greater sensitivity to inflammation compared to conF1 mice, we challenged MPV-free mice with a low dose of LPS during late pregnancy. Specifically, we subjected conF1 mice and F1 mice with a history of in utero TCDD exposure to 200µg/kg LPS on E16.5 of gestation and monitored all animals for timing of delivery. As shown in Table 3, control mice provided PBS only or 200µg/kg LPS delivered healthy pups at term. In contrast, 100% of pregnant mice with a history of in utero TCDD exposure (F1) delivered within 16 hours of LPS treatment, further signifying an increased sensitivity to an inflammatory stressor among these mice.
Table 3.
Impact of LPS-Induced Preterm Birth in Adult C57bl/6 Mice with a History of Developmental TCDD Exposure
| Mouse Phenotype and Treatment | Pregnancy Rate | Pregnancy Outcome | |
|---|---|---|---|
| Full-Term | Preterm | ||
| Vehicle Control + 200 uL PBS1 | |||
| conF1 | 7/7 (100%) | 7/7 | 0/7 |
| Vehicle Control + 200 ug/kg LPS2 | |||
| conF1 | 5/5 (100%) | 5/5 | 0/5 |
| TCDD in utero + 200 ug/kg LPS3 | |||
| F1 | 3/6 (50%) | 0/3 | 3/3 |
Pregnant, control mice (conF1) were provided 200 uL PBS by intraperitoneal injection under anesthesia on E16.5 and monitored until delivery.
Pregnant, control mice (conF1) were provided 200 mg/kg LPS in 200 uL PBS by intraperitoneal injection under anesthesia on E16.5 and monitored until delivery.
Pregnant, adult mice which were exposed to TCDD during their own in utero development (F1), were provided 200 mg/kg LPS in 200 uL PBS by intraperitoneal injection under anesthesia on E16.5 and monitored until delivery.
4. Discussion
Developmental plasticity is the concept that an organism adapts epigenetically during in utero development to the anticipated external environment via cues available within the maternal-fetal microenvironment. As a consequence, the developing fetus is exquisitely sensitive to not only nutritional factors within the maternal circulation, but also to toxicants such as heavy metals, pesticides and endocrine-disrupting compounds [37]. Among these endocrine disruptors, TCDD and structurally-related compounds have been known to disturb steroid action related to maintenance of pregnancy following adult exposures [10, 38, 39]. However, early life exposure to this toxicant may have an even greater potential to alter reproductive success by disrupting reproductive tract development via epigenetic modification of critical genes. Thus, there is growing concern that prenatal and neonatal toxicant exposure may promote reproductive disorders which do not become apparent for many years [2] and perhaps persist for multiple generations.
Although a better understanding of human health issues related to early life toxicant exposure will be critical for making future policy decisions related to exposure risks, we must first utilize animal models that can reveal potential toxicant-associated epigenetic changes under controlled laboratory conditions. In this manner, using a murine model, we have previously shown that developmental exposure to TCDD leads to reduced uterine sensitivity to progesterone in adult female animals [19], suggesting that reproductive success may be compromised in exposed animals. In the current study, we have examined whether the TCDD-mediated loss of progesterone responsiveness in similarly exposed mice acts to disrupt either fertility or maintenance of pregnancy. In addition, we examined whether the phenotype previously observed in TCDD exposed F1 mice persists in future generations, which would suggest that early life toxicant exposure results in heritable epigenetic alterations that can negatively affect fertility.
Within our current TCDD exposure studies, we found that singly-exposed F1 mice and dually exposed F1 mice exhibited similar reproductive tract defects with regard to fertility and maintenance of pregnancy. Specifically, both treatment groups exhibited reduced fertility compared to control mice, with approximately half of the mice in each exposure group able to achieve visible pregnancy (Table 1). Infertility correlated with diminished uterine PR expression as determined by immunohistochemistry. Significantly, in the first series of studies we also found that the majority of TCDD exposed F1 mice which were able to become pregnant in either treatment group failed to sustain their pregnancy and delivered preterm. Although most pups appeared to be viable at birth, (due to direct visualization or the presence of a milkspot), all premature mice died within 24 hours of birth. Surviving (full-term) female offspring were mated at adulthood and were similarly examined for fertility and pregnancy outcome. With the exception of the F2 mice, which were descended from the fertile but dually TCDD-exposed dams, we found that each successive generation of animals exhibited similar fertility and pregnancy outcomes as the F1 mice. All toxicant exposed mice, or descendants of exposed mice, fared worse with regard to fertility or pregnancy outcomes compared to any generation of control mice.
Not surprisingly, F1 mice in the current study which were exposed to TCDD at multiple times during development (in utero, 4 weeks and 9 weeks) exhibited complete infertility, likely due in part to their profound loss of PR expression described within in our previous report [19]. However, it is also probable that multiple exposures to TCDD impact other reproductive organs and systems which can affect fertility. For example, defects in ovarian steroid synthesis [39] and neural development [40] have also been noted following exposure to this toxicant. For these reasons, our ongoing murine studies will focus primarily on the impact of a single in utero TCDD exposure since our data suggests that a single exposure more closely mimics the spectrum of reproductive phenotypes observed in TCDD-exposed human populations [11, 41].
Perhaps, the most profound observation of our study came following the realization that our colony had been unexpectedly exposed to mouse parvovirus (MPV). MPV is a DNA virus of the Parvoviridae family; although common in laboratory animal facilities, this virus does not usually cause overt reproductive failure [33,42]. Since the virus is shed in urine and feces, it is highly contagious and spreads quickly within a mouse colony. MPV infection is known to impede development of grafted tumors in mice [43], but little is currently known about the impact of this virus on reproductive tract function. Our data, presented herein, suggests that although MPV infection alone did not affect fertility or pregnancy outcomes, this virus dramatically affected the length of pregnancy in mice with a history of developmental toxicant exposure.
Similar to MPV infections in mice, chronic viral infection is common among humans; however, the inflammatory stress induced by such infection may have little impact on the length of pregnancy in women with a robust responsiveness to progesterone at the maternal-fetal interface. Our MPV data, albeit unintentional, suggests that the TCDD-mediated decrease in progesterone responsiveness may heighten sensitivity to inflammation, leading to PTB in mice additionally exposed to this virus. Specifically, the incidence of PTB among singly-exposed F1 mice in the presence and absence of MPV was 86% and 36%, respectively. Thus, we surmise that the higher incidence of PTB observed in the first study was due to the combination of increased sensitivity to inflammation in the presence of an underlying, occult inflammatory stressor (MPV). In order to explore this hypothesis, we exposed pregnant, virus-free control (conF1) and virus-free TCDD exposed F1 mice to a low dose of LPS that would not independently affect pregnancy length. As expected, control mice were resistant to this inflammatory stressor while all mice with a history of developmental toxicant exposure delivered within 16 hours of LPS treatment. Although speculative at this juncture, humans commonly are infected with numerous viruses that do not cause reproductive failure (i.e., herpes simplex [cold sores] and herpes zoster [shingles]); thus a dormant viral infection or other minor stressor (smoking, depression, allergies) might be risk factors only in susceptible women (i.e., with a genetic polymorphism or history of toxicant exposure).
The rate of PTB in women in industrialized countries continues to increase despite better health care and patient awareness. Our data supports the possibility of environmental factors which lead to reduced endometrial progesterone responsiveness and an increased sensitivity to inflammation as a risk for reproductive tract failure. More specifically, known risk factors for PTB among women (e.g. stress, dental caries, asthma) may only truly be risk factors for women with a heightened sensitivity to inflammation. Although our study did not assess the potential role of genetic polymorphisms, our data certainly supports a possible role of gene-environment interactions in the loss of reproductive tract function observed in certain women. Understanding the impact of “minor” stressors on inflammatory processes which lead to reproductive dysfunction, such as PTB, should allow development of better management strategies for both clinicians and patients.
Acknowledgements
We gratefully acknowledge Dr. Tianbing Ding, Ms. Melinda McConaha, Ms. Dana Glore, Dr. Jennifer Herington and Ms. Ashley Emerson for assistance with the studies described herein and the preparation of this manuscript.
This work was supported by NIEHS #5R01ES14942 and The Endometriosis Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heindel JJ. Role of exposure to environmental chemicals in the developmental basis of reproductive disease and dysfunction. Semin Reprod Med. 2006;24:168–177. doi: 10.1055/s-2006-944423. [DOI] [PubMed] [Google Scholar]
- 3.Anway MD, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors. Endocrinology. 2006;47:S43–S49. doi: 10.1210/en.2005-1058. [DOI] [PubMed] [Google Scholar]
- 4.Anway MD, Skinner MK. Epigenetic programming of the germ line: effects of endocrine disruptors on the development of transgenerational disease. Reprod Biomed Online. 2008;16:23–25. doi: 10.1016/s1472-6483(10)60553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skinner MK. Endocrine disruptors and epigenetic transgenerational disease etiology. Pediatr Res. 2007;61:48R–50R. doi: 10.1203/pdr.0b013e3180457671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hombach-Klonisch S, Pocar P, Kietz S, Klonisch T. Molecular actions of polyhalogenated arylhydrocarbons (PAHs) in female reproduction. Curr Med Chem. 2005;12:599–616. doi: 10.2174/0929867310504050599. [DOI] [PubMed] [Google Scholar]
- 7.Bruner-Tran KL, Ding T, Osteen KG. Dioxin and endometrial progesterone resistance. Semin Reprod Med. 2010;28:59–68. doi: 10.1055/s-0029-1242995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zook D, Rappe C. Environmental sources, distribution, and fate of polycholorinated dibenzodioxins, dibenzofurans and related organochlorines. In: Schecter A, editor. Dioxins and Health. New York: Plenum Press; 1994. pp. 79–113. [Google Scholar]
- 9.McNulty WP. Toxicity and fetotoxicity of TCDD, TCDF and PCB isomers in rhesus macaques (Macaca mulatta) Environ Health Perspect. 1985;60:77–88. doi: 10.1289/ehp.856077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sever LE, Arbuckle TE, Sweeney A. Reproductive and developmental effects of occupational pesticide exposure: the epidemiologic evidence. Occup Med. 1997;12:305–325. [PubMed] [Google Scholar]
- 11.Birnbaum LS, Tuomisto J. Non-carcinogenic effects of TCDD in animals. Food Addit Contam. 2000;17:275–288. doi: 10.1080/026520300283351. [DOI] [PubMed] [Google Scholar]
- 12.Le TN, Johansson A. Impact of chemical warfare with agent orange on women's reproductive lives in Vietnam: a pilot study. Reprod Health Matters. 2001;9:156–164. doi: 10.1016/s0968-8080(01)90102-8. [DOI] [PubMed] [Google Scholar]
- 13.Birnbaum LS. The mechanism of dioxin toxicity: relationship to risk assessment. Environ Health Perspect. 1994;102:157–167. doi: 10.1289/ehp.94102s9157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schecter A, Wallace D, Pavuk M, Piskac A, Papke O. Dioxins in commercial United States baby food. J Toxicol Environ Health A. 2002;65:1937–1943. doi: 10.1080/00984100290071450. [DOI] [PubMed] [Google Scholar]
- 15.Harrad S, Wang Y, Sandaradura S, Leeds A. Human dietary intake and excretion of dioxin-like compounds. J Environ Monit. 2003;5:224–228. doi: 10.1039/b211406b. [DOI] [PubMed] [Google Scholar]
- 16.Pompa G, Caloni F, Fracchiolla ML. Dioxin and PCB contamination of fish and shellfish: assessment of human exposure. Review of the international situation. Vet Res Commun. 2003;27:159–167. doi: 10.1023/b:verc.0000014134.23782.10. [DOI] [PubMed] [Google Scholar]
- 17.Domingo JL, Bocio A. Levels of PCDD/PCDFs and PCBs in edible marine species and human intake: a literature review. Environ Int. 2007;33:397–405. doi: 10.1016/j.envint.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Dewailly E, Ryan JJ, Laliberte′ C, Bruneau S, Weber JP, Gingras S, et al. Exposure of remote maritime populations to coplanar PCBs. Environ Health Perspect. 1994;102:205–209. doi: 10.1289/ehp.94102s1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nayyar T, Bruner-Tran KL, Piestrzeniewicz-Ulanska D, Osteen KG. Developmental exposure of mice to TCDD elicits a similar uterine phenotype in adult animals as observed in women with endometriosis. Reprod Toxicol. 2007;23:326–336. doi: 10.1016/j.reprotox.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Virgo BB, Bellward GD. Serum progesterone levels in the pregnant and postpartum laboratory mouse. Endocrinology. 1974;95:1486–1490. doi: 10.1210/endo-95-5-1486. [DOI] [PubMed] [Google Scholar]
- 21.Lydon JP, DeMayo FJ, Conneely OM, O'Malley BW. Reproductive phenotpes of the progesterone receptor null mutant mouse. J Steroid Biochem Mol Biol. 1996;56:67–77. doi: 10.1016/0960-0760(95)00254-5. [DOI] [PubMed] [Google Scholar]
- 22.Brown AG, Leiter RS, Strauss JF., 3rd Mechanisms underlying "functional" progesterone withdrawal at parturition. Ann N Y Acad Sci. 2004;1034:36–49. doi: 10.1196/annals.1335.004. [DOI] [PubMed] [Google Scholar]
- 23.Druckmann R, Druckmann MA. Progesterone and the immunology of pregnancy. J Steroid Biochem Mol Biol. 2005;97:389–396. doi: 10.1016/j.jsbmb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Miniero R, De Felip E, Ferri F, di Domenico A. An overview of TCDD half-life in mammals and its correlation to body weight. Chemosphere. 2001;43:839–844. doi: 10.1016/s0045-6535(00)00442-2. [DOI] [PubMed] [Google Scholar]
- 25.Vogel CF, Zhao Y, Wong P, Young NF, Matsumura F. The use of c-src knockout mice for the identification of the main toxic signaling pathway of TCDD to induce wasting syndrome. J Biochem Mol Toxicol. 2003;17:305–315. doi: 10.1002/jbt.10096. [DOI] [PubMed] [Google Scholar]
- 26.Lanman JT, Seidman L. Length of Gestation in Mice Under a 21-Hour Day. Biol Reprod. 1977;17:224–227. doi: 10.1095/biolreprod17.2.224. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Xie H, Dey SK. Loss of Cannabinoid Receptor CB1 Induces Preterm Birth. PLoS ONE. 2008;3(10):e3320. doi: 10.1371/journal.pone.0003320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roizen JD, Asada M, Tong M, Tai HH, Muglia LJ. Preterm birth without progesterone withdrawal in 15-hydroxyprostaglandin dehydrogenase hypomorphic mice. Mol Endocrinol. 2008;22:105–112. doi: 10.1210/me.2007-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorivodsky M, Torchinsky A, Zemliak I, Savion S, Fein A, Toder V. TGF beta 2 mRNA expression and pregnancy failure in mice. Am J Reprod Immunol. 1999;42:124–133. [PubMed] [Google Scholar]
- 30.Clark DA, Croitoru K. TH1/TH2,3 imbalance due to cytokine-producing NK, gammadelta T and NK-gammadelta T cells in murine pregnancy decidua in success or failure of pregnancy. Am J Reprod Immunol. 2001;45:257–265. doi: 10.1111/j.8755-8920.2001.450501.x. [DOI] [PubMed] [Google Scholar]
- 31.Meis PJ. 17 hydroxyprogesterone for the prevention of preterm delivery. Obstet Gynecol. 2005;105:1128–1135. doi: 10.1097/01.AOG.0000160432.95395.8f. [DOI] [PubMed] [Google Scholar]
- 32.McKisic MD, Lancki DW, Otto G, et al. Identification and propagation of a putative immunosuppressive orphan parvovirus in cloned T cells. J Immunol. 1993;150:419–428. [PubMed] [Google Scholar]
- 33.Jacoby RO, Ball-Goodrich LJ, Besselsen DG, McKisic MD, Riley LK, Smith AL. Rodent parvovirus infections. LabAnim Sci. 1996;46:370–380. [PubMed] [Google Scholar]
- 34.Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol. 2003;163:2103–2111. doi: 10.1016/S0002-9440(10)63567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elovitz M, Wang Z. Medroxyprogesterone acetate, but not progesterone, protects against inflammation-induced parturition and intrauterine fetal demise. Am J Obstet Gynecol. 2004;190:693–701. doi: 10.1016/j.ajog.2003.10.693. [DOI] [PubMed] [Google Scholar]
- 36.Bruner-Tran KL, Osteen KG. Omega-3 Fatty Acids Prevent Inflammation-Mediated Preterm Birth in a Mouse Model; 57th Annual Society of Gynecologic Investigation Meeting; Orlando, Fl: 2010. [Google Scholar]
- 37.Environmental Working Group. Body Burden—The Pollution in Newborns: A benchmark investigation of industrial chemicals, pollutants and pesticides in umbilical cord blood. [Internet] [Cited; Aug 6, 2010] Available from: http://www.ewg.org/reports/bodyburden2/execsumm.php.
- 38.Heimler I, Rawlins RG, Owen H, Hutz RJ. Dioxin perturbs, in a dose- and time-dependent fashion, steroid secretion, and induces apoptosis of human luteinized granulosa cells. Endocrinology. 1998;139:4373–4379. doi: 10.1210/endo.139.10.6264. [DOI] [PubMed] [Google Scholar]
- 39.Gregoraszczuk EL, Zabielny E, Ochwat D. Aryl hydrocarbon receptor (AhR)-linked inhibition of luteal cell progesterone secretion in 2,3,7,8-tetrachlorodibenzo-p-dioxin treated cells. J Physiol Pharmacol. 2001;52:303–311. [PubMed] [Google Scholar]
- 40.Kakeyama M, Tohyama C. Developmental neurotoxicity of dioxin and its related compounds. Ind Health. 2003;41:215–230. doi: 10.2486/indhealth.41.215. [DOI] [PubMed] [Google Scholar]
- 41.Eskenazi B, Warner M, Marks AR, Samuels S, Needham L, Brambilla P, et al. Serum dioxin concentrations and time to pregnancy. Epidemiology. 2010;21:224–231. doi: 10.1097/EDE.0b013e3181cb8b95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ball-Goodrich LJ, Johnson E. Molecular characterization of a newly recognized mouse parvovirus. J Virol. 1994;68:6476–6486. doi: 10.1128/jvi.68.10.6476-6486.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKisic MD, Paturzo FX, Smith AL. Mouse parvovirus infection potentiates rejection of tumor allografts and modulates T cell effector functions. Transplantation. 1996;61:292–299. doi: 10.1097/00007890-199601270-00022. [DOI] [PubMed] [Google Scholar]