Abstract
Pre-pandemic intravenous immunoglobulin (IVIG) and sera from Kawasaki disease (KD) patients treated with this IVIG were analyzed for 2009 H1N1-specific microneutralization and hemagglutination inhibition antibodies. All six different IVIG preparations tested had significant levels of cross-reactive specific antibody at a concentration of 2.0 g/dL of immunoglobulin. Sera from 18/19 of KD patients had significant increases of cross-reactive specific antibody after 2.0 g/kg of pre-pandemic IVIG. These results suggest a role for adjunctive IVIG therapy for severe and/or drug-resistant 2009 H1N1 virus and other highly antigenically drifted influenza strains, particularly in the immunocompromised.
Keywords: 2009 H1N1, influenza A, neutralizing antibody, intravenous immunoglobulin, Kawasaki disease
INTRODUCTION
H1N1 swine-origin influenza A virus (2009 H1N1) initiated a human pandemic in April, 2009.1 Although by the spring of 2010, the activity of this virus declined below pandemic levels in most parts of the world, it is likely to re-emerge during the next seasonal epidemic, as a substantial proportion of the population were not immunized with 2009 H1N1 monovalent vaccine. For example, in the U.S., only 40% of children between 6 months and 14 years of age were immunized.2 Interestingly, early in the pandemic, older individuals appeared to be relatively protected from severe disease from 2009 H1N1, with most infection-associated hospitalizations occurring in young adults (<49 years of age) and children.3 This contrasts with seasonal influenza epidemics, for which morbidity and mortality is concentrated in the elderly.4 The relative protection in older adults may be due to pre-existing cross-reactive neutralizing antibody from prior natural infection with H1N1, particularly with 1918 H1N1 and its derivatives,5 which caused seasonal outbreaks between 1918–1957. The formation of protective cross-reactive antibodies from natural infection with H1N1 is plausible, as the hemagglutinin glycoprotein of 2009 H1N1 and the 1918 H1N1 virus and its early derivatives are structurally similar,6 and hemagglutinin is a well-established target for antibody-mediated protection in humans.7
Given the existence of cross-reactive antibodies to 2009 H1N1 in a substantial number of adults, we reasoned that commercial intravenous immunoglobulin (IVIG) products produced prior to the 2009 pandemic, which combine plasma from thousands of adult donors, might contain significant levels of these antibodies. We tested IVIG preparations for cross-reactive microneutralization (MN) and hemagglutination inhibition (HI) antibody against 2009 H1N1, and we also determined if administration of high-dose IVIG to patients with Kawasaki Disease (KD) patients treated prior to 2009 significantly raised their serum titers of these antibodies.
MATERIALS AND METHODS
Immunoglobulin Preparations
IVIG solutions of 10% (g/dL) immunoglobulin concentration [Gamunex (3 lots), Talecris Biotherapeutics; Gammagard, Baxter Pharmaceuticals; Cytogam and Privigen, CSL Behring], all of which were produced prior to the emergence of 2009 H1N1 in April, 2009, were tested. These preparations were diluted in phosphate-buffered saline (PBS) to final concentrations of 1.0, 2.0, and 4.0 g/dL, which encompass the normal IgG concentration in human serum and the peak achievable serum IgG concentration after a 2.0 g/kg dose of IVIG (approximately 3.0 g/dL).8
Serum Samples
Sera were obtained from KD patients, aged 10 months to 10 years, treated in San Diego County, California, USA from December, 2007 to March, 2009 with IVIG (Gammagard), following parental informed consent and Institutional Review Board of the University of California at San Diego approval. One group of sera were collected immediately prior to and 1–3 days after KD patients received a single 2.0 g/kg dose of IVIG. A second group of sera were collected immediately prior to and 5–13 days after other KD patients received two 2.0 g/kg doses of IVIG. Aliquots of sera were frozen at −80°C until thawed for later analysis.
Viral Isolation and Propagation
The 2009 H1N1 influenza A strain, which was obtained from a de-identified primary clinical sample of bronchoalveolar lavage fluid, was grown in Madin-Darby canine kidney (MDCK) cells.9 This isolate was confirmed as 2009 H1N1 by the California State Department of Public Health using a reverse-transcriptase real-time polymerase chain reaction assay.
Hemagglutination inhibition and microneutralization assays
Hemagglutination inhibition (HI) and microneutralization (MN) assays were performed as previously described.5,10 For the HI assay, serum samples treated with receptor-destroying enzyme (Denka Seiken) were diluted 10-fold (vol/vol) in PBS from which serial two-fold dilutions were prepared. Diluted sera were mixed with 2009 H1N1 influenza, turkey red blood cells (0.5% vol/vol) were added (Rockland Immunochemicals), and hemagglutination was noted after 30 minutes of incubation. The HI titer was identified as the last dilution that prevented hemagglutination of red blood cells. For the MN assay, heat-inactivated samples were initially diluted 10-fold in PBS and mixed with live 2009 H1N1 influenza. MDCK infection was determined by an influenza A nucleoprotein ELISA using a mouse anti-nucleoprotein antibody (Millipore). The MN antibody titer was identified as the last serum dilution that prevents infection by 50% as measured by ELISA. For calculation of geometric mean titers (GMT), titers were expressed as reciprocal titers (e.g., 1:40 was expressed as 40) and an undetectable MN or HI titer was recorded as a reciprocal titer of 5 or one-half the lowest dilution tested.10 Both the HI and MN assays were validated with negative control serum from an unvaccinated, uninfected volunteer and with positive control serum from an individual who had been infected with H1N1.
Statistical Analysis
Paired, two-tailed Student’s t-test were used to compare: 1) MN and HI titers measured in the same IVIG preparation or serum sample; and 2) pre- and post-treatment MN or HI antibody titers for sera from the same individual. Pearson r values were used to calculate the correlation between MN and HI titers. Prism 5 software (GraphPad) was used for statistical calculations.
RESULTS
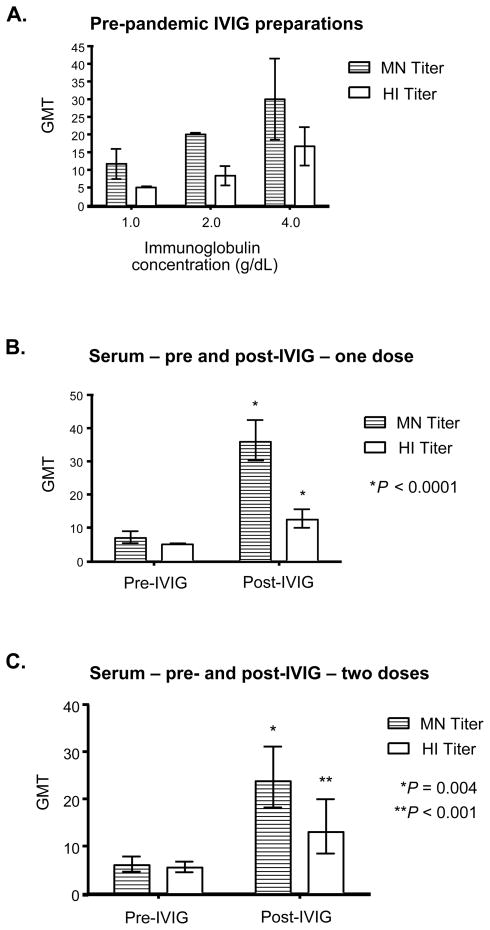
Six different commercial preparations of IVIG were tested for the presence of MN and HI titers. All IVIG preparations had detectable levels of MN antibody to 2009 H1N1 at the lowest concentration tested of 1.0 g/dL, which increased in a dose-dependent manner (Fig. 1A). The MN GMT of all preparations was 28.3 at the highest dilution tested of 4.0 g/dL, with some variation between different commercial brands (See Table, Supplementary Digital Content 1 for titers from individual IVIG preparations). All IVIG brands tested achieved a MN titer of 1:20 at 2.0 g/dL, a concentration that can be readily achieved with the high-doses of IVIG given in Kawasaki’s disease.8 Similarly, all IVIG preparations demonstrated dose-dependent increases in HI titers, with a HI GMT of 15.9 at the highest concentration tested of 4.0 g/dL, though there was variation between different lots of the same brand in addition to between brands. There was a significant correlation between the MN titer and HI titer of the IVIG samples at each concentration (Pearson r = 0.64, 95% confidence interval (CI) 0.25–0.85, P = 0.004), with the MN titer approximately two-fold higher than the HI titer.
FIGURE 1.
(A) MN and HI antibody titers against 2009 H1N1 in commercial preparations of IVIG. GMT of MN and HI titers against 2009 H1N1 were determined at three different concentrations. Error bars represent 95% CI. (B) MN and HI antibody titers against 2009 H1N1 before and after treatment one treatment of 2.0 g/kg of IVIG are shown using serum samples that were drawn 1–3 days apart. Error bars represent 95% CI. P values were determined using the two-tailed, paired Student’s t-test with respect to the pre-IVIG sera sample from the same subject. (C) GMT for both MN and HI assays against 2009 H1N1 at baseline, and after two treatments of 2.0 g/kg of IVIG, are shown using serum samples that were drawn 5 to 13 days apart following the second dose. Error bars represent 95% CI. P values were determined using the two-tailed, paired Student’s t-test with respect to the pre-IVIG sera sample from the same subject.
We next determined whether high-dose therapy using pre-pandemic produced IVIG raised the serum titer of HI and MN antibodies in patients. A single treatment of KD patients with 2.0 g/kg of IVIG increased MN titers against 2009 H1N1 in 18 out of 19 subjects (95%) when measured one to three days post-infusion. Similarly, HI titers also increased in 17/19 (89%) of subjects after one dose of IVIG (See Table, Supplementary Digital Content 2, individual patient titers). In KD patients who received a single dose of IVIG, both MN and HI titers increased significantly after treatment (Fig. 1B). MN GMT increased from 6.9 to 35.9 (p<0.0001), whereas HI GMT increased from 5.0 to 12.5 (P <0.0001). Again, as in the IVIG preparations, the GMT for MN antibody was approximately two times the titer of HI antibody in post-IVIG sera, and this difference was significant (P < 0.0001).
In KD patients receiving two doses of IVIG, there was also significant increase in MN and HI titers measured within 14 days after the second dose (Fig. 1C). All 8 subjects had increases in the MN titer while 7/8 (88%) of subjects had increases in the HI titer (See Table, Supplementary Digital Content 3 for individual patient titers). The MN GMT increased from 5.9 to 23.8 (P = 0.0005), whereas HA GMTs increased from 5.4 to 13.0 (P = 0.0038).
DISCUSSION
Although the 2009 H1N1 influenza A viral pandemic spread rapidly due to the lack of pre-existing immunity in much of the human population, older adults appeared to be relatively protected due to pre-existing cross-protective antibodies that were most likely generated in response to natural infection with 1918 H1N1 and its early derivatives.5 Here, we show that commercial preparations of IVIG, produced prior to the 2009 H1N1 pandemic, contain cross-reactive antibodies against 2009 H1N1 as assessed by both HI and MN assays. In addition, we found that administration of high-dose IVIG significantly increased the levels of cross-reactive HI and MN antibodies against 2009 H1N1. Thus, commercially available IVIG produced prior to the pandemic could potentially be used as an adjunctive treatment for 2009 H1N1 infection in severe cases or in patients with limitations in adaptive immunity. Since newer IVIG preparations will likely include plasma from donors who have been vaccinated for 2009 H1N1 or who were infected with 2009 H1N1, the HI and MN antibody titers will likely increase. However, our results indicate that such inclusion is not necessary for IVIG to provide substantial passive immunity to 2009 H1N1, and suggest that the approach of using existing IVIG preparations might also be considered in future influenza pandemics or if highly drifted strains circulate.
Although there has been no reported clinical experience with the use of passive antibodies in treating 2009 H1N1, there were many attempts to use convalescent blood products in treating acute influenza during the influenza pandemic in 1918. The therapeutic administration of blood products enriched in anti-influenza antibodies appeared to confer a survival advantage particularly if the treatment was given within the first four days of illness.11 In addition, convalescent plasma has been used anecdotally for severe H5N1 infection.12 Furthermore in animal models, therapeutic monoclonal anti-influenza IgG antibodies have been shown to clear influenza virus after infection in SCID mice, which lack B cells and T cells.13 Thus, it is plausible that the therapeutic administration of immunoglobulin containing neutralizing antibody against 2009 H1N1 could aid in halting the progression of infection as well as in viral clearance.
Passive immunotherapy for 2009 H1N1 influenza and other pandemic influenza A strains may be of particular importance in cases of anti-viral resistance. While the 2009 H1N1 virus has remained largely susceptible to the neuraminidase inhibitors, oseltamivir and zanamivir, there have been sporadic reports of oseltamivir-resistant strains. Resistance has been particularly frequent in hosts with impaired adaptive immunity who shed virus for prolonged periods,14 and for whom adjunctive therapy with IVIG could be particularly useful.
In summary, we have identified significant titers of cross-reactive antibody against 2009 H1N1 in commercial preparations of IVIG despite the low prevalence of pre-existing cross-reactive immunity in the general population. Administration of high-dose IVIG increased the serum titer of these antibodies, and this may be a useful adjunctive therapy in severe infections with 2009 H1N1 influenza, particularly in the immunocompromised, those who are refractory to neuraminidase inhibitor therapy, and immunologically naïve children.
Supplementary Material
Acknowledgments
We thank DeeAnna Scherrer (UCSD) and Kira Dionis (Stanford University) for technical support, Benjamin Pinsky, Stanford Clinical Microbiology Laboratory, for providing clinical samples, and Roshni Mathew and David Nguyen for critique of the manuscript.
Supported by grants from the National Institute of Allergy and Infectious Disease (NIAID) (K08-AI-079269 to D.K.H and U01-AI-074512 to D.B.L.), the National Heart, Lung, and Blood Institute (NHLBI) (R01 HL-69413 to J.C.B.), a Medimmune Research Development/Pediatric Infectious Disease Society Award (to D.K.H.), and the Jeffrey Modell Foundation (to D.B.L.).
References
- 1.Dawood FS, Jain S, Finelli L, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 2.Interim results: State-specific seasonal influenza vaccine coverage - United States, August 2009–January 2010. MMWR. 2010;59:477–484. [PubMed] [Google Scholar]
- 3.Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med. 2009;361:1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 4.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 5.Hancock K, Veguilla V, Lu X, et al. Cross-Reactive Antibody Responses to the 2009 Pandemic H1N1 Influenza Virus. N Engl J Med. 2009;361:1945–1952. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 6.Xu R, Ekiert DC, Krause JC, et al. Structural basis of preexisting immunity to the 2009 H1N1 pandemic influenza virus. Science. 2010;328:357–360. doi: 10.1126/science.1186430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Jong JC, Palache AM, Beyer WE, et al. Haemagglutination-inhibiting antibody to influenza virus. Dev Biol (Basel) 2003;115:63–73. [PubMed] [Google Scholar]
- 8.Lee KY, Han JW, Lee JS, Whang KT. Alteration of biochemical profiles after high-dose intravenous immunoglobulin administration in Kawasaki disease. Acta Paediatr. 2002;91:164–167. doi: 10.1080/080352502317285153. [DOI] [PubMed] [Google Scholar]
- 9.Webster R, Cox N, Stohr K. WHO Manual on Animal Influenza Diagnosis and Surveillance. World Health Organization; 2002. [Google Scholar]
- 10.Rowe T, Abernathy RA, Hu-Primmer J, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999;37:937–943. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145:599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 12.Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med. 2007;357:1450–1451. doi: 10.1056/NEJMc070359. [DOI] [PubMed] [Google Scholar]
- 13.Palladino G, Mozdzanowska K, Washko G, Gerhard W. Virus-neutralizing antibodies of immunoglobulin G (IgG) but not of IgM or IgA isotypes can cure influenza virus pneumonia in SCID mice. J Virol. 1995;69:2075–2081. doi: 10.1128/jvi.69.4.2075-2081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oseltamivir-resistant novel influenza A (H1N1) virus infection in two immunosuppressed patients - Seattle, Washington, 2009. MMWR. 2009;58:893–896. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.