Abstract
Objective
Cervical Dystonia (CD) lacks an objective quantitative measure. Electrical impedance myography (EIM) is a non-invasive assessment method sensitive to changes in muscle structure and physiology. We evaluate the potential role of EIM in quantifying CD, hypothesizing that patients would demonstrate differences in the symmetry of muscle electrical resistance compared to controls, and that this asymmetry would decrease after botulinum neurotoxin (BoNT) treatment.
Methods
EIM was performed on the sternocleidomastoid (SCM) and cervical paraspinal (PS) muscles of CD patients and age-matched controls. 50kHz Resistance was analyzed, comparing side-to-side asymmetry in patients and controls, and, in patients, before and after BoNT treatment.
Results
16 patients and 10 controls were included. Resistance asymmetry was on average 3-5 times higher in patients than controls. Receiver operating characteristic analysis demonstrated 91% accuracy of discriminating CD from normal. From pre-treatment to maximum BoNT effect, asymmetry decreased from 20.8 (13.9-26.1)% to 6.2 (3.1-9.9)% (SCM), and from 16.0(14.3-16.0)% to 8.4(7.0-9.2)% (PS), p<0.05 (median, interquartile range).
Conclusions
EIM effectively differentiates normal subjects from CD patients by revealing asymmetries in resistance values and detects improvement in muscle symmetry after treatment.
Significance
These results suggest that EIM, a painless, non-invasive measure, can provide a useful quantitative metric in CD evaluation and deserves further study.
Keywords: Dystonia, electrical impedance myography, diagnosis, botulinum toxin
Introduction
Cervical dystonia (CD) is the most common focal dystonia (Stacy, 2008). Prevalence estimates vary from 5.7/100000 (ESDE Collaborative, 2000) to 0.4% of the population (Jankovic et al., 2007). Widespread abnormal excitability in the brainstem, basal ganglia and cortical circuits is associated with CD (Tolosa and Marti, 2004; Singer and Velickovic, 2008). At muscular level, the end result is abnormal activation, with prominent EMG bursts and co-contraction of agonist and antagonist (Deuschl et al., 1992). This abnormal activation is almost always asymmetric side-to-side, resulting in prominent asymmetric head/neck/shoulder position and muscle hypertrophy. Muscle activation on EMG can be used to guide botulinum neurotoxin (BoNT) injections and to identify the most affected muscles, but not routinely for quantifying dystonia or its response to treatment.
The most effective therapy for CD remains BoNT injections (Simpson et al., 2008). Deep Brain Stimulation (DBS) therapy is another valid option for intractable CD, with less predictable effects (Ostrem and Starr, 2008; Pretto et al., 2008). Since effective therapies are available, objective measures are very important but generally lacking for CD monitoring and could play a role in more effectively tailoring therapy in a given patient or in clinical trials. To date, dystonia evaluation has been performed semi-quantitatively on a clinical basis, using rating scales. The Burke-Fahn-Marsden (BFM) scale (Burke et al., 1985) has been utilized in clinical and research contexts since the 1980s, and the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) (Consky et al., 1990; Comella et al., 1997) is designed specifically for CD.
Electrical impedance myography (EIM) is a relatively new, non-invasive technology that to date is primarily being studied in the evaluation of neuromuscular diseases, including myositis and amyotrophic lateral sclerosis (Tarulli et al., 2005; Rutkove, 2005; Rutkove et al., 2007). It is based on the application of low-intensity, high-frequency electrical current to localized areas of muscle using surface electrodes and measuring the resulting electrical voltages from which an impedance can be calculated (Rutkove et al., 2002). The two components of impedance are the resistance (R) and reactance (X). R is inversely proportional to the cross-sectional area of the conductor (the muscle), so it is expected to be sensitive to changes in the muscle volume as a result of hypertrophy and atrophy, but also to muscle composition (e.g., it will be influenced by the size of individual muscle fibers, the amount of free water, and the presence of connective tissue). X is inversely proportional to capacitance, but has a considerably more complex response to the status of the muscle, also being impacted by size and composition of the tissue. A third parameter can be calculated from the R and X values, the phase angle (θ), using the formula θ = arctan (X/R), which has been used as the major outcome measure to date in neuromuscular disease work (Rutkove, 2009; Rutkove et al., 2002; Tarulli et al., 2009B).
In this study we preliminarily evaluate the use of EIM in the quantification of muscle hypertrophy due to CD (and by extension illustrating the severity of CD) and its potential as an outcome measure with therapy. We do this by investigating the technique's ability to differentiate between subjects with and without CD and by assessing its ability to detect the effect of BoNT in a subgroup of patients with CD. Since asymmetric muscle hypertrophy is a major feature of CD and since electrical resistance is the value most closely related to muscle size, we hypothesized that patients with CD would demonstrate substantial differences in the symmetry of the electrical resistance of muscles compared to normal subjects and that this asymmetry would decrease after BoNT.
Methods
Study subjects: Patients were recruited from the therapeutic injections clinic in our Movement Disorders center on a consecutive basis. All patients had a diagnosis of CD made by a neurologist specializing in movement disorders; no specific attempt was made to include any one particular type (e.g., rotational, laterocollis, etc). In addition, 10 normal subjects without history of cervical dystonia or other movement disorders were also recruited. The study was approved by the Beth Israel Deaconess Medical Center Institutional Review Board and all subjects provided written informed consent.
EIM: Measurements were performed with either a commercially available Imp SFB7® (Impedimed, Inc, San Diego, CA) or a wide range lock-in amplifier (Signal Recovery Model 7280, Advanced Measurement Technology Inc., Oak Ridge, TN, USA) as previously described (Esper et al., 2006; Shiffman et al., 1999). The signal from the voltage electrode pair is fed into the input channel, and digital outputs representing R and X are transmitted to the analysis stage. We used disposable 5.5-mm wide × 9 cm long Ag/AgCl strip electrodes (Viasys Healthcare, Madison, WI 019-766400) cut to 2.25 cm in length as both current-injecting and voltage-sensing electrodes. For each muscle the electrodes were placed linearly, with the two current injecting electrodes outside and the two voltage sensing electrodes inside. Two muscle groups were studied: sternocleidomastoid (SCM) and the longitudinal paraspinal muscle group (PS), which includes semispinalis and the upper portion of trapezius. Specific locations for the placement of the electrodes were established based on the relation to bony landmarks. For SCM, the lowest electrode was placed 2 cm above the clavicle and the other 3 at 2 cm intervals above (see Figure 1). For PS, the lowest electrode was 2 cm lateral and 1 cm above the C7 spinous process, and the others above at 2 cm intervals. All recordings were performed with the subject's head in consistent positions. For dystonia patients, this was the “neutral”, most comfortable position that minimized the subjective strain. Both systems provide raw resistance (R) and reactance (X) values, though for this study we only used the measured muscle resistance. For both systems, although a spectrum of frequencies was obtained, for purposes of simplifying the analysis, only the 50 kHz data was used (Rutkove et al., 2006). The advantage of using this frequency is that other relatively inexpensive impedance devices perform measurements only at this one frequency and thus could also be utilized in future work.
BoNT injections: Six of the CD patients received BoNT injections using BoNT type A (Botox ®, Onabotulinum toxin A, Allergan) or type B (Myobloc ®, Rimabotulinum toxin B, Solstice) during the study. All patients had a perceived duration of benefit of 3 months, receiving regularly scheduled injections. The injections were performed based on anatomical markers and in accordance with accepted standards of care. EIM was performed at or near the estimated nadir of their symptoms a minimum of 3 months after the last BoNT injection and at the point of maximum benefit from BoNT therapy approximately 4 weeks after injection.
Data analysis: Percent side-to-side asymmetry was calculated for each muscle by subtracting the smaller from the larger resistance values and dividing by the larger of the values and multiplying by 100. Comparison of categorical values (i.e., sex distribution between groups) was made using Fisher's exact test. Given the small number of subjects in both groups, summary statistics and group comparisons were performed using non-parametric tests (Mann-Whitney and Wilcoxon signed rank test for unpaired and paired analyses, respectively), with alpha = 0.05, two-tailed. To assess the ability of side-to-side asymmetry to distinguish normal from CD patients, a receiver operating characteristic (ROC) analysis was completed for each muscle based on the calculated per cent side-to-side asymmetries (Zweig and Campbell, 1993). To test a possible effect of gender on the results, we checked for an interaction between disease group and gender. Univariate analyses using gender and dystonic status as covariates were performed for each of the two tested muscle groups. Data analysis was performed using SPSS ® software (SPSS, inc., Chicago, IL).
Figure 1. Measurement setup.
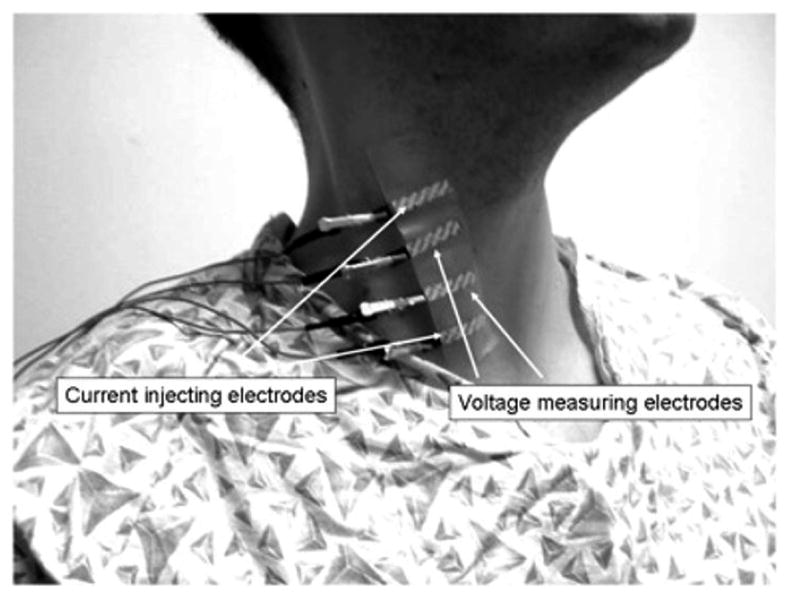
Electrical impedance myography being performed on right sternocleidomastoid. The two outer electrodes inject the electrical current; the two inner electrodes measure the resulting voltages, from which the impedance values are calculated.
Results
16 patients and 10 normal volunteer subjects were enrolled in the study. The median age and interquartile range (IQR) for CD patients was 51.5 (48.1-57.9) years, and for the normal volunteers 46.9 (42.3-52.7) years, a non-significant difference. 4 men and 12 women were included in the CD group, and 7 men and 3 women were included in the normal control group. This difference in sex distribution was significant (p = 0.042).
Table 1 provides median resistance data for bilateral SCM and for PS in each patient and the percent asymmetry expressed as the median, IQR. As shown, there was no significant difference between the raw R values for the CD patients and for healthy subjects. However, marked differences in the percent asymmetries of R were observed, with values being nearly 3-fold greater in the SCM and more than 5-fold greater in the PS in the CD patients as compared to the healthy subjects. Similarly, Figures 2a and b show receiver operating characteristic (ROC) analyses for SCM and PS respectively, demonstrating the ability of the resistance asymmetry alone to accurately discriminate CD patients from normal subjects, with both muscles providing a very high sensitivity and specificity, with an overall accuracy of approximately 91% (as calculated from the area under the ROC curve). No specific attempt was made to identify an abnormal side in these calculations; this issue is explored further in the discussion.
Table 1. Comparison of resistance values in healthy subjects and CD patients.
| Muscle | Resistance (ohms) Normal | Resistance (ohms) CD | Sig. | % resistance asymmetry Normal | % resistance asymmetry CD | Sig. |
|---|---|---|---|---|---|---|
| SCM | 37.3, 30.4-43.8 | 39.8, 33.8-50.4 | p = 0.166 | 7.5, 5.4-8.2 | 19.1, 12.9-29.0 | p < 0.001 |
| PS | 47.5, 38.9-65.4 | 57.0, 43.7-69.7 | p = 0.298 | 4.4, 1.4-5.5 | 23.0, 9.1-15.2 | p < 0.001 |
Data reported as median value, interquartile range.
Figure 2. ROC resistance plots.
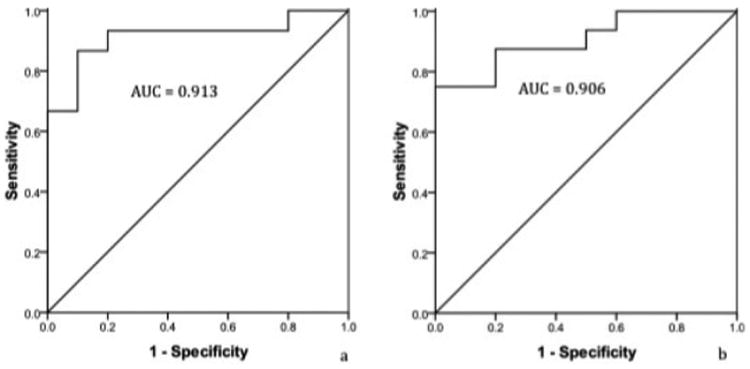
ROC plots of SCM resistance (A) and PS resistance (B). AUC, area under the curve or the accuracy of the test in discriminating normal subjects from CD patients.
Although there were a greater proportion of women in the CD group than in the control group, there was no significant difference between the sexes in the raw measured resistance values or in the calculated percent asymmetries. Moreover, we performed a multivariate analysis which confirmed a significant relationship between resistance asymmetry and disease status (beta = 0.55, p = 0.006) but not between resistance asymmetry and gender (beta = .204, p = 0.272). No interaction between disease group and gender was found (p = 0.86 for SCM and p = 0.57 for PS).
Of the six patients who underwent impedance measurements before and after BoNT injection, baseline resistance asymmetry for SCM was 20.8 (13.9-26.1)% and for PS was 16.0(14.3-16.0)%. After treatment with BoNT, these asymmetries decreased in each patient, changing to 6.2 (3.1- 9.9)% in SCM (p = 0.028) and 8.4(7.0-9.2)% in PS (p = 0.043). Figure 3 provides a graphical presentation of the effect of BoNT on the asymmetry of these muscles.
Figure 3. Before and after BoNT asymmetry.
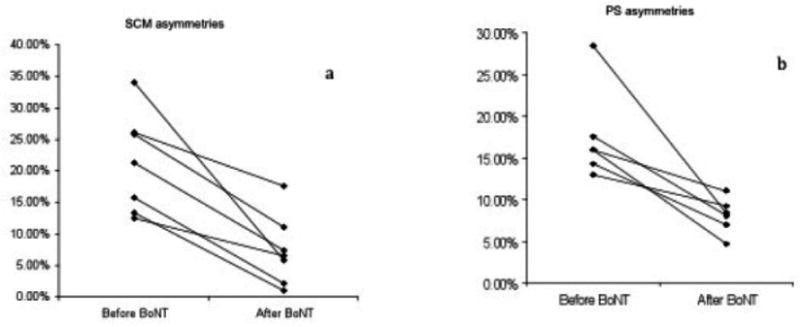
Absolute asymmetries before and after BoNT treatment in six subjects.
Discussion
A clear need exists, in both the clinical and the research contexts, for a reliable objective measure of severity and response to treatment of dystonia in general and of CD in particular. While validated clinical scales are in use (Consky et al., 1990; Tarsy, 1997; Vercueil, 2003), there is agreement within the Movement Disorders community that these subjective history- and examination-based scales are at best imperfect tools, leading to attempts to develop improved scales (Comella et al., 2003; Cano et al., 2006).
Needle EMG can be employed for treatment guidance and attempts have been made to use it for quantification of dystonia or for measuring response to treatment (Finsterer, 2001), but these have not been widely accepted. Other neurophysiological methods are exceedingly complex and can be employed in a research context (with unproved efficacy and reliability) and are not suitable for routine clinical use (Tinazzi et al., 2009).
In this initial application of EIM to CD, we have shown that the electrical resistance of muscle appears to effectively differentiate normal subjects from CD patients by revealing marked asymmetries in the resistance values in the two muscles studied. Indeed, the ROC analysis demonstrated that resistance values of either muscle alone could effectively discriminate the CD patients from the normal subjects with greater than 90% accuracy. In addition, as demonstrated in the pre- and post-BoNT therapy studies, the asymmetries in resistance decreased in all subjects after treatment, supporting the concept that EIM can detect the improvement in muscle symmetry as a consequence of BoNT therapy and highlighting its potential use in therapy monitoring.
In pursuing this analysis, we specifically avoided identifying an affected and an unaffected side since CD is a complex disorder which results in excessive activation not only of unilateral or sometimes bilateral agonist muscles, but often also of the antagonist muscles attempting to inhibit or correct the abnormal movement (Deuschl et al., 1992). Thus the search for asymmetry in the overall values simplified the analysis considerably.
In most studies evaluating electrical impedance methods for the assessment of neuromuscular disease, the data analysis has focused on the phase angle, a value obtained by calculating the ratio of the measured reactance of the tissue to its resistance (Tarulli et al., 2005; Rutkove et al., 2002; Rutkove et al., 2005; Tarulli et al., 2009A). An advantage of the phase angle is that it is typically less affected by muscle size than the raw resistance values (Rutkove, 2009). Indeed, of all three variables, the raw resistance values are likely to have the greatest sensitivity to muscle size, and it is for this reason that we hypothesized that the electrical resistance would provide the best measure of asymmetry. Preliminary analyses of both phase and reactance failed to reveal consistent alterations and thus both measures were omitted from this report. The reason for the expected reduction in resistance in dystonic/hypertrophic muscle is simply that greater muscle size implies a lower resistance to electrical current flow (analogous to a larger pipe allowing a greater flow of water) (Tarulli et al., 2009A).
This study has two important limitations. First, in this most basic analysis, we have not attempted a specific clinical correlate. For example, we have omitted the use of the TWSTRS scale or any other standard approach for quantifying the degree of dystonia. Similarly, we have not attempted to relate the abnormalities to hyperactivity of specific individual muscles, and we did not analyze the size and degree of hypertrophy of the muscles. Although these are obviously important issues, the main purpose of this study was to determine whether we could detect a disease “signal” of any sort. Second, all of our CD patients had received BoNT at some point prior to our initial measurements (both those who received BoNT during this study and those who only had a one time measurement). One advantage of looking at this specific population is that it is one in which EIM might most likely be utilized. Still, it would have been preferable to study a group of untreated CD patients before and then again after their first treatment with BoNT. With chronic BoNT therapy, complex changes occur in the muscle, which can contribute to changes detectable by EIM (Borodic and Ferrante, 1992; Matic et al., 2007; Schroeder et al., 2009). Future studies in BoNT-naïve patients are therefore planned.
A third more minor limitation is the disproportionate number of women in the CD group as compared to the control group. Whereas this is not ideal, there is no reason to suspect that gender should play a major role in CD; moreover, our post hoc analyses failed to show a significant influence of gender on the raw resistance values or the percent asymmetries.
Another important consideration is the methodology we used to obtain the data. Both impedance measuring systems used here are not specifically tailored for these kinds of measurements. For example, the commercially available Imp SFB7® is specifically designed for measuring whole body impedance for nutritional and lymphedema assessment. The application of adhesive electrodes can also be slow and prone to error. However, work is currently ongoing to develop a handheld system (Ogunnika et al., 2008) and an early prototype is now being tested. It is therefore likely that our techniques will gradually improve over time, allowing this approach to be applied more easily and accurately in a busy clinical setting. Still, relatively inexpensive commercial 50 kHz bioimpedance devices are available and could also be employed using an approach identical to the one described here.
These early results provide initial evidence that electrical resistance values are sensitive to the muscle asymmetries that occur in CD and offer the possibility of providing an innovative metric for use in both the care of individual patients and clinical trials work. The method is painless and well tolerated and lends itself well to potential standardization and automation. Future studies will attempt to address more specifically the relationship between EIM parameters and standard clinical measures.
Acknowledgments
This project is supported by NIH grants K24NS060951; RO1NS042037.
Footnotes
The work was performed in the Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA. All authors were affiliated with this institution at the time the work was performed.
Financial disclosures: Nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Borodic GE, Ferrante R. Effects of repeated botulinum toxin injections on orbicularis oculi muscle. J Clin Neuroophthalmol. 1992;12(2):121–127. doi: 10.3109/01658109209058127. [DOI] [PubMed] [Google Scholar]
- Burke RE, Fahn S, Marsden CD, Bressman SB, Moskowitz C, Friedman J. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology. 1985;35(1):73–77. doi: 10.1212/wnl.35.1.73. [DOI] [PubMed] [Google Scholar]
- Cano SJ, Hobart JC, Edwards M, Fitzpatrick R, Bhatia K, Thompson AJ, et al. CDIP-58 can measure the impact of botulinum toxin treatment in cervical dystonia. Neurology. 2006;67(12):2230–2232. doi: 10.1212/01.wnl.0000249310.25427.f2. [DOI] [PubMed] [Google Scholar]
- Comella CL, Leurgans S, Wuu J, Stebbins GT, Chmura T, Dystonia Study Group Rating scales for dystonia: a multicenter assessment. Mov Disord. 2003;18(3):303–312. doi: 10.1002/mds.10377. [DOI] [PubMed] [Google Scholar]
- Comella CL, Stebbins GT, Goetz CG, Chmura TA, Bressman SB, Lang AE. Teaching tape for the motor section of the Toronto Western Spasmodic Torticollis Scale. Mov Disord. 1997;12(4):570–575. doi: 10.1002/mds.870120414. [DOI] [PubMed] [Google Scholar]
- Consky E, Basinski A, Belle L, Ranawaya R, Lang AE. The Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS): assessment of validity and inter-rater reliability. Neurology. 1990;40(suppl 1):445. [Google Scholar]
- Deuschl G, Heinen F, Kleedorfer B, Wagner M, Lücking CH, Poewe W. Clinical and polymyographic investigation of spasmodic torticollis. J Neurol. 1992;239:9–15. doi: 10.1007/BF00839204. [DOI] [PubMed] [Google Scholar]
- Epidemiological Study of Dystonia in Europe (ESDE) Collaborative Group. A prevalence study of primary dystonia in eight European countries. J Neurol. 2000;247(10):787–792. doi: 10.1007/s004150070094. [DOI] [PubMed] [Google Scholar]
- Esper GJ, Shiffman CA, Aaron R, Lee KS, Rutkove SB. Assessing neuromuscular disease with multifrequency electrical impedance myography. Muscle Nerve. 2006;34(5):595–602. doi: 10.1002/mus.20626. [DOI] [PubMed] [Google Scholar]
- Finsterer J. EMG-interference pattern analysis. J Electromyogr Kinesiol. 2001;11(4):231–246. doi: 10.1016/s1050-6411(01)00006-2. [DOI] [PubMed] [Google Scholar]
- Jankovic J, Tsui J, Bergeron C. Prevalence of cervical dystonia and spasmodic torticollis in the United States general population. Parkinsonism Relat Disord. 2007;13(7):411–416. doi: 10.1016/j.parkreldis.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Matic DB, Lee TY, Wells RG, Gan BS. The effects of botulinum toxin type A on muscle blood perfusion and metabolism. Plast Reconstr Surg. 2007;120(7):1823–1833. doi: 10.1097/01.prs.0000287135.17291.2f. [DOI] [PubMed] [Google Scholar]
- Ogunnika OT, Scharfstein M, Cooper RC, Ma H, Dawson JL, Rutkove SB. Conf Proc IEEE Eng Med Biol Soc. Vol. 2008. 2008. Handheld Electrical Impedance Myography probe for the assessment of neuromuscular disease; pp. 3566–3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrem JL, Starr PA. Treatment of dystonia with deep brain stimulation. Neurotherapeutics. 2008;5(2):320–330. doi: 10.1016/j.nurt.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretto TE, Dalvi A, Kang UJ, Penn RD. A prospective blinded evaluation of deep brain stimulation for the treatment of secondary dystonia and primary torticollis syndromes. J Neurosurg. 2008;109(3):405–409. doi: 10.3171/JNS/2008/109/9/0405. [DOI] [PubMed] [Google Scholar]
- Rutkove SB, Aaron R, Shiffman CA. Localized bioimpedance analysis in the evaluation of neuromuscular disease. Muscle Nerve. 2002;25:390–397. doi: 10.1002/mus.10048. [DOI] [PubMed] [Google Scholar]
- Rutkove SB, Esper GJ, Lee KS, Aaron R, Shiffman CA. Handheld Electrical impedance myography in the detection of radiculopathy. Muscle Nerve. 2005;32(3):335–341. doi: 10.1002/mus.20377. [DOI] [PubMed] [Google Scholar]
- Rutkove SB, Lee KS, Shiffman CA, Aaron R. Test-retest reproducibility of 50 kHz linear-electrical impedance myography. Clin Neurophysiol. 2006;117(6):1244–1248. doi: 10.1016/j.clinph.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Rutkove SB, Zhang H, Schoenfeld DA, Raynor EM, Shefner JM, Cudkowicz ME, et al. Electrical impedance myography to assess outcome in ALS clinical trials. Clin Neurophysiol. 2007;118:2413–2418. doi: 10.1016/j.clinph.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkove SB. Electrical impedance myography: Background, current state, and future directions. Muscle Nerve. 2009;40(6):936–946. doi: 10.1002/mus.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder AS, Ertl-Wagner B, Britsch S, Schröder JM, Nikolin S, Weis J, et al. Muscle biopsy substantiates long-term MRI alterations one year after a single dose of botulinum toxin injected into the lateral gastrocnemius muscle of healthy volunteers. Mov Disord. 2009;24(10):1494–1503. doi: 10.1002/mds.22661. [DOI] [PubMed] [Google Scholar]
- Shiffman CA, Aaron R, Amoss V, Therrien J, Coomler K. Resistivity and phase in localized BIA. Phys Med Biol. 1999;44:2409–2429. doi: 10.1088/0031-9155/44/10/304. [DOI] [PubMed] [Google Scholar]
- Simpson DM, Blitzer A, Brashear A, Comella C, Dubinsky R, Hallett M, et al. Assessment: Botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;70(19):1699–1706. doi: 10.1212/01.wnl.0000311389.26145.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer C, Velickovic M. Cervical dystonia: etiology and pathophysiology. Neurol Clin. 2008;26 1:9–22. doi: 10.1016/s0733-8619(08)80002-3. [DOI] [PubMed] [Google Scholar]
- Stacy M. Epidemiology, clinical presentation, and diagnosis of cervical dystonia. Neurol Clin. 2008;26 1:23–24. doi: 10.1016/s0733-8619(08)80003-5. [DOI] [PubMed] [Google Scholar]
- Tarsy D. Comparison of clinical rating scales in treatment of cervical dystonia with botulinum toxin. Mov Disord. 1997;12(1):100–102. doi: 10.1002/mds.870120117. [DOI] [PubMed] [Google Scholar]
- Tarulli A, Esper GJ, Lee KS, Aaron R, Shiffman CA, Rutkove SB. Electrical impedance myography in the bedside assessment of inflammatory myopathy. Neurology. 2005;65(3):451–452. doi: 10.1212/01.wnl.0000172338.95064.cb. [DOI] [PubMed] [Google Scholar]
- Tarulli AW, Duggal N, Esper GJ, Garmirian LP, Fogerson PM, Lin CH, et al. Electrical impedance myography in the assessment of disuse atrophy. Arch Phys Med Rehabil. 2009A;90(10):1806–1810. doi: 10.1016/j.apmr.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarulli AW, Garmirian LP, Fogerson PM, Rutkove SB. Localized muscle impedance abnormalities in amyotrophic lateral sclerosis. Clin Neuromuscul Dis. 2009B;10(3):90–96. doi: 10.1097/CND.0b013e3181934423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinazzi M, Squintani G, Berardelli A. Does neurophysiological testing provide the information we need to improve the clinical management of primary dystonia? Clin Neurophysiol. 2009;120(8):1424–1432. doi: 10.1016/j.clinph.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Tolosa ES, Marti MJ. Adult-Onset Idiopathic Torsion Dystonias. In: Watts RL, Koller WC, editors. Movement Disorders, Neurologic Principles and Practice. 2nd. McGraw-Hill; 2004. pp. 511–526. [Google Scholar]
- Vercueil L. Les échelles cliniques de la dystonie. Rev Neurol (Paris) 2003;159(10 Pt 1):906–915. [PubMed] [Google Scholar]
- Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39(4):561–77. Erratum in: Clin Chem 1993;39(8):1589. [PubMed] [Google Scholar]


