Abstract
Nestling birds have been hypothesized to be important hosts for mosquito-borne arboviruses, but the role of nestlings for West Nile virus (WNV) amplification remains unclear. We sampled open-cup and cavity-nesting passerines in Chicago, Illinois, an area of intense WNV transmission, to determine infection rates in nestlings and mosquitoes, and to test whether mosquitoes are attracted to nesting birds. Analysis of Culex pipiens mosquito populations demonstrated WNV amplification to high mosquito infection rates during both years of the study near the locations where nestlings were sampled. Nevertheless, of 194 nestlings representing 12 species, only one 8-day-old house wren was positive for WNV RNA, and only one 10-day-old mourning dove was seropositive for antibodies to WNV, but at a low titer (1:20). The number of mosquitoes captured in nest box traps and control traps was not significantly different. These combined results suggest that nestling passerines play no evident role in WNV amplification and transmission in the Chicago area.
Key Words: birds, Culex, Field studies, Mosquito(es), West Nile virus
Introduction
West Nile virus (WNV) is now endemic throughout temperate North America. Periodic epidemics cause human illness and mortality, while epizootics can lead to regional declines of bird populations (LaDeau et al. 2007). Whereas much has been learned about the transmission dynamics of WNV, the role of nestling birds for WNV transmission remains unclear. Nestlings have been hypothesized to be important hosts for mosquito-borne arboviruses that are closely related to WNV. Modeling studies have suggested that nesting birds and first-year birds are important for viral amplification of eastern equine encephalomyelitis (Unnasch et al. 2006). Hayes et al. (1967) have suggested that nestling house sparrows (Passer domesticus) played a basic role in transmission of western equine encephalomyelitis. Nestlings have also been found to be competent hosts for St. Louis encephalitis (SLE), since they displayed elevated viremia after experimental infection (Mahmood et al. 2004). While adult birds may be more attractive than nestlings to mosquitoes in some cases, potentially due to pheromonal cues (Scott et al. 1990), nestlings are seemingly more susceptible to mosquito bites than adult birds due to minimal feather coverage (Blackmore and Dow 1958) and lack of defensive behavior (Kale et al. 1972). Furthermore, increased duration or intensity of viremia in nestlings, compared to adult birds, may facilitate mosquito infection (Mahmood et al. 2004).
High WNV seroprevalence in nestling herons (Reisen et al. 2005) and raptors (Stout et al. 2005) likely reflects antibodies of maternal origin. However, high mortality in nestling pelicans was due to WNV infection during an epizootic (Rocke et al. 2005). In previous studies, Culex spp. mosquitoes, primary vectors of WNV, displayed high landing rates on nesting American robins (Turdus migratorius), (Griffing et al. 2007). Savage et al. (2007) further suggested that feeding patterns of mosquitoes were consistent with a preference for nesting robins. Our own previous work in Chicago shows that WNV amplification coincides with the appearance of first-year birds that apparently provide a large population of susceptible hosts (Hamer et al. 2008). Whether nestlings or post-fledged juvenile birds contribute differentially to amplification remains unknown. Indeed, determining the relative competence of nestling, juvenile, and adult birds has been identified as a priority for research on WNV transmission (Kilpatrick et al. 2007). Therefore, we conducted a study of open-cup and cavity-nesting passerines in an area of intense WNV transmission to determine infection rates and seroprevalence in nestlings, infection rates in mosquitoes, and to make inferences about the role of nestlings in WNV amplification.
Materials and Methods
Study area
We conducted field research in 2006 and 2007 in the southwest suburbs of Chicago, Illinois. We sampled four residential sites and four natural areas, a subset of the sites previously described in Hamer et al. (2008). Residential sites included Palos Hills—North (Site ID 1), Oak Lawn—Central (5), Evergreen Park (7), and Alsip (11). Natural areas included Holy Sepulchre (HS), Saint Casimir (SC), and Evergreen Cemetery (EC) and Wolfe Wildlife Refuge (WW). In 2006, we also sampled nestlings at North Shore Country Club (NSCC) in Glenview, Illinois, a northern suburb of Chicago that was near a WNV “hotspot” of human cases during epidemics in 2002 and 2005 (Ruiz et al. 2004, 2007).
Collection of nestling blood samples
We erected five nest boxes in each residential site and two nest boxes in each natural area to attract cavity-nesting songbirds. Boxes followed the eastern/western bluebird design provided by the North American Bluebird Society (www.nabluebirdsociety.org/nestboxplans.htm) and were mounted 5 ft above the ground on steel posts. Nest boxes were placed in public parks and in yards of private residents within 10 ft of edge habitat (i.e., hedges and small groves of trees) and facing mowed lawns. Nest boxes were evenly spaced across each site with at least 200 m of separation. At NSCC, we sampled nestlings from an established “trail” of more than 50 nest boxes of various designs and uneven spacing. We also searched for open-cup nests in the southwest study area, targeting northern cardinals (Cardinalis cardinalis) and American robins, species displaying high seroprevalence in 2005 (unpublished data). We located these additional nests by searching systematically in appropriate habitat and opportunistically while conducting other fieldwork.
We inspected nest boxes weekly for avian activity throughout the breeding season (May through August). We checked active nests every 5 days to determine nest status and nestling age. Nestling age was estimated based upon degree of feather emergence and whether eyes were open or closed (Weaver 1942). Since NSCC was distant from our central study area, we sampled nestlings twice in June 2006 to collect blood samples from as many nestlings as possible. Nestlings >5 days old were bled using jugular venipuncture. Each nestling was bled only once. Nestlings <5 days old were not bled due to unacceptable risk for mortality, except at NSCC where some nestling house sparrows <5 days old were bled and then removed from nest boxes managed for eastern bluebirds (Sialia sialis). Blood samples were kept on ice and then centrifuged at the field laboratory within 4 h after collection. Nestling blood samples were tested for WNV antibodies using inhibition enzyme-linked immunosorbent assay (ELISA) and for virus using reverse transcription-polymerase chain reaction (RT-PCR) (Hamer et al. 2008).
Collection of mosquitoes at nest boxes and determination of WNV infection rates
We used a modified CDC miniature light trap to collect host-seeking mosquitoes from nest boxes in 2006 and 2007. Traps were mounted on the sides of nest boxes to permit access by adult birds, the light bulb was shut off, and no dry ice attractant was provided. We placed traps on active nests with nestlings, and we only trapped once for each brood. As a control treatment, we mounted an identical mosquito trap in vegetation at the same height at a location ∼10 m from the nest box. Mosquitoes from these traps were identified to species and tested individually (i.e., not pooled) for WNV using quantitative RT-PCR (Hamer et al. 2008).
To determine WNV amplification at the same sites where we erected nest boxes (i.e., sites 1, 5, 7, 11, EC, HS, SC, WW, but not NSCC), we collected adult female mosquitoes using CO2-baited CDC miniature light traps, CDC gravid traps baited with rabbit pellet infusion, and battery-powered backpack aspirators. Mosquito traps were placed an average of 166.4 m (range, 11–401 m) from nest boxes. We sampled each site once every 2 weeks from mid-May to mid-October in 2006 and 2007. Mosquitoes were identified to species (Andreadis et al. 2005) and grouped into pools of 25 or fewer individuals. We used morphological traits to identify Cx. pipiens and Cx. restuans mosquitoes. Mosquitoes were stored at −20°C or −80°C, and tested for WNV RNA using RT-PCR. Maximum likelihood estimates for Culex spp. infection rates were calculated using the Pooled Infection Rate Version 3.0 Add-In (Biggerstaff 2006) in the program Excel (Microsoft, Redmond, WA). All fieldwork was carried out under appropriate collecting permits with approvals from the University of Illinois Animal Use Protocol 03034 and the Institutional Animal Care and Use Committee at Michigan State University, Animal Use Form 12/03-152-00.
Results
We sampled 151 nestlings from 50 nests in 2006 and 43 nestlings from 11 nests in 2007. House sparrows dominated samples of cavity-nesting species (Table 1).The most commonly sampled open-cup nesters were American robins and red-winged blackbirds (Agelaius phoeneceus). Of all nestlings sampled during both years, only two tested positive: one 10-day-old mourning dove (Zenaida macroura) tested seropositive on 31 July 2006 (CDC Week 31), and one 8-day-old house wren (Troglodytes aedon) tested virus positive on 9 August 2006 (CDC Week 32). The seropositive dove, the only nestling present at the time of sampling, had a low antibody titer (endpoint titer 1:20). These positive samples were collected from two natural sites in the southwest study area. Due to the small sample of positive nestlings, we were unable to test for a relationship between nestling age and infection status. Despite sampling 41 nestlings from NSCC, none tested positive for antibodies or viral RNA.
Table 1.
Serology and Virus Testing Results from Investigation of WNV Exposure and Infection in Nestlings from Cavity Nests and Open-Cup Nests, Chicago Metropolitan Area, 2006–2007
| |
|
2006 |
2007 |
||||
|---|---|---|---|---|---|---|---|
| Species | Nest type | Nests | Individuals tested | No. positive | Nests | Individuals tested | No. positive |
| American robin (Turdus migratorius) | Open | 5 | 13 | 0 | 1 | 2 | 0 |
| Barn swallow (Sterna hirundo) | Open | 1 | 5 | 0 | 0 | 0 | 0 |
| Brown thrasher (Toxostoma rufum) | Open | 2 | 4 | 0 | 0 | 0 | 0 |
| European starling (Sturnus vulgaris) | Cavity | 2 | 8 | 0 | 0 | 0 | 0 |
| House finch (Carpodacus mexicanus) | Open | 1 | 1 | 0 | 0 | 0 | 0 |
| House sparrow (Passer domesticus) | Cavity | 22 | 54 | 0 | 5 | 20 | 0 |
| House wren (Troglodytes aedon) | Cavity | 4 | 23 | 1a | 4 | 17 | 0 |
| Mourning dove (Zenaida macroura) | Open | 2 | 3 | 1b | 0 | 0 | 0 |
| Northern cardinal (Cardinalis cardinalis) | Open | 3 | 7 | 0 | 0 | 0 | 0 |
| Purple martin (Progne subis) | Cavity | 1 | 4 | 0 | 0 | 0 | 0 |
| Red-winged blackbird (Agelaius phoeneceus) | Open | 4 | 12 | 0 | 0 | 0 | 0 |
| Tree swallow (Tachycineta bicolor) | Cavity | 3 | 17 | 0 | 1 | 4 | 0 |
| Total | 50 | 151 | 2 | 11 | 43 | 0 | |
Tested positive for WNV RNA.
Tested seropositive for WNV antibodies.
Mosquitoes were trapped at nest boxes from 15 broods of nestlings; collection dates ranged from 13 May to 27 July. We collected 32 female mosquitoes of 3 species (Cx. pipiens, Cx. restuans, and Aedes vexans), though most were Culex spp. (n = 30). All individual mosquitoes tested negative for WNV. Normality was not achieved for the mosquito count data; therefore, we used a Wilcoxon paired rank test to compare the group means. There was no significant difference between numbers of mosquitoes captured in nest box traps (0 ± 0.85, median ± SE) and control traps (0 ± 0.58; Wilcoxon test; W = 137; p = 0.219).
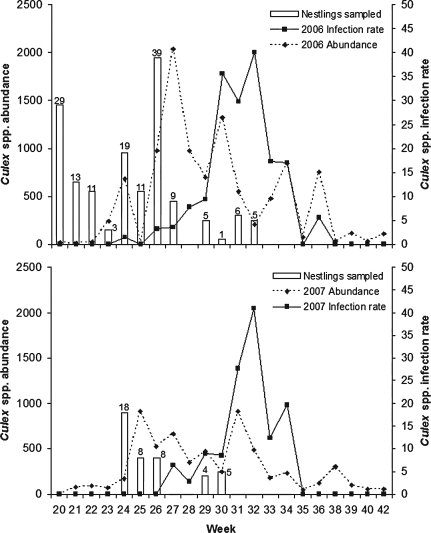
We collected 10,158 individual Culex spp. mosquitoes in 2006 and 6097 in 2007 from traps not associated with nest boxes. Captures were dominated by Cx. pipiens. The overall Culex spp. infection rates per 1000 female mosquitoes were 10.75 ± 2.3 in 2006 and 8.85 ± 2.8 in 2007. We observed rapid WNV amplification in late July 2006 and in early August 2007 (Fig. 1). The first positive pools were collected on 13 June 2006 and 5 July 2007.
FIG. 1.
Temporal patterns of Culex spp. mosquito abundance and infection rates per 1000 female mosquitoes from late May to mid-October at study sites with nest boxes (1, 5, 7, 11, EC, SC, HS, WW) in southwest suburban Chicago, Illinois, 2006 and 2007. Vertical bars indicate seasonal distribution of nestling blood samples collected during each year. Weeks used are defined by the Centers for Disease Control for use in the Mortality and Morbidity Weekly Report.
Discussion
The importance of a specific avian host to WNV transmission depends upon individual contact rates with mosquito vectors and competence to support viremia sufficient to infect mosquitoes (Kilpatrick et al. 2007). Our results suggest that nestling passerines are not major hosts for WNV in the Chicago area. Despite extensive sampling of nestlings during two nesting seasons when virus transmission was ongoing and amplifying seasonally, we detected viral RNA and WNV antibodies in only one bird each. In contrast, our results documented widespread amplification of WNV and high virus infection rates in mosquitoes that were captured in close proximity (average distance, 166.4 m) to sampled nestlings. Adult birds of the nestling species sampled (see Table 1 for species) also had high seroprevalence (27.0% in 2005; 4.7% in 2006) in the same study areas (unpublished data). Furthermore, nestlings of cavity-nesting bird species did not strongly attract host-seeking Culex spp. mosquitoes. Previous research suggests a role for nestling birds (Hayes et al. 1967) in transmission of encephalitide diseases. Experimental evidence also indicates higher host competence of nestling passerines for SLE (Mahmood et al. 2004), a closely related flavivirus to WNV. Based on our study, however, nestling passerines appear to experience low WNV infection and seroprevalence, thus precluding them from a role as focal amplification hosts.
Low WNV exposure and infection rates in nestlings may reflect a time lag between the peak avian nesting season and the onset of rapid WNV amplification in our study area (Fig. 1). For many passerine species, nesting activity in the mid-western United States typically decreases by the end of July. Even species that lay multiple broods, such as house sparrows, exhibit decreased breeding activity in late summer (Anderson 1994). We observed rapid WNV amplification in adult mosquitoes in late July 2006 and in early August 2007. This discrepancy in timing of nesting activity compared to arbovirus transmission was also highlighted for SLE transmission in Texas (Hayes et al. 1967) and California (Mahmood et al. 2004). Some passerine species, including American goldfinches (Carduelis tristis) and indigo buntings (Passerina cyanea), commonly nest in the Chicago area during July and August. We were unable to collect blood samples from nestlings of these species; therefore, it remains unclear whether nestlings of late-nesting passerine species experience greater WNV infection rates than the species we tested.
The combined evidence that large populations of susceptible first-year birds trigger amplification events (Hamer et al. 2008) and that nestlings are not central to WNV transmission implicates recently fledged birds as important hosts for WNV transmission and amplification. Most juvenile birds have left the nest by July, when WNV amplification typically commences. Though fledged birds have more extensive feather cover and greater defensive ability than nestlings, fledglings lack the acquired anti-mosquito defense behavior known to increase with age (Kale et al. 1972, Darbro and Harrington 2006). In our study area, we observed large flocks of juvenile American robins, house sparrows, and house finches (Carpodacus mexicanus) from mid-July through August. Such groups may serve as attractive sources of blood meals for Culex spp. mosquitoes, which are more likely to be WNV infected at that time.
While evidence for maternal transfer of WNV antibodies exists for birds (Gibbs et al. 2005, Reisen et al. 2005, Stout et al. 2005), the origin of antibodies in the seropositive mourning dove sampled during this study remains uncertain. With large sample sizes of seropositive birds, maternal origin of antibodies can be inferred based on clumped occurrence of seropositives within nests, decreased antibody titers as nestlings age, or detection of neutralizing antibodies that are not WNV-specific (Reisen et al. 2005). Furthermore, duration of maternally acquired antibodies in nestling birds is short in comparison to antibodies developed through viral challenge (Gibbs et al. 2005). Our sample of seropositive birds, however, was too small to observe any of these phenomena. If antibodies were found to be of maternal origin, our conclusion that nestlings are not important for vector-borne WNV transmission would be further strengthened.
We did not sample nestling corvids (crows and jays) or larids (gulls and terns), families that are known to be highly WNV competent (Kilpatrick et al. 2007). Therefore, our results cannot exclude the possibility that nestlings of these families and other nonpasserine groups may be important for WNV transmission. Nevertheless, our spatially and temporally intensive study strongly suggests that nestling passerines are not central to transmission of WNV in the study region. Our study emphasizes the potential importance of fledgling and post-fledged juvenile birds for arbovirus transmission in the upper Midwest.
Acknowledgments
We thank L. Abernathy, G. Amore, B. Bullard, S. Dallman, D. Gohde, M. Goshorn, J. McClain, M. Neville, B. Pultorak, E. Secker, and T. Thompson for field and laboratory assistance. We thank D. Dinelli and others at North Shore Country Club for providing access and generous assistance during fieldwork, as well as all residents of the Chicago area who provided access to their yards. We thank the Village of Oak Lawn for providing field lab space. This research was supported by the NSF/NIH program in the Ecology of Infectious Diseases (grant 04-29124) and by a fellowship from the University of Illinois, College of Agriculture, Consumer, and Environmental Sciences.
References
- Anderson TR. Breeding biology of house sparrows in northern lower Michigan. Wilson Bulletin. 1994;106:537–548. [Google Scholar]
- Andreadis TG. Thomas MC. Shepard JJ. Identification Guide to the Mosquitoes of Connecticut. New Haven: The Connecticut Agricultural Experiment Station; 2005. [Google Scholar]
- Biggerstaff BJ. Redmond, WA: Microsoft; 2006. PooledInfRate, Version 3.0: A Microsoft Excel Add-In to Compute Prevalence Estimates from Pooled Samples. [Google Scholar]
- Blackmore JS. Dow RP. Differential feeding of Culex tarsalis on nestling and adult birds. Mosq News. 1958;18:15–17. [Google Scholar]
- Darbro JML. Harrington C. Bird-baited traps for surveillance of West Nile mosquito vectors: effect of bird species, trap height, and mosquito escape rates. J Med Entomol. 2006;43:83–92. doi: 10.1093/jmedent/43.1.83. [DOI] [PubMed] [Google Scholar]
- Gibbs SEJ. Hoffman DM. Stark LM. Marlenee NL, et al. Persistence of antibodies to West Nile virus in naturally infected rock pigeons (Columba liva) Vector Borne Zoonotic Dis. 2005;12:665–667. doi: 10.1128/CDLI.12.5.665-667.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffing SM. Kilpatrick AM. Clark L. Marra PP. Mosquito landing rates on nesting American robins (Turdus migratorius) Vector Borne Zoonotic Dis. 2007;7:437–443. doi: 10.1089/vbz.2006.0560. [DOI] [PubMed] [Google Scholar]
- Hamer GH. Walker ED. Brawn JD. Loss SR, et al. Rapid amplification of West Nile virus: the role of hatch year birds. Vector Borne Zoonotic Dis. 2008;8:57–68. doi: 10.1089/vbz.2007.0123. [DOI] [PubMed] [Google Scholar]
- Hayes RO. LaMotte LC. Holden P. Ecology of arboviruses in Hale County, Texas, during 1965. Am J Trop Med Hyg. 1967;16:675–687. doi: 10.4269/ajtmh.1967.16.675. [DOI] [PubMed] [Google Scholar]
- Kale HW. Edman JD. Webber LA. Effect of behavior and age of individual ciconiiform birds on mosquito feeding success. Mosq News. 1972;32:343–350. [Google Scholar]
- Kilpatrick AM. LaDeau SL. Marra PP. Ecology of West Nile virus transmission and its impact on birds in the western hemisphere. Auk. 2007;124:1121–1136. [Google Scholar]
- LaDeau SL. Kilpatrick AM. Marra PP. West Nile virus emergence and large-scale declines of North American bird populations. Nature. 2007;447:710–713. doi: 10.1038/nature05829. [DOI] [PubMed] [Google Scholar]
- Mahmood F. Chiles RE. Fang Y. Barker CM, et al. Role of nestling mourning doves and house finches as amplifying hosts of St. Louis encephalitis virus. J Med Entomol. 2004;41:965–972. doi: 10.1603/0022-2585-41.5.965. [DOI] [PubMed] [Google Scholar]
- Reisen WK. Wheeler SS. Yamamoto S. Fang Y, et al. Nesting ardeid colonies are not a focus of elevated West Nile virus activity in southern California. Vector Borne Zoonotic Dis. 2005;6:248–260. doi: 10.1089/vbz.2005.5.258. [DOI] [PubMed] [Google Scholar]
- Rocke T. Converse K. Meteyer C. McLean B. The impact of disease in the American white pelican in North America. Anderson DW, editor; King DT, editor; Coulson J, editor. The biology and conservation of the American white pelican. Waterbirds. 2005;28:87–94. Special Publ 1. [Google Scholar]
- Ruiz MO. Tedesco C. McTighe TJ. Austin C, et al. Environmental and social determinants of human risk during a West Nile virus outbreak in the greater Chicago area, 2002. Int J Health Geogr. 2004;3:11. doi: 10.1186/1476-072X-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz MO. Walker ED. Foster ES. Haramis LD, et al. Association of West Nile illness and urban landscapes in Chicago and Detroit. Int J Health Geogr. 2007;6:10. doi: 10.1186/1476-072X-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage HM. Aggarwal D. Apperson CS. Katholi CR, et al. Host choice and West Nile virus infection rates in blood-fed mosquitoes, including members of the Culex pipiens complex, from Memphis and Shelby County, Tennessee, 2002–2003. Vector Borne Zoonotic Dis. 2007;7:365–386. doi: 10.1089/vbz.2006.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott TW. Lorenz LH. Edman JD. Effects of house sparrow age and arbovirus infection on attraction of mosquitoes. J Med Entomol. 1990;27:856–863. doi: 10.1093/jmedent/27.5.856. [DOI] [PubMed] [Google Scholar]
- Stout WE. Cassini AG. Meece JK. Papp JM, et al. Serologic evidence of West Nile virus infection in three wild raptor populations. Avian Dis. 2005;49:371–375. doi: 10.1637/7335-012805R1.1. [DOI] [PubMed] [Google Scholar]
- Unnasch RS. Sprenger T. Katholi CR. Cupp EW, et al. A dynamic transmission model of eastern equine encephalitis virus. Ecol Model. 2006;192:425–440. doi: 10.1016/j.ecolmodel.2005.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver RL. Growth and development of English sparrows. Wilson Bulletin. 1942;54:183–191. [Google Scholar]