Abstract
Two ordinary green light-emitting diodes used as light emitter and detector coupled with simple voltmeter form a complete, cost-effective prototype of a photometric hemoglobinometer. The device has been optimized for cuvette assays of total hemoglobin (Hb) in diluted blood using three different chemical methods recommended for the needs of clinical analysis (namely Drabkin, lauryl sulfate, and dithionite methods). The utility of developed device for real analytics has been validated by the assays of total Hb content in human blood. The results of analysis are fully compatible with those obtained using clinically recommended method and clinical analyzer.
Keywords: Hemoglobin, Blood, Photometry, Paired emitter detector diode
Introduction
Economic, miniature, low-power, and field-deployable analytical tools are required for many kinds of analyses performed outside specialized laboratories like biomedical tests at home or surgery, food quality control, environmental screening and monitoring, etc. [1–5]. As a large number of analytes are colored or could be easily and selectively converted into colored species, the predominant position of photometric methods in this field of modern analytics as well as the reasons for investigations and development of novel systems for optical measurements are obvious. For many analytical uses, simple dedicated photometric devices based on economic optoelectronic components are sufficient.
Light-emitting diodes (LEDs) are the simplest and cheapest optoelectronic components, mass-produced in semiconductor technology. These extremely robust, low-power, and intensive light emitters, offering narrow emission spectra covering broad spectral range from UV to NIR, are widely applied in photometric systems as effective light sources [6, 7]. On the other hand, it is known that the internal photoelectric effect (opposite to electroluminescence phenomenon) allows the use of LEDs as light detectors [8–11]. Thus, the pairing of two LEDs, playing the roles of light emitter and detector, leads to construction of a complete photometric device useful in analytical chemistry. Such paired emitter detector diode (PEDD) photometers generate reproducible, hundred-millivolts-large analytical signals [9–11]. As an example, a complete PEDD-based photometer made of two red LEDs coupled with an ordinary voltmeter, developed for education needs and useful as an efficient analytical tool for determination of blue dyes, pH, and pH-indicators as well as for kinetic assays of enzymes activity, has been demonstrated only recently [11].
In this note, a very simple PEDD-based device dedicated for photometric determination of hemoglobin (Hb) is presented. Contrary to other prototypes of Hb meters recently reported in the literature [12, 13], this fibreless device is fabricated without the use of any advanced technologies, electronics nor optoelectronics. The utility of the developed device for real analytics will be validated by the assays of total Hb content in human blood.
Experimental
Lyophilized hemoglobin, sodium lauryl sulfate (SLS) and sodium dithionite (Na2S2O4), were obtained from Sigma-Aldrich (USA). Other reagents of analytical grade were obtained from POCh (Poland). Doubly distilled water was used throughout.
Six kinds of 5-mm diameter LEDs (525 nm/10 Cd, 565 nm/0.8 Cd, 570 nm/1.5 Cd, 600 nm/1.5 Cd; 630 nm/1.5 Cd and 650 nm/4 Cd) used in these investigations were obtained from Optosupply (Hong Kong). Paired LEDs were mounted into cuvette holder made of Lego bricks, shown elsewhere [9, 11]. Electromotive force generated by tested PEDDs, treated as an analytical signal, was measured and recorded with Axiomet multimeter (model AX-18B, China).
Reference spectrophotometric measurements were performed using Shimadzu spectrophometer (model 2401/PC, Japan). For all experiments, disposable 1 × 1 × 4 cm3 polystyrene cuvettes (Sarstedt, Germany) were used.
Results and discussion
Spectrochemistry of Hb
Figure 1 presents color and corresponding spectra for 100 to 500 fold diluted blood and different forms of Hb at comparable concentrations. Hemoglobin forms an unstable, reversible bond with oxygen. This oxygen-loaded form named oxyhemoglobin (HbO2) is bright red. In the oxygen-unloaded form, it is called deoxyhemoglobin (Hb) and is purple-blue. Additionally, blood samples may contain carbaminohemoglobin (unstable compound of Hb and carbon dioxide) which is bluish in color and carboxyhemoglobin (a stable complex of Hb and carbon monoxide) which is bright, cherry red. Lyophilized Hb available as reagent, similarly as the native forms of Hb, is readily oxidized in air so its preparations predominantly contain methemoglobin (MetHb) forming brownish solutions having four absorbance peaks in visible range of spectrum. Simultaneous multiwavelength absorbance measurements permitted the composition of Hb pigment mixtures to be determined. However, such approach is easily implemented only with a precise microprocessor-controlled spectrophotometer [14–16].
Fig. 1.
Color and spectra of selected Hb forms. Description in the text
An alternative approach useful for determination of total Hb is based on chemical conversion of all Hb fractions into one stable form [16]. The most popular and widely accepted in clinical settings is the cyanmethemoglobin (hemiglobincyanide, HiCN) method used as the international standard procedure for hemoglobin (Hb) determinations due to the accuracy, stability and availability of a stable reference standard/calibrator [16, 17]. HiCN has one absorbance peak at 542 nm (Fig. 1). Unfortunately, the presence of potassium cyanide and potassium ferricyanide in the reagents constitutes a potential toxic hazard and problems of laboratory and environmental pollution. As an alternative, conversion of hemoglobin to a sulfate derivative by non-toxic SLS has been proposed [18, 19]. This reagent converts all fractions of Hb into stable SLS-MetHb form exhibiting maximal absorbance at 536 nm (Fig. 1). Another methodology is based on deoxygenation/reduction of all Hb fractions (including MethHb) with sodium dithionite [20, 21] to Hb form exhibiting one absorbance peak at 555 nm (Fig. 1).
In the course of primary measurements performed with spectrophotometer (Fig. 1), it has been found that Hb standards prepared using lyophilized powder are stable over time, whereas the spectra of blood standards slightly change in a random way. The predominant forms in reagent and blood solutions are MetHb and HbO2, respectively. In both cases, absorbances of diluted solutions stay in agreement with Lambert–Beer’s law (Fig. 1).
Both kinds of original standards were modified with Drabkin reagent (1.5 mM KCN + 1.0 mM K3Fe(CN)6), SLS (0.35%), and dithionite (0.1 M) leading to HiCN, SLS-MetHb, and Hb forms, respectively. The stable standards prepared by HiCN, SLS, and dithionite methods were obtained within 20, 5, and 2 min, respectively. The color and corresponding spectra of modified standards are shown in Fig. 1. The spectra of modified standards are identical, stable over many hours and consistent with Lambert–Beer’s law, independently of kind of used original standard (lyophilized reagent or blood). It means that in case of the mentioned methods, the standards prepared of lyophilized Hb are useful for real blood analysis.
Hb-PEDD characteristics
All 36 PEDD combinations possible to construct using LEDs itemized in the “Experimental” section were tested as potential photometric Hb detectors. For PEDDs where the wavelength of LED emitter was significantly shorter than for the LED used as light detector, the lack of sensitivity was observed, which is in agreement with earlier findings [9, 10]. Taking into account the sensitivity criterion, the best results are offered by PEDDs constructed using 565- and 570-nm LEDs as their emission spectra are the most compatible with absorption spectra of various Hb forms. The calibrations graphs for such four PEDD combinations (Fig. 2) have been obtained using Hb standards modified with dithionite. In the course of analogous experiments with standards prepared using SLS and HiCN method the same PEDDs were found to be the most promising. The sensitivities for the linear range of detection (0–1,000 mg/L), obtained at 15 mA PEDD driving current for SLS, HiCN, and dithionite methods were 1.03 ± 0.04, 1.11 ± 0.07, and 1.25 ± 0.08 mV/mg/L, respectively. As expected, the highest sensitivity was observed for dithionite method, because in this case the absorption spectrum of analyte (Fig. 1) is the most compatible with the emission spectrum of used LED.
Fig. 2.
The effect of PEDD driving current on sensitivity of Hb determination. Results for four possible PEDD combinations constructed of 565 and 570 nm LEDs
The calibration graphs shown in Fig. 2 were obtained at various currents driving tested PEDDs. As it was reported earlier [10], it is possible to adjust the range of maximal sensitivity to the required range of detection by the appropriate choice of power supplying this device. The optimal PEDD driving currents for investigated range of Hb concentration (consistent with Hb levels in 100–500-fold-diluted blood samples) are lower than maximal currents (30 mA) recommended for LED supplying. It is worth noticing that at currents lower than 20 mA where no saturation of analytical signal for diluted samples is observed, it is possible to detect Hb concentrations lower than 0.01 g/dl. Normal Hb content in human blood varies from 11 to 16 g/dl and decreases in case of anemia diseases.
Blood analysis
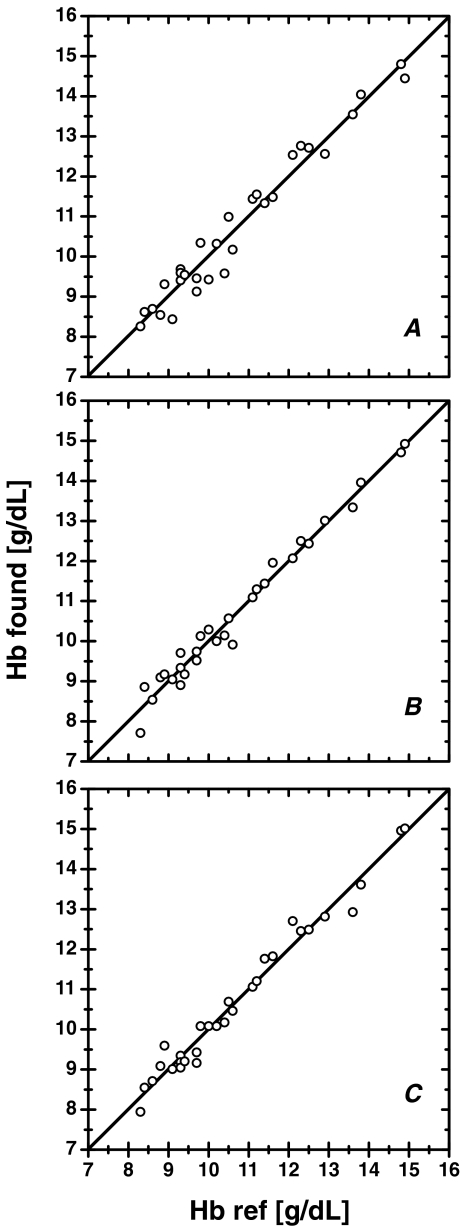
The developed prototype of hemoglobinometer based on 570/570 nm PEDD operating at 15-mA driving current was validated with 30 real samples of human blood containing Hb at physiological as well as pathological levels. Before measurements, blood samples were 200-fold-diluted with reagent solutions for HiCN, SLS, or dithionite methods. Thus, for single determination, less than 0.02 mL of blood was required. Reference determinations of total Hb were performed by hospital laboratory using clinical photometric HiCN analyzer XT-2000i/XT (SYSMEX, Japan). As can be seen from Fig. 3, the results of analysis with the developed detector are fully compatible with those obtained using the reference method. The regression coefficient for HiCN, SLS, and dithionite methods are 0.9580, 0.9782, and 0.9752, respectively. Such results are acceptable in case of manual analysis.
Fig. 3.
Correlation between results of blood analysis obtained using developed 570/570 nm PEDD detector and reference method recommended for clinical uses. Results for 30 human blood samples 200-fold-diluted with Drabkin reagent (a), SDS (b), and dithionite (c)
Conclusions
In this communication, a complete, cost-effective prototype of hemoglobinometer constructed of only two ordinary green LEDs coupled with simple voltmeter is presented. The price of two LEDs is lower than a half euro whereas the total cost of electronics for PEDD supplying and signal recording is only a few euros. Its practical utility for real analytics has been confirmed by the results of human blood analysis fully compatible with those obtained using clinically recommended method and analyzer.
Although the presented PEDD detector is extremely simple, its potential applications for development of advanced bioanalytical systems dedicated for more specific clinical applications are attractive. As shown elsewhere [22, 23], PEDDs are easily applicable as flow-through detectors in various flow analysis systems. Thus, PEDD-based detectors could be easily miniaturized and integrated with separation chromatographic or electrophoretic devices potentially leading to dedicated analytical tools useful for determination of selected Hb fractions recognized as specific markers for diabetes [24, 25], thalassemia [26, 27], etc.
Acknowledgments
The investigations were performed in the frame of undergraduate student project (EM licentiate) supported by the Ministry of Science and Higher Education (Project no. N N204 029636). Analyzed samples of human blood were obtained from Medical University of Warsaw (MUW). Helpful assistance and comments from Dr. Agnieszka Wiśniewska (Department of Laboratory Diagnostics, MUW) are kindly acknowledged.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Published in the special issue Focus on Analytical Science in Poland (VIIIth Polish Conference on Analytical Chemistry) with Guest Editor Pawel Koscielniak.
References
- 1.Kostov Y, Rao G. Rev Sci Instum. 2000;71:4361–4374. doi: 10.1063/1.1319859. [DOI] [Google Scholar]
- 2.Wang J. Trend Anal Chem. 2002;21:226–232. doi: 10.1016/S0165-9936(02)00402-8. [DOI] [Google Scholar]
- 3.Wang J. Anal Chim Acta. 2004;507:3–10. doi: 10.1016/j.aca.2003.08.031. [DOI] [Google Scholar]
- 4.Weigl B, Domingo G, La Barre P, Gerlach J. Lab Chip. 2008;8:1999–2014. doi: 10.1039/b811314a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kieckle FL, Holland CA. Clin Lab Med. 2009;29:555–560. doi: 10.1016/j.cll.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Dasgupta PK, Eom IY, Morris KJ, Li J. Anal Chim Acta. 2003;500:337–364. doi: 10.1016/S0003-2670(03)00575-0. [DOI] [Google Scholar]
- 7.O’Toole M, Diamond D. Sensors. 2008;8:2453–2479. doi: 10.3390/s8042453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau KT, Baldwin S, O’Toole M, Shepherd R, Yerazunis WJ, Izuo S, Ueyama S, Diamond D. Anal Chim Acta. 2006;557:111–116. doi: 10.1016/j.aca.2005.10.046. [DOI] [Google Scholar]
- 9.Tymecki Ł, Pokrzywnicka M, Koncki R. Analyst. 2008;133:1501–1504. doi: 10.1039/b807127f. [DOI] [PubMed] [Google Scholar]
- 10.Tymecki Ł, Koncki R. Anal Chim Acta. 2009;639:73–77. doi: 10.1016/j.aca.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Pokrzywnicka M, Koncki R, Tymecki Ł. Chem Anal. 2009;54:427–435. [Google Scholar]
- 12.Noda T, Takao H, Yoshioka K, Oku N, Ashiki M, Sawada K, Matsumoto K, Ishida M. Sens Act B. 2005;119:245–250. doi: 10.1016/j.snb.2005.12.017. [DOI] [Google Scholar]
- 13.Weiwei Y, Aiyu Z, Baoshan H, Xinxia C. Sens Act B. 2008;130:21–24. doi: 10.1016/j.snb.2007.07.075. [DOI] [Google Scholar]
- 14.Zwart A, Buursma A, van Kampen EJ, Zijlstra WG. Clin Chem. 1984;30:373–379. [PubMed] [Google Scholar]
- 15.Zijlstra WG, Buursma A, Zwart A. Clin Chem. 1988;34:149–152. [PubMed] [Google Scholar]
- 16.Zijlstra WG, Buursma A, van Assendelft OW. Visible and near infrared absorption spectra of human and animal haemoglobin: determination and application. Boston: VSP; 2000. [Google Scholar]
- 17.van Kampen EJ, Zijlstra WG. Clin Chim Acta. 1961;6:538–544. doi: 10.1016/0009-8981(61)90145-0. [DOI] [PubMed] [Google Scholar]
- 18.Oshiro I, Takenaka T, Maeda J. Clin Biochem. 1982;15:83–88. doi: 10.1016/S0009-9120(82)91069-4. [DOI] [PubMed] [Google Scholar]
- 19.Lewis SM, Garvey B, Manning R, Sharp SA, Wardle J. Clin Lab Haematol. 1991;13:279–290. doi: 10.1111/j.1365-2257.1991.tb00283.x. [DOI] [PubMed] [Google Scholar]
- 20.Dalziel K, O'Brien JRP. Biochem J. 1957;67:119–124. [PMC free article] [PubMed] [Google Scholar]
- 21.Hamada K, Okazaki T, Shukuya R, Kaziro K. J Biochem. 1962;52:374–376. doi: 10.1093/oxfordjournals.jbchem.a127630. [DOI] [PubMed] [Google Scholar]
- 22.Tymecki Ł, Brodacka L, Rozum B, Koncki R. Analyst. 2009;134:1333–1337. doi: 10.1039/b822025e. [DOI] [PubMed] [Google Scholar]
- 23.Tymecki Ł, Strzelak K, Koncki R. Talanta. 2009;79:205–210. doi: 10.1016/j.talanta.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 24.Halwachs-Baumann G, Katzensteiner S, Schnedl W, Purstner P, Pieber T, Wilders-Truschnig M. Clin Chem. 1997;43:511–517. [PubMed] [Google Scholar]
- 25.Bay L, Chen PC, Sacks DB. Clin Chem. 2001;47:153–163. [PubMed] [Google Scholar]
- 26.Srisawang B, Kongtawelert P, Hartwell SK, Jakmunee J, Grudpan K. Talanta. 2003;60:1163–1170. doi: 10.1016/S0039-9140(03)00219-4. [DOI] [PubMed] [Google Scholar]
- 27.Hartwell SK, Srisawang B, Kongtawelert P, Christian GD, Grudpan K. Talanta. 2005;65:1149–1161. doi: 10.1016/j.talanta.2004.09.013. [DOI] [PubMed] [Google Scholar]