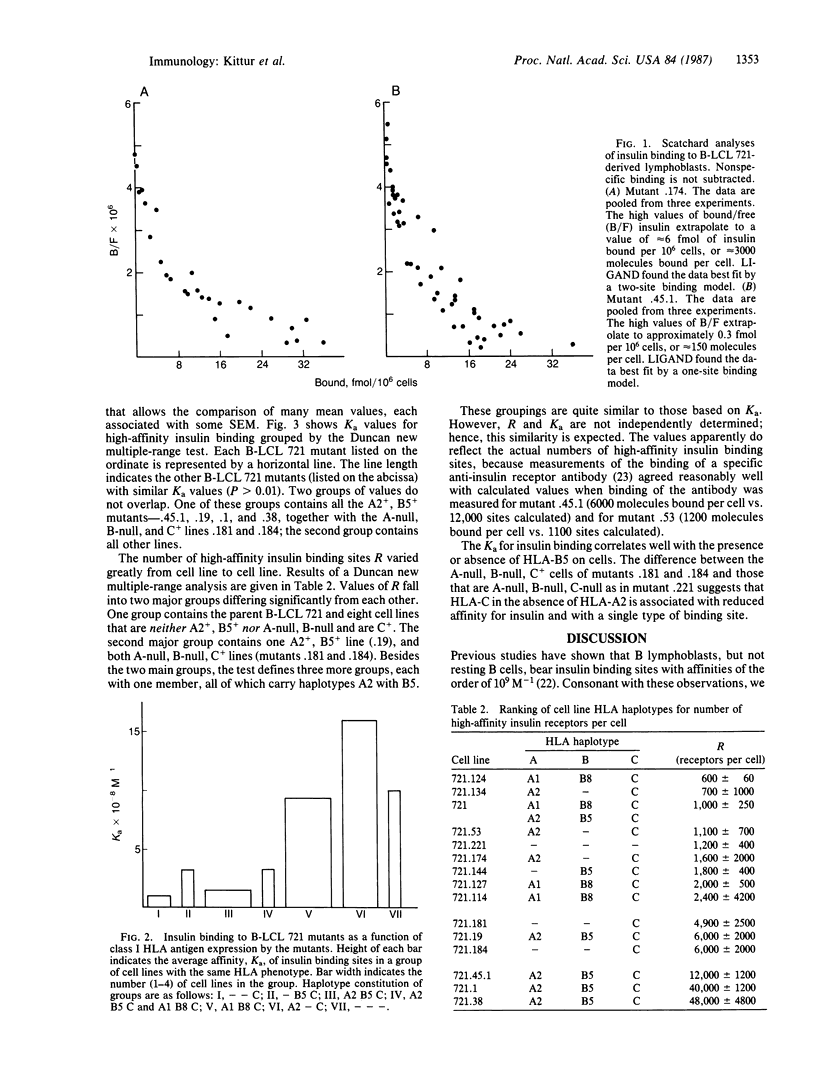
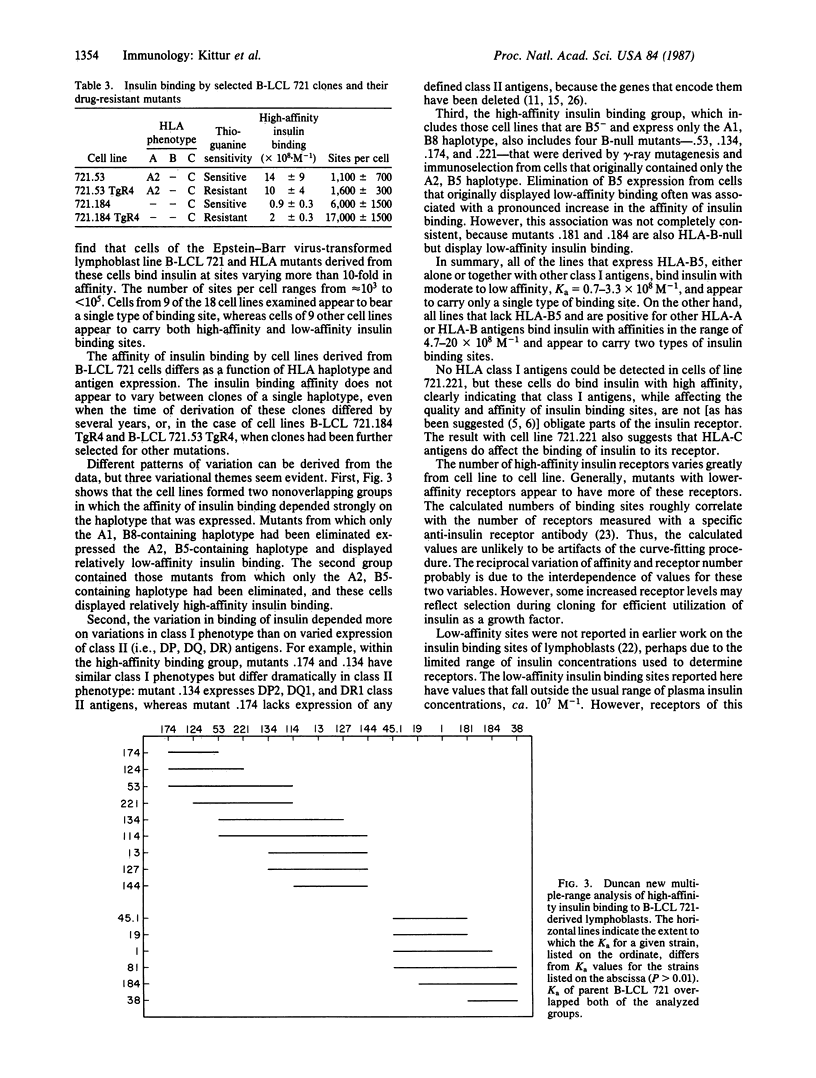
Abstract
A variety of genetic and biochemical evidence points to an association between major histocompatibility complex (MHC) haplotype and several types of cell surface receptors including epidermal growth factor and insulin receptors. We report evidence for such associations between human class I MHC antigens, HLA antigens, and specific insulin binding sites on human B lymphoblasts. We have measured insulin binding to cells of an HLA-heterozygous, Epstein-Barr virus-transformed B-cell line, LCL 721, and to derivative mutants from which all or part of the HLA complex had been deleted. The affinity, Ka, of insulin binding sites is approximately 10(8) M-1 in mutants expressing antigen HLA-B5 together with other HLA antigens and in mutants expressing only HLA-C. HLA-A1; HLA-A1,B8; HLA-A2,C; and HLA null mutants (not expressing any HLA antigens) bind insulin to sites with an affinity of approximately 10(9) M-1.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DeMars R., Chang C. C., Rudersdorf R. A. Dissection of the D-region of the human major histocompatibility complex by means of induced mutations in a lymphoblastoid cell line. Hum Immunol. 1983 Oct;8(2):123–139. doi: 10.1016/0198-8859(83)90008-3. [DOI] [PubMed] [Google Scholar]
- DeMars R., Chang C. C., Shaw S., Reitnauer P. J., Sondel P. M. Homozygous deletions that simultaneously eliminate expressions of class I and class II antigens of EBV-transformed B-lymphoblastoid cells. I. Reduced proliferative responses of autologous and allogeneic T cells to mutant cells that have decreased expression of class II antigens. Hum Immunol. 1984 Oct;11(2):77–97. doi: 10.1016/0198-8859(84)90047-8. [DOI] [PubMed] [Google Scholar]
- DeMars R., Rudersdorf R., Chang C., Petersen J., Strandtmann J., Korn N., Sidwell B., Orr H. T. Mutations that impair a posttranscriptional step in expression of HLA-A and -B antigens. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8183–8187. doi: 10.1073/pnas.82.23.8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Due C., Simonsen M., Olsson L. The major histocompatibility complex class I heavy chain as a structural subunit of the human cell membrane insulin receptor: implications for the range of biological functions of histocompatibility antigens. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6007–6011. doi: 10.1073/pnas.83.16.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel J., Reinauer H. Effect of EDTA on insulin binding and insulin action in isolated cardiocytes from adult rat. Evidence for a functional role of low-affinity insulin receptors. Diabetes. 1984 Mar;33(3):214–218. doi: 10.2337/diab.33.3.214. [DOI] [PubMed] [Google Scholar]
- Erlich H., Lee J. S., Petersen J. W., Bugawan T., DeMars R. Molecular analysis of HLA class I and class II antigen loss mutants reveals a homozygous deletion of the DR, DQ, and part of the DP region: implications for class II gene order. Hum Immunol. 1986 Jun;16(2):205–219. doi: 10.1016/0198-8859(86)90049-2. [DOI] [PubMed] [Google Scholar]
- Fehlmann M., Peyron J. F., Samson M., Van Obberghen E., Brandenburg D., Brossette N. Molecular association between major histocompatibility complex class I antigens and insulin receptors in mouse liver membranes. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8634–8637. doi: 10.1073/pnas.82.24.8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helderman J. H., Strom T. B. Specific insulin binding site on T and B lymphocytes as a marker of cell activation. Nature. 1978 Jul 6;274(5666):62–63. doi: 10.1038/274062a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iványi P. Some aspects of the H-2 system, the major histocompatibility system in the mouse. Proc R Soc Lond B Biol Sci. 1978 Jun 5;202(1146):117–158. doi: 10.1098/rspb.1978.0060. [DOI] [PubMed] [Google Scholar]
- Kavathas P., Bach F. H., DeMars R. Gamma ray-induced loss of expression of HLA and glyoxalase I alleles in lymphoblastoid cells. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4251–4255. doi: 10.1073/pnas.77.7.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J., Juretic A., Baxevanis C. N., Nagy Z. A. The traditional and a new version of the mouse H-2 complex. Nature. 1981 Jun 11;291(5815):455–460. doi: 10.1038/291455a0. [DOI] [PubMed] [Google Scholar]
- Koller B. H., Sidwell B., DeMars R., Orr H. T. Isolation of HLA locus-specific DNA probes from the 3'-untranslated region. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5175–5178. doi: 10.1073/pnas.81.16.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafuse W., Edidin M. Influence of the mouse major histocompatibility complex, H-2, on liver adenylate cyclase activity and on glucagon binding to liver cell membranes. Biochemistry. 1980 Jan 8;19(1):49–54. doi: 10.1021/bi00542a008. [DOI] [PubMed] [Google Scholar]
- Meruelo D., Edidin M. Association of mouse liver adenosine 3':5'-cyclic monophosphate (cyclic AMP) levels with histocompatibility-2 genotype. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2644–2648. doi: 10.1073/pnas.72.7.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Orr H. T., Bach F. H., Ploegh H. L., Strominger J. L., Kavathas P., DeMars R. Use of HLA loss mutants to analyse the structure of the human major histocompatibility complex. Nature. 1982 Apr 1;296(5856):454–456. doi: 10.1038/296454a0. [DOI] [PubMed] [Google Scholar]
- Orr H. T., DeMars R. Mapping of class I DNA sequences within the human major histocompatibility complex. Immunogenetics. 1983;18(5):489–502. doi: 10.1007/BF00364390. [DOI] [PubMed] [Google Scholar]
- Phillips M. L., Moule M. L., Delovitch T. L., Yip C. C. Class I histocompatibility antigens and insulin receptors: evidence for interactions. Proc Natl Acad Sci U S A. 1986 May;83(10):3474–3478. doi: 10.1073/pnas.83.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R. A., Cassell D. J. Insulin receptor: evidence that it is a protein kinase. Science. 1983 Jan 21;219(4582):299–301. doi: 10.1126/science.6849137. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Koller B., Geraghty D., Orr H., Shaw S., Kavathas P., DeMars R. Transfer of cloned human class I major histocompatibility complex genes into HLA mutant human lymphoblastoid cells. Mol Cell Biol. 1986 Apr;6(4):1074–1087. doi: 10.1128/mcb.6.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen M., Skjødt K., Crone M., Sanderson A., Fujita-Yamaguchi Y., Due C., Rønne E., Linnet K., Olsson L. Compound receptors in the cell membrane: ruminations from the borderland of immunology and physiology. Prog Allergy. 1985;36:151–176. [PubMed] [Google Scholar]
- Sodoyer R., Damotte M., Delovitch T. L., Trucy J., Jordan B. R., Strachan T. Complete nucleotide sequence of a gene encoding a functional human class I histocompatibility antigen (HLA-CW3). EMBO J. 1984 Apr;3(4):879–885. doi: 10.1002/j.1460-2075.1984.tb01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



