Abstract
Ag persistence during high-titer chronic viral infections induces CD8 T cell dysfunction and lack of Ag-independent CD8 T cell memory formation. However, we have a poor understanding of the generation and maintenance of CD8 T cell memory during asymptomatic persistent viral infections, particularly γ-herpesvirus infections. In this study, we demonstrate that the continuous presence of cognate Ag in the host is not required for the maintenance of CD8 T cell memory during a persistent γ-herpesvirus infection. Importantly, the Ag-independent CD8 T cell memory that is maintained during γ-herpesvirus persistence has the capacity to survive long-term under homeostatic conditions and to mount a protective recall response to a secondary encounter with the pathogen. These data highlight the ability of the immune system to maintain a population of protective memory CD8 T cells with capacity for long-term Ag-independent survival in the presence of systemic virus persistence.
Immunological memory represents the ability of the adaptive immune system to remember a previously encountered Ag and protect the host against secondary infection with that pathogen. The formation of immunological memory allows the immune system to mount a rapid, robust, more effective recall response to a secondary challenge (1, 2). After the resolution of an infection, the expanded pathogen-specific T cell population contracts via programmed cell death, leaving only a small population of long-lived memory T cells (3). These memory T cells are maintained at increased frequencies than their Ag-specific naive repertoire, and have reduced costimulatory requirements and a higher activation status than their naive counterparts (1, 4, 5). Memory T cells persist independently of Ag and self renew by homeostatic proliferation in response to the cytokines IL-7 and IL-15 (6–8).
Although the process of maintenance of memory CD8 T cells during an acute resolving infection is starting to be understood, the fate of CD8 T cell memory during persistent infections is still a matter of debate. Substantial evidence from studies of persistent infection with lymphocytic choriomeningitis virus (LCMV)3 clone 13 demonstrates that Ag persistence leads to progressive effector CD8 T cell dysfunction and to lack of Ag-independent memory formation (9–11). Cognate viral Ag is required for the maintenance of virus-specific CD8 T cells during LCMV persistence (12). These findings have been partially extended to human infections with HIV and hepatitis C virus (13–16). Persistent virus infections can appear in two different forms, as follows: those that lead to progressive pathogen spread, viremia, and clinical disease (high-titer infections such as LCMV or HIV), and those that are immunologically contained after a generally asymptomatic infection (low-titer infections such as γ-herpesviruses). γ-Herpesviruses establish a systemic infection with a perpetual dynamic state of latency/reactivation that shapes the ongoing T cell response (17–21). Viral Ags are continuously presented during long-term persistence (21) and readily induce naive and memory CD8 T cell proliferation (22). Despite Ag persistence, immune surveillance maintains herpesviruses in check and CD8 T cell responses are not globally compromised (20, 21, 23–26). Altogether, these results highlight that persistent viral infections can last for the life of the host without apparent T cell dysfunction.
A key unanswered question that these findings elicit is whether memory CD8 T cells generated during a low-titer persistent viral infection can survive independently of cognate Ag and are capable of eliciting long-term protection. This issue is critical for the maintenance of memory after antiviral treatment during persistent infections. Some studies suggest that virus persistence may be necessary to maintain a CD8 T cell response (27–29), whereas others show stable or elevated T cell responses after antiviral drug therapy (30, 31). Whether these sustained or contracted T cell responses are protective to virus reinfection remains unknown and can only be tested in an animal model. We have addressed these issues using a model of γ-herpesvirus infection.
In the present study, we sought to analyze the capacity of Ag-experienced CD8 T cells from murine γ-herpesvirus-68 (γHV68) persistently infected mice to survive and proliferate in the absence of viral Ags and to subsequently mount a protective recall response. Our data show that memory CD8 T cells isolated from mice persistently infected with γHV68 have the ability to survive and homeostatically proliferate in vivo in the absence of cognate viral Ags. Furthermore, γHV68-specific memory CD8 T cells elicit a recall response by proliferating during secondary γHV68 infection after being rested in a recipient mouse in the absence of cognate virus. Finally, the data show that recall of Ag-independent γHV68-specific memory CD8 T cells confers enhanced protection against a virus challenge. Altogether, these data demonstrate that protective Ag-independent memory CD8 T cell responses are generated and maintained during herpesvirus persistence.
Materials and Methods
Mice and viral infection
C57BL/6J and B6.PL-Thy1a/CyJ mice were obtained from The Jackson Laboratory and Harlan Farms, or were bred at the Research Institute at Nationwide Children’s Hospital. γHV68, clone WUMS, was propagated and titered on a monolayer of NIH3T3 fibroblasts. Mice were housed in BL2 containment under pathogen-free conditions. The Institutional Animal Care and Use Committee approved all of the animal studies described in this work. Mice were anesthetized with 2,2,2,-tribromoethanol and intranasally inoculated with 1000 PFU γHV68 in 30 μl of HBSS.
Adoptive cell transfers and CFSE staining
Splenocytes from C57BL/6 mice at 3 mo post-γHV68 infection and non-infected age-matched controls were processed into single-cell suspensions, as described above. Cells were plated in flasks coated with anti-mouse IgG plus IgM Abs (Jackson ImmunoResearch Laboratories) for 1 h to enrich for T cells. The nonadherent cells were incubated with Fc block (CD16/32) and washed and stained with anti-CD44, anti-CD19, and anti-CD8 Abs. Cells were purified using a FACSVantage with Diva option; the CD19+ cells were gated out; and CD8+CD44+ cells were purified (purity 98%). The purified cells were labeled with 0.5 μM CFSE for 15 min at 37°C in HBSS. The cells were washed thoroughly, and 1 × 106 purified cells were injected i.v. into the recipient B6.PL mice. The presence of CD8+CD90.2+ CFSE-positive cells was analyzed in spleen, lung, and bone marrow cell suspensions from the recipient mice at different time points after transfer. When indicated, B6.PL recipient mice were exposed to a sublethal dose of irradiation. Briefly, the recipient B6.PL mice were exposed to a contained cesium source to receive 5.5 Gy (550 rad). The presence of γHV68 was routinely analyzed in the spleen of lymphopenic and nonlymphopenic host mice after adoptive transfer by limiting dilution nested PCR (21) and infective center assays (32).
Virus titers
The number of latently infected cells was determined on splenic cell suspensions on day 14 after infection using a a standard infectious center assay (32).
Flow cytometry analysis
Single-cell suspensions were obtained from the following organs: bronchial alveolar lavage, lung parenchyma, mediastinal lymph node (MLN), peripheral blood, spleen, and bone marrow. Cells were stained with Fc block (CD16/32), and then washed and stained with a combination of the following γHV68-specific MHC tetramers: open reading frame (ORF)6487– 495/Db, ORF61524–531/Kb, and Abs against CD8 (53-6.7) and CD90.2 (53.2-1). MHC tetramers were generated, as described (33), or obtained from the National Institutes of Health Tetramer Core Facility. Flow cytometry data were acquired on a BD LSR (BD Biosciences) and analyzed using FlowJo software. Gates were set using negative controls and isotype controls.
BrdU administration and staining
Recipient B6.PL mice were administered BrdU at 0.8 mg/ml in their drinking water for 14 days, changing out for fresh BrdU water every other day. Single-cell suspensions of the bone marrow and spleen were prepared at day 14 of BrdU administration. The BD Pharmingen BrdU flow kit was used to stain the samples following the manufacturer instructions.
Results
Memory CD8 T cells undergo homeostatic proliferation and mediate protective recall responses in lymphopenic hosts
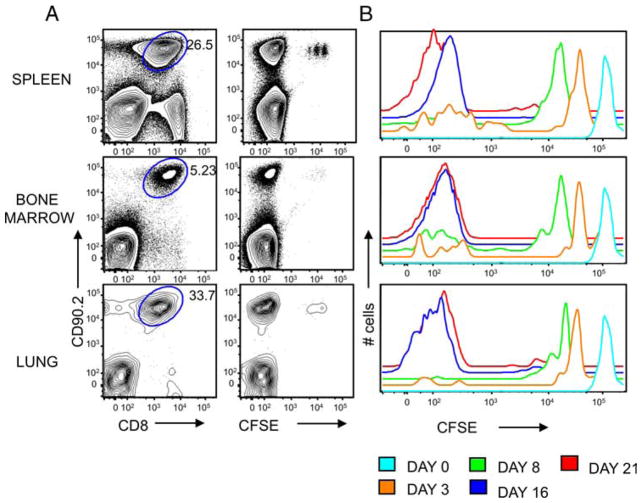
The continuous presence of persistent viral Ags is thought to maintain virus-specific CD8 T cells during persistent infections (9, 12). Using γHV68, it has been shown that memory CD8 T cells can undergo IL-15-independent proliferation and that they fail to proliferate in a naive secondary host (34). Thus, to test whether Ag-experienced CD8 T cells isolated from long-term γHV68-infected hosts can survive and proliferate in the absence of cognate viral Ags, we used an adoptive transfer system into lymphopenic hosts. The recipient mice were previously sublethally irradiated to create space for the newly transferred cells and to facilitate their engraftment (35, 36). We FACS purified Ag-experienced CD8 T cells (CD44+CD8+) from the spleens of mice infected with γHV68 for at least 3 mo, labeled them with CFSE, and i.v. transferred 1 × 106 cells per mouse into naive CD90.1 congenic recipients. This cell transfer represents an average frequency of 5,000 ORF6487–495/Db-specific CD8 T cells and 50,000 ORF61524–531/Kb-specific CD8 T cells transferred per mouse. To ensure that no γHV68 virus was transferred, B cells and macrophages were depleted by panning and plastic adherence. In addition, CD19+ cells were gated out during the FACS-sorting procedure. The presence of γHV68 was routinely analyzed in the spleen of adoptively transferred mice by limiting dilution nested PCR (21) and infective center assays (32), and no γHV68 was detected (data not shown). In addition, no features of γHV68-associated infectious mononucleosis (splenomegaly, selective expansion of virus-specific or Vβ4CD8 T cells) (37) were found in mice recipient of memory CD8 T cells during the course of the experiments presented in this study. The data show that we could detect the presence of donor CD8 T cells in the spleen, bone marrow, and lung of the recipient mice at different times after adoptive transfer (Fig. 1). Donor CD8 T cells constituted between 5 and 30% of the total number of cells in each recipient tissue on day 16, which suggests that they have dramatically expanded in the recipient mouse. The analysis of CFSE dilution shows that the transferred CD8 T cells proliferated in response to homeostatic cytokines in the absence of cognate viral Ags, as typical of Ag-independent memory T cells. High-CFSE fluorescence intensity could still be detected in a small fraction of donor CD8 T cells that had not proliferated on day 16 after adoptive transfer (Fig. 1), indicating that the decrease in CFSE signal intensity described above could not be due to a global decay in CFSE fluorescence intensity over time. Altogether, these data indicate that Ag-experienced CD8 T cells from mice latently infected with γHV68 have the ability to survive and proliferate in the absence of cognate viral Ag in a lymphopenic host.
FIGURE 1.
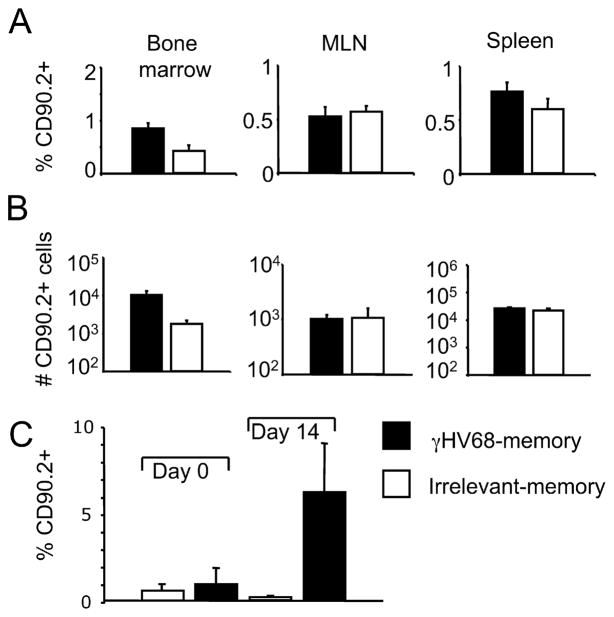
Ag-experienced CD8 T cells isolated from γHV68 persistently infected mice survive and proliferate in the absence of cognate viral Ags in lymphopenic hosts. Sublethally irradiated B6.PL mice received 1 × 106 CFSE-labeled CD44+CD8+ T cells isolated from the spleens of B6 mice that have been infected with γHV68 for at least 3 mo. A, Representative FACS plots of the spleen, bone marrow, and lung 16 days after adoptive cell transfer. Left column, Shows CD8 and CD90.2 staining. Numbers show the frequency of CD8+ CD90.2+ cells. Right column, Shows representative plots of the CFSE and CD90.2 staining of CD8 T cells. B, Analysis of the CFSE fluorescent intensity of donor CD90.2+CD8+ T cells in the spleen on days 0, 3, 8, 16, and 21 after adoptive transfer. Similar results were obtained in two independent experiments.
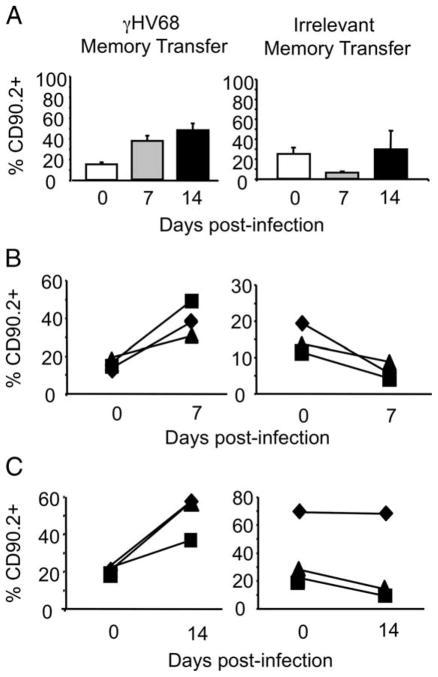
Next, we addressed whether the adoptively transferred CD8 T cells could mount a recall response to γHV68 after being rested in the absence of cognate viral Ags. We compared the recall capacity of Ag-experienced CD8 T cells purified from mice latently infected with γHV68 (termed γHV68 memory) with that of Ag-experienced CD8 T cells purified from noninfected control donor mice (termed irrelevant memory). After FACS purification and adoptive transfer, the γHV68 memory CD8 T cells or irrelevant memory cells were rested for 30 days in their respective naive congenic recipients. The data show that both populations of donor CD8 T cells could be detected in the peripheral blood of their respective recipients at least 1 mo after adoptive transfer (day 0; Fig. 2A). On day 30 after adoptive transfer, all of the mice were intranasally infected with γHV68. The percentage of donor CD8 T cells in the peripheral blood was analyzed before infection (day 0) and then on days 7 and 14 after γHV68 infection. The data show that γHV68 memory CD8 T cells of donor origin expand from 20% of the total CD8 T cell population (day 30 posttransfer, day 0 of infection) to 40 and 50% of the total CD8 T cells on days 7 and 14 after infection, respectively (Fig. 2A). The donor CD8 T cells did not expand in response to a γHV68 challenge in the recipient mice that have received irrelevant memory CD8 T cells. These data strongly suggest that the recall response was specific to γHV68 and not due to nonspecific inflammation or to an artifact of adoptively transferring cells into a lymphopenic environment, because irrelevant memory donor cells did not proliferate in response to γHV68 infection under similar conditions. Due to the high variability of the response in mice that received irrelevant memory, we performed an analysis of the recall response in individual mice. The data corroborate that there was a 2-fold increase in the frequency of donor CD8 T cells transferred from γHV68 memory mice on day 7 (Fig. 2B) or on day 14 (Fig. 2C) after γHV68 recall. The frequency of CD8 T cells of donor origin isolated from irrelevant memory mice either stayed the same or went down in their respective host after γHV68 infection. Taken together, these data indicate that memory CD8 T cells from mice latently infected with γHV68 can mount a recall response to γHV68.
FIGURE 2.
Rested memory CD8 T cells from γHV68 persistently infected mice proliferate in response to γHV68 virus recall in lymphopenic hosts. Sublethally irradiated B6.PL mice received 1 × 106 CFSE-labeled CD44+CD8+ T cells isolated from the spleens of B6 mice that have been infected with γHV68 for at least 3 mo or from noninfected age-matched controls. After adoptive cell transfer, the recipient B6.PL mice were rested for 30 days and subsequently infected with γHV68. A, Bar diagrams show the frequency of CD90.2+CD8+ T cells of donor origin in the peripheral blood on days 0, 7, and 14 after γHV68 challenge. The analysis of B6.PL mice that received γHV68 memory is on the left, and the analysis of B6.PL mice that received control irrelevant memory CD8 T cells is on the right. Bars represent the mean value of three experimental animals, and error bars indicate SEM. B, The graphs show the frequency of CD90.2+CD8+ T cells in PBLs of the same individual B6.PL recipient mouse before γHV68 infection and at day 7 after infection. Left panel, B6.PL mice that received γHV68 memory; right panel, B6.PL mice that received control irrelevant memory CD8 T cells. C, The graphs show the frequency of CD90.2+CD8+ T cells in the PBLs of the same individual recipient B6.PL mice before γHV68 infection and at day 14 after infection. Left panel, B6.PL mice that received γHV68 memory; right panel, B6.PL mice that received control irrelevant memory CD8 T cells. Similar results were obtained in two independent experiments.
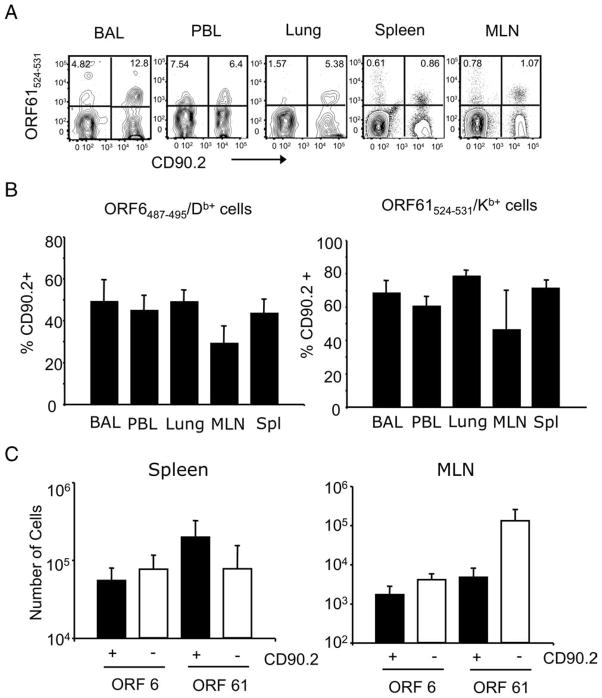
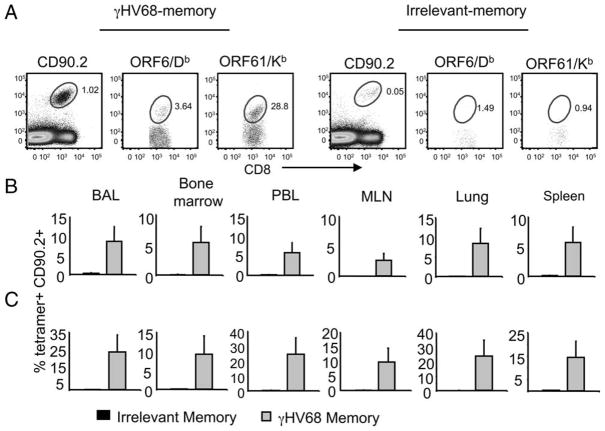
To test whether the recall response was mediated by γHV68-specific CD8 T cells of donor origin, we analyzed the frequency and number of Ag-specific cells of donor (CD90.2) and host (CD90.1) origin on day 14 after γHV68 infection (day 44 after adoptive transfer). The tetramer analysis shows that ORF6487–495/Db- and ORF61524–531/Kb-specific CD8 T cell populations of donor origin were conspicuous and could be detected in every organ analyzed (bronchoalveolar lavage, lung tissue, PBL, MLN, spleen, and bone marrow) (Fig. 3A). The data show that donor ORF6487–495/Db-specific CD8 T cells constitute 40 –50% of the total epitope-specific pool, and that 60–70% of the ORF61524–531/Kb-specific CD8 T cells are also of donor origin (Fig. 3B). The analysis of the total numbers of epitope-specific CD8 T cells of donor and host origin in the spleen and MLN of the adoptively transferred γHV68-infected mice shows that memory CD8 T cells of donor origin make a substantial contribution to the total antiviral T cell response (Fig. 3C). Whether this process is due to the faster proliferation of memory cells of donor origin than naive host cells or to donor T cell quenching of the host response via Ag or cytokine competition remains unclear.
FIGURE 3.
γHV68-specific memory CD8 T cells mount a recall response to γHV68 infection. Sublethally irradiated B6.PL mice received 1 × 106 CFSE-labeled CD44+CD8+ T cells isolated from the spleens of B6 mice that have been infected with γHV68 for at least 3 mo. The recipient B6.PL mice were rested for 30 days after adoptive cell transfer and subsequently infected with γHV68. On day 14 after infection, we analyzed bronchoalveolar lavage (BAL), peripheral blood lymphocytes (PBL), lung, MLN, and spleen of individual mice. A, Representative FACS plots showing tetramer-positive (ORF61524–531/Kb) cells of donor origin (CD90.2+) after gating on CD8+ T cells. Numbers indicate the frequency of tetramer-positive cells of donor (upper right quadrant) and host (upper left quadrant) origin. B, Bar diagrams show the frequency of cells of donor origin (CD90.2+) among virus-specific ORF6487–495/Db+ CD8+ T cells (left panel) or ORF61524–531/Kb+ CD8+ T cells (right panel). C, Bar diagrams represent the number of virus-specific CD8 T cells (ORF6487–495/Db+ and ORF61524–531/Kb+) that are of donor origin (CD90.2+) or host cells (CD90.2−). Left panel, Spleen; right panel, MLN. Bars represent the mean value of three experimental animals, and error bars indicate SEM. Similar results were obtained in two independent experiments.
Next, we assessed the ability of these rested memory CD8 T cells isolated from mice latently infected with γHV68 to protect against secondary infection. After FACS purification and adoptive transfer, the γHV68 memory CD8 T cells or irrelevant memory cells were rested for 30 days in their respective naive congenic recipients. On day 30 after adoptive transfer, the mice were intranasally infected with γHV68. At the peak of viral infection in the spleen (14 days postinfection), we analyzed the frequency of γHV68 latently infected cells by quantifying the ability of the virus to reactivate and cause plaques. The frequency of infected cells in the spleen was compared between mice that received γHV68 memory or irrelevant memory CD8 T cells. The data in Fig. 4 illustrate that the mice receiving CD8 T cells from γHV68 persistently infected donors had significant lower levels of splenic viral latency than the mice that received control Ag-experienced CD8 T cells (p ≤ 0.0001). These data indicate that γHV68-specific memory CD8 T cells preserve their capacity to mediate protective responses after Ag withdrawal. Altogether, these data demonstrate that after adoptive transfer into a lymphopenic environment free of cognate Ag, herpesvirus-specific memory CD8 T cells have the capacity to survive and to mount proliferative and protective recall responses.
FIGURE 4.
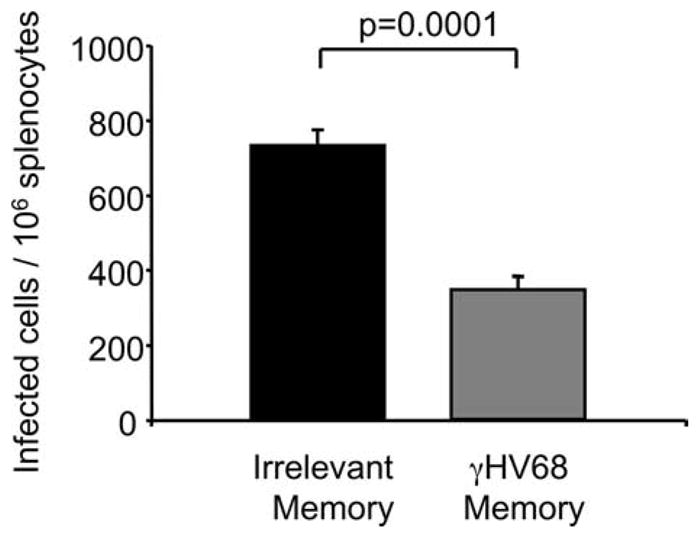
γHV68-specific memory CD8 T cells protect a γHV68 challenge. Sublethally irradiated B6.PL mice received 1 × 106 CFSE-labeled CD44+CD8+ T cells isolated from the spleens of B6 mice that have been infected with γHV68 for at least 3 mo (γHV68 memory;  ) or from noninfected age-matched controls (irrelevant memory; ■). The recipient B6.PL mice were rested for 30 days after adoptive cell transfer and subsequently infected with γHV68. The bar diagram shows the number of γHV68-infected cells in the spleen 14 days after infection. Bars represent the mean value of three experimental animals, and error bars indicate SEM. Similar results were obtained in two independent experiments.
) or from noninfected age-matched controls (irrelevant memory; ■). The recipient B6.PL mice were rested for 30 days after adoptive cell transfer and subsequently infected with γHV68. The bar diagram shows the number of γHV68-infected cells in the spleen 14 days after infection. Bars represent the mean value of three experimental animals, and error bars indicate SEM. Similar results were obtained in two independent experiments.
Survival and proliferation of memory CD8 T cells in γHV68-free mice
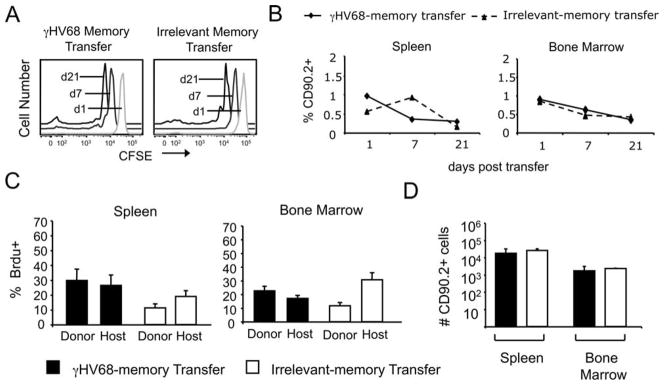
The survival of γHV68-specific CD8 T cells in adoptive hosts was unexpected in light of the previous literature (34), and thus, we considered the possibility that it was an artifact due to the lymphopenic environment. Sublethal irradiation creates an environment that enhances adoptive immunity (36). A potential concern with homeostatic proliferation during lymphopenia is that it can produce memory T cells with protective capacity (38, 39). We next addressed whether CD8 T cells from γHV68 latently infected mice could survive and proliferate without cognate Ags in a normal environment in which they have to compete with normal memory T cells from the host. We FACS purified Ag-experienced CD8 T cells (CD44+CD8+) from the spleens of mice that had been infected with γHV68 for at least 3 mo, labeled them with CFSE, and i.v. transferred 1 × 106 cells per mouse into recipient naive congenic B6.PL mice (CD90.1). CD8 T cells of donor origin could be detected at days 1, 7, and 21 after transfer in the spleen, bone marrow, and lung of mice that received γHV68 memory or irrelevant memory transfers (Fig. 5A). The analysis of CFSE dilution in the transferred cells suggests that they could proliferate at a slow rate (Fig. 5A). The frequency of CD8 T cells of donor origin decreased over time, but their values were similar between the γHV68 memory and irrelevant memory transfers in both the bone marrow and spleen (Fig. 5B). Next, to determine the ability of the donor CD8 T cells to proliferate in the naive recipients, we performed BrdU assays. At the time of adoptive transfer, the recipient mice were administered BrdU in their drinking water for 14 days. The percentage of cells that incorporated BrdU was analyzed by intracellular staining (Fig. 5C). The data show that in the mice that received the γHV68 memory transfer, the percentage of donor cells that have incorporated BrdU in the spleen was 30% and was nearly identical with the percentage of host CD8 T cells that incorporate BrdU. In the irrelevant memory transfer group, the frequency of cells that incorporated BrdU in the spleen was 15–20% and also similar between donor and host CD8 T cells. Similar data were obtained in the bone marrow. On day 14 post-transfer, the total number of donor CD8 T cells in the spleen and bone marrow was assessed in both the irrelevant memory transfer and the γHV68 memory transfer mice. The data show that the total number of donor cells is identical between the irrelevant memory and the γHV68 memory transfer mice (Fig. 5D). Because our data show that adoptively transferred memory CD8 T cells from latently infected mice incorporated BrdU, these indicate that they can survive and proliferate in the absence of cognate viral Ags by homeostatic proliferation. Their ability to survive and proliferate is similar to that of memory CD8 T cells isolated from noninfected control mice.
FIGURE 5.
Memory CD8 T cell isolated from γHV68-infected mice survive and proliferate in the absence of cognate viral Ags. B6.PL mice received 1 × 106 CFSE-labeled CD44+CD8+ T cells isolated from the spleens of B6 mice that have been infected with γHV68 for at least 3 mo (γHV68 memory transfer) or from noninfected age-matched controls (irrelevant memory transfer). A, FACS histograms show a temporal kinetic analysis of the CFSE intensity of CD90.2+CD8+ T splenocytes on days 1, 7, and 21 after adoptive transfer. Left panel, B6.PL mice that received γHV68 memory; right panel, B6.PL mice that received control irrelevant memory CD8 T cells. B, Frequency of CD8 T cells of donor origin (CD90.2+) on days 1, 7, and 21 after adoptive cell transfer in the spleen (left panel) and bone marrow (right panel). The dotted lines represent B6.PL mice that received control irrelevant memory CD8 T cells, and the solid line represents B6.PL mice that received γHV68 memory. C, On the day of adoptive cell transfer, B6.PL recipient mice were administered BrdU in their drinking water for 14 days. Bar diagrams display the frequency of donor CD90.2+CD8+ T cells and host CD90.2−CD8+ T cells that incorporated BrdU in the spleen (left panel) and bone marrow (right panel). D, Bar diagram shows the total number of donor CD90.2+CD8+ T cells in the spleen and bone marrow that have incorporated BrdU 14 days after adoptive cell transfer. ■, Represent B6.PL mice that received γHV68 memory CD8 T cells; □, represent B6.PL mice that received control irrelevant memory CD8 T cells. Bars represent the mean value of three experimental animals, and error bars indicate SEM.
γHV68-specific memory CD8 T cells mediate recall responses
To address whether memory CD8 T cells can mount an effective recall response after cognate Ag withdrawal, the adoptively transferred CD8 T cells were rested in naive recipients for 30 days, and the donor cell frequency among the total CD8 T cell population was determined in the bone marrow, MLN, and spleen on day 30 after transfer. The donor cell frequencies in the lymphoid organs of mice that received γHV68 memory cells were between 0.5 and 2% of the total CD8 T cell population, and ~0.5% in the mice that received irrelevant memory (Fig. 6A). The total donor cell numbers in the recipient mice on day 30 after transfer show no differences between mice that receive γHV68 memory or irrelevant memory (Fig. 6B). To examine the donor cell response to γHV68 infection, we analyzed the frequency of donor cells before and after infection with γHV68. The data show that 14 days after infection, the CD8 T cells of donor origin from recipient mice that received γHV68 memory expanded ~10-fold (Fig. 6C). The donor CD8 T cells from the mice that received irrelevant memory cells did not increase their frequency after γHV68 infection, suggesting that bystander activation or inflammation from the infection does not play a role in the cell expansion observed in mice recipient of γHV68 memory cells. Altogether, these data suggest that γHV68-specific memory CD8 T cells can mount a proliferative response during virus re-encounter after being rested in the absence of cognate Ags for up to 1 mo.
FIGURE 6.
Memory CD8 T cells from γHV68 persistently infected mice survive in an environment free of cognate γHV68 Ags, and mount a proliferative response during recall to γHV68. B6.PL mice (CD90.1) received 1 × 106 CFSE-labeled CD44+ CD8+ T cells isolated from the spleens of B6 mice (CD90.2) that have been infected with γHV68 for at least 3 mo (γHV68 memory; ■) or from noninfected age-matched controls (irrelevant memory; □). A, Bar diagrams represent the frequency of CD90.2+CD8+ T cells in the bone marrow, MLN, and spleen 30 days after adoptive cell transfer. B, Bar diagrams show the total cell number of CD90.2+CD8+ T cells in the bone marrow, MLN, and spleen 30 days after adoptive transfer. Recipient mice were rested for 30 days without Ag after adoptive cell transfer and subsequently infected with γHV68. C, Bar diagrams represent the frequency of donor CD90.2+CD8+T cells in PBLs on day 0 (before γHV68 infection) and on day 14 after γHV68 infection. Bars represent the mean value of three experimental animals, and error bars indicate SEM. Similar results were obtained in two independent experiments.
Next, to address whether the recall response of the adoptively transfer cells was virus specific, we analyzed the presence of virus-specific cells in the donor CD8 T cell populations using γHV68-specific tetrameric reagents. On day 14 after infection, we determined the frequency of ORF6487–495/Db and ORF61524–531/Kb CD8 T cells of donor origin in mice that had received γHV68 or irrelevant memory transfers 44 days before (Fig. 7). The results show that in every tissue analyzed, the frequency of γHV68-specific cells was 5- to 25-fold higher in the mice that received the γHV68 memory donor cells than in the mice that received irrelevant memory donor cells. The analysis of the total cell numbers corroborates these differences and shows that the magnitude of the Ag-specific response is dramatically enhanced in mice that received γHV68 memory cells compared with that of control mice that received irrelevant memory (Table I). Altogether, these data demonstrate that γHV68-specific memory CD8 T cells can survive in the absence of cognate Ag, and upon virus recall mount an efficient Ag-specific proliferative response. We considered the possibility that 30 days was not enough time for the γHV68-specific transferred cell to rest in the absence of cognate viral Ags. Thus, we performed the transfer experiments, waiting 60 days after adoptive transfer of γHV68 memory or irrelevant memory cells before infecting the recipient mice with γHV68. The data show that CD8 T cells of donor origin could be detected 60 days after transfer in both experimental groups (Fig. 8). Furthermore, the donor CD8 T cells from the mice that received γHV68 memory expanded 10-fold after exposure to γHV68 infection, whereas the donor cells from the mice that received an irrelevant memory transfer did not expand. These data corroborate that memory CD8 T cells purified from persistently γHV68-infected mice can survive long-term without cognate Ag and mount a proliferative recall response against γHV68.
FIGURE 7.
Rested γHV68-specific memory CD8 T cells mount a proliferative response upon Ag recall. B6.PL mice received 1 × 106 CFSE-labeled CD44+CD8+ T cells isolated from the spleens of B6 mice that have been infected with γHV68 for at least 3 mo (γHV68 memory) or from noninfected age-matched controls (irrelevant memory). The recipient B6.PL mice were rested for 30 days after adoptive cell transfer and subsequently infected with γHV68. All data presented in this figure are from day 14 after γHV68 infection. A, Representative FACS plots from mice that received γHV68 memory CD8 T cells (left panel) or control irrelevant memory T cells (right panel) by adoptive cell transfer. Left plot, CD8 vs CD90.2; shows the frequency of donor CD90.2+CD8+ T cells. Middle plot, CD8 vs ORF6487–495/Db; shows the frequency of tetramer-positive cells among donor CD90.2+CD8+ T cells. Right plot, CD8 vs ORF61524–531/Kb; shows the frequency of tetramer-positive cells among donor CD90.2+CD8+ T cells. B, Bar diagrams indicate the frequency of donor CD90.2+ ORF6487–495/Db+ cells among CD8 T cells. C, Bar diagrams represent the frequency of donor CD90.2+ ORF61524–531/Kb+ cells among CD8 T cells. ■, Indicate B6.PL mice that received control irrelevant memory CD8 T cells;  , indicate B6.PL mice that received γHV68 memory CD8 T cell transfer. Bars represent the mean value of six experimental animals, and error bars indicate SEM. Similar results were obtained in two independent experiments.
, indicate B6.PL mice that received γHV68 memory CD8 T cell transfer. Bars represent the mean value of six experimental animals, and error bars indicate SEM. Similar results were obtained in two independent experiments.
Table I.
Number of Ag-specific CD8 T cells of donor origin on day 14 after γ HV68 infection (day 44 after adoptive cell transfer)
| Antigen Specificityb | Transferc | Tissue Analyzeda |
||||
|---|---|---|---|---|---|---|
| Spleen | MLN | PBLs | Airways | Lung | ||
| ORF6487–495/Db | γHV68 memory | 35,015 | 353 | 107 | 631 | 2,528 |
| Irrelevant memory | 893 | 15 | 4 | 42 | 20 | |
| ORF61524–531/Kb | γHV68 memory | 220,674 | 2,934 | 755 | 1,966 | 17,230 |
| Irrelevant memory | 3,309 | 61 | 10 | 62 | 130 | |
Tissues from six individual mice were analyzed per group.
Tetramer-positive lymphocytes were gated as CD8+CD90.2+.
A total of 1 × 106 CD8+CD44+ T lymphocytes was purified from γHV68 persistently infected mice or irrelevant naive controls, and transferred on day 0 of the experiment, as described in Material and Methods.
FIGURE 8.
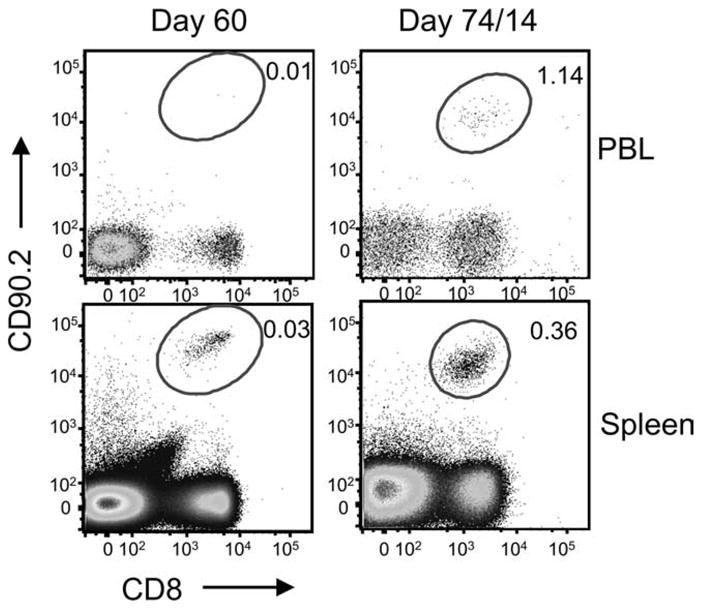
γHV68-specific memory CD8 T cells survive long-term without cognate Ag and mount a recall response. B6.PL mice received 1 × 106 CFSE-labeled CD44+CD8+ T cells isolated from the spleens of B6 mice that have been infected with γHV68 for at least 3 mo or from non-infected age-matched controls. The recipient B6.PL mice were rested for 60 days after adoptive cell transfer and subsequently infected with γHV68. FACS plots show the frequency of CD90.2+CD8+ T cells in PBLs and spleen at day 60 after adoptive transfer (left panel) and at day 14 after γHV68 infection (day 74 after adoptive cell transfer; right panel).
Rested memory CD8 T cells mediate antiviral protective responses
Having established that γHV68-specific memory cells can survive in the absence of cognate Ag and that they can mount a proliferative recall response against γHV68, we wanted to examine the ability of these rested γHV68-specific memory CD8 T cells to protect against a secondary infection with γHV68. Therefore, we adoptively transferred memory CD8 T cells from γHV68 persistently infected mice or from naive controls into naive congenic recipients. Thirty days after transfer, the mice were intranasally infected with γHV68, and the number of γHV68-infected cells was determined on day 14 after infection. The data in Fig. 9 show that the mice that received Ag-experienced CD8 T cells purified from persistently infected mice had significant lower numbers of infected cells than the mice that received control memory CD8 T cells (p ≤ 0.0079). These data demonstrate that γHV68-specific memory CD8 T cells preserve their protective capacity after prolonged cognate Ag withdrawal in a normal host.
FIGURE 9.
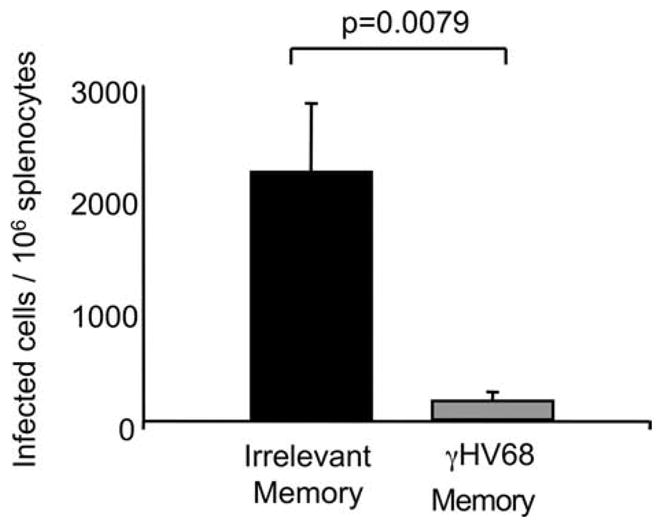
Rested γHV68-specific memory CD8 T cells mediate a protective recall response. B6.PL mice received 1 × 106 CFSE-labeled CD44+CD8+ T cells isolated from the spleens of B6 mice that have been infected with γHV68 for at least 3 mo (γHV68 memory; ■) or from non-infected age-matched controls (irrelevant memory;  ). The recipient B6.PL mice were rested for 30 days after adoptive cell transfer and subsequently infected with γHV68. Bar diagrams show the number of infected cells and splenocytes 14 days after infection. Bars represent the mean value of six experimental animals, and error bars indicate SEM. Similar results were obtained in two independent experiments.
). The recipient B6.PL mice were rested for 30 days after adoptive cell transfer and subsequently infected with γHV68. Bar diagrams show the number of infected cells and splenocytes 14 days after infection. Bars represent the mean value of six experimental animals, and error bars indicate SEM. Similar results were obtained in two independent experiments.
Discussion
The generation and maintenance of memory CD8 T cell responses during persistent viral infections are poorly understood. The current line of thought is that persistent infection leads to progressive CD8 T cell dysfunction and lack of memory formation. Our data show that after adoptive transfer into an Ag-free environment, γ-herpesvirus-specific memory CD8 T cells have the capacity to survive and homeostatically proliferate in the absence of cognate antigenic peptide, and to mount a proliferative and protective recall response. Importantly, these responses occur both in lymphopenic mice, in which the adoptively transferred memory CD8 T cells encounter an environment that enhances survival and memory generation, and in normal mice, in which the adoptively transferred memory CD8 T cells must compete with the host cells for limited resources and space. These results demonstrate that self-renewing memory CD8 T cells with antiviral protective capacity can develop during persistent viral infection.
T cells are critical in controlling γ-herpesvirus infections (25, 40 – 42). γ-Herpesviruses establish a lifelong infection that constantly influences the maintenance and function of the T cell response during virus persistence both in humans (24, 43) and mice (19, 20). The capacity of γHV68-specific memory CD8 T cells to survive and proliferate in vivo in the absence of persistent viral Ags represents the maintenance of a crucial attribute of a functional CD8 T cell response. These findings contrast with the maintenance of CD8 T cell memory against γHV68 in absence of IL-15 in IL-15-deficient mice (34), when IL-15 is critical for the generation and survival of memory T cells (44). It is possible that in the absence of IL-15, the survival of γHV68-specific memory CD8 T cells is mediated by viral-persistent Ags or other cytokines. However, this finding in a transgenic model does not exclude the possibility that a population of memory CD8 T cells with their capacity to homeostatically proliferate intact is maintained during long-term γ-herpesvirus persistence in immunocompetent hosts. It is possible that the γHV68-specific CD8 T cells that survive and proliferate in cognate Ag-free recipients represent a robust expansion of an otherwise small subpopulation of CD8 T cells, or it is also possible that a large pool of γHV68 memory CD8 T cells maintained their homeostatic proliferative capacity intact. Our data showing a global decay in CFSE intensity and the incorporation of BrdU in 20 –30% of the transferred CD8 T cells appear to support this second alternative. It should be noted that previous studies have indicated that a population of central-memory CD8 T cells is maintained during γHV68 infection (21) and the expression of IL-7R and IL-15R on γHV68-specific CD8 T cells does not show major deficiencies (20, 21). Furthermore, addition of IL-15 increased the survival of γHV68-specific CD8 T cells (19) and induced proliferation of a subpopulation of memory cells (20). Although the CD8 T cell responses against γ-herpesvirus infections can be very heterogeneous and epitope specific (19, 45), antiviral CD8 T cell functions are not apparently compromised during γ-herpesvirus persistence (21, 23).
Persistent Ag is required for the maintenance of CD8 T cell responses during persistent infection with LCMV-clone 13 (9, 12). Our data show that γHV68-specific CD8 T cells maintain the central-memory property of long-term Ag-independent survival. Can these two independent findings obtained with two different persistent infections be reconciled? First, it is possible that the form of virus persistence in the host can influence the maintenance of T cell memory. γHV68 persists in a latent state in multiple anatomical locations (46, 47), and, although the number of infected cells is low, lytic viral Ags are continuously being presented by professional APCs during long-term infection (21). Importantly, this Ag presentation during the persistent phase of infection is capable of inducing the proliferation of naive and memory T cells (22). Thus, γ-herpesvirus lytic Ags are continuously priming CD8 T cells during viral persistence, but they do not appear to be sufficient to induce global CD8 T cell dysfunction or lack of memory formation. Second, it is possible that there is a threshold of persistent Ag for maintaining memory CD8 T cells that proliferate in response to homeostatic cytokines. The antigenic load, not merely persistence, will be a crucial factor in determining the outcome of a memory CD8 T cell response (48). During γHV68 persistence, the number of infected cells is close to 1000 per spleen (21, 46, 49), whereas during LCMV-clone 13 persistence, viral titers are 104–106 PFU/g tissue (11, 50). γHV68 represents a persistent systemic infection in which the viral and antigenic load is relatively low and, thus, CD8 T cell function and memory are maintained.
We have modeled the elimination of the persistent pathogen using an adoptive transfer strategy because herpesviruses cannot be readily eliminated from the host. Our findings show that γHV68-specific memory CD8 T cells do not require cognate viral Ag for survival. Our experimental approach employs memory CD8 T cells from mice at 3 mo after infection, and different results may be obtained at earlier or later times after infection. Continuous exposure to low levels of persistent Ag for longer periods of time may alter the properties of γHV68-specific memory cells, although previous analyses at 8–9 mo after infection have shown maintenance of IL-7R expression and of cytotoxic capacity (21). The adoptively transferred memory CD8 T cells are efficiently maintained in lymphoid organs and in the periphery of naive recipient mice, despite the lack of cognate viral Ags. Two mutually nonexclusive mechanisms can facilitate this process: cell survival and cell proliferation. Through the use of CFSE and BrdU, we observed that cell proliferation is an important component for the maintenance of the transferred memory. These observations were made in the absence of cognate Ag stimulation, suggesting that homeostatic cytokines play a crucial role in this process. Importantly, we observed the existence of long-term memory CD8 T cells with antiviral protective capacity both in lymphopenic and normal hosts. This finding indicates that lymphopenia is not inducing a nonphysiological property on γHV68-specific memory CD8 T cells, but merely amplifying a pre-existent property of a subset of γHV68-specific memory CD8 T cells. Our findings appear to be reminiscent of those of Trypanosoma cruzi persistence in mice, in which stable CD8 T cell function and memory have been reported (51, 52). Drug-induced elimination of T. cruzi parasites results in the induction of a protective central-memory CD8 T cell response (53). Along the same lines, antiviral therapy during chronic hepatitis C virus or HIV infection can induce enhanced antiviral T cell responses (30, 31). Thus, data from several persistent infections support the idea that pathogen persistence is not sufficient to induce lack of memory CD8 T cell maintenance. Other parameters such as Ag load, pathogen tropism, immune evasion mechanisms, and quality of the CD4 T cell help are likely to play a fundamental role in shaping the memory CD8 T cell response to a persistent pathogen.
A critical aspect of our findings is that the memory CD8 T cell response generated during a persistent γ-herpesvirus infection has the capacity to mediate antiviral protection during a recall response. These data support the previous observation that γHV68-specific CD8 T cells maintain antiviral functions and mediate protection during the persistent phase of infection (21). Our data demonstrate that memory CD8 T cells that have been rested in the absence of cognate γHV68 Ags have the capacity to mount a proliferative response following secondary encounter with γHV68. In addition, these rested persistent memory CD8 T cells enhance the protection to a γHV68 challenge by reducing the number of infected cells in both lymphopenic and normal hosts. On day 14 after challenge, mice that received CD8 T cells isolated from γHV68-asymptomatic donors more than 1 mo before had significantly lower viral titers than control mice that received irrelevant CD8 T cells (p ≤ 0.0001 in lymphopenic hosts; p ≤ 0.0079 in normal hosts). Useful cellular memory must be long-lived and able to mount an effective recall response, and γHV68-specific CD8 T cell memory shows these two characteristics. These findings suggest a modified model of T cell memory generation during virus persistence in which, at least under conditions of controlled virus spread, a population of memory CD8 T cells with capacity for long-term Ag-independent survival and for mediating protection during secondary virus encounter can be maintained in the host. Protective memory T cell responses might be achieved by bringing or maintaining the persistent viral or antigenic load below a certain threshold that prevents triggering of progressive T cell dysfunction.
Acknowledgments
We thank J. Weslow-Schmidt for technical assistance, J. Durbin for critically reviewing the manuscript, and the Flow Cytometry Core Laboratory at the Research Institute for assistance with flow cytometry and sorting.
Footnotes
This work was supported in part by National Institutes of Health Grant AI-59603 and by the Research Institute.
Abbreviations used in this paper: LCMV, lymphocytic choriomeningitis virus; γHV68, murine γ-herpesvirus-68; MLN, mediastinal lymph node; ORF, open reading frame.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 2.Sprent J, Surh CD. T cell memory. Annu Rev Immunol. 2002;20:551–579. doi: 10.1146/annurev.immunol.20.100101.151926. [DOI] [PubMed] [Google Scholar]
- 3.Sprent J, Tough DF. T cell death and memory. Science. 2001;293:245–248. doi: 10.1126/science.1062416. [DOI] [PubMed] [Google Scholar]
- 4.Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Curr Opin Immunol. 2005;17:326–332. doi: 10.1016/j.coi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Lefrancois L. Development, trafficking, and function of memory T-cell subsets. Immunol Rev. 2006;211:93–103. doi: 10.1111/j.0105-2896.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 7.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 9.Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci USA. 2004;101:16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 12.Shin H, Blackburn SD, Blattman JN, Wherry EJ. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J Exp Med. 2007;204:941–949. doi: 10.1084/jem.20061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wherry EJ, Day CL, Draenert R, Miller JD, Kiepiela P, Woodberry T, Brander C, Addo M, Klenerman P, Ahmed R, Walker BD. HIV-specific CD8 T cells express low levels of IL-7Rα: implications for HIV-specific T cell memory. Virology. 2006;353:366–373. doi: 10.1016/j.virol.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al. Up-regulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 15.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 16.Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Up-regulation of PD-1 expression on circulating and intra-hepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249–9258. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen G, Shankar P, Lange C, Valdez H, Skolnik PR, Wu L, Manjunath N, Lieberman J. CD8 T cells specific for human immunodeficiency virus, Epstein-Barr virus, and cytomegalovirus lack molecules for homing to lymphoid sites of infection. Blood. 2001;98:156–164. doi: 10.1182/blood.v98.1.156. [DOI] [PubMed] [Google Scholar]
- 18.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 19.Obar JJ, Crist SG, Gondek DC, Usherwood EJ. Different functional capacities of latent and lytic antigen-specific CD8 T cells in murine γ herpesvirus infection. J Immunol. 2004;172:1213–1219. doi: 10.4049/jimmunol.172.2.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obar JJ, Fuse S, Leung EK, Bellfy SC, Usherwood EJ. γ Herpesvirus persistence alters key CD8 T-cell memory characteristics and enhances antiviral protection. J Virol. 2006;80:8303–8315. doi: 10.1128/JVI.00237-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cush SS, Anderson KM, Ravneberg DH, Weslow-Schmidt JL, Flano E. Memory generation and maintenance of CD8+ T cell function during viral persistence. J Immunol. 2007;179:141–153. doi: 10.4049/jimmunol.179.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kupresanin F, Chow J, Mount A, Smith CM, Stevenson PG, Belz GT. Dendritic cells present lytic antigens and maintain function throughout persistent γ-herpesvirus infection. J Immunol. 2007;179:7506–7513. doi: 10.4049/jimmunol.179.11.7506. [DOI] [PubMed] [Google Scholar]
- 23.Hislop AD, Annels NE, Gudgeon NH, Leese AM, Rickinson AB. Epitope-specific evolution of human CD8+ T cell responses from primary to persistent phases of Epstein-Barr virus infection. J Exp Med. 2002;195:893–905. doi: 10.1084/jem.20011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hislop AD, Kuo M, Drake-Lee AB, Akbar AN, Bergler W, Hammerschmitt N, Khan N, Palendira U, Leese AM, Timms JM, et al. Tonsillar homing of Epstein-Barr virus-specific CD8+ T cells and the virus-host balance. J Clin Invest. 2005;115:2546–2555. doi: 10.1172/JCI24810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rickinson AB, Callan MF, Annels NE. T-cell memory: lessons from Epstein-Barr virus infection in man. Philos Trans R Soc London B Biol Sci. 2000;355:391–400. doi: 10.1098/rstb.2000.0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simon CO, Holtappels R, Tervo HM, Bohm V, Daubner T, Oehrlein-Karpi SA, Kuhnapfel B, Renzaho A, Strand D, Podlech J, et al. CD8 T cells control cytomegalovirus latency by epitope-specific sensing of transcriptional reactivation. J Virol. 2006;80:10436–10456. doi: 10.1128/JVI.01248-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalams SA, Goulder PJ, Shea AK, Jones NG, Trocha AK, Ogg GS, Walker BD. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J Virol. 1999;73:6721–6728. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casazza JP, Betts MR, Picker LJ, Koup RA. Decay kinetics of human immunodeficiency virus-specific CD8+ T cells in peripheral blood after initiation of highly active antiretroviral therapy. J Virol. 2001;75:6508–6516. doi: 10.1128/JVI.75.14.6508-6516.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sester U, Sester M, Kohler H, Pees HW, Gartner BC, Wain-Hobson S, Bocharov G, Meyerhans A. Maintenance of HIV-specific central and effector memory CD4 and CD8 T cells requires antigen persistence. AIDS Res Hum Retroviruses. 2007;23:549–553. doi: 10.1089/aid.2006.0234. [DOI] [PubMed] [Google Scholar]
- 30.Pillai V, Lee WM, Thiele DL, Karandikar NJ. Clinical responders to antiviral therapy of chronic HCV infection show elevated antiviral CD4+ and CD8+ T-cell responses. J Viral Hepatitis. 2007;14:318–329. doi: 10.1111/j.1365-2893.2006.00804.x. [DOI] [PubMed] [Google Scholar]
- 31.Baker CA, Emenyonu N, Ssewanyana I, Jones NG, Elrefaei M, Nghania F, Nakiwala J, Andia I, Clark R, Martin J, et al. Profile of immunologic recovery in HIV-infected Ugandan adults after antiretroviral therapy. AIDS Res Hum Retroviruses. 2007;23:900–905. doi: 10.1089/aid.2006.0309. [DOI] [PubMed] [Google Scholar]
- 32.Flano E, Woodland DL, Blackman MA. Requirement for CD4+ T cells in Vβ4+CD8+ T cell activation associated with latent murine γ herpes-virus infection. J Immunol. 1999;163:3403–3408. [PubMed] [Google Scholar]
- 33.Altman JD, Moss PH, Goulder PR, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 34.Obar JJ, Crist SG, Leung EK, Usherwood EJ. IL-15-independent proliferative renewal of memory CD8+ T cells in latent γ herpesvirus infection. J Immunol. 2004;173:2705–2714. doi: 10.4049/jimmunol.173.4.2705. [DOI] [PubMed] [Google Scholar]
- 35.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 36.Singh NJ, Schwartz RH. The lymphopenic mouse in immunology: from patron to pariah. Immunity. 2006;25:851–855. doi: 10.1016/j.immuni.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Blackman MA, Flano E, Usherwood E, Woodland DL. Murine γ-herpesvirus-68: a mouse model for infectious mononucleosis? Mol Med Today. 2000;6:488–490. doi: 10.1016/s1357-4310(00)01813-x. [DOI] [PubMed] [Google Scholar]
- 38.Cho BK, V, Rao P, Ge Q, Eisen HN, Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J Exp Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- 40.Khanna R, Burrows SR. Role of cytotoxic T lymphocytes in Ep-stein-Barr virus-associated diseases. Annu Rev Microbiol. 2000;54:19–48. doi: 10.1146/annurev.micro.54.1.19. [DOI] [PubMed] [Google Scholar]
- 41.Moore PS, Chang Y. Kaposi’s sarcoma-associated herpesvirus immunoevasion and tumorigenesis: two sides of the same coin? Annu Rev Microbiol. 2003;57:609–639. doi: 10.1146/annurev.micro.57.030502.090824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doherty PC, Christensen JP, Belz GT, Stevenson PG, Sangster MY. Dissecting the host response to a γ-herpesvirus. Philos Trans R Soc London B Biol Sci. 2001;356:581–593. doi: 10.1098/rstb.2000.0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hislop AD, Gudgeon NH, Callan MF, Fazou C, Hasegawa H, Salmon M, Rickinson AB. EBV-specific CD8+ T cell memory: relationships between epitope specificity, cell phenotype, and immediate effector function. J Immunol. 2001;167:2019–2029. doi: 10.4049/jimmunol.167.4.2019. [DOI] [PubMed] [Google Scholar]
- 44.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 45.Callan MF, Fazou C, Yang H, Rostron T, Poon K, Hatton C, McMichael AJ. CD8+ T-cell selection, function, and death in the primary immune response in vivo. J Clin Invest. 2000;106:1251–1261. doi: 10.1172/JCI10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flano E, I, Kim J, Moore J, Woodland DL, Blackman MA. Differential γ-herpesvirus distribution in distinct anatomical locations and cell subsets during persistent infection in mice. J Immunol. 2003;170:3828–3834. doi: 10.4049/jimmunol.170.7.3828. [DOI] [PubMed] [Google Scholar]
- 47.Tibbetts SA, Loh J, Van Berkel V, McClellan JS, Jacoby MA, Kapadia SB, Speck SH, Virgin HWt. Establishment and maintenance of γ herpesvirus latency are independent of infective dose and route of infection. J Virol. 2003;77:7696–7701. doi: 10.1128/JVI.77.13.7696-7701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klenerman P, Hill A. T cells and viral persistence: lessons from diverse infections. Nat Immunol. 2005;6:873–879. doi: 10.1038/ni1241. [DOI] [PubMed] [Google Scholar]
- 49.Flano E, I, Kim J, Woodland DL, Blackman MA. γ-Herpesvirus latency is preferentially maintained in splenic germinal center and memory B cells. J Exp Med. 2002;196:1363–1372. doi: 10.1084/jem.20020890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wherry EJ, Blattman JN, Ahmed R. Low CD8 T-cell proliferative potential and high viral load limit the effectiveness of therapeutic vaccination. J Virol. 2005;79:8960–8968. doi: 10.1128/JVI.79.14.8960-8968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bixby LM, Tarleton RL. Stable CD8+ T cell memory during persistent Trypanosoma cruzi infection. J Immunol. 2008;181:2644–2650. doi: 10.4049/jimmunol.181.4.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin DL, Tarleton RL. Antigen-specific T cells maintain an effector memory phenotype during persistent Trypanosoma cruzi infection. J Immunol. 2005;174:1594–1601. doi: 10.4049/jimmunol.174.3.1594. [DOI] [PubMed] [Google Scholar]
- 53.Bustamante JM, Bixby LM, Tarleton RL. Drug-induced cure drives conversion to a stable and protective CD8+ T central memory response in chronic Chagas disease. Nat Med. 2008;14:542–550. doi: 10.1038/nm1744. [DOI] [PMC free article] [PubMed] [Google Scholar]