Abstract
Objective
We recently identified a population of small Sca-1+/Lin−/CD45− cells in adult murine bone marrow that express several epiblast/germ line and pluripotent stem cell markers (e.g., Oct-4 and SSEA-4) that we named “very small embryonic-like stem cells” (VSELs). In this report, we test the hypothesis that VSELs can differentiate along the hemato/lymphopoietic lineage.
Material and Methods
Purified from BM VSELs were primed/co-cultured over OP9 stroma cell line and subsequently tested in vitro and in vivo assays for their hematopoietic potential. In parallel cells derived from VSELs were evaluated for expression of hematopoietic genes and surface markers.
Results
While we observed that freshly isolated VSELs do not exhibit in vitro and in vivo hematopoietic potential, they may, after co-culture over OP9 stromal cells, differentiate along the hematopoietic lineage in a similar way as embryonic stem cells or inducible pluripotent stem cells. “OP9-primed,” VSEL-derived cells acquired expression of several hemato/lymphopoiesis-specific genes and markers, gave rise to hematopoietic colonies in vitro, and protected lethally irradiated mice in both primary and secondary transplant models upon transplantation. We also observed that, compared to hematopoietic stem/progenitor cells (HSCs), VSELs are highly resistant to total body irradiation.
Conclusions
Based on these observations, we postulate that VSELs are the most primitive murine BM-residing population of stem cells that have the potential to become specified into the hematopoietic lineage and thus may share some of the characteristics of long-term repopulating HSCs.
Keywords: VSELs, OP9 stroma, long term repopulating hematopoietic stem cells
Introduction
In addition to hematopoietic stem/progenitor cells (HSPCs), bone marrow contains other types of developmentally early stem/progenitor cells, including mesenchymal stem cells (MSCs) and endothelial progenitor cells (EPCs) [1, 2]. Cells with the ability to differentiate into multiple germ layers were recently identified in BM tissue, including multipotent adult progenitor cells (MAPC) [3], marrow-isolated adult multipotent-induced (MIAMI) cells [4], as well as a population of so-called very small embryonic/epiblast-like stem cells (VSELs) identified by us [5].
We have hypothesized that VSELs are deposited at the beginning of gastrulation in developing tissues and play an important role as a backup population for tissue-committed stem cells (TCSCs) [5]. We envision that during steady-state conditions these cells may be involved in tissue rejuvenation and participate in tissue repair after organ injury [6, 7]. Molecular analysis of adult bone marrow (BM)-derived, purified VSELs revealed that they i) express pluripotent stem cell (PSC) genes (e.g., Oct-4, Nanog, Klf-4, and SSEA-1), ii) share several markers characteristic of epiblast as well as migratory primordial germ cells (PGCs), and iii) possess a unique pattern of genomic imprinting (namely erasure of differently methylated regions at the Igf2-H19 and Rasgrf1 loci and hypermethylation at the KCNQ1 and Igf2R loci) [5-7]. These characteristics suggest that VSELs are related to epiblast-derived migrating PGC-like cells and, despite their PSC character, changes in the epigenetic signature of imprinted genes keep these cells quiescent in adult tissues and prevent them from uncontrolled proliferation (e.g., teratoma formation). On the other hand, epigenetic changes or mutations that lead to activation of imprinted genes in these cells could potentially lead to tumorogenesis [8]. Using appropriate experimental models, we have already demonstrated that VSELs may give rise to MSCs [9], neural cells, cardiomyocytes, and insulin-producing cells [4].
It is well known that murine and human BM contain a population of long-term repopulating hematopoietic stem cells (LT-HSCs); however, the exact phenotype of these cells is not well defined and varies between reports [10]. For example, based on surface expression markers, the phenotype of murine long-term engrafting LT-HSCs is described as Thy 1.1lo Lin- Scahigh Mac1- CD4-or Thy1.1low Flk-2- [11] or CD150high CD34-/low c-Kit+ Sca-1+ Lin- [12]. However, evidence is accumulating that these surface markers do not identify all the cells that reside in BM and are endowed with long-term hematopoietic potential [13-15].
Because of this problem, we decided to evaluate whether BM-residing, developmentally early VSELs isolated by our team can give rise to the hemato/lymphopoietic lineage. When employing in vitro and in vivo assays in co-cultures with OP9 stromal cells, we observed that VSELs may become specified into the hemato/lymphopoietic lineage. Thus, our data suggest that VSELs may share some characteristics with the most primitive LT-HSCs.
Material and Methods
Isolation and FACS sorting of VSELs from murine bone marrow
This study was performed in accordance with the guidelines of the Animal Care and Use Committee of the University of Louisville School of Medicine and with the Guide for the Care and Use of Laboratory Animals (Department of Health and Human Services, Publication No. NIH 86-23).
VSELs were isolated from BM of adult male or female transgenic (with enhanced green fluorescence protein [EGFP]) C57BL/6 mice (4–8 weeks old; Jackson Laboratory, Bar Harbor, ME, USA). Briefly, BM was flushed from tibias and femurs and the population of total nucleated cells (TNCs) was obtained after lysis of RBCs using 1× BD Pharm Lyse Buffer (BD Pharmingen, San Jose, CA, USA). TNCs were subsequently stained for CD45, hematopoietic lineage markers (Lineage [Lin]), and Sca-1 antigen for 30 minutes in medium containing 2% FBS. The following anti-mouse antibodies (mAbs; BD Pharmingen, San Jose, CA, USA) were employed for staining: rat anti-CD45 (allophycocyanin-Cy7 [APC-Cy7]; clone 30-F11), anti-CD45R/B220 (phycoerythrin [PE]; clone RA3-6B2), anti-Gr-1 (PE; clone RB6-8C5), anti-TCRαβ (PE; clone H57-597), anti-TCRγδ (PE; clone GL3), anti-CD11b (PE; clone M1/70), anti-Ter119 (PE; clone TER-119), and anti-Ly-6A/E (Sca-1) (biotin; clone E13-161.7, with streptavidin conjugated to PE-Cy5). Cells were then washed, re-suspended in RPMI 1640 medium with 10% FBS, and sorted by MoFlo cell sorter (Dako, Carpinteria, CA, USA). The Sca-1+Lin−CD45− cells (VSELs) and control Sca-1+Lin−CD45+ cells (HSCs) were isolated according to the gating and sorting strategy described below (Figure 1).
Figure 1. Gating strategy for sorting VSELs and HSCs by FACS.
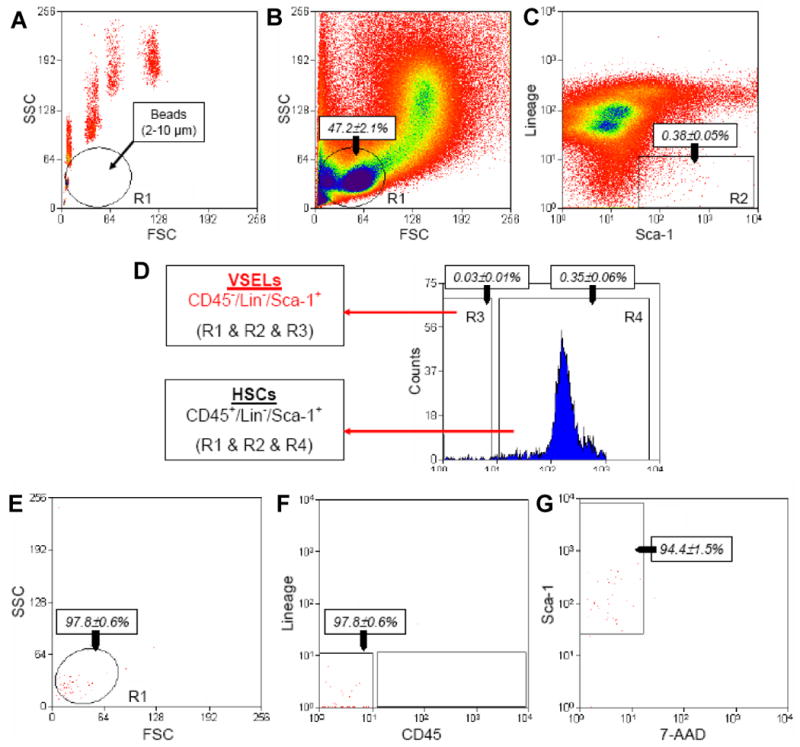
BM-derived VSELs and HSCs were isolated from murine BM total nucleated cells (TNCs) following immunostaining for Sca-1, CD45, and hematopoietic lineages markers (Lineage [Lin]). Panel A: Agranular, small events between 2–10 μm in diameter were assigned to gate R1 after comparison with predefined-size beads with standard diameters of 1, 2, 4, 6, 10, and 15 μm (Flow Cytometry Size beads, Invitrogen; Molecular Probes, Carlsbad, Ca, USA). Panel B: BM-derived TNCs were visualized by dot plot showing forward scatter (FSC) vs. side scatter (SSC) signals, which are related to the size and granularity/complexity of cells, respectively. Panel C: Cells from region R1 were further analyzed for Sca-1 and Lineage marker expression. The population of Sca-1+/Lin− objects was included in region R2 and subsequently sorted into CD45− and CD45+ subpopulations based on CD45 marker expression. Panel D shows regions R3 and R4, respectively. Sca-1+/Lin−/CD45- cells (VSELs) were sorted as cells from the logical gate including regions R1, R2, and R3, while Sca-1+/Lin−/CD45+ cells (HSCs) were from the gate including regions R1, R2, and R4. Percentages show the average content of each cellular subpopulation (±SEM) in total BM nucleated cells. Panels E and F show the purity of sorted VSELs, while panel G presents their viability following staining with 7-aminoactinomycin D (7-AAD).
Ex vivo differentiation of VSELs into hematopoietic cells in primary co-cultures over OP9 stromal cells
Freshly sorted Sca-1+Lin−CD45− VSELs and Sca-1+Lin−CD45+ HSCs from BM were plated over OP9 cells in α-MEM with 20% FBS (Molecular Probes®, Invitrogen) for 5 days and subsequently trypsinized, washed by centrifugation in α-MEM, and replated in methylcellulose-based medium (StemCell Tech, Vancouver, BC, CAN).
Evaluation of the clonogenic potential of sorted cells in methylcellulose cultures
VSELs or HSCs freshly isolated from BM or cells harvested from OP9 cultures (primary cultures) were plated in methylcellulose-based medium (StemCell Tech, Vancouver, BC, CAN) supplemented with murine stem cell growth factor (SCF), interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF), FLT3, thrombopoietin (TpO), erythropoietin (EpO), and insulin growth factor-2 (IGF-2). Cells were cultured for 5 days and the colonies formed were scored. Subsequently, methylcellulose cultures were solubilized and trypsinized and the resulting cells were washed by centrifugation in α-MEM and plated into secondary methylcellulose cultures. Cells were grown in the presence of the same growth factors and replated after 5 days into new methylcellulose cultures. The same procedure was repeated for the next 2 passages.
PCR analysis of gene expression in freshly sorted cells and OP9-expanded cells
Total RNA from various cells (approximately 20,000 cells) was isolated using the RNeasy Mini Kit (Qiagen Inc., Valencia, CA) and genomic DNA removed using the DNA-free™ Kit (Applied Biosystems, Foster City, CA). Isolated messenger (m)RNA was reverse-transcribed with Taqman Reverse Transcription Reagents (Applied Biosystems) according to the manufacturer's instructions. RT-PCR was performed using Amplitaq Gold (Applied Biosystems) with 1 cycle of 8 min at 95°C; 2 cycles of 2 min at 95°C, 1 min at 62°C, and 1 min at 72°C; 38 cycles of 30 sec at 95°C, 1 min at 62°C, and 1 min at 72°C; and 1 cycle of 10 min at 72°C using sequence-specific primers. Quantitative measurement of target transcript expression was performed by RQ-PCR using an ABI Prism 7500 Sequence Detection System (Applied Biosystems). Complementary (c)DNA from indicated cells was amplified using SYBR Green PCR Master Mix (Applied Biosystems) and specific primers. All primers were designed with Primer Express software (Applied Biosystems), with at least one primer in each pair containing an exon-intron boundary. The threshold cycle (Ct) was determined and relative quantification of the expression level of target genes was obtained with the 2−ΔΔCt method, using β2-microglobulin (β2mg) as an endogenous control gene and mononuclear cell (MNC) genes as calibration controls. All primers used in RT- and RQ-PCR are shown in Table I.
Table I. Sequences of primers employed for RT-PCR analysis.
| Gene | Forward | Reverse |
|---|---|---|
| Ikaros | CACTACCTCTGGAGCACAGC | TCTGAGGCATAGAGCTCTTA |
| Lmo2 | ATGTCCTCGGCCATCGAAAGG | AGATGATCCCATTGATCTTGG |
| GATA-2 | CATTGGCCCCTTGTGAGGCCAG | CGCTCCAGCCAGATTCGACCC |
| HOXB4 | GCACGGTAAACCCCAATTA | GGCAACTTGTGGTCTTTTTT |
| PU.1 | ATGGAAGGGTTTTCCCTCACCGCC | GTCCACGCTCTGCAGCTCTGTGAA |
| SCL | TCCCCATATGAGATGGAGATTTC | ATTGATGTACTTCATGGCAAGG |
| c-myb | GAGCTTGTCCAGAAATATGGTCCGAAG | GGCTGCCGCAGCCGGCTGAGGGAC |
| Oct-4 | ACATCGCCAATCAGCTTGG | AGAACCATACTCGAACCACATCC |
| βActin | CGACGATGCTCCCCGGGCTGTA | CTCTTTGATGTCACGCACGATTTCCCTCT |
FACS analysis of OP9-expanded cells
Cells were OP9 primed/expanded and plated in methylcellulose to grow hematopoietic colonies. Subsequently, colonies were solubilized and evaluated by FACS (LSRII, BD Biosciences) for expression of CD45, CD41, and Gr-1. For detection of CD45, we employed clone 30-F11, rat APC-Cy7-conjugated Abs; to detect CD41, we employed rat PE-conjugated Abs; and to detect Gr-1, we employed clone RB6-8C5, rat PE-conjugated Abs. All antibodies were obtained from BD Biosciences.
Hematopoietic transplantation studies
For transplantation experiments, mice were irradiated with a lethal dose of γ-irradiation (950 cGy). After 24 hours, mice were transplanted with cells (freshly isolated VSELs or HSCs, or OP9-“primed” expanded cells from secondary methylcellulose cultures) with or without wt BM MNCs by tail vein or intra-femoral injection. Anesthetized transplanted mice were sacrificed 3 or 6 months after transplantation to evaluate chimerism. In some of the experiments, secondary transplants were performed with GFP+-sorted BM cells isolated from chimeric mice transplanted with GFP+ cells.
Irradiation and BrdU staining
To evaluate the resistance of VSELs to radiation, adult C57BL/6 mice (4–8 weeks old; Jackson Laboratory, Bar Harbor, ME, USA) underwent whole-body irradiation with the following doses of gamma radiation: 0, 250, 500, 1000, and 1500 cGy. Mice were sacrificed after 4 days post irradiation and the content of VSELs (Sca-1+Lin−CD45−) and HSCs (Sca-1+Lin−CD45+) in BM was evaluated by flow cytometry. Proliferation events in BM-derived VSEL and HSC populations were examined by bromedeoxyuridine (BrdU) incorporation following whole-body irradiation by flow cytometry. Briefly, mice were gamma irradiated (0, 500, and 1000 cGy) and daily intraperitoneally injected with 5 mg BrdU (Sigma Aldrich, St. Louis, Mo, USA) for 4 days. BM was subsequently isolated and TNCs were immunostained for CD45, Lin markers, Sca-1 (as described above), as well as for BrdU (FITC BrdU Flow Kit; BD Pharmingen, San Jose, CA, USA). Samples were analyzed with the LSR II flow cytometer (BD Biosciences; San Jose, CA, USA).
Statistical analysis
All data in quantitative (q)ChIP and gene expression analyses were analyzed using one-factor Analysis of Variance (ANOVA) with Bonferroni's Multiple Comparison Test. We used the Instat1.14 program (GraphPad Software, La Jolla, CA) and statistical significance was defined as p<0.05 or p<0.01.
Results
Size marker bead-directed sorting strategy for murine BM-derived VSELs
By employing flow cytometry and size marker beads, we have confirmed that the majority of Sca-1+Lin−CD45− cells (VSELs) isolated from adult BM are unusually small (<6 μm); larger than peripheral blood platelets, but smaller than erythrocytes [16].
As shown in Figure 1, panel A, we employed a novel size-based approach using defined-size marker beads for isolating rare and small VSELs from murine BM by FACS. The overall sorting strategy is based on gating in regions containing small-diameter events (2–10 μm), as indicated in the dot plot (region R1) (Figure 1, panel B). This region contains mostly cell debris, but also rare nuclear cell events, including the population of VSELs. It is well known that most of the sorting protocols exclude events smaller than erythrocytes (<6 μm in diameter) as debris or platelets, which may explain why unusually small VSELs have, in the past, been excluded from sorted cell populations [17].
Figure 1 (panels A and B) shows that the size of the sorted cells from region R1 was determined very well by comparing them with the mixture of beads with predefined sizes (1, 2, 4, 6, 10 and 15 μm in diameter). The events enclosed in this region R1 include an average of 47.2 ± 2.1% total events, which were further analyzed for the expression of Sca-1 and lineage markers (Lin). The Sca-1+/Lin− events shown in region R2 (Figure 1, panel C) consisted on average 0.38 ± 0.05% of total analyzed nucleated cells (TNCs). Since we employed the antibodies against Ter119 in our “lineage cocktail”, small CD45− cells from the erythroid lineage (e.g., erythroblasts) were excluded from our sorting populations. Cells from region R2 were subsequently sorted according to the expression of CD45 antigen and divided into Sca-1+/Lin−/CD45− (region R3) and Sca-1+Lin−CD45+ (region R4) subpopulations (Figure 1, panel D) that contain, as we reported previously, VSELs and HSPCs, respectively [1, 5-7]. We found that VSELs comprised an average 0.030 ± 0.008% and HSPCs an average 0.347 ± 0.057% of TNCs (Figure 1, panel D).
Figure 1, panels E and F shows the post-sort reanalysis of sorted VSELs, which revealed their high purity (97.83 ± 0.63%). At the same time, we demonstrated that 94.43 ± 1.49% of these cells were negative for staining with 7-AAD dye, confirming that “contamination” by anucleated debris was excluded from the population of viable cells in this step (Figure 1, panel G). In sum, Figure 1, panels E–G shows that VSELs sorted from murine BM via this novel sizing and staining strategy are a homogenous and viable population of small nucleated cells.
Lack of hematopoietic potential in vitro and acquisition of hematopoietic potential after expansion over OP9 cells
Initially, we became interested in the hematopoietic potential of highly purified murine BM-derived Sca-1+Lin−CD45+ HSPCs and Sca-1+Lin−CD45− VSELs and employed several in vitro and in vivo assays to evaluate the hematopoietic potential of these cells.
Figure 2 panel A shows the in vitro clonogenic potential of both cell populations plated in methylcellulose cultures after stimulation with a cocktail of growth factors and cytokines (KL, EpO, TpO, IL-3, GM-CSF, FLT-3, and IGF-II), and we observed that, in contrast to HSPCs, VSELs freshly isolated from BM did not grow any hematopoietic colonies. Specifically, freshly sorted 104 Sca-1+Lin−CD45+ HSPCs and 104 Sca-1+Lin−CD45- VSELs formed, on average, ∼380–430 and 1–3 colonies per plating, respectively.
Figure 2. VSELs are specified into HSCs in co-cultures over OP9 stromal cells.
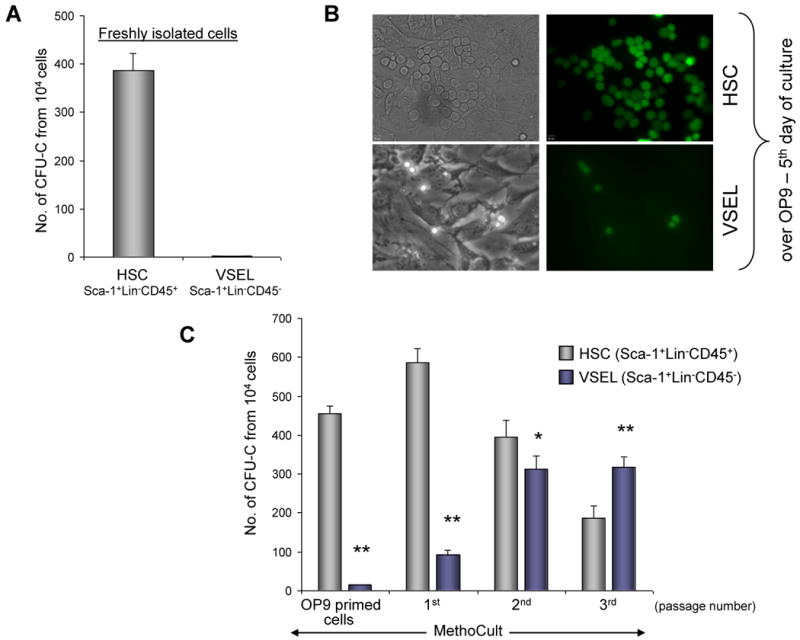
Panel A: In contrast to HSCs, VSELs freshly isolated from murine BM do not grow hematopoietic colonies. Panel B: Day 5 cultures over OP9 stromal cells initiated by HSCs and VSELs isolated from GFP+ mice (representative pictures are shown; magnification × 20). Panel C: The number of colonies formed in methylcellulose by OP9-primed VSELs and HSCs, as well as VSEL- and HSC-derived cells replated every 5 days in methylcellulose cultures (1st, 2nd, and 3rd passage). The data shown in Panels A and C represent the combined results from four independent experiments carried out in triplicate per group (n=12). * p<0.05, ** p<0.0001.
Taking into consideration the primitive embryonic/epiblast/germ line phenotype of VSELs, we assumed that an in vitro clonogenic test would not detect their hematopoietic potential. Therefore, we switched to the OP9 co-culture expansion system that is widely employed to grow hematopoietic cells from ESCs and iPS cells [18-20]. The same numbers of sorted VSELs and HSPCs (104) from GFP+ transgenic mice were plated into glass-bottom Petri dishes that contained OP9 stromal support. Five days latter, we observed some green cobblestone-area colonies (CAFCs) formed by Sca-1+Lin−CD45+ HSPCs and once-dividing green Sca-1+Lin−CD45− VSELs (Figure 2 panel B).
Subsequently, these cultures were trypsinized and all cells from trypsinized dishes, including GFP+ HSPCs or VSELs and OP9 cells, after washing away old methylcellulose, were replated into new methylcellulose cultures supplemented with a cocktail of growth factors and cytokines. Five days later, the total number of growing colonies was scored again. Figure 2 panel C shows that after 5 days of co-culture, there was high clonogenicity of OP9-primed HSPCs, while only rare colonies of OP9-primed VSELs were formed.
These methylcellulose cultures were again solubilized and trypsinized; after washing, all cells were again re-plated into fresh methylcellulose and cultured for the next 5 days. This replating strategy was repeated after 5 days, and again after the following 5 days (Figure 2 panel C). We noticed that the clonogenic potential of OP9-primed, VSEL-derived cells gradually increased in the 1st, 2nd, and 3rd methylcellulose passage, and 25 days from the time of initial isolation, significantly more colonies were formed by VSEL-derived cells than HSPC-derived cells (Figure 2 panel C). Significantly, colonies formed by VSEL-derived cells were also 2–3 times larger in size (data not shown) than the original VSELs.
Both RT-PCR and FACS analysis suggest that CD45− VSELs expanded over OP9 cells become CD45+ hematopoietic progenitors
Figure 3 Panel A shows expression of selected hematopoietic genes (Ikaros, Lmo2, GATA-2, HoxB4, PU.1, SCL, and c-myb) as well as the PSC marker Oct-4 in freshly isolated HSPCs and VSELs (left panel) and in cells expanded first for 5 days on OP9 cells and plated for two rounds of passaging to grow hematopoietic colonies in methylcellulose (right panel). As shown, VSEL-derived cells acquire expression of several hematopoietic genes; however, they lose expression of Oct-4.
Figure 3. Hematopoietic differentiation of VSELs.
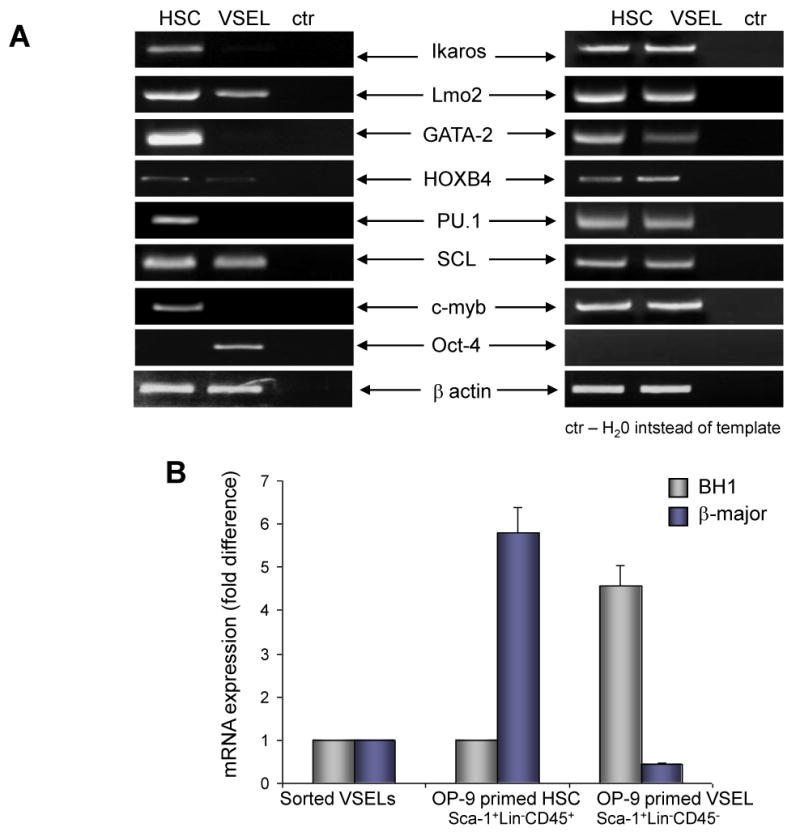
Panel A: RT-PCR analysis of the expression of hematopoietic genes in freshly isolated VSELs and HSCs (left panel) and OP9-primed and methylcellulose-expanded VSEL- and HSC- derived cells. A representative gel out of three independent experiments performed is shown. Panel B: Expression of mRNA for hemoglobin BH1 and β-major in freshly sorted VSELs, OP9-primed and methylcellulose-expanded HSC- and VSEL-derived cells. The data shown represent the combined results from three independent experiments. Panel C: FACS analysis of CD45, CD41, and Gr-1 expression of OP9-primed and methylcellulose-expanded HSC- and VSEL-derived cells. Representative FACS analysis from one of four independent experiments is shown.
To evaluate erythroid differentiation of VSEL-derived cells, we measured by RQ-PCR expression of hemoglobin-βH1 and β-major hemoglobin in freshly isolated VSELs, as well as OP9-primed and methylcellulose-expanded HSPC- and VSEL-derived cells (Figure 3 panel B). While freshly isolated VSELs do not express mRNA for hemoglobin genes, OP9 primed, VSEL-derived cells cultured in methylcellulose first show expression of hemogblobin-βH1 (Figure 3 panel B), which, following passages in methylcellulose, switch to β-major hemoglobin expression (not shown). Thus, VSEL-derived cells are able to differentiate into erythropoietic lineage based on pattern of hemoglobin expression similar to that of differentiating hematopoietic cells derived from murine embryonic stem cells (ES) or induced pluripotent stem cells (iPS) [17, 21]. At the same time, OP9-primed and methylcellulose-expanded HSPCs begin to express β-major hemoglobin.
Figure 3 panel C shows FACS analysis of GFP+-sorted cells derived after the 2nd passage in methylcellulose initiated by BM-derived, GFP+, OP9-primed HSPCs and VSELs. As shown, CD45− VSEL-derived cells acquire expression of CD45, CD41, and Gr-1 antigens over time. Thus, our data show that VSELs primed over OP9 stroma, in a similar way as differentiated murine ESCs and iPS, become specified into the hemato/lymphopoietic lineage.
In contrast to OP9 stroma-primed/methylcellulose-expanded VSEL-derived cells, freshly isolated VSELs do not radioprotect lethally irradiated mice
Next, we evaluated the hematopoietic potential of freshly isolated VSELs and OP9-primed and methylcellulose-expanded VSEL-derived cells in lethally irradiated syngeneic mice to see whether they show radioprotection.
To address this question, C57Bl/6 recipients (10 per group) were lethally irradiated (950 cGy) and transplanted (104 each) with freshly sorted HSPCs or VSELs isolated from GFP+ transgenic C57Bl6 animals. We observed that, while all mice transplanted with purified VSELs died within 2–3 weeks after transplantation, mice transplanted with HSPCs survived and become fully chimeric (data not shown). Moreover, in a set of additional experiments, VSELs freshly isolated from BM, in contrast to HSPCs, also did not form CFU-S colonies in lethally irradiated syngeneic recipients (data not shown). Thus, the freshly isolated VSELs lack radioprotective hematopoietic progenitor potential as well as in vitro colony forming ability.
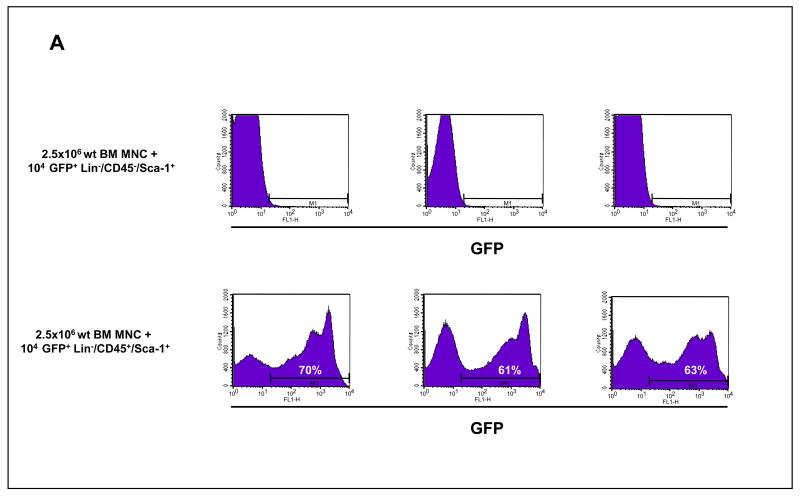
In the next experiment, we co-transplanted lethally irradiated (950 cGy) C57Bl/6 recipients (10 per group) each with 104 freshly isolated HSPCs or VSELs purified from GFP+ transgenic C57Bl6 animals together with 2.5 × 106 wt BMMNC. We observed that, while three months after transplantation all animals transplanted with GFP+ HSPCs established chimerism (∼60–75% of GFP+ cells in PB), only wt cell chimerism was displayed in the PB in mice that received freshly isolated GFP+ VSELs co-transplanted with wt BMMNCs (Figure 4 panel A). Of note, we kept some of these GFP+ VSEL transplanted animals up to six months with no emergence of GFP chimerism observed.
Figure 4. In vivo transplants of freshly sorted BM-derived GFP+ VSELs and HSCs.
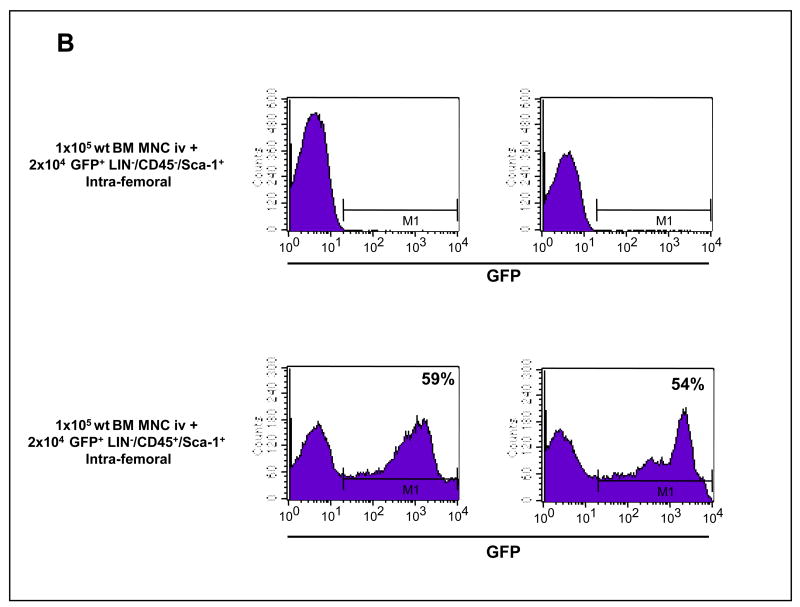
Panel A: Analysis of GFP+ MNC in PB of lethally irradiated mice transplanted intravenously with 104 sorted GFP+ VSELs and 2.5 × 106 wild type BMMNC (upper panel) and 104 sorted GFP+ HSCs and 2.5 × 106 wild type BMMNC (lower panel). Representative results showing the percentage of GFP+ cells in peripheral blood three months after transplantation are shown. Panel B: Analysis of GFP+ MNC in PB of lethally irradiated mice transplanted intra-femorally with 2 × 104 sorted GFP+ VSELs and 105 wild type BMMNC (upper panel) and 2 × 104 sorted GFP+ HSCs and 105 wild type BMMNC (lower panel). Representative results showing the percentage of GFP+ cells in peripheral blood three months after transplantation are shown.
Similarly, we also did not observe any GFP chimerism (for up to six months) when syngeneic recipients were transplanted with freshly sorted GFP+ 2 × 104 VSELs and 105 wt BMMNC by intra-femoral injection (3 mice/per group, Figure 4 panel B). Again, as expected, intra-bone injection of GFP+ HSPCs with 105 wt BMMNC gave rise to GFP+ chimeric mice in control experiments.
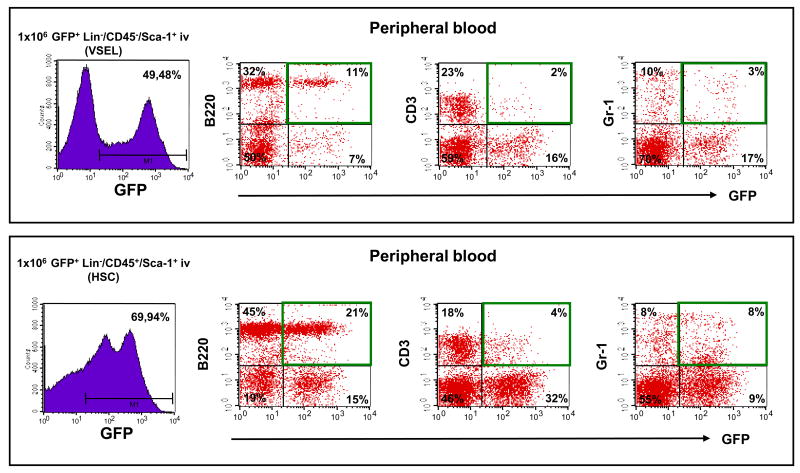
Based on our in vitro data (Figures 2 and 3) showing that VSELs may become specified to the hematopoietic lineage after first co-culturing over OP9 cells, we repeated transplants experiments with OP9-primed, methylcellulose culture-expanded cells (Figure 2 panel C). Lethally irradiated (950 cGy) C57Bl/6 recipients (6/group) were transplanted with 106 FACS-purified GFP+ cells isolated from OP9 primed cells, methylcellulose-expanded for 10 days. Table II and Figure 5 shows that 3 months after transplantation into lethally irradiated animals, both populations of cells were able to establish chimerism in all three major hematopoietic lineages (myeloid, B-lymphocytic, and T-lymphocytic). The overall GFP chimerism achieved in PB varied from 50–80% for HSPC-expanded cells and from 30–55% for VSEL-expanded cells (Table II). Chimerism in BM of transplanted animals was lower and varied between 22–51% and 12–26% for HSPC- and VSEL-expanded cells, respectively. More importantly, 105 GFP+ cells sorted from BM from these animals provided radioprotection and established full long-term chimerism in secondary transplantations (3 mice per group, repeated in duplicate experiments) as evaluated 6 months after secondary transplantation (data not shown).
Table II.
Chimerism 3 months after transplantation with GFP+ HSCs- or VSELs- expanded cells.
| Cells expanded/transplanted | Total GFP+ chimerism [PB/BM in %] | GFP+Gr-1+ cells [PB/BM in %] | GFP+ CD3+ cells [PB/BM in %] | GFP+ B220+ cells [PB/BM in %] |
|---|---|---|---|---|
| HSCs expanded | 50/22 | 16/11 | 8/1 | 16/10 |
| HSCs expanded | 80/51 | 31/44 | 9/1 | 40/6 |
| HSCs expanded | 86/44 | 23/30 | 10/1 | 53/13 |
| HSCs expanded | 70/34 | 19/18 | 8/1 | 19/22 |
| HSCs expanded | 63/31 | 17/22 | 9/1 | 37/8 |
| HSCs expanded | 81/47 | 43/32 | 11/2 | 25/14 |
| VSELs expanded | 46/26 | 13/18 | 5/1 | 20/6 |
| VSELs expanded | 51/22 | 19/14 | 4/1 | 26/7 |
| VSELs expanded | 31/15 | 13/9 | 3/1 | 15/5 |
| VSELs expanded | 50/12 | 17/9 | 10/1 | 14/3 |
| VSELs expanded | 46/23 | 27/13 | 8/1 | 17/10 |
| VSELs expanded | 53/26 | 19/11 | 7/1 | 26/16 |
Figure 5. In vivo PB chimerism of mice transplanted with OP9-primed and methylcellulose-expanded GFP+ VSEL- and HSC-derived cells.
Lethally irradiated mice were transplanted with 106 GFP+ VSEL- and HSC-derived cells (upper and lower panel respectively). Chimeric GFP+ cells were detected among B220-, CD3- and Gr-1-positive cell populations. Representative data from one of the experiments summarized in Table II are shown.
Significantly, since neither freshly isolated VSELs nor VSEL-derived HSPCs grew teratomas in transplanted animals, these cells seem to be safe from a therapeutic point of view. This finding is explained by our molecular studies, which have revealed an erasure of the imprint of some paternally imprinted genes in these cells [6].
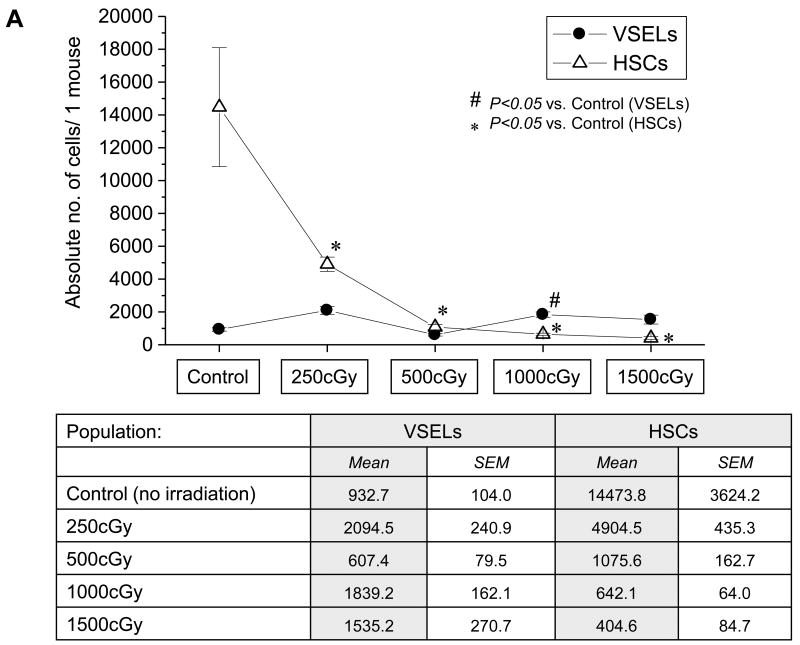
VSELs are highly resistant to irradiation and respond by proliferation to BM irradiation
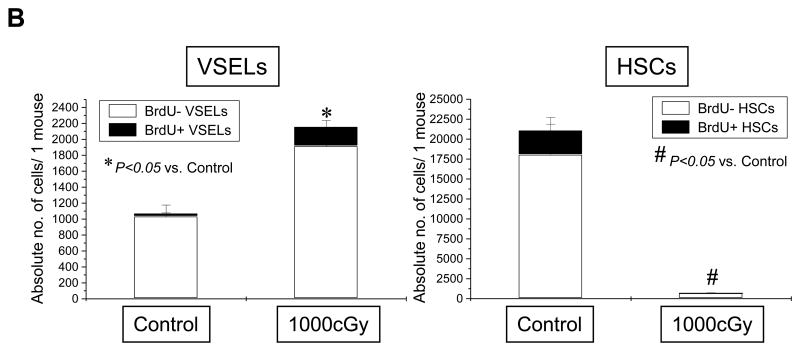
Finally, we evaluated radioresistance of BM-residing HSPCs and VSELs in irradiated mice (250–1500 cGy). HSPCs and VSELs in BM of irradiated animals were evaluated via FACS 24 hrs after total body irradiation. Figure 6, panel A shows that, as expected, BM-residing HSPCs were eliminated after irradiation by 1000–1500 cGy. In contrast, VSELs remain resistant and their number even increased slightly after irradiation by 1000–1500 cGy. This was confirmed by high expression of Oct-4 mRNA in cells isolated from lethally irradiated BM (not shown). Most importantly, we observed that, following lethal irradiation, mice injected for 4 days with BrdU displayed 12±4% BrdU-positive VSELs in BM, which suggests that some of these cells began to respond to lethal irradiation by proliferation. As expected, such an effect was not observed in the case of HSPCs (Figure 6, panel B). However, significant percentage of VSELs entered cell cycle, these cells if isolated from BM did not grow hematopoietic colonies in vitro without prior activation/co-culture over OP-9 cells (not show).
Figure 6. Resistance of VSELs to gamma radiation.
The content of VSELs and HSCs was evaluated in murine BM following whole-body irradiation with different doses of gamma radiation (250, 500, 1000, and 1500cGy) when compared to control (no irradiation). Panel A: Absolute numbers of VSELs (Sca-1+/Lin−/CD45−) and HSCs (Sca-1+/Lin−/CD45+) in BM after 4 days post irradiation. The table presents mean numbers of VSELs and HSCs per mouse (±SEM). Panel B: Absolute numbers of VSELs and HSCs incorporating BrdU following whole-body irradiation. The data are presented as mean absolute numbers of VSELs and HSCs per mouse (±SEM).
These in vivo and in vitro experiments along with radioresistance experiments allow us to propose that VSELs represent the most primitive population of stem cells in BM, which becomes specified into the hematopoietic lineage under appropriate conditions (e.g., OP9 support).
Discussion
The phenotype of most primitive LT-HSCs is still not well defined. Several potential candidate cells have been described based on cell-surface antigen expression and exclusion of fluorescent dyes [22]. On the other hand, evidence is accumulating that BM may contain some rare primitive cells that do not exhibit either in vitro or in vivo hematopoietic activity immediately after purification [14, 15]. Accordingly, a population of quiescent Sca-1+Lin-c-kit-CD45+ cells that do not proliferate in vitro and do not form CFU-S colonies upon transplantation can be expanded toward erythroid/myeloid progenitors after prolonged stimulation by Wnt3a [15].
Similarly, another group of investigators has isolated by elutriation from BM a population of very small FR25 (flow rate 25 ml/min) ALDHhigh Lin− AA4.1- stem cells that do not radioprotect lethally irradiated mice and lack spleen colony-forming (CFU-S) activity, but if co-transplanted with short-term engrafting HSCs produce delayed multilineage engraftment [13]. Interestingly, these cells are similar to VSELs in their small size of less than 5 μm in diameter [13]. While they were not characterized for expression of PSC markers, it is very likely that these two cell populations (FR25, ALDHhigh, lin−, AA4.1− stem cells and VSELs) are closely related.
In addition, evidence has accumulated that some primitive CD45-negative stem cells isolated from BM and other tissues exhibit hematopoietic potential under specific treatment conditions. Accordingly, murine BM-derived SSEA-1+ MSCs [23], skeletal muscle isolated Sca-1+c-kit−CD45− cells [24], MAPC [25], murine testis-derived multipotent germ cells (mGCs) [21], and fat tissue-derived CD45 negative cells [26] have been shown to acquire hematopoietic potential under appropriate experimental settings. The relationship of these cells to VSELs also requires further study.
Because of these findings, we became interested in the hematopoietic potential of CD45-negative VSELs. We observed that, similar to FR25 ALDHhigh lin− AA4.1− stem cells, VSELs freshly isolated from BM do not show any in vitro or in vivo hematopoietic activity. They do, however, acquire hematopoietic potential after co-culture over OP9 stroma, similar to ESCs, iPS or mGCs [18-21]. Interestingly, we observed that freshly isolated VSELs already highly express the HoxB-4 gene, which is required for hematopoietic expansion of embryonic cells [27]. However, VSELs, in striking contrast to mGCs, differentiate over OP9 stroma, not only into clonogenic hematopoietic progenitors, but more importantly, also into HSCs, which are able to establish long-term chimerism both in primary as well as in secondary transplants. This is possible because VSELs and VSEL-derived cells [5-7], in contrast to mGS derived hematopoietic cells [21], express high levels of the homing receptor CXCR4.
Recently we reported that VSELs share several characteristics with migratory primordial germ cells (PGCs). Thus, the hematopoietic potential of VSELs that we demonstrate in this paper suggests that HSCs are somehow developmentally related to a population of early PGCs [7]. The following evidence supports this hypothesis: i) a tight spatio-temporal overlap exists between the migration route of PGCs and the developmental origin of HSCs, first in extra-embryonic tissues in yolk sac blood islands and then in the aorta-gonad-mesonephros (AGM) region [22, 28]; ii) PGCs isolated from murine embryos have been described as being able to grow HSC colonies [29]; and iii) robust hematopoietic differentiation has been observed in many classical germ tumors [30, 31]. Furthermore, we have already reported that E12.5 fetal liver-derived Oct4+ VSELs, similar to their BM-derived counterparts described in this study, may differentiate over OP9 cells into CD45+ hematopoietic progenitors, which form myeloid colonies in secondary methylcellulose cultures [32]. To better link developmental migration of PGCs/VSELs and HSCs, it will be important to evaluate whether VSELs are present in yolk sac blood islands and are detectable in hematopoietic organs in Ncx1−/− embryos that do not initiate a heart beat and thus lack definitive HSCs in embryonic tissues [33].
Altogether, based on molecular analysis of purified VSELs and the finding that FL-derived epiblast/PGC-like VSELs follow the developmental migratory pathway of HSCs on their way to colonizing BM, we suggests that VSELs may, perhaps via circulation, spread in addition to BM to other organs as well [7, 32]. However, we cannot exclude the possibility that some VSELs originate via a different mechanism in situ during specification of EpiSCs during gastrulation [34]. The high radioresistance of VSELs, their ability to acquire hematopoietic potential, and the results of our BrdU-irradiation experiments further support the notion that these cells are most likely deposited in BM as a reserve pool for HSCs and may become specified along the hemato/lymphopoietic lineage [7, 22].
These data also have implications for human HSCs. As with murine HSCs, the phenotype of most primitive human HSCs is not well defined [22]. Accordingly, human BM also contains a population of rare CD34−lin−CD38− HSCs that show poor clonogenic activity in vitro, but in vivo engraft robustly in immunodeficient mice [35]. Similarly, human umbilical cord blood (UCB) has been reported to contain rare primitive CD34−flt−Lin- cells that, in contrast to normal adult HSCs, do not engraft after intravenous injection; only when transplanted directly into the bones do they exhibit hematopoietic potential [36]. Similar cells were recently also found among the human BM-derived CD33+lin− ALDHhigh cell population [37]. Thus, further studies are needed to answer whether the UCB CD133+lin−CD45− VSELs identified by us in human cord blood are also able to differentiate into the hematopoietic lineage, indicating that they constitute a population of human LT-HSCs.
Furthermore, we have demonstrated here that murine VSELs are highly resistant to irradiation. This may imply that human VSELs can survive myeloablative conditioning therapy for transplantation as well and could be responsible for the emergence of recipient hematopoiesis after unsuccessful or partial engraftment of transplanted cells. Taking into consideration the hematopoietic potential of VSELs, it would be interesting to see whether the number of these cells changes in aplastic anemia patients. If VSELs are protected from damage, they could, after expansion, offer an alternative source of autologous HSCs for transplantation in these patients.
Based on the foregoing, we postulate that VSELs are the most primitive murine BM-residing population of stem cells that possess the potential to become specified into the hematopoietic lineage and thus may share some of the characteristics of LT-HSCs. However, future studies are needed to compare several populations of putative LT-HSCs in parallel experiments to determine the similarities and differences between these cell populations.
Acknowledgments
This work was supported by NIH R01 CA106281-01, NIH R01 DK074720, EU structural funds, Innovative Economy Operational Program POIG.01.01.01-00-109/09-01 and the Henry M. and Stella M. Hoenig Endowment to MZR. Current address of EKZ is Department of Medical Biotechnology, Faculty of Biochemistry, Biophysics and Biotechnology, Jagiellonian University, Krakow, Poland.
Footnotes
Conflict of Interest Disclosure: No financial interest/relationships with financial interest relating to the topic of this article have been declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- 1.Kucia M, Reca R, Jala VR, Dawn B, Ratajczak J, Ratajczak MZ. Bone marrow as a home of heterogenous populations of nonhematopoietic stem cells. Leukemia. 2005;19:1118–1127. doi: 10.1038/sj.leu.2403796. [DOI] [PubMed] [Google Scholar]
- 2.Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- 3.Verfaillie CM. Multipotent adult progenitor cells: an update. Novartis Found Symp. 2005;265:55–61. discussion 61-55, 92-57. [PubMed] [Google Scholar]
- 4.D'Ippolito G, Diabira S, Howard GA, Menei P, Roos BA, Schiller PC. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117:2971–2981. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- 5.Kucia M, Reca R, Campbell FR, et al. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- 6.Shin DM, Zuba-Surma EK, Wu W, et al. Novel epigenetic mechanisms that control pluripotency and quiescence of adult bone marrow-derived Oct4(+) very small embryonic-like stem cells. Leukemia. 2009;23:2042–2051. doi: 10.1038/leu.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin DM, Liu R, Klich I, et al. Molecular signature of adult bone marrow-purified very small embryonic-like stem cells supports their developmental epiblast/germ line origin. Leukemia. 2010;24:1450–1461. doi: 10.1038/leu.2010.121. [DOI] [PubMed] [Google Scholar]
- 8.Ratajczak MZ, Shin DM, Kucia M. Very small embryonic/epiblast-like stem cells: a missing link to support the germ line hypothesis of cancer development? Am J Pathol. 2009;174:1985–1992. doi: 10.2353/ajpath.2009.081143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taichman RS, Wang Z, Shiozawa Y, et al. Prospective Identification and Skeletal Localization of Cells Capable of Multilineage Differentiation In Vivo. Stem Cells Dev. 2010;19:1–14. doi: 10.1089/scd.2009.0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forsberg EC, Bhattacharya D, Weissman IL. Hematopoietic stem cells: expression profiling and beyond. Stem Cell Rev. 2006;2:23–30. doi: 10.1007/s12015-006-0005-z. [DOI] [PubMed] [Google Scholar]
- 11.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci U S A. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morita Y, Ema H, Nakauchi H. Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. J Exp Med. 2010;207:1173–1182. doi: 10.1084/jem.20091318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones RJ, Collector MI, Barber JP, et al. Characterization of mouse lymphohematopoietic stem cells lacking spleen colony-forming activity. Blood. 1996;88:487–491. [PubMed] [Google Scholar]
- 14.Randall TD, Weissman IL. Characterization of a population of cells in the bone marrow that phenotypically mimics hematopoietic stem cells: resting stem cells or mystery population? Stem Cells. 1998;16:38–48. doi: 10.1002/stem.160038. [DOI] [PubMed] [Google Scholar]
- 15.Trowbridge JJ, Guezguez B, Moon RT, Bhatia M. Wnt3a activates dormant c-Kit(-) bone marrow-derived cells with short-term multilineage hematopoietic reconstitution capacity. Stem Cells. 2010;28:1379–1389. doi: 10.1002/stem.457. [DOI] [PubMed] [Google Scholar]
- 16.Ratajczak MZ, Zuba-Surma EK, Wysoczynski M, Ratajczak J, Kucia M. Very small embryonic-like stem cells: characterization, developmental origin, and biological significance. Exp Hematol. 2008;36:742–751. doi: 10.1016/j.exphem.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howell JC, Lee WH, Morrison P, Zhong J, Yoder MC, Srour EF. Pluripotent stem cells identified in multiple murine tissues. Ann N Y Acad Sci. 2003;996:158–173. doi: 10.1111/j.1749-6632.2003.tb03244.x. [DOI] [PubMed] [Google Scholar]
- 18.Orlovskaya I, Schraufstatter I, Loring J, Khaldoyanidi S. Hematopoietic differentiation of embryonic stem cells. Methods. 2008;45:159–167. doi: 10.1016/j.ymeth.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Choi KD, Yu J, Smuga-Otto K, et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009;27:559–567. doi: 10.1634/stemcells.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji J, Vijayaragavan K, Bosse M, Menendez P, Weisel K, Bhatia M. OP9 stroma augments survival of hematopoietic precursors and progenitors during hematopoietic differentiation from human embryonic stem cells. Stem Cells. 2008;26:2485–2495. doi: 10.1634/stemcells.2008-0642. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimoto M, Heike T, Chang H, et al. Bone marrow engraftment but limited expansion of hematopoietic cells from multipotent germline stem cells derived from neonatal mouse testis. Exp Hematol. 2009;37:1400–1410. doi: 10.1016/j.exphem.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratajczak MZ. Phenotypic and functional characterization of hematopoietic stem cells. Curr Opin Hematol. 2008;15:293–300. doi: 10.1097/MOH.0b013e328302c7ca. [DOI] [PubMed] [Google Scholar]
- 23.Anjos-Afonso F, Bonnet D. Nonhematopoietic/endothelial SSEA-1+ cells define the most primitive progenitors in the adult murine bone marrow mesenchymal compartment. Blood. 2007;109:1298–1306. doi: 10.1182/blood-2006-06-030551. [DOI] [PubMed] [Google Scholar]
- 24.Howell JC, Yoder MC, Srour EF. Hematopoietic potential of murine skeletal muscle-derived CD45(-)Sca-1(+)c-kit(-) cells. Exp Hematol. 2002;30:915–924. doi: 10.1016/s0301-472x(02)00872-x. [DOI] [PubMed] [Google Scholar]
- 25.Serafini M, Dylla SJ, Oki M, et al. Hematopoietic reconstitution by multipotent adult progenitor cells: precursors to long-term hematopoietic stem cells. J Exp Med. 2007;204:129–139. doi: 10.1084/jem.20061115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minana MD, Carbonell-Uberos F, Mirabet V, Marin S, Encabo A. IFATS collection: Identification of hemangioblasts in the adult human adipose tissue. Stem Cells. 2008;26:2696–2704. doi: 10.1634/stemcells.2007-0988. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Yates F, Naveiras O, Ernst P, Daley GQ. Embryonic stem cell-derived hematopoietic stem cells. Proc Natl Acad Sci U S A. 2005;102:19081–19086. doi: 10.1073/pnas.0506127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kritzenberger M, Wrobel KH. Histochemical in situ identification of bovine embryonic blood cells reveals differences to the adult haematopoietic system and suggests a close relationship between haematopoietic stem cells and primordial germ cells. Histochem Cell Biol. 2004;121:273–289. doi: 10.1007/s00418-004-0629-5. [DOI] [PubMed] [Google Scholar]
- 29.Rich IN. Primordial germ cells are capable of producing cells of the hematopoietic system in vitro. Blood. 1995;86:463–472. [PubMed] [Google Scholar]
- 30.Woodruff K, Wang N, May W, Adrone E, Denny C, Feig SA. The clonal nature of mediastinal germ cell tumors and acute myelogenous leukemia. A case report and review of the literature. Cancer Genet Cytogenet. 1995;79:25–31. doi: 10.1016/0165-4608(94)00109-o. [DOI] [PubMed] [Google Scholar]
- 31.Saito A, Watanabe K, Kusakabe T, Abe M, Suzuki T. Mediastinal mature teratoma with coexistence of angiosarcoma, granulocytic sarcoma and a hematopoietic region in the tumor: a rare case of association between hematological malignancy and mediastinal germ cell tumor. Pathol Int. 1998;48:749–753. doi: 10.1111/j.1440-1827.1998.tb03977.x. [DOI] [PubMed] [Google Scholar]
- 32.Zuba-Surma EK, Kucia M, Rui L, et al. Fetal liver very small embryonic/epiblast like stem cells follow developmental migratory pathway of hematopoietic stem cells. Ann N Y Acad Sci. 2009;1176:205–218. doi: 10.1111/j.1749-6632.2009.04562.x. [DOI] [PubMed] [Google Scholar]
- 33.Lux CT, Yoshimoto M, McGrath K, Conway SJ, Palis J, Yoder MC. All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood. 2008;111:3435–3438. doi: 10.1182/blood-2007-08-107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Miguel MP, Arnalich Montiel F, Lopez Iglesias P, Blazquez Martinez A, Nistal M. Epiblast-derived stem cells in embryonic and adult tissues. Int J Dev Biol. 2009;53:1529–1540. doi: 10.1387/ijdb.072413md. [DOI] [PubMed] [Google Scholar]
- 35.Kimura T, Asada R, Wang J, et al. Identification of long-term repopulating potential of human cord blood-derived CD34-flt3- severe combined immunodeficiency-repopulating cells by intra-bone marrow injection. Stem Cells. 2007;25:1348–1355. doi: 10.1634/stemcells.2006-0727. [DOI] [PubMed] [Google Scholar]
- 36.Bhatia M, Bonnet D, Murdoch B, Gan OI, Dick JE. A newly discovered class of human hematopoietic cells with SCID-repopulating activity. Nat Med. 1998;4:1038–1045. doi: 10.1038/2023. [DOI] [PubMed] [Google Scholar]
- 37.Hess DA, Wirthlin L, Craft TP, et al. Selection based on CD133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells. Blood. 2006;107:2162–2169. doi: 10.1182/blood-2005-06-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]