Abstract
Background
The diagnosis and treatment of indeterminate dominant strictures (DS) in patients with primary sclerosing cholangitis (PSC) is challenging and the literature on the subject is scarce.
Objectives
This review aims to appraise and synthesize the evidence published in the English-language medical literature on this topic.
Methods
Scientific papers published from 1950 until week 4 of July 2010 were extracted from MEDLINE, Ovid Medline In-Process, the Cochrane Database of Systematic Reviews, the Database of Systematic Reviews, the Database of Abstracts of Reviews of Effects, EMBASE, PubMed and the National Library of Medicine Gateway.
Results
Strategies for the optimal management of DS in PSC patients are supported only by level II and III evidence. Intraductal endoscopic ultrasound appears to be the most sensitive (64%) and specific (95%) diagnostic test for the evaluation of DS in PSC. Endoscopic and percutaneous dilatations achieve 1- and 3-year palliation in 80% and 60% of patients, respectively. Although dilatation and stenting are the most common palliative interventions in DS, no randomized trials on the optimal duration of treatment have been conducted.
Conclusions
In benign DS, endoscopic dilatation with short-term stenting seems to be effective and safe and does not increase the risks for malignant transformation or complications after liver transplantation. Surgical bile duct resection and/or bilioenteric bypass are indicated only in patients with preserved liver function.
Keywords: primary sclerosing cholangitis, dominant stricture, cholangiocarcinoma, DIA, FISH, endoscopic ultrasound, ERCP MRCP CT cholangiography, biliary stenting, biliary dilatation
Introduction
Primary sclerosing cholangitis (PSC) is a progressive cholestatic liver disease with patchy inflammatory fibrosis and strictures of the intra- and extrahepatic bile ducts.1 The presence of T lymphocyte infiltrates in areas of severe inflammation2 would suggest an autoimmune pathogenesis.3 Recent American and European studies show the prevalence of PSC to be 20.9 per 100 000 men and 6.3 per 100 000 women4 and the incidence to be 0.9–1.3 per 100 000 individuals per year.5,6
The diagnosis of PSC requires the exclusion of secondary causes of sclerosing cholangitis, such as surgical trauma, presence of intraductal stones or recurrent episodes of inflammation.7 The management of early PSC is controversial and no therapy has been proven to prolong survival except for orthotopic liver transplantation (OLT).8 Ursodeoxycholic acid,8–12 corticosteroids,13–15 endoscopic or percutaneous dilatation of biliary strictures,16,17 and drainage or surgical resection of isolated extrahepatic stenosis18–20 have been used to slow the progression of the disease, with limited success.
In 15–20% of patients with PSC, a localized high-grade stenosis is the most prominent feature at the time of presentation.21,22 These dominant strictures (DS) can be located in the intrahepatic ducts but, more frequently, they are extrahepatic.
The radiological characteristics of benign DS in patients with PSC mimic those of cholangiocarcinoma and the limitations of current diagnostic modalities make the differential diagnosis quite difficult.
The management of patients with PSC and DS is challenging because treatments depend on the nature of the DS. Cholestasis is the principal cause of morbidity; it causes acute deterioration of liver function and should be addressed promptly.23 Limited knowledge on how to differentiate benign from malignant DS, on which treatment options are best and on how to optimize symptoms of PSC while waiting for an OLT24 led us to perform a systematic review of the literature. The aim of this review is to provide an evidence-based synthesis of modern diagnostic strategies and management of DS in patients affected by PSC.
Materials and methods
Evidence acquisition
Studies reporting epidemiology, diagnosis and therapy of DS in patients affected by PSC were sought. Preference was given to randomized controlled trials (RCTs) and prospective observational studies. MEDLINE, Ovid Medline In-Process, the Cochrane Database of Systematic Reviews, the Database of Systematic Reviews, the Database of Abstracts of Reviews of Effects, EMBASE, PubMed and the National Library of Medicine Gateway were searched using established systematic review methods (Jadad Scale for Randomized Controlled Studies, Downs and Black checklist for observational studies).25–27 In addition, relevant general radiological, medical and surgical journals were hand-searched, as were the reference lists of all included articles for other relevant evidence and seminar and conference abstracts. The reference search was limited to English-language articles published from January 1950 until the 4th week of July 2010. Boolean logic methods were used to combine medical subject heading terms (MeSH) used to identify the articles referenced in this paper (Table 1). Two authors (MA and MM) independently selected articles based on the content of titles and abstracts. In cases of doubt, the article was reviewed in its entirety. Decisions to include articles in this review were reached by consensus.
Table 1.
Summary of all MeSH headings and terms used to identify publications on dominant strictures in patients affected by primary sclerosing cholangitis in the MEDLINE, EMBASE and Cochrane Library databases
| Primary MeSH terms | Secondary MeSH terms (epidemiology, diagnosis) | Secondary MeSH terms (treatment, palliation) |
|---|---|---|
| Cholangitis | Epidemiology | Resection |
| Sclerosing cholangitis | Classification | Therapeutic(s) |
| Primary sclerosing cholangitis | Diagnosis | Treatment outcome(s) |
| Biliary tract disease(s) | Differential diagnosis | Operation |
| Bile duct disease(s) | Early diagnosis | Transplantation |
| Common bile duct | Risk factor(s) | Biliary tract surgical |
| Bile duct(s) | Diagnostic imaging | Procedures liver |
| Intrahepatic bile duct(s) | MRI | Organ transplantation |
| Extrahepatic bile duct(s) | Endosonography | Clinical trial |
| Dominant or single or predominant | Ultrasonography | Controlled clinical trial(s) |
| Stricture or stenosis or narrowing | Emission CT | Random allocation |
| Radionuclide imaging | Randomized controlled trial(s) | |
| PET | Clinical trial (phase I) | |
| X-ray CT | Clinical trial (phase II) | |
| Cytology | Clinical trial (phase III) | |
| Cytodiagnosis | Clinical trial (phase IV) | |
| Tumour markers (biological) | Drug therapy | |
| Antigen(s) | Modality therapy | |
| Carcinoembryonic antigen | Drainage | |
| CA 19-9 antigen | Cholestasis | |
| ERCP | Obstructive jaundice | |
| Cholangiography | Pathology | |
| In situ hybridization | Treatment outcome | |
| Fluorescence in situ hybridization | Outcome assessment | |
| Nucleic acid hybridization | Prognosis | |
| Computer-assisted image processing | Therapy | |
Boolean logic methods were used to combine MeSH headings and terms
MeSH, medical subject headings; MRI, magnetic resonance imaging; CT, computed tomography; PET, positron emission tomography; ERCP, endoscopic retrograde cholangiopancreatography
Inclusion criteria
Studies on diagnostic tests were required to report at least one of the following outcomes: sensitivity; specificity; positive and negative predictive values, and accuracy.
Studies describing clinical outcomes were required to report one or more of the following variables: success rate; failure rate; morbidity or mortality; time to recurrence, and time to death or liver transplantation.
Exclusion criteria
Articles published only as abstracts, papers in languages other than English, studies on DS after liver transplantation, editorials, letters, case reports describing fewer than 10 patients, reviews and reports of in vitro or animal experiments were excluded.
In addition, as DS in patients with PSC is predominantly managed by endoscopic or radiological interventions such as stenting or dilatation or by surgical therapy, studies on medical treatments were also excluded.
Definitions of terms
Definition of dominant strictures
Dominant strictures (DS) are discrete narrowings of the common bile duct (CBD) or the common hepatic duct (CHD) that prevent the normal flow of bile into the duodenum.28,29 In the current literature, DS are defined as: (i) strictures of the CBD measuring ≤1.5 mm in diameter, or (ii) a diameter of the CHD measuring ≤1.0 mm within 2 cm of the bifurcation at the hilum.28,29
Figure 1 represents a schematic diagram of the main features of DS in patients with PSC.
Figure 1.
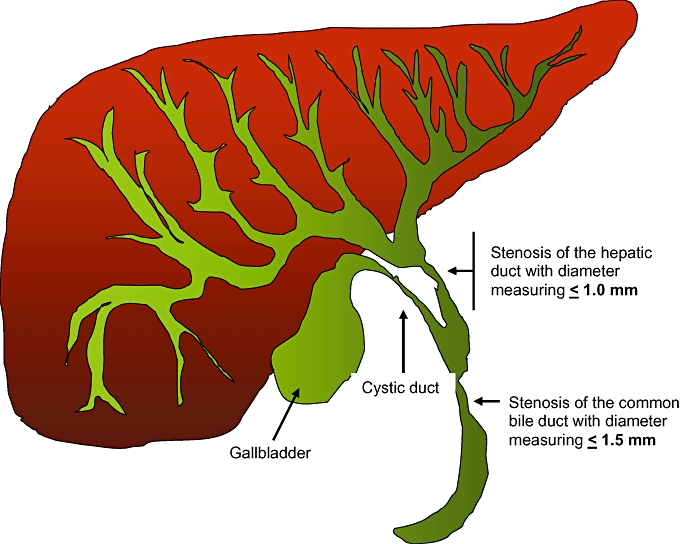
Graphic representation of the intra- and extrahepatic biliary system. Dominant strictures are defined as stenosis of the common bile duct with a diameter of ≤1.5 mm and/or stenosis of the hepatic duct with a diameter of ≤1.0 mm. The hepatic duct is the biliary tract distal to the bifurcation of the right and left biliary systems and proximal to the cystic duct junction
Definition of primary sclerosing cholangitis
The included papers defined PSC as a chronic and progressive disease conditioning sclerosis of the biliary ducts. PSC was diagnosed on the basis of either liver biopsy or typical cholangiographic findings in the presence of compatible biochemical abnormalities and the absence of secondary causes of sclerosing cholangitis.30
Definition of diagnostic modalities
Modalities used to diagnose PSC and DS included the full array of imaging technologies, such as ultrasound (US), computed tomography (CT), magnetic resonance imaging (MRI) and percutaneous transhepatic cholangiography, endoscopic studies such as endoscopic retrograde cholangiography (ERC) and cholangioscopy, serological markers such as liver function tests and tumour markers (e.g. CA19-9) and cytological and bioptic investigations.
Definition of interventions
Papers on treatments were divided into two groups based on the nature of the interventions according to whether they described non-surgical or surgical therapies. Non-surgical therapies were defined as all interventions that did not require resection of the biliary system (stenting or dilatation or both). All interventions that required resection of the DS or liver transplantation were defined as surgical.
Data extraction and quality control
The literature retrieval and the initial screening of relevant studies were performed by MA and MM. Material was reviewed and coded as per the study protocol. Studies were scrutinized to exclude any duplicate reports of the same group of patients. The abstract of each article was appraised and if there was any suggestion of relevant data, the full text was retrieved. Two doctors (MA and MM) checked data from a random sample of studies for reliability; discrepancies were resolved by consensus.
Aims of the study
The aims of this study were: (i) to report the epidemiology of DS in patients affected by PSC; (ii) to assess the sensitivity and specificity of imaging modalities, serum biomarkers, cytology and histology in differentiating benign from malignant DS, and (iii) to assess the safety and effectiveness of non-invasive and invasive therapies for DS.
Analysis
Statistical analysis was not possible as the data could not be pooled because the variables of interest (e.g. diagnostic modalities, medical interventions, endoscopic or radiology procedures, surgical therapy) were frequently not available for all patients in the same study. Furthermore, we observed significant heterogeneity in terms of the quality and design of the various studies, which included, for example, retrospective or prospective observational studies, semi-randomized trials and randomized trials.
Results
A total of 272 abstracts of studies published from 1950 through 2010 that appeared to meet the criteria for inclusion in this review were identified. The selected studies were appraised and 94 were approved for inclusion in this review according to the criteria described earlier. The most common reasons for excluding studies were that they reported outcomes of PSC patients with multiple strictures or that they included patients with PSC and DS but did not allow for the extrapolation of results of interest as the methods described in the papers were not sufficiently clear. The studies selected for the review did not include any RCTs as they were not available; therefore only 76 retrospective observational studies and 18 prospective observational studies were included in this review.
Epidemiology
Dominant strictures of the extrahepatic bile ducts were observed in 10–20% of patients with PSC.31–34 At the time of presentation, approximately 75% of DS were benign, whereas 25% were malignant.35 A large, prospective, cohort study of 159 Scandinavian patients with a mean follow-up of 113 months (range: 3–345 months) showed that DS at the CBD were present in 23% of participants. Dominant strictures were present in the right and left hepatic ducts in 19% and 17% of cases, respectively.22 Overlap among groups occurred frequently, as 12% of patients had multiple strictures.34
PSC was the most common risk factor for cholangiocarcinoma in Western countries.36,37 The prevalence of cholangiocarcinoma in patients with PSC ranged from 8% to 25%,35,37 with an annual incidence of 1.2%.38 In a study investigating the natural history of PSC, 62% of patients who developed cholangiocarcinoma had a DS of the biliary tree.11 Furthermore, the presence of DS was an independent factor for poor prognosis in patients who develop cholangiocarcinoma.11 About 30% of cholangiocarcinomas in patients with PSC occurred within 2 years of the diagnosis of PSC10,39 and several findings seemed to be predictive for malignant transformation, including: rapid clinical and biochemical deterioration of liver function and presence of jaundice; pruritus; weight loss; marked proximal ductal dilatation with progressive distal strictures; elevated serum levels of CA 19-9 (>100 U/ml), and the presence of dysplasia in bile duct brush cytology.40,41
Symptoms
In the early stage, DS were usually asymptomatic. As the disease progressed, DS of extrahepatic bile ducts presented with an acute exacerbation of jaundice, deterioration of liver function tests and/or cholangitis.32,34 In a prospective study, enteric bacteria were detected in the bile of 40% of patients affected by symptomatic DS.42 Despite in vitro susceptibility, antibiotic treatment was not effective in eradicating bacteria from the bile ducts suggesting that chronic bacterial infection may contribute to the progression of the disease.42
Diagnostic modalities
Differentiation between malignant and benign DS in patients with PSC required the combination of several diagnostic modalities as they complemented one another. Despite extensive investigations, DS remained a very challenging condition to diagnose as each test carried a risk for false negative or false positive results. Despite the use of predictive criteria, differential diagnosis between a benign DS and cholangiocarcinoma in PSC was challenging and most cases of cholangiocarcinoma in PSC were detected at an advanced stage and were unresectable; overall median survival was only 5 months.43
Laboratory tests
Table 2 summarizes the sensitivity, specificity, accuracy and positive and negative predictive values of the most common diagnostic laboratory tests.
Table 2.
Summary of the sensitivity, specificity, accuracy and positive and negative predictive values of laboratory tests used to discriminate between benign and malignant dominant strictures in patients with primary sclerosing cholangitis
| Author(s) (year) | Patients, n | Diagnostic test | Sensitivity | Specificity | Accuracy | PPV | NPV |
|---|---|---|---|---|---|---|---|
| Levy et al. (2008)49 | 36 | Serum CA 19-9 ≥100 ng/ml in 36 patients with PSC among 86 patients enrolled in the study | 14% | 95% | 61% | 67% | 60% |
| Nakeeb et al. (1996)50 | 34 | Bile CEA >30 ng/ml in all patients with DS | 72% | 84% | NA | 72% | 84% |
| Levy et al. (2008)49 | 86 | Endoscopic luminal biopsy for all 86 patients with DS enrolled in the study | 29% | 100% | 71% | 100% | 67% |
| Levy et al. (2008)49 | 36 | Brushings for 36 patients with PSC among 86 patients enrolled in the study | 29% | 100% | 71% | 100% | 67% |
| Levy et al. (2008)49 | 36 | FISH in 36 patients with PSC among 86 patients enrolled in the study | 64% | 70% | 68% | 60% | 74% |
| Levy et al. (2008)49 | 36 | DIA in 36 patients with PSC among 86 patients enrolled in the study | 21% | 95% | 65% | 75% | 63% |
PPV, positive predictive value; NPV, negative predictive value; NA, not available; PSC, primary sclerosing cholangitis; CEA, carcinoembryonic antigen; DS, dominant strictures; FISH, fluorescence in situ hybridization; DIA, digital image analysis
Serum laboratory tests
In the majority of patients affected by DS and PSC, serum laboratory tests were neither sensitive nor specific enough for either diagnosis or the evaluation of disease progression.22,44 Often, patients had elevated levels of serum alkaline phosphatase (ALP) and gamma-glutamyl transpeptidase (GGT), and the serum total bilirubin level was elevated in only about 40% of patients at presentation.6,7 Non-specific auto-antibodies, such as antinuclear and antineutrophil cytoplasmic immunoglobulins, were detected in 50–80% of patients45,46 and elevated serum IgM levels were observed in only 45% of patients.45
Serum tumour markers
A prospective study of 75 patients affected by PSC evaluated the use of serial levels of four serum tumour markers (CEA [carcinoembryonic antigen], CA 19-9, CA 50 and CA 242) for the diagnosis of cholangiocarcinoma in the presence of DS.47 Over a period of 3 years, two patients (3%) developed cholangiocarcinoma, but only one had elevated levels of at least one of the serum tumour markers. Serum tumour markers CEA, CA 19-9, CA 50 and CA 242 appeared to be of limited value because of their low sensitivity and specificity. One of the criticisms of this study was that the number of patients who developed cholangiocarcinoma was too small to allow the drawing of generalizable conclusions.
A second prospective observational study from Germany included a cohort of 106 patients treated with ursodeoxycholic acid and biliary endoscopic dilatations of DS over a median of 5 years.48 CA 19-9 was elevated (>100 ng/ml) in 23.5% of patients, but cholangiocarcinoma developed in only three individuals (3%). In 14 of 25 patients with elevated CA 19-9 levels, DS were diagnosed and treated by endoscopic dilatations. In 71.4% of the endoscopically treated patients, CA 19-9 levels decreased following dilatation. In a prospective study from the Mayo Clinic,49 serum CA 19-9 was evaluated in 86 patients with indeterminate DS. For the diagnosis of cholangiocarcinoma, serum levels of CA 19-9 ≥100 ng/ml had sensitivity, specificity, accuracy, and positive and negative predictive values of 42%, 91%, 64%, 86% and 55%, respectively. In this cohort, 36 patients had been previously diagnosed with PSC; in this selected group, CA 19-9 ≥100 ng/ml had sensitivity, specificity, accuracy, and positive and negative predictive values of 14%, 95%, 61%, 67% and 60%, respectively.49 At present, data from the current literature suggest that an increased serum level of CA 19-9 in PSC patients has unsatisfactory sensitivity and positive predictive values for the diagnosis of cholangiocarcinoma.
Biliary tumour markers
A prospective cohort of 34 patients affected by benign DS due to ischaemia or chronic pancreatitis underwent bile collection via transhepatic biliary drainage at the Johns Hopkins Hospital.50 Bile levels of CEA were compared with those in a group of 16 individuals at high risk for cholangiocarcinoma because they had choledochal cysts (n= 5), PSC with the presence of DS (n= 6) or intrahepatic bile duct stones (n= 5). Levels of CEA in bile collected from individuals with benign biliary strictures were significantly lower than in bile from patients with histologically confirmed cholangiocarcinoma (10.1 ± 3.9 ng/ml vs. 50.2 ± 5.8 ng/ml; P < 0.02). The majority of patients with benign strictures secondary to pancreatitis, PSC, ischaemia and stones had bile CEA levels of <30 ng/ml, whereas most patients with cholangiocarcinoma had levels above this value. A bile CEA level >30 ng/ml resulted in sensitivity of 72%, specificity of 84%, a positive predictive value of 72% and a negative predictive value of 84%. Although the measurement of CEA levels in the bile of patients with PSC was promising, the test is only available in selected medical centres and requires validation in a larger population before it can be recommended as a diagnostic tool for differentiation between benign and malignant DS.
Routine cytology
In PSC patients, the majority of cases of malignant DS presented in the perihilar region,51 which was accessible for cytology sampling during ERC. In a prospective study of 61 PSC patients evaluated at the University of Oslo (Norway), the sensitivity, specificity, accuracy, and positive and negative predictive values of brush cytology were 100%, 84%, 88%, 68% and 100%, respectively, for the combination of low- and high-grade dysplasia/adenocarcinoma.52 When only patients with high-grade dysplasia/adenocarcinoma were included, the sensitivity, specificity, accuracy, and positive and negative predictive values of the test decreased to 73%, 95%, 90%, 85% and 91%, respectively.52 In a more recent prospective study including 36 patients affected by PSC and indeterminate DS,49 brush cytology had significantly lower sensitivity (29%), excellent specificity (100%) and positive predictive value (100%), and comparable accuracy (71%) and negative predictive value (67%).
Endoscopic intraluminal biopsy
Endoscopic intraluminal biopsies are obtained from abnormal segments of the bile duct using fluoroscopic guidance of regular bioptic forceps or during cholangioscopy. In a recent prospective study of 86 patients affected by DS,49 intraductal biopsies could not be obtained in only seven individuals (8.1%). When these cases were excluded, the sensitivity, specificity and accuracy of histology were 41%, 100% and 67%, respectively. Among the 86 patients enrolled in this study, 34 (39%) were affected by PSC and intraluminal biopsy provided histological samples with slightly lower sensitivity and similar specificity, accuracy, and positive and negative predictive values (29%, 100%, 71%, 100% and 67%, respectively).49
Fluorescence in situ hybridization
Fluorescence in situ hybridization (FISH) utilizes fluorescent labelled DNA probes that bind only to parts of chromosomes with a high degree of sequence similarity and thus detects cells that have abnormalities indicative of malignancy. In a prospective study, FISH was used to investigate the nature of cytological samples obtained from PSC patients with indeterminate strictures.49 DNA probes used targeted centromeres of chromosome 3 (CEP3), chromosome 7 (CEP7), chromosome 17 (CEP17) and band 9p21 (P16/CDKN2A gene). Two general types of chromosomal abnormalities were observed by FISH: polysomy and trisomy of chromosomes 7 or 3. In 36 patients affected by PSC with cholangiocarcinoma, FISH was 64% sensitive, 70% specific, 68% accurate and had positive and negative predictive values of 60% and 74%, respectively.49
Digital image analysis
Digital image analysis (DIA) is a form of cytological analysis that requires spectrophotometry to quantify cellular constituents.53 Small foci of tumour cells can be analysed54 by computer vision techniques and cell nuclei are classified as diploid, aneuploid or tetraploid. Aneuploid and tetraploid specimens are considered malignant.55 In a prospective study comparing different modalities,49 DIA had overall sensitivity, specificity, accuracy and positive and negative predictive values of 38%, 95%, 64%, 90% and 56%, respectively. Unfortunately, when DIA was evaluated in 36 patients with PSC, its sensitivity, specificity, accuracy and positive and negative predictive values decreased to 21%, 95%, 65%, 75% and 63%, respectively.49
Non-invasive imaging modalities
Table 3 summarizes the sensitivity, specificity, accuracy and positive and negative predictive values of the non-invasive imaging modalities most commonly used to discriminate patients with benign from malignant DS in the presence of PSC.
Table 3.
Summary of the sensitivity, specificity, accuracy, positive and negative predictive values of non-invasive imaging modalities used to discriminate between benign and malignant dominant strictures in patients with primary sclerosing cholangitis
| Author(s) (year) | Patients, n | Diagnostic test | Sensitivity | Specificity | Accuracy | PPV | NPV |
|---|---|---|---|---|---|---|---|
| Majoie et al. (1995)56 | 23 | US (intrahepatic DS) | 77% | NA | NA | NA | NA |
| Lee et al. (1995)57 | 85 | US (extrahepatic DS) | 94% | NA | NA | NA | NA |
| Macchi et al. (2004)59 | 16 | CT cholangiography (extrahepatic DS) | 94% | NA | NA | NA | NA |
| Eracleous et al. (2005)60 | 31 | CT cholangiography (intrahepatic DS) | 100% | NA | NA | NA | NA |
| Silverman et al. (1994)61 | 34 | MRC | 85% | 92% | NA | 85% | NA |
| Moff et al. (2006)62 | 36 | MRC | 85–91% | 85–96% | NA | NA | NA |
| Berstad et al. (2006)68 | 66 | MRC | 77–82% | 81–93% | 79–86% | 86–94% | 71–78% |
PPV, positive predictive value; NPV, negative predictive value; NA, not available; US, ultrasound; DS, dominant strictures; CT, computed tomography; MRC, magnetic resonance cholangiography
Ultrasonography
Abdominal US seemed to be very sensitive in detecting extrahepatic DS. In a prospective observational study from the Netherlands, 23 consecutive patients with previously established diagnoses of PSC underwent trans-abdominal US to evaluate the intra- and extrahepatic biliary ducts.56 The major limitation of US was its inability to exclude intrahepatic duct disease: in 23% of patients the diagnosis of PSC was missed but confirmed with ERC.56 By contrast, extrahepatic disease was accurately diagnosed by US,57 that showed mural thickening of the CBD, in 94% of patients and subsequently confirmed by cholangiography.
Computerized tomography
Technological advances in CT scanning have improved the ability to evaluate the biliary system. Recent reports have shown that CT cholangiography, defined as CT scanning with i.v. administration of a contrast medium selectively excreted in the biliary system, can be used to generate three-dimensional images of the biliary tract.58 In a prospective study carried out at the University of Padua (Italy),59 CT scan findings were compared with magnetic resonance cholangiography (MRC) images showing sensitivity values of 94% and 63% for CT cholangiography and MRC, respectively. Extrahepatic disease was diagnosed in 69% of patients using CT cholangiography in comparison with 25% with magnetic resonance cholangiopancreatography (MRCP) (P < 0.05). These findings were confirmed by a more recent retrospective study that showed that CT cholangiography provided better delineation of the biliary system in terms of intrahepatic segmental strictures than MRC (100% vs. 80%, respectively; P= 0.03).60
Magnetic resonance imaging
Magnetic resonance cholangiography has been extensively used for the diagnosis of PSC.59 In a prospective case–control study of 34 patients, Silverman et al. reported that MRC had sensitivity of 85–88%, specificity of 92–97% and positive predictive values of 85–94%.61 A recent retrospective study comparing MRC with ERC in 36 patients showed that extrahepatic and intrahepatic ductal visualization of MRC was excellent in 64% and 66% of patients, respectively, compared with 86% and 74% for ERC.62 Sensitivity and specificity in MRC were 81–91% and 85–96%, respectively. Although the receiver operating curve values were excellent for both MRC and ERC (0.9), interobserver agreement was only good for identification of intrahepatic stenosis (MRC, 0.64; ERC, 0.86) and only ERC achieved good interobserver agreement for extrahepatic strictures (MRC, 0.36; ERC, 0.55). Interobserver agreement was poor for both MRC and ERC for the assessment of the degree of severity of PSC.62
Invasive diagnostic tests
Table 4 summarizes the sensitivity, specificity, accuracy and positive and negative predictive values of some of the invasive imaging modalities commonly used to diagnose patients with DS and PSC.
Table 4.
Summary of the sensitivity, specificity, accuracy, positive and negative predictive values of invasive imaging modalities used to discriminate between benign and malignant dominant strictures in patients with primary sclerosing cholangitis
| Author(s) (year) | Patients, n | Diagnostic test | Sensitivity | Specificity | Accuracy | PPV | NPV |
|---|---|---|---|---|---|---|---|
| Berstad et al. (2006)68 | 66 | ERC | 85–92% | 78–81% | 83–86% | 86–87% | 79–88% |
| Tischendorf et al. (2006)35 | 53 | Transpapillary cholangioscopy | 92% | 93% | 93% | 79% | 97% |
| Levy et al. (2008)49 | 36 | Intraductal US | 64–71% | 55–95% | 62–82% | 53–90% | 73–79% |
PPV, positive predictive value; NPV, negative predictive value; ERC, endoscopic retrograde cholangiography; US, ultrasound
Cholangiography
In the majority of patients, the diagnosis of DS was established with endoscopic retrograde cholangiopancreatography (ERCP) and less often with percutaneous transhepatic cholangiography. Strictures were typically short and nearly all patients had both intra- and extrahepatic duct involvement (98.8%).63 Only in 20% of patients PSC involved only the intrahepatic and proximal extrahepatic ducts.63 ERC and percutaneous transhepatic cholangiography provided high-quality images of the biliary tract, but both were associated with risks for cholangitis,64 haemorrhage65 and pancreatitis and bowel perforation.66 For these reasons, MRC has become more popular for patients with suspected PSC. In single-institution studies, ERC and MRC have shown similar rates of sensitivity (82% and 80%, respectively), and specificity (96% and 88%, respectively) for the diagnosing of PSC. However, MRC demonstrated inferior performance in detecting extrahepatic DS compared with ERC or CT cholangiography.67,68
Transpapillary cholangioscopy
In patients with DS and PSC, non-invasive techniques had low sensitivity for detection of cholangiocarcinoma. Patients with DS should be referred for endoscopic brushing or biopsy to exclude cholangiocarcinoma as the results of these tests influence clinical management and prognosis.52,69 In a well-designed, prospective, observational study from Germany, 53 patients with PSC and DS underwent transpapillary cholangioscopy and endoscopic tissue sampling in addition to ERC.35 In comparison with ERC alone, cholangioscopy had higher sensitivity (92% vs. 66%; P= 0.25), specificity (93% vs. 51%; P < 0.001), accuracy (93% vs. 55%; P < 0.001), and positive (79% vs. 29%; P < 0.001) and negative (97% vs. 84%; P < 0.001) predictive values.
Intraductal ultrasound
In a prospective study from the Mayo Clinic, 86 patients were enrolled to assess the use of advanced molecular and imaging techniques in the presence of indeterminate DS.49 Intraductal ultrasound (IDUS) was performed by using a 20-MHz US probe advanced over a guidewire at least 2 cm proximal to the upper border of the stricture. The sensitivity, specificity, accuracy, and positive and negative predictive values of IDUS to detect cholangiocarcinoma in patients affected by PSC (n= 36) were 71%, 55%, 62%, 53% and 73%, respectively. Except for sensitivity, IDUS improved significantly in performance when the operators used their clinical impressions rather than formal criteria to diagnose cholangiocarcinoma as the sensitivity, specificity, accuracy and positive and negative predictive values of the test changed to 64%, 95%, 82%, 90% and 79%, respectively.
Summary of findings for diagnostic modalities
In the majority of PSC patients, diagnosis of DS requires a combination of non-invasive and invasive imaging modalities. Although US has high sensitivity for extrahepatic DS, it is still used only as a first-line modality for screening. Endoscopic retrograde cholangiography and MRC are the most commonly used imaging modalities as they are relatively sensitive and specific. Of the two, ERC is the more sensitive for extrahepatic DS and is useful for obtaining cytology specimens and performing IDUS and endoscopic intraluminal biopsies. However, ERC is disadvantaged by its invasive characteristics and the associated morbidity and mortality and therefore has become a second-line modality after MRC. Unfortunately, both MRC and ERC have relatively low sensitivity and specificity for differentiating between benign and malignant DS. During the last decade, several new modalities have been introduced for the diagnosis of cholangiocarcinoma in the presence of DS. The use of molecular markers and imaging techniques such as IDUS, FISH, DIA or a combination of multiple modalities has enhanced diagnostic accuracy, achieving sensitivity rates of up to 71% in the best series. However, no single test can be considered as a reference standard for the differentiation of benign from malignant DS. Combining several modalities with the clinical characteristics of each patient is therefore necessary in PSC patients.
Treatments
Orthotopic liver transplantation remains the only cure for advanced PSC. Therefore, the most important considerations to guide therapeutic decisions should refer to the impact that each treatment might have on the feasibility of future OLT. As many aspects must be considered, DS in PSC should be managed at centres in which it is possible to take a multidisciplinary approach to the problem. In the past, cases of DS were mainly managed surgically, but, in view of the advances made in non-operative modalities, management trends have shifted towards endoscopic techniques as they are less invasive, can be performed in outpatient settings and can be repeated whenever necessary.70
Primary sclerosing cholangitis is a slowly progressive disease and patients survive for several years before requiring OLT. The early management of DS is aimed at improving the symptoms and quality of life of these patients without jeopardizing the possibility of future OLT.
Minimally invasive procedures such as percutaneous or endoscopic dilatation with or without the use of stents have come to represent first-line interventions. Resection or bypass surgery has been performed only in a small and selected group of patients with DS in the extrahepatic duct without signs of cirrhosis.71
Non-operative therapies
Endoscopic therapy
Non-operative therapies have several advantages: they have lower complication rates, can be performed under sedation and can be repeated several times if necessary without altering the biliary anatomy or compromising the possibility of future OLT.
Endoscopic and percutaneous biliary dilatations in benign DS could achieve clinical and biochemical response or improvement in approximately 80% of non-cirrhotic patients.28,43,72–75 In the majority of cases, more than one session of dilatation was necessary to achieve the desired results.74,75 Stents were often used with the aim of improving patency after dilatation. However, recent studies have reported that biliary stents were associated with an increased risk for cholangitis as a result of their occlusion over time.74,75 Because of the small numbers of subjects, and the differences in stenting protocols, interpretation of the outcomes after endoscopic therapy for DS in PSC patients was somewhat difficult. In a retrospective study of 63 patients, repeated endoscopic dilatation (a mean of 2.3 times per patient) with concomitant intake of ursodeoxycholic acid was associated with a 5-year survival of 83%, which was higher than predicted survival without treatment.73 The deployment of temporary stents was necessary in 50% of patients following satisfactory dilatation of the DS. In a prospective study of 106 patients with PSC treated with ursodeoxycholic acid, Stiehl et al.74 performed interval ERCPs and documented the occurrence of benign DS in 52 patients (49%) who subsequently underwent endoscopic dilatation. Common bile duct stenoses were dilated up to a diameter of 24 French, whereas stenoses of the intrahepatic ducts up to 2 cm proximal to the bifurcation were dilated to a diameter of 18–24 French. Balloon dilatations were repeated at 4-week intervals until satisfactory dilatation was obtained. Only five individuals (10%) required temporary stents and underwent a mean of 4.5 dilatations per patient over 5 years. Similarly to the patients reported by Hazel et al.,64 patients affected by DS had a 5-year survival of 70%, which was higher than that predicted by the Mayo Clinic PSC survival model.76
As these are observational studies, it was not possible to assess whether ursodeoxycholic acid alone or its combination with aggressive endoscopic therapy was responsible for the improved survival. One of the most important drawbacks of stent therapy alone has been identified as the risk (up to 50%) for cholangitis and sepsis associated with clogging of the stents.28 In a retrospective study of 32 PSC patients with symptomatic DS treated with endoscopic stents for a mean of 11 days, short-term stenting was used to avoid occlusion.28 When the stent was left in situ for only 1–2 weeks (median: 9 days), 81% of patients became asymptomatic and remained so without stent occlusions for a median follow-up of 19 months.28 Improvements in clinical and biochemical parameters were observed in 83% of patients, and 80% and 60% of individuals remained free of re-intervention for 1 and 3 years, respectively. However, these studies did not examine the effect of endoscopic stenting on survival or the eventual need for OLT. A retrospective study compared two groups of PSC patients with DS treated with balloon dilatation alone, and balloon dilatation plus stenting.77 Stent placement did not achieve additional benefit after dilatation and was associated with further complications caused by infections.77 Randomized controlled trials are necessary to determine whether stent therapy following dilatation is superior to dilatation alone.
Percutaneous transhepatic therapies
Percutaneous transhepatic dilatation and/or stenting produced clinical and biochemical improvements similar to those brought about by endoscopic therapy.78 This approach was usually used for symptomatic patients in whom endoscopic management had failed because percutaneous treatments have potential serious side-effects and the discomfort caused by external stents decreases quality of life.
Surgical interventions
Liver transplantation
Orthotopic liver transplantation is the best treatment option for patients with PSC conditioning liver failure. Overall 5-year survival rates in PSC patients treated by OLT were 70–85%, and recurrence rates were 20–30% at 5 years.79,80 Although OLT provided the best survival for patients with PSC, the number of grafts available was insufficient to match the number of potential recipients.81 Therefore, OLT was performed only when PSC was associated with liver failure.
Biliary resection or biliary bypass
In PSC patients with DS but without cirrhosis, the non-transplant surgical therapies available included biliary resection or bypass with bilioenteric anastomosis.73,82,83 Bypass procedures were technically less demanding than bile duct resection and reconstruction, but they had the disadvantages of leaving the stricture of the bile duct in situ with the potential for malignant transformation. Pitt et al. reported results of bilioenterostomy in the form of hepaticojejunostomy, choledochojejunostomy or choledochoduodenostomy in PSC patients with DS of the extrahepatic bile ducts.83 The overall survival of the 22 patients managed with this approach was 82% with a median follow-up of 5 years. In the same context, Myburgh reported an actuarial survival of 100% with a median follow-up of 6.5 years in 16 non-cirrhotic PSC patients with DS managed with hepaticojejunostomy.82 Similar results were achieved by surgical resection.73 In a retrospective series from John Hopkins University, most PSC patients with DS were managed surgically prior to 1990.20 The authors of this study reported that 50 patients with DS of the extrahepatic biliary tree secondary to PSC were treated with resection of the extrahepatic bile ducts and longterm transhepatic stenting.20 This approach achieved an overall 5-year survival of 85% among non-cirrhotic patients.20 After 1990 the percentage of patients who underwent biliary tract surgery at Johns Hopkins Hospital decreased in favour of endoscopic therapy.20 Among 54 patients who underwent endoscopic or percutaneous dilatation with or without stenting for DS, overall 5-year survival in non-cirrhotic patients reached only 59%.20 Although survival in these patients was inferior to that obtained by surgical resection, procedure-related morbidity and mortality rates were higher in patients who were treated surgically than in those treated endoscopically.20
Several reports have reported that surgical therapy on the bile system prior to OLT was associated with increased morbidity and mortality at the time of transplant.84–87 Therefore, concerns have been raised about the performance of any biliary tract operation in patients who may need OLT.88–91 One study from Norway reported that previous biliary surgery in PSC patients was associated with a higher rate of retransplantation.91 Generally, surgical resection of DS in non-cirrhotic PSC patients should be strongly considered for strictures that persist, recur soon after non-operative therapy or are highly suspicious for malignancy. Cirrhotic patients are best managed with OLT because non-transplant surgical treatment in these patients was associated with high operative mortality rates and poor longterm survival.73,82
Summary of findings for treatments
Orthotopic liver transplantation is the treatment of choice in patients with PSC and cirrhosis. In this group of patients, endoscopic or percutaneous dilatation with or without stenting provide symptomatic relief and act as a bridge to organ replacement.
Symptomatic non-cirrhotic PSC patients with DS can be treated both endoscopically and surgically. Repeated endoscopic dilatations of DS improve survival over predicted survival without treatment. Patients in whom non-operative techniques fail and those in whom DS is suspicious for malignancy are best managed by surgical therapy.
Although some studies have reported that treating DS in PSC patients non-operatively is associated with an increased risk for cholangiocarcinoma,92,93 others have failed to find any increased risk for cholangiocarcinoma in patients undergoing endoscopic therapy.73,94 Currently, there is insufficient evidence to suggest that manipulation of the biliary system with interval dilatations and/or stenting is associated with increased risk for cholangiocarcinoma.
There are no RCTs on the short- and longterm outcomes of endoscopic vs. surgical therapy in PSC patients affected by symptomatic DS. In addition, no prospective studies have compared the optimal duration of stent therapy and the frequency of endoscopic dilatation of DS.
Over the last decades, the use of multidisciplinary approaches in PSC patients has improved their survival. Nevertheless, the management of indeterminate DS remains a clinical challenge as there is no strong evidence to guide clinicians in their decisions. Controlled studies are needed to determine which therapeutic options are most beneficial. Because the incidence of DS in PSC patients who are not in need of OLT is low, such studies should be performed in multicentre contexts.
Conflicts of interest
None declared.
References
- 1.Lee YM, Kaplan MM. Medical progress: primary sclerosing cholangitis. N Engl J Med. 1995;332:924–993. doi: 10.1056/NEJM199504063321406. [DOI] [PubMed] [Google Scholar]
- 2.Nakanuma Y, Hirai N, Kono N, Ohta G. Histological and ultrastructural examination of the intrahepatic biliary tree in primary sclerosing cholangitis. Liver. 1986;6:317–325. doi: 10.1111/j.1600-0676.1986.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 3.Broome U, Grunewald J, Scheynius A, Olerup O, Hultcrantz R. Preferential V beta3 usage by hepatic T lymphocytes in patients with primary sclerosing cholangitis. J Hepatol. 1997;26:527–534. doi: 10.1016/s0168-8278(97)80417-5. [DOI] [PubMed] [Google Scholar]
- 4.Bambha K, Kim WR, Talwalkar J, Torgerson H, Benson JT, Therneau TM, et al. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology. 2003;125:1364–1369. doi: 10.1016/j.gastro.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Boberg KM, Aadland E, Jahnsen J, Raknerud N, Stiris M, Bell H. Incidence and prevalence of primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis in a Norwegian population. Scand J Gastroenterol. 1998;33:99–103. doi: 10.1080/00365529850166284. [DOI] [PubMed] [Google Scholar]
- 6.Kingham JGC, Kochar N, Gravenor MB. Incidence, clinical pattern, and outcomes of primary sclerosing cholangitis in South Wales, United Kingdom. Gastroenterology. 2004;126:1929–1930. doi: 10.1053/j.gastro.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 7.Gossard AA, Angulo P, Lindor KD. Secondary sclerosing cholangitis: a comparison to primary sclerosing cholangitis. Am J Gastroenterol. 2005;100:1330–1333. doi: 10.1111/j.1572-0241.2005.41526.x. [DOI] [PubMed] [Google Scholar]
- 8.Smith T, Befeler AS. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Curr Gastroenterol Rep. 2007;9:54–59. doi: 10.1007/s11894-008-0021-z. [DOI] [PubMed] [Google Scholar]
- 9.Charatcharoenwitthaya P, Lindor KD. Primary sclerosing cholangitis: diagnosis and management. Curr Gastroenterol Rep. 2006;8:75–82. doi: 10.1007/s11894-006-0067-8. [DOI] [PubMed] [Google Scholar]
- 10.Fickert P, Wagner M, Marschall HU, Fuchsbichler A, Zollner G, Tsybrovskyy O, et al. 24-norUrsodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2006;130:465–481. doi: 10.1053/j.gastro.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Beuers U, Spengler U, Kruis W, Aydemir U, Wiebecke B, Heldwein W, et al. Ursodeoxycholic acid for treatment of primary sclerosing cholangitis: a placebo-controlled trial. Hepatology. 1992;16:707–714. doi: 10.1002/hep.1840160315. [DOI] [PubMed] [Google Scholar]
- 12.Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis–Ursodeoxycholic Acid Study Group. N Engl J Med. 1997;336:691–695. doi: 10.1056/NEJM199703063361003. [DOI] [PubMed] [Google Scholar]
- 13.van Hoogstraten HJ, Vleggaar FP, Boland GJ, van Steenbergen W, Griffioen P, Hop WC, et al. Budesonide or prednisone in combination with ursodeoxycholic acid in primary sclerosing cholangitis: a randomized double-blind pilot study. Belgian–Dutch PSC Study Group. Am J Gastroenterol. 2000;95:2015–2022. doi: 10.1111/j.1572-0241.2000.02267.x. [DOI] [PubMed] [Google Scholar]
- 14.Vacca M, Krawczyk M, Petruzzelli M, Sasso RC, van Erpecum KJ, Palasciano G, et al. Current treatments of primary sclerosing cholangitis. Curr Med Chem. 2007;14:2081–2094. doi: 10.2174/092986707781368388. [DOI] [PubMed] [Google Scholar]
- 15.Ishibashi H, Komori A, Shimoda S, Gershwin ME. Guidelines for therapy of autoimmune liver disease. Semin Liver Dis. 2007;27:214–226. doi: 10.1055/s-2007-979472. [DOI] [PubMed] [Google Scholar]
- 16.Stiehl A. Primary sclerosing cholangitis: the role of endoscopic therapy. Semin Liver Dis. 2006;26:62–68. doi: 10.1055/s-2006-933564. [DOI] [PubMed] [Google Scholar]
- 17.Stiehl A, Rost D. Endoscopic treatment of dominant stenoses in patients with primary sclerosing cholangitis. Clin Rev Allergy Immunol. 2005;28:159–165. doi: 10.1385/CRIAI:28:2:159. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto T, Hirohashi K, Kubo S, Tsukamoto T, Uenishi T, Shuto T, et al. Surgery for segmental primary sclerosing cholangitis. Hepatogastroenterology. 2004;51:668–671. [PubMed] [Google Scholar]
- 19.Hirai I, Ishiyama S, Fuse A, Kuzu H, Sakurai F, Kimura S, et al. Primary sclerosing cholangitis successfully treated by resection of the confluence of the hepatic duct. J Hepatobiliary Pancreat Surg. 2001;8:169–173. doi: 10.1007/s005340170042. [DOI] [PubMed] [Google Scholar]
- 20.Ahrendt SA, Pitt HA, Kalloo AN, Venbrux AC, Klein AS, Herlong HF, et al. Primary sclerosing cholangitis: resect, dilate, or transplant? Ann Surg. 1998;227:412–423. doi: 10.1097/00000658-199803000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Laethem JL, Devière J, Bourgeois N, Love J, Gelin M, Cremer M, et al. Cholangiographic findings in deteriorating primary sclerosing cholangitis. Endoscopy. 1995;27:223–228. doi: 10.1055/s-2007-1005675. [DOI] [PubMed] [Google Scholar]
- 22.Björnsson E, Lindqvist-Ottosson J, Asztely M, Olsson R. Dominant strictures in patients with primary sclerosing cholangitis. Am J Gastroenterol. 2004;99:502–508. doi: 10.1111/j.1572-0241.2004.04106.x. [DOI] [PubMed] [Google Scholar]
- 23.Meenan J, Rauws EA, Huibregtse K. Benign biliary strictures and sclerosing cholangitis. Gastrointest Endosc Clin N Am. 1996;6:127–138. [PubMed] [Google Scholar]
- 24.Olsson R, Boberg KM, de Muckadell OS, Lindgren S, Hultcrantz R, Folvik G, et al. High-dose ursodeoxycholic acid in primary sclerosing cholangitis: a 5-year multicentre, randomized, controlled study. Gastroenterology. 2005;129:1464–1472. doi: 10.1053/j.gastro.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 25.Cook DJ, Mulrow CD, Haynes RB. Systematic reviews: synthesis of best evidence for clinical decisions. Ann Intern Med. 1997;126:376–380. doi: 10.7326/0003-4819-126-5-199703010-00006. [DOI] [PubMed] [Google Scholar]
- 26.Mulrow CD, Oxman AD. Cochrane Collaboration Handbook. Oxford: Cochrane Library, Update Software; 1997. [Google Scholar]
- 27.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomized and non-randomized studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ponsioen CY, Lam K, van Milligen de Wit AW, Huibregtse K, Tytgat GN. Four years experience with short-term stenting in primary sclerosing cholangitis. Am J Gastroenterol. 1999;94:2403–2407. doi: 10.1111/j.1572-0241.1999.01364.x. [DOI] [PubMed] [Google Scholar]
- 29.Stiehl A, Rudolph G, Klöters-Plachky P, Sauer P, Walker S. Development of dominant bile duct stenoses in patients with primary sclerosing cholangitis treated with ursodeoxycholic acid: outcome after endoscopic treatment. J Hepatol. 2002;36:151–156. doi: 10.1016/s0168-8278(01)00251-3. [DOI] [PubMed] [Google Scholar]
- 30.LaRusso NF, Shneider BL, Black D, Gores GJ, James SP, Doo E, et al. Primary sclerosing cholangitis: summary of a workshop. Hepatology. 2006;44:746–764. doi: 10.1002/hep.21337. [DOI] [PubMed] [Google Scholar]
- 31.MacCarty RL, LaRusso NF, Wiesner RH. Primary sclerosing cholangitis: findings on cholangiography and pancreatography. Radiology. 1983;149:39–44. doi: 10.1148/radiology.149.1.6412283. [DOI] [PubMed] [Google Scholar]
- 32.May GR, Bender CE, LaRusso NF, Wiesner RH. Non-operative dilation of dominant strictures in primary sclerosing cholangitis. AJR Am J Roentgenol. 1985;145:1061–1064. doi: 10.2214/ajr.145.5.1061. [DOI] [PubMed] [Google Scholar]
- 33.van Milligen de Wit AW, Rauws EA, van Bracht J, Mulder CJ, Jones EA, Tytgat GN, et al. Endoscopic stent therapy for dominant extrahepatic bile duct strictures in primary sclerosing cholangitis. Gastrointest Endosc. 1996;44:293–299. doi: 10.1016/s0016-5107(96)70167-0. [DOI] [PubMed] [Google Scholar]
- 34.Olsson RG, Asztély MS. Prognostic value of cholangiography in primary sclerosing cholangitis. Eur J Gastroenterol Hepatol. 1995;7:251–254. [PubMed] [Google Scholar]
- 35.Tischendorf JJ, Krüger M, Trautwein C, Duckstein N, Schneider A, Manns MP, et al. Cholangioscopic characterization of dominant bile duct stenoses in patients with primary sclerosing cholangitis. Endoscopy. 2006;38:665–669. doi: 10.1055/s-2006-925257. [DOI] [PubMed] [Google Scholar]
- 36.Bergquist A, Broome U. Hepatobiliary and extrahepatic malignancies in primary sclerosing cholangitis. Best Pract Res Clin Gastroenterol. 2001;15:643–656. doi: 10.1053/bega.2001.0210. [DOI] [PubMed] [Google Scholar]
- 37.Farrant JM, Hayllar KM, Wilkinson ML, Karni J, Portmann BC, Westaby D, et al. Natural history and prognostic variables in primary sclerosing cholangitis. Gastroenterology. 1991;100:1707–1710. doi: 10.1016/0016-5085(91)90673-9. [DOI] [PubMed] [Google Scholar]
- 38.Charatcharoenwitthaya P, Enders FB, Halling KC, Lindor KD. Cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology. 2008;48:1106–1117. doi: 10.1002/hep.22441. [DOI] [PubMed] [Google Scholar]
- 39.Bergquist A, Ekbom A, Olsson R, Kornfeldt D, Loof L, Danielsson A, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36:321–327. doi: 10.1016/s0168-8278(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 40.Kaya M, de Groen P, Angulo P, Nagorney DM, Gunderson LL, Gores GJ, et al. Treatment of cholangiocarcinoma complicating primary sclerosing cholangitis: the Mayo Clinic experience. Am J Gastroenterology. 2001;96:1164–1169. doi: 10.1111/j.1572-0241.2001.03696.x. [DOI] [PubMed] [Google Scholar]
- 41.Rosen CB, Nagorney DM, Wiesner RH, Coffey RJ, LaRusso NF. Cholangiocarcinoma complicating primary sclerosing cholangitis. Ann Surg. 1991;213:21–25. doi: 10.1097/00000658-199101000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pohl J, Ring A, Stremmel W, Stiehl A. The role of dominant stenoses in bacterial infections of bile ducts in primary sclerosing cholangitis. Eur J Gastroenterol Hepatol. 2006;18:69–74. doi: 10.1097/00042737-200601000-00012. [DOI] [PubMed] [Google Scholar]
- 43.Lee JG, Schutz SM, England RE, Leung JW, Cotton PB. Endoscopic therapy of sclerosing cholangitis. Hepatology. 1995;21:661–667. [PubMed] [Google Scholar]
- 44.Bjornsson E, Boberg KM, Cullen S, Fleming K, Clausen OP, Fausa O, et al. Patients with small duct primary sclerosing cholangitis have favourable longterm prognosis. Gut. 2002;51:731–735. doi: 10.1136/gut.51.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Angulo P, Peter JB, Gershwin ME, DeSotel CK, Shoenfeld Y, Ahmed AE, et al. Serum autoantibodies in patients with primary sclerosing cholangitis. J Hepatol. 2000;32:182–187. doi: 10.1016/s0168-8278(00)80061-6. [DOI] [PubMed] [Google Scholar]
- 46.Mulder AH, Horst G, Haagsma EB, Limburg PC, Kleibeuker JH, Kallenberg CG. Prevalence and characterization of neutrophil cytoplasmic antibodies in autoimmune liver disease. Hepatology. 1993;17:411–417. [PubMed] [Google Scholar]
- 47.Hultcranz R, Olsson R, Danielsson A, Jarnerot G, Loof L, Ryden BO, et al. A 3-year prospective study on serum tumour markers used for detecting cholangiocarcinoma in patients with primary sclerosing cholangitis. J Hepatol. 1999;30:669–673. doi: 10.1016/s0168-8278(99)80198-6. [DOI] [PubMed] [Google Scholar]
- 48.Petersen-Benz C, Stiehl A. Impact of dominant stenoses on the serum level of the tumour marker CA 19-9 in patients with primary sclerosing cholangitis. Z Gastroenterol. 2005;43:587–590. doi: 10.1055/s-2005-858105. [DOI] [PubMed] [Google Scholar]
- 49.Levy MJ, Baron TH, Clayton AC, Enders FB, Gostout CJ, Halling KC, et al. Prospective evaluation of advanced molecular markers and imaging techniques in patients with indeterminate bile duct strictures. Am J Gastroenterol. 2008;103:1263–1273. doi: 10.1111/j.1572-0241.2007.01776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakeeb A, Lipsett PA, Lillemoe KD, Fox-Talbot MK, Coleman J, Cameron JL, et al. Biliary carcinoembryonic antigen levels are a marker for cholangiocarcinoma. Am J Surg. 1996;171:147–153. doi: 10.1016/S0002-9610(99)80090-7. [DOI] [PubMed] [Google Scholar]
- 51.Ahrendt SA, Pitt HA, Nakeeb A, Klein AS, Lillemoe KD, Kalloo AN, et al. Diagnosis and management of cholangiocarcinoma in primary sclerosing cholangitis. J Gastrointest Surg. 1999;3:357–368. doi: 10.1016/s1091-255x(99)80051-1. [DOI] [PubMed] [Google Scholar]
- 52.Ponsioen CY, Vrouenraets SM, van Milligen de Wit AW, Sturm P, Tascilar M, Offerhaus GJ, et al. Value of brush cytology for dominant strictures in primary sclerosing cholangitis. Endoscopy. 1999;31:305–309. doi: 10.1055/s-1999-18. [DOI] [PubMed] [Google Scholar]
- 53.Sebo TJ. Digital image analysis. Mayo Clin Proc. 1995;70:81–82. doi: 10.1016/S0025-6196(11)64670-3. [DOI] [PubMed] [Google Scholar]
- 54.Pearsons DL, Takai K, Gibney DJ, Katzmann JA, Lieber MM, Jenkins RB, et al. Comparison of fluorescence in situ hybridization with flow cytometry and static image analysis in ploidy analysis of paraffin-embedded prostate adenocarcinoma. Hum Pathol. 1994;25:678–683. doi: 10.1016/0046-8177(94)90301-8. [DOI] [PubMed] [Google Scholar]
- 55.Moreno Luna LE, Kipp B, Halling KC, Sebo TJ, Kremers WK, Roberts LR, Barr Fritcher EG, Levy MJ, Gores GJ, et al. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology. 2006;131:1064–1072. doi: 10.1053/j.gastro.2006.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Majoie CBLM, Smits NJ, Phoa SSKS, Reeders JWAJ, Jansen PLM. Primary sclerosing cholangitis: sonographic findings. Abdom Imaging. 1995;20:109–112. doi: 10.1007/BF00201514. [DOI] [PubMed] [Google Scholar]
- 57.Lee JG, Schutz SM, England RE, Leung JW, Cotton PB. Endoscopic therapy of sclerosing cholangitis. Hepatology. 1995;21:661–667. [PubMed] [Google Scholar]
- 58.Schulte SJ, Baron RL, Teefey SA, Rohrmann CA, Freeney PC, Shuman WP, et al. CT of the extrahepatic bile ducts: wall thickening and contrast enhancement in normal and abnormal ducts. AJR Am J Roentgenol. 1990;154:79–85. doi: 10.2214/ajr.154.1.2104731. [DOI] [PubMed] [Google Scholar]
- 59.Macchi V, Floreani A, Marchesi P, Pasini R, Zuliani M, Feltrin GP, et al. Imaging of primary sclerosing cholangitis: preliminary results by two new non-invasive techniques. Dig Liver Dis. 2004;36:614–621. doi: 10.1016/j.dld.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 60.Eracleous E, Genagritis M, Papanikolaou N, Kontou AM, Prassopoulos H, Allan P, et al. Complementary role of helical CT cholangiography to MR cholangiography in the evaluation of biliary function and kinetics. Eur Radiol. 2005;15:2130–2139. doi: 10.1007/s00330-005-2809-7. [DOI] [PubMed] [Google Scholar]
- 61.Silverman WB, Kaw M, Rabinovitz M, Schade RR, Bates BL. Complication rate of endoscopic retrograde cholangiopancreatography (ERCP) in patients with primary sclerosing cholangitis (PSC): is it safe? Gastroenterology. 1994;106:A359. [Google Scholar]
- 62.Moff SL, Kamel IR, Eustace J, Lawler LP, Kantsevoy S, Kalloo AN, et al. Diagnosis of primary sclerosing cholangitis: a blinded comparative study using magnetic resonance cholangiography and endoscopic retrograde cholangiography. Gastrointest Endosc. 2006;64:219–223. doi: 10.1016/j.gie.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 63.MacCarty RL, LaRusso NF, Wiesner RH. Primary sclerosing cholangitis: findings on cholangiography and pancreatography. Radiology. 1983;149:39–44. doi: 10.1148/radiology.149.1.6412283. [DOI] [PubMed] [Google Scholar]
- 64.Hazel SJ, Wolfhagen FHJ, Buuren HR, van de Meeberg PC, van Leeuwen DJ. Prospective risk assessment of endoscopic retrograde cholangiography in patients with primary sclerosing cholangitis. Endoscopy. 2000;32:779–782. doi: 10.1055/s-2000-7708. [DOI] [PubMed] [Google Scholar]
- 65.Harbin WP, Mueller PR, Ferrucci JT. Transhepatic cholangiography: complications and use patterns of the fine-needle technique. Radiology. 1980;135:15–22. doi: 10.1148/radiology.135.1.6987704. [DOI] [PubMed] [Google Scholar]
- 66.Cohen SA, Siegel JH, Kasmin FE. Complications of diagnostic and therapeutic ERCP. Abdom Imaging. 1996;21:385–394. doi: 10.1007/s002619900089. [DOI] [PubMed] [Google Scholar]
- 67.Moff SL, Kamel IR, Eustace J, Lawler LP, Kantsevoy S, Kalloo AN, et al. Diagnosis of primary sclerosing cholangitis: a blinded comparative study using magnetic resonance cholangiography and endoscopic retrograde cholangiography. Gastrointest Endosc. 2006;64:219–223. doi: 10.1016/j.gie.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 68.Berstad AE, Aabakken L, Smith HJ, Aasen S, Boberg KM, Schrumpf E. Diagnostic accuracy of magnetic resonance and endoscopic retrograde cholangiography in primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2006;4:514–520. doi: 10.1016/j.cgh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 69.Boberg KM, Jebsen P, Clausen OP, Foss A, Aabakken L, Schrumpf E. Diagnostic benefit of biliary brush cytology in cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol. 2006;45:568–574. doi: 10.1016/j.jhep.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 70.Ahrendt SA. Surgical approaches to strictures in primary sclerosing cholangitis. J Gastrointest Surg. 2008;12:423–425. doi: 10.1007/s11605-007-0342-5. [DOI] [PubMed] [Google Scholar]
- 71.Tsai S, Pawlik TM. Primary sclerosing cholangitis: the role of extrahepatic biliary resection. Adv Surg. 2009;43:175–188. doi: 10.1016/j.yasu.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 72.May GR, Bender CE, LaRusso NF, Wiesner RH. Non-operative dilatation of dominant strictures in primary sclerosing cholangitis. AJR Am J Roentgenol. 1985;145:1061–1064. doi: 10.2214/ajr.145.5.1061. [DOI] [PubMed] [Google Scholar]
- 73.Baluyut AR, Sherman S, Lehman GA, Hoen H, Chalasani N. Impact of endoscopic therapy on the survival of patients with primary sclerosing cholangitis. Gastrointest Endosc. 2001;53:308–312. doi: 10.1016/s0016-5107(01)70403-8. [DOI] [PubMed] [Google Scholar]
- 74.Stiehl A, Rudolph G, Kloters-Plachky P, Sauer P, Walker S. Development of dominant bile duct stenoses in patients with primary sclerosing cholangitis treated with ursodeoxycholic acid: outcome of endoscopic treatment. J Hepatol. 2002;36:151–156. doi: 10.1016/s0168-8278(01)00251-3. [DOI] [PubMed] [Google Scholar]
- 75.Van Milligen de Wit AWM, Van Bracht J, Rauws EAJ, Jones EA, Tytgat GNJ, Huibregtse K. Endoscopic stent therapy for dominant extrahepatic bile duct strictures in primary sclerosing cholangitis. Gastrointest Endosc. 1996;44:293–299. doi: 10.1016/s0016-5107(96)70167-0. [DOI] [PubMed] [Google Scholar]
- 76.Dickson ER, Murtaugh PA, Wiesner RH, Grambsch PM, Flemming TR, Ludwig J, et al. Primary sclerosing cholangitis: refinement and validation of survival models. Gastroenterology. 1992;103:1893–1901. doi: 10.1016/0016-5085(92)91449-e. [DOI] [PubMed] [Google Scholar]
- 77.Kaya M, Petersen BT, Angulo P, Baron TH, Andrews JC, Gostout CJ, et al. Balloon dilation compared to stenting of dominant strictures in primary sclerosing cholangitis. Am J Gastroenterol. 2001;96:1059–1066. doi: 10.1111/j.1572-0241.2001.03690.x. [DOI] [PubMed] [Google Scholar]
- 78.Skolkin MD, Alspaugh JP, Casarella WJ, Chuang VP, Galambos JT. Sclerosing cholangitis: palliation with percutaneous cholangioplasty. Radiology. 1989;170:199–206. doi: 10.1148/radiology.170.1.2462261. [DOI] [PubMed] [Google Scholar]
- 79.Graziadei IW, Wiesner RH, Marotta PJ, Porayko MK, Hay JE, Charlton MR, et al. Longterm results of patients undergoing liver transplantation for primary sclerosing cholangitis. Hepatology. 1999;30:1121–1127. doi: 10.1002/hep.510300501. [DOI] [PubMed] [Google Scholar]
- 80.Vera A, Moledina S, Gunson B, Hubscher S, Mirza D, Olliff S, et al. Risk factors for recurrence of primary sclerosing cholangitis of liver allograft. Lancet. 2002;360:1943–1944. doi: 10.1016/S0140-6736(02)11861-7. [DOI] [PubMed] [Google Scholar]
- 81.United Network for Organ Sharing (UNOS. UNOS Database. 2002. http://www.unos.org. [Accessed 29 February 2008.
- 82.Myburgh JA. Surgical biliary drainage in primary sclerosing cholangitis: the role of the Hepp–Couinaud approach. Arch Surg. 1994;129:1057–1062. doi: 10.1001/archsurg.1994.01420340071012. [DOI] [PubMed] [Google Scholar]
- 83.Pitt HA, Thompson HH, Tompkins RK, Longmire WP. Primary sclerosing cholangitis: results of an aggressive surgical approach. Ann Surg. 1982;196:259–268. doi: 10.1097/00000658-198209000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martin FM, Rossi RL, Nugent FW, Scholz FJ, Jenkins RL, Lewis WD, et al. Surgical aspects of sclerosing cholangitis. Results in 178 patients. Ann Surg. 1990;212:551–556. doi: 10.1097/00000658-199010000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ismail T, Angrisani L, Powell JE, Hübscher S, Buckels J, Neuberger J, et al. Primary sclerosing cholangitis: surgical options, prognostic variables, and outcome. Br J Surg. 1991;78:564–567. doi: 10.1002/bjs.1800780515. [DOI] [PubMed] [Google Scholar]
- 86.Farges O, Malassagne B, Sebaugh M, Bismuth H. Primary sclerosing cholangitis: liver transplantation or biliary surgery. Surgery. 1995;117:146–155. doi: 10.1016/s0039-6060(05)80078-9. [DOI] [PubMed] [Google Scholar]
- 87.Brandsaeter B, Friman S, Broomé U, Isoniemi H, Olausson M, Bäckman L, et al. Outcome following liver transplantation for primary sclerosing cholangitis in the Nordic countries. Scand J Gastroenterol. 2003;38:1776–1783. doi: 10.1080/00365520310006009. [DOI] [PubMed] [Google Scholar]
- 88.Martin FM, Rossi RL, Nugent FW, Scholz FJ, Jenkins RL, Lewis WD, et al. Surgical aspects of sclerosing cholangitis. Results in 178 patients. Ann Surg. 1990;212:551–556. doi: 10.1097/00000658-199010000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ismail T, Angrisani L, Powell JE, Hübscher S, Buckels J, Neuberger J, et al. Primary sclerosing cholangitis: surgical options, prognostic variables, and outcome. Br J Surg. 1991;78:564–567. doi: 10.1002/bjs.1800780515. [DOI] [PubMed] [Google Scholar]
- 90.Farges O, Malassagne B, Sebaugh M, Bismuth H. Primary sclerosing cholangitis: liver transplantation or biliary surgery. Surgery. 1995;117:146–155. doi: 10.1016/s0039-6060(05)80078-9. [DOI] [PubMed] [Google Scholar]
- 91.Brandsaeter B, Friman S, Broomé U, Isoniemi H, Olausson M, Bäckman L, et al. Outcome following liver transplantation for primary sclerosing cholangitis in the Nordic countries. Scand J Gastroenterol. 2003;38:1776–1783. doi: 10.1080/00365520310006009. [DOI] [PubMed] [Google Scholar]
- 92.Ahrendt SA, Pitt HA, Kalloo AN, Venbrux AC, Klein AS, Herlong HF, et al. Primary sclerosing cholangitis: resect, dilate, or transplant? Ann Surg. 1998;227:412–423. doi: 10.1097/00000658-199803000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Linder S, Söderlund C. Endoscopic therapy in primary sclerosing cholangitis: outcome of treatment and risk of cancer. Hepatogastroenterology. 2001;48:387–392. [PubMed] [Google Scholar]
- 94.Chalasani N, Baluyut A, Ismail A, Zaman A, Sood G, Ghalib R, et al. Cholangiocarcinoma in patients with primary sclerosing cholangitis: a multicentre case–control study. Hepatology. 2000;31:7–11. doi: 10.1002/hep.510310103. [DOI] [PubMed] [Google Scholar]


