Abstract
Objectives
Obesity has been associated with worse postoperative outcomes. No data are available regarding short-term results after liver resection (LR). The aim of this study was to analyse outcomes in obese patients (body mass index [BMI] > 30 kg/m2) undergoing LR.
Methods
85 consecutive obese patients undergoing LR between 1998 and 2008 were matched on a ratio of 1:2 with 170 non-obese patients. Matching criteria were diagnosis, ASA score, METAVIR fibrosis score, extent of LR, and Child–Pugh score in patients with cirrhosis.
Results
Operative time, blood loss and blood transfusions were similar in the two groups. Mortality was 2.4% in both groups. Morbidity was significantly higher in the obese group (32.9% vs. 21.2%; P= 0.041). However, only grade II morbidity was increased in obese patients (14.1% vs. 1.8%; P < 0.001) and this was mainly related to abdominal wall complications (8.2% vs. 2.4%; P= 0.046). No differences were encountered in terms of grade III or IV morbidity. The same results were observed in major LR and cirrhotic patients. When patients were stratified by BMI (<20, 20–25, 25–30 and >30 kg/m2), progressive increases in overall and infectious morbidity were observed (5.6%, 22.4%, 23.7%, 32.9%, and 5.6%, 11.8%, 14.5%, 18.8%, respectively). Rates of grade III and IV morbidity did not change.
Discussion
Obese patients have increased postoperative morbidity after LR in comparison with non-obese patients, but this is mainly related to minor abdominal wall complications. Severe morbidity rates and mortality are similar to those in non-obese patients, even in cirrhosis or after major LR.
Keywords: liver surgery, liver resection, obesity, BMI, case-control study, steatosis, ASA score, morbidity, postoperative outcomes, laparoscopic liver surgery, cirrhosis
Introduction
The prevalence of obesity is continuously increasing worldwide.1 In the USA more than one-fourth of the population has a body mass index (BMI) > 30 kg/m2.2 In Europe the situation is less pronounced, but the incidence of obesity is advancing rapidly and current prevalences range between 6% and 27%.3 Even in Asia, obesity is becoming a significant problem.4
Obesity may negatively impact on surgical outcomes through associated co-morbidities, such as cardiovascular and pulmonary diseases and diabetes,5,6 and increased technical difficulties. With respect to liver surgery, obesity may be associated with liver parenchymal disease such as steatosis and non-alcoholic fatty liver disease,7,8 which can further increase postoperative morbidity.7,9–12
Outcomes in obese patients in different surgical fields have been analysed, but data are controversial. Several studies have reported no increased risk outside that for minor complications,13–17 whereas other reports have found obesity to be associated with high morbidity, mortality and poor longterm outcomes.18–23 Recently, a large series compared 808 obese with 5528 non-obese patients undergoing elective general surgery and reported similar morbidity rates between the two groups, except for wound infections.24 However, few liver resections were included. At present, no study specifically focuses on liver surgery in obese patients with a BMI > 30 kg/m2.
The aim of the present study was to compare outcomes in obese and non-obese patients undergoing liver surgery in a case–control setting. Matching was intended to limit confounding factors, such as co-morbidities and liver function, in order to focus specifically on the impact of obesity in liver surgery.
Materials and methods
Between January 1998 and July 2008, 719 patients underwent liver resection at Henri Mondor University Hospital. All patients were considered for the present study. On the basis of the World Health Organization (WHO) definition of obesity (BMI > 30 kg/m2),1 85 (11.8%) consecutive obese patients were identified. They included 67 (78.8%) patients with class I obesity (BMI 30–35 kg/m2), 13 (15.3%) with class II obesity (35–40 kg/m2), and five (5.9%) with class III obesity (>40 kg/m2).1 Obese patients were matched at a ratio of 1:2 with 170 non-obese patients (BMI ≤ 30 kg/m2). Matching criteria were diagnosis (benign vs. malignant, the same diagnosis whenever possible), American Society of Anesthesiologists (ASA) score (I, II and III), METAVIR F score for liver fibrosis25 (0–1, 2, 3 and 4), extent of liver resection (major vs. minor hepatectomy) and, in cirrhotic patients (METAVIR F4), the Child–Pugh score.26 The median BMI was 32.6 kg/m2 (range: 31.0–62.4 kg/m2) in the obese group and 24.5 kg/m2 (range: 16.9–28.9 kg/m2) in the non-obese group. The strict matching policy is detailed in Table 1.
Table 1.
Matching criteria
| Obese patientsBMI > 30 kg/m2 (n= 85) | Non-obese patientsBMI ≤ 30 kg/m2 (n= 170) | |
|---|---|---|
| Median BMI, kg/m2 (range) | 32.6 (31.0–62.4) | 24.5 (16.9–28.9) |
| 1Diagnosis | ||
| Malignant lesions | 74 (87.1%) | 148 (87.1%) |
| Hepatocellular carcinoma | 30 (35.3%) | 68 (40.0%) |
| Peripheral cholangiocarcinoma | 7 (8.2%) | 3 (1.8%) |
| Colorectal metastases | 25 (29.4%) | 60 (35.3%) |
| Non-colorectal metastases | 5 (5.9%) | 8 (4.7%) |
| Hilar cholangiocarcinoma | 3 (3.5%) | 6 (3.5%) |
| Gallbladder cancer | 2 (2.4%) | 3 (1.8%) |
| Benign lesions | 11 (12.9%) | 22 (12.9%) |
| Liver adenoma | 5 (5.9%) | 6 (3.5%) |
| Haemangioma | 2 (2.4%) | 3 (1.8%) |
| Focal nodular hyperplasia | 1 (1.2%) | 6 (3.5%) |
| Other lesions | 3 (3.5%) | 7 (4.1%) |
| 2ASA score | ||
| I | 3 (3.5%) | 6 (3.5%) |
| II | 57 (67.1%) | 114 (67.1%) |
| III | 25 (29.4%) | 50 (29.4%) |
| 3METAVIR F score for liver fibrosis25 | ||
| 0–1 | 54 (63.5%) | 108 (63.5%) |
| 2 | 9 (10.6%) | 18 (10.6%) |
| 3 | 5 (5.9%) | 10 (5.9%) |
| 4 | 17 (20.0%) | 34 (20.0%) |
| 4Extent of liver resection | ||
| Major hepatectomy | 42 (49.4%) | 84 (49.4%) |
| Right hepatectomy ± segment I | 19 (22.4%) | 42 (24.7%) |
| Right trisectionectomy ± segment I | 9 (10.6%) | 18 (10.6%) |
| Left hepatectomy ± segment I | 10 (11.8%) | 17 (10.0%) |
| Left hepatectomy ± segment VIII | 1 (1.2%) | – |
| Left hepatectomy + segments V–VIII | – | 1 (0.6%) |
| Central hepatectomy | 3 (3.5%) | 6 (3.5%) |
| 5Child–Pugh class in cirrhotic patients | n = 17 | n = 34 |
| A | 16 | 32 |
| B | 1 | 2 |
BMI, body mass index; ASA, American Society of Anesthesiologists
Preoperative characteristics, intraoperative data, pathological results concerning both primary parenchymal disease and the pathological lesion, and postoperative outcomes were compared between the two groups. Subgroup analysis of postoperative outcomes was performed for major liver resections, cirrhotic patients, diabetic patients and those with moderate or severe steatosis. Correlations between BMI and outcomes were further studied by stratifying patients by BMI values (<20, 20–25, 25–30 and >30 kg/m2). Among the obese patients, outcomes according to the severity of obesity were analysed by stratifying patients according to obesity classes I (BMI 30–35 kg/m2), II (35–40 kg/m2) and III (>40 kg/m2).1
Preoperative patient evaluation
Preoperative patient selection systematically relied on liver function tests, computed tomography (CT) volumetry of the future liver remnant if major liver resection was planned, and anesthesiological evaluation based on case history, physical examination, biochemistry, arterial blood gas, electrocardiography (ECG) and chest X-ray. Further evaluations, such as cardiological and pulmonary tests, were required in selected patients only. No specific preoperative work-up was considered for obese patients. Obesity per se was never considered as a contraindication for liver surgery. Liver surgery was contraindicated in patients with poor liver function, inadequate future liver remnant (even after portal vein embolization or ligation) and severe co-morbidities that exposed the patient to unacceptable operative risk (usually corresponding to ASA score IV patients).
Surgical technique
The surgical techniques for both open and laparoscopic liver resections have been previously reported.27–30 Indications for the laparoscopic approach have been detailed elsewhere.29,31 Obesity has never been considered a contraindication to laparoscopic liver resection. The laparoscopic approach was primarily considered for lesions of ≤5 cm located in the anterolateral segments (segments II–VI) and requiring limited resection, although a few major resections were performed. Resection of larger lesions, those located close to the liver hilus or the hepatocaval junction and the vast majority of major resections were performed using the conventional open approach.
Intraoperative ultrasonography was routinely performed to confirm the number of lesions, their size and their relationship with the intrahepatic vascular structures. Extrahepatic inflow and outflow vascular control was regularly performed for anatomic resections, with ligation and division of appropriate portal and arterial branches and hepatic veins. Bile duct division was usually performed during the parenchymal transection. Generally, a tape was placed around the hepatic pedicle and the intermittent Pringle manoeuvre was used only if significant bleeding occurred. Total vascular exclusion was considered for lesions invading the inferior vena cava. In open resections, parenchymal transection was performed with an ultrasonic dissector (CUSA Dissectron [Integra Life Sciences Corp., Plainsboro, NJ, USA]; SonoSurg [Olympus Corp., Tokyo, Japan]). In laparoscopic cases, the harmonic scalpel (Ethicon Endo-Surgery, Inc., Cincinnati, OH, USA) was used for superficial layers and the ultrasonic dissector for deeper transection. Bipolar forceps with continuous irrigation were used to divide small vessels, whereas absorbable clips or ligatures were preferred for larger vessels or bile ducts. Abdominal drainage was used in right or extended right hepatectomies, but usually omitted in other types of resection.
Definitions
Types of hepatectomy were classified according to Brisbane 2000 terminology.32 Major hepatectomy was defined as the resection of three or more Couinaud segments. Fibrosis was classified according to METAVIR F score.25 Steatosis was estimated as the percentage of involved hepatocytes, and was categorized as follows: absent (0%); mild (1–30%); moderate (31–60%), and severe (>60%).33 Operative mortality was defined as death within 90 days after surgery or before discharge from hospital. Morbidity included all postoperative complications and was classified according to the Clavien classification.34 Liver dysfunction was defined as both prothrombin time (PT) <50% and serum bilirubin level >50 µmol/l on postoperative day (PoD) 5.35
Statistical analysis
Continuous variables were compared between groups by the unpaired t-test or Mann–Whitney U-test, as appropriate; categorical variables were compared using the chi-squared test or Fisher's exact test, as appropriate. A P-value <0.050 was considered significant for all tests.
Results
Patient characteristics
Demographic data were similar between the two groups except that the obese patient group included a higher proportion of females (51.8% [44/85] vs. 33.5% [57/170]; P= 0.005). Associated co-morbidities, including cardiovascular and pulmonary diseases and diabetes, were not increased in obese patients. Liver function, even in patients with cirrhosis, was similar between the two groups (Table 2).
Table 2.
Preoperative characteristics and histopathological steatosis severity
| Obese patientsBMI > 30 kg/m2 (n= 85) | Non-obese patientsBMI ≤ 30 kg/m2 (n= 170) | P-value | |
|---|---|---|---|
| Median age (range), years | 62.5 (24–83) | 62.5 (15–84) | 0.918 |
| Male sex | 41 (48.2%) | 113 (66.5%) | 0.005 |
| Preoperative chemotherapy | 15 (17.6%) | 35 (20.6%) | 0.577 |
| Co-morbidities | |||
| Arterial hypertension | 38 (44.7%) | 64 (37.6%) | 0.278 |
| Diabetes | 18 (21.2%) | 28 (16.5%) | 0.357 |
| COPD | 6 (7.1%) | 15 (8.8%) | 0.629 |
| Cardiovascular disease | 6 (7.1%) | 10 (5.9%) | 0.715 |
| Steatosis | |||
| Absent (0%) | 16 (18.9%) | 62 (36.5%) | 0.004 |
| Mild (1–30%) | 45 (52.9%) | 101 (59.4%) | 0.325 |
| Moderate (31–60%) | 20 (23.5%) | 6 (3.5%) | <0.001 |
| Severe (>60%) | 4 (4.7%) | 1 (0.6%) | 0.044 |
| Liver function tests, median (range) | |||
| AST, U/l | 26 (8–568) | 26 (10–253) | 0.849 |
| ALT, U/l | 27 (7–535) | 23 (8–411) | 0.154 |
| GGT, U/l | 69 (4–1229) | 57.5 (5–1384) | 0.799 |
| Total bilirubin, µmol/l | 11 (4–56) | 11 (4–499) | 0.252 |
| PT, % | 90 (43–100) | 92 (63–110) | 0.086 |
| Creatinine, µmol/l | 86 (48–168) | 89 (40–259) | 0.886 |
| Liver function tests in cirrhotic patients, median (range) | n = 17 | n = 34 | |
| Albumin, g/l | 40 (25–48) | 41.4 (25.8–53.9) | 0.391 |
| Total bilirubin, µmol/l | 14 (8–39) | 12 (5–26) | 0.163 |
| PT, % | 76.5 (50–110) | 83 (63–110) | 0.115 |
| Ascites | – | 2 | 0.547 |
| Encephalopathy | – | 3 | 0.542 |
| Platelet count, 103/mm3 | 123.5 (63–279) | 154 (47–315) | 0.353 |
COPD, chronic pulmonary obstructive disease; AST, aspartate aminotranferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transferase; PT, prothrombin time
Parenchymal steatosis was related to BMI: it was very frequent in obese patients, especially in its moderate (31–60%) and severe (>60%) forms (23.5% [20/85] vs. 3.5% [6/170], P < 0.001 and 4.7% [4/85] vs. 0.6% [1/170], P= 0.044, respectively).
Intraoperative and pathological data
Laparoscopic resection was attempted in about one-third of patients in both groups; the rate of conversion to hand assistance or laparotomy was not increased in obese patients. Pedicle clamping was used in similar proportions of patients in the two groups. Obesity was not associated with increased blood loss, and transfusion rate was low in both groups, at about 5% (4/85 vs. 9/170 patients). Median operative time was longer in obese patients (210 min vs. 180 min), but the difference was not significant (Table 3).
Table 3.
Intraoperative data
| Surgical approach | Obese patientsBMI > 30 kg/m2 (n= 85) | Non-obese patientsBMI ≤ 30 kg/m2 (n= 170) | P-value |
|---|---|---|---|
| Laparoscopy | 28 (32.9%) | 57 (33.5%) | 0.925 |
| Hand-assisted | 4 (14.3%) | 7 (12.3%) | 0.797 |
| Conversion | 2 (7.1%) | 3 (5.3%) | 0.885 |
| Vascular clamping | |||
| No clamping | 28 (32.9%) | 65 (38.2%) | 0.408 |
| Pedicle clamping | 53 (62.4%) | 97 (57.1%) | 0.418 |
| Total vascular exclusion | 4 (4.7%) | 8 (4.7%) | 0.754 |
| Blood loss, ml | 300 (0–1500) | 300 (0–2000) | 0.367 |
| Operative time, min | 210 (60–360) | 180 (80–420) | 0.931 |
| Blood transfusions | 4 (4.7%) | 9 (5.3%) | 0.841 |
| Plasma transfusions | 1 (1.2%) | 1 (0.6%) | 1.000 |
Continuous variables are reported as median value (range)
In terms of pathological data, no differences were encountered between the two groups. Median lesion diameter and number were 40 mm (range: 10–190 mm) and one (range: 1–17) in the obese group vs. 40 mm (range 5–300 mm) and one (range: 1–13) in the non-obese group (P= 0.939 and P= 0.235, respectively). In malignant lesions, median surgical margin and complete resection rates were similar, 10 mm and 83.8% (62/74) vs. 8 mm and 85.1% (126/148), respectively (P= 0.183 and P= 0.792, respectively).
Postoperative outcomes
The mortality rate was 2.4% in both groups (i.e. two obese and four non-obese patients). Causes of death were as follows: three patients, of whom two had hepatocellular carcinoma (HCC) (BMI 33.1 kg/m2 and 28.0 kg/m2) and one had a Klatskin tumour (BMI 25.9 kg/m2), died of liver failure and sepsis on PoDs 11, 20 and 45, respectively; one patient (Klatskin tumour, BMI 32.9 kg/m2) had Roux-en-Y jejunal necrosis and died of sepsis on PoD 28; one patient (HCC, BMI 26.1 kg/m2) had liver failure secondary to portal vein thrombosis and died on PoD 29 after an operative attempt at thrombectomy, and one patient (HCC, BMI 28.4 kg/m2) died of pulmonary distress on PoD 8 (Table 4).
Table 4.
Overall postoperative morbidity, mortality and length of stay
| Obese patientsBMI > 30 kg/m2 (n= 85) | Non-obese patientsBMI ≤ 30 kg/m2 (n= 170) | P-value | |
|---|---|---|---|
| Mortality | 2 (2.4%) | 4 (2.4%) | 0.603 |
| Overall morbidity | 28 (32.9%) | 36 (21.2%) | 0.041 |
| Grade I | 5 (5.9%) | 4 (2.4%) | 0.280 |
| Grade II | 12 (14.1%) | 3 (1.8%) | <0.001 |
| Grade III (a/b) | 6 (7.1%) (4/2) | 17 (10.0%) (13/4) | 0.440 |
| Grade IV (a/b) | 3 (3.5%) (3/0) | 8 (4.7%) (9/0) | 0.913 |
| Liver dysfunction | 4 (4.7%) | 10 (5.9%) | 0.923 |
| Bile leak | 2 (2.4%) | 7 (4.1%) | 0.719 |
| Haemoperitoneum | 2 (2.4%) | 3 (1.8%) | 0.873 |
| Portal vein thrombosis | 1 (1.2%) | 1 (0.6%) | 1.000 |
| Intra-abdominal collection | 3 (3.5%) | 4 (2.4%) | 0.892 |
| Ascites | 4 (4.7%) | 5 (2.9%) | 0.719 |
| Pulmonary | 6 (7.1%) | 12 (7.1%) | 0.576 |
| Infectious | 16 (18.8%) | 21 (12.4%) | 0.167 |
| Abdominal wall morbiditya | 7 (8.2%) | 4 (2.4%) | 0.046 |
| Transient renal dysfunction | 1 (1.2%) | 1 (0.6%) | 1.000 |
| Cardiac arrhythmia | 1 (1.2%) | 2 (1.2%) | 1.000 |
| Reoperation | 3 (3.5%) | 6 (3.5%) | 0.719 |
| Hospital stay, median (range), days | 9 (2–50) | 8 (2–115) | 0.907 |
Abdominal wall morbidity includes abscess, haematoma and wound dehiscence
Morbidity was significantly higher in the obese group (32.9% [28/85] vs. 21.2% [36/170]; P= 0.041). Only grade II morbidities were increased (14.1% [12/85] in obese patients vs. 1.8% [3/170] in non-obese patients; P < 0.001), but no differences occurred in severe complications (grades III and IV). With regard to complication types, abdominal wall morbidities (abscess, haematoma or wound dehiscence) were more frequent in obese patients (8.2% [7/85] vs. 2.4% [4/170]; P= 0.046). A non-significant increase was observed for infectious complications (18.8% [16/85] vs. 12.4% [21/170]; P= 0.167). Liver-specific morbidity, such as liver dysfunction, bile leak, portal vein thrombosis, ascites and abdominal collection, occurred at similar incidences in both groups.
In the 26 obese patients who underwent laparoscopic liver resection (excluding patients who were converted to open resection), abdominal wall morbidity was low (one patient) and lower than in obese patients who underwent open resection (10.1% [6/59]; P= 0.431). These findings were similar to those in non-obese patients. Moreover, this complication occurred in the early part of the laparoscopic experience (2000) and no further cases were subsequently observed.
Outcomes in four subgroups were analysed separately. These subgroups included: patients who underwent major hepatectomy; patients with cirrhosis; patients with diabetes, and patients with moderate or severe steatosis (Table 5). Results in these subgroups were similar to those for the whole series: mortality was low and similar in obese and non-obese patients, whereas overall morbidity tended to be higher in obese patients through all subgroups. Incidences of grade II complications were higher in obese patients, with significant differences in cirrhotic and diabetic patients (4/17 vs. 0/34 [P= 0.018] and 5/18 vs. 1/28 [P= 0.028], respectively). Obesity was not associated with increased rates of severe morbidity (grades III and IV) in any of the four subgroups.
Table 5.
Morbidity and mortality subgroup analysis
| Obese patientsBMI > 30 kg/m2 | Non-obese patientsBMI ≤ 30 kg/m2 | P-value | |
|---|---|---|---|
| Major hepatectomy | n= 42 | n= 84 | |
| Mortality | 2 | 4 | 1.000 |
| Overall morbidity | 18 | 29 | 0.362 |
| Grade I | 3 | 2 | 0.420 |
| Grade II | 6 | 3 | 0.059 |
| Grade III | 4 | 13 | 0.519 |
| Grade IV | 3 | 7 | 0.816 |
| Cirrhotic patients (F4) | n= 17 | n= 34 | |
| Mortality | 1 | 2 | 1.000 |
| Overall morbidity | 6 | 8 | 0.375 |
| Grade I | – | 1 | 1.000 |
| Grade II | 4 | – | 0.018 |
| Grade III | 1 | 4 | 0.868 |
| Grade IV | – | 1 | 1.000 |
| Diabetic patients | n= 18 | n= 28 | |
| Mortality | – | 1 | 1.000 |
| Overall morbidity | 8 | 5 | 0.051 |
| Grade I | 1 | – | 0.391 |
| Grade II | 5 | 1 | 0.028 |
| Grade III | 1 | 1 | 1.000 |
| Grade IV | 1 | 2 | 1.000 |
| Moderate/severe steatosis | n= 24 | n= 7 | |
| Mortality | – | – | 1.000 |
| Overall morbidity | 8 | 1 | 0.615 |
| Grade I | 2 | – | 1.000 |
| Grade II | 4 | – | 0.550 |
| Grade III | 1 | 1 | 0.407 |
| Grade IV | 1 | – | 1.000 |
When patients were stratified according to their BMI (<20, 20–25, 25–30 and >30 kg/m2), a progressive increase in overall morbidity rates was observed (5.6% [1/18], 22.4% [17/76], 23.7% [18/76] and 32.9% [28/85], respectively) (Fig. 1). Grade I complications increased from 0% (0/18) to 5.9% (5/85) and grade II from 0% (0/18) to 14.1% (12/85). Grade III and IV morbidity rates remained stable. Infectious complication rates progressively increased (5.6% [1/18], 11.8% [9/76], 14.5% [11/76], and 18.8% [16/85], respectively).
Figure 1.
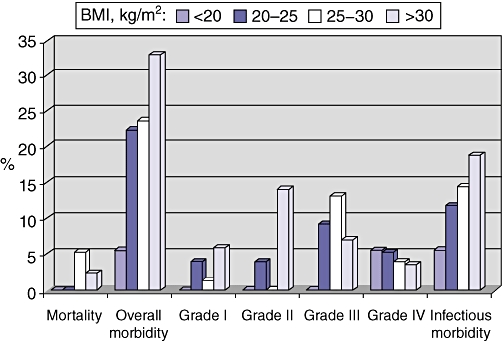
Mortality and morbidity according to body mass index (BMI). Patients were stratified into four groups on the basis of their BMI: < 20 kg/m2 (n= 18); 20–25 kg/m2 (n= 76); 25–30 kg/m2 (n= 76), and > 30 kg/m2 (n= 85)
Among the obese patients classified according to WHO criteria, those with a BMI of 35–40 kg/m2 (class II obesity,1 13 patients) or >40 kg/m2 (class III obesity 1, five patients) did not have worse outcomes. Both postoperative deaths occurred in patients with BMIs of <35 kg/m2 (33.1 kg/m2 and 32.9 kg/m2). Morbidity rates, both globally and when stratified according to the Clavien classification, did not increase. Among five morbidly obese patients (BMI > 40 kg/m2), only one case of mild renal dysfunction occurred, which was treated with diuretics.
Discussion
Increased surgical risk has been anticipated for obese patients undergoing liver surgery because of associated co-morbidities, underlying liver disease and technical difficulties.5–7,9–12 The present series did not confirm this hypothesis and demonstrated that liver surgery can be safely performed even in patients with a BMI > 30 kg/m2. Overall morbidity was increased, but was related to minor complications, especially abdominal wall issues. Severe and liver-specific complications, such as liver failure and bile leak, occurred at similar frequencies in obese and non-obese patients. Even in patients with cirrhosis, mild or severe steatosis, diabetes and those undergoing major hepatectomy, similar results were observed. Obesity of WHO grades II and III1 was not associated with increased surgical risk.
The impact of obesity on surgical outcomes is achieving significant attention because the prevalence of this condition is rapidly increasing worldwide.1 In our centre, about 12% of patients who underwent liver surgery during the last 10 years were obese. This percentage reflects the prevalence of obesity in France, but a significant increase is expected within a few years.3 Furthermore, obesity has been reported to be a risk factor for development of primary liver tumours, such as liver adenoma and HCC.8,36
Surgical outcomes in obese patients have been studied in different surgical fields. The largest available series, published by Dindo et al.,24 compared 808 obese with about 5500 non-obese patients undergoing elective general surgery. They reported no difference between the two groups except for an increased rate of wound infections in the obese group. Despite the large number of included patients, only a minority underwent liver surgery. In fact, the authors classified the surgical procedures into three groups, with group C including oesophageal, rectal, pancreatic and liver operations. Group C, when considered globally, included only 32 obese patients. Therefore, these conclusions cannot be generalized to liver surgery. To date, only one paper has specifically focused on liver surgery, but its definition of obesity was BMI > 25 kg/m2 and only eight patients met the WHO definition (BMI > 30 kg/m2).18
The present paper is the first to specifically report on this topic using standardized definitions of obesity that can be generalized across Western countries. We decided to match patients in a case–control fashion in order to limit the impact of confounding factors on surgical outcomes. The main anticipated confounding factors were associated co-morbidities such as cardiovascular and pulmonary disease and diabetes, which are common in obese patients and are known to negatively impact surgical outcomes.5,6,24 The ASA score identifies the presence of associated co-morbidities well and is a known independent predictor for postoperative morbidity.19,24 By matching patients according to their ASA scores, we eliminated differences among key co-morbidities between the two groups and better analysed the impact of obesity on surgical outcomes. Underlying liver parenchymal disease may represent a further factor influencing outcomes. Steatosis and non-alcoholic fatty liver disease are common in obese patients7,8 and are associated with worse postoperative outcomes.7,9–12 This is why patients were matched for fibrosis, but not for steatosis. As expected, steatosis was more common and more severe in the obese group. Finally, a higher proportion of female patients was observed in the obese group. This reflects the higher prevalence of obesity among women.1 Similar data were reported by Dindo et al.24 This difference between the two groups should not influence the results of the present study because sex usually does not impact on surgical outcomes.24,37,38
In obese patients, technical intraoperative difficulties are anticipated. Longer operative times and higher transfusion rates have been observed in obese patients undergoing rectal19 and pancreatic surgery.22 Similar data were reported by Utsunomiya et al.18 in overweight patients (BMI > 25 kg/m2) treated for recurrent HCC. These differences were not confirmed by the present series. Only operative time tended to be longer, but the difference was not significant. Despite a higher degree of steatosis in obese patients, bleeding risk was not increased, as was clearly depicted by similar rates of blood loss, transfusion and need for pedicle clamping between the two groups. Further, there was no significant difference in resection margins for malignant lesions, suggesting that even oncological principles are not compromised in obese patients.
The impact of obesity on postoperative morbidity is still debated. Results of published papers are extremely heterogeneous: some suggest there are no differences and others report a high mortality rate and poor longterm outcomes in obese subjects.13–23 The large series reported by Dindo et al.24 apparently refuted these differences, reporting similar morbidity rates in obese and non-obese patients, except for wound infections. However, because the study by Dindo et al.24 included few liver resections, its findings cannot be generalized to this population. Prior to the present study, only Utsunomiya et al.18 had analysed the impact of obesity on liver surgery. These authors reported higher morbidity and infection rates and lower survival in overweight patients(BMI > 25 kg/m2) undergoing repeat hepatectomy for recurrent HCC. In the present series, obese patients had increased morbidity rates. However, these reflected minor complications, typically related to the abdominal wall. Liver-related morbidity, such as liver failure and bile leak, and severe complications occurred at similar frequencies in both groups. The same results were observed in patients at increased risk as a result of the extent of liver resection (major hepatectomies), co-morbidities (cirrhosis, diabetes) and primary liver parenchymal disease (mild or severe steatosis). Interestingly, morbidity rates progressively increased in line with BMI. This reflected progressive increases in Clavien grade I and II complications (typically infectious), whereas severe morbidity remained stable. However, patients with WHO grade II and III obesity1(BMI > 35 kg/m2) did not show an increase in morbidity over patients with grade I obesity (BMI 30–35 kg/m2). Based on these results, we can infer that obesity in patients undergoing liver surgery is not associated with increased surgical risk and severe complications. A strict patient selection policy based on liver function, volume and ASA score is mandatory and allows for good outcomes in liver surgery, even in obese patients.
In obese patients, a further decrease in morbidity can be achieved by using a laparoscopic approach. This was attempted in about one-third of patients in both groups, and obesity did not affect the success of a minimally invasive approach. Randomized controlled trials comparing laparoscopic and open bariatric operations demonstrated a significant reduction in abdominal wall morbidity in the first group.39,40 Similar results were reported in the present series: among obese patients, abdominal wall morbidity was observed in only one patient undergoing laparoscopic liver resection, but in 10% of patients undergoing open resection. Moreover, as previously reported,31 technical refinements of our laparoscopic technique allowed for a further reduction in abdominal wall morbidity and no more cases were observed in the later years. According to the present findings, laparoscopic liver surgery is feasible in obese patients and should be considered whenever indications are consistent.
The present results have some limitations. Firstly, although BMI is the most common way to evaluate obesity, more accurate indexes such as waist and hip circumferences or waist : hip ratio may better integrate the proportion of muscle mass when identifying obese patients.1,3 Secondly, few patients with grade III obesity (n= 5) were present in our database, reflecting the low occurrence of grade III obesity in Europe.3 Therefore, the generalizability of these findings to morbidly obese patients may be limited. Finally, increased risk for late complications such as embolism and incisional hernia can be expected in obese patients. Our retrospective analysis was focused on in-hospital morbidity and late complications may be underestimated. Prospective studies are needed to better assess these issues. Despite these limitations, our data accurately depict the outcomes of liver surgery in obese patients because of the large number of patients included and the case–control design in a population that cannot be randomized.
In conclusion, obesity should not be considered a risk factor for liver surgery. Obesity was not associated with significantly longer operative time or increased bleeding, and oncological principles were upheld. Laparoscopic liver surgery was equally feasible in obese patients. Higher morbidity rates were observed in obese patients, but were mainly related to minor complications, such as abdominal wall infections. Liver-specific morbidity and severe complications were not related to BMI. Good patient selection in terms of liver function, liver volume and associated co-morbidities is the key to good outcomes after liver surgery, independent of BMI.
Conflicts of interest
None declared.
References
- 1.World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Geneva: WHO; 1997. Report of a WHO Consultation on Obesity. [PubMed] [Google Scholar]
- 2.National Center for Chronic Disease Prevention and Health Promotion. Behavioral Risk Factor Surveillance System. 2008. Prevalence and Trends Data Overweight and Obesity (BMI) – 2008 http://www.cdc.gov/brfss/index.htm. [Accessed 15 December 2009.
- 3.Branca F, Nikogosian H, Lobstein T. The Challenge of Obesity in the WHO European Region and the Strategies for Response. Copenhagen: World Health Organization Regional Office for Europe; 2007. [Google Scholar]
- 4.McCurry J. Japan battles with obesity. Lancet. 2007;369:451–452. doi: 10.1016/S0140-6736(07)60214-1. [DOI] [PubMed] [Google Scholar]
- 5.Berkalp B, Cesur V, Corapcioglu D, Erol C, Baskal N. Obesity and left ventricular diastolic dysfunction. Int J Cardiol. 1995;52:23–26. doi: 10.1016/0167-5273(95)02431-u. [DOI] [PubMed] [Google Scholar]
- 6.Pi-Sunyer FX. Medical hazards of obesity. Ann Intern Med. 1993;119:655–660. doi: 10.7326/0003-4819-119-7_part_2-199310011-00006. [DOI] [PubMed] [Google Scholar]
- 7.Veteläinen R, van Vliet A, Gouma DJ, van Gulik TM. Steatosis as a risk factor in liver surgery. Ann Surg. 2007;245:20–30. doi: 10.1097/01.sla.0000225113.88433.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regimbeau JM, Colombat M, Mognol P, Durand F, Abdalla E, Degott C, et al. Obesity and diabetes as a risk factor for hepatocellular carcinoma. Liver Transpl. 2004;10(Suppl 1):69–73. doi: 10.1002/lt.20033. [DOI] [PubMed] [Google Scholar]
- 9.McCormack L, Petrowsky H, Jochum W, Furrer K, Clavien PA. Hepatic steatosis is a risk factor for postoperative complications after major hepatectomy: a matched case–control study. Ann Surg. 2007;245:923–930. doi: 10.1097/01.sla.0000251747.80025.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 11.Behrns KE, Tsiotos GG, DeSouza NF, Krishna MK, Ludwig J, Nagorney DM. Hepatic steatosis as a potential risk factor for major hepatic resection. J Gastrointest Surg. 1998;2:292–298. doi: 10.1016/s1091-255x(98)80025-5. [DOI] [PubMed] [Google Scholar]
- 12.Kooby DA, Fong Y, Suriawinata A, Gonen M, Allen PJ, Klimstra DS, et al. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034–1044. doi: 10.1016/j.gassur.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Moulton MJ, Creswell LL, Mackey ME, Cox JL, Rosenbloom M. Obesity is not a risk factor for significant adverse outcomes after cardiac surgery. Circulation. 1996;94(Suppl):87–92. [PubMed] [Google Scholar]
- 14.Shapiro M, Muñoz A, Tager IB, Schoenbaum SC, Polk BF. Risk factors for infection at the operative site after abdominal or vaginal hysterectomy. N Engl J Med. 1982;307:1661–1666. doi: 10.1056/NEJM198212303072701. [DOI] [PubMed] [Google Scholar]
- 15.Miles RH, Carballo RE, Prinz RA, McMahon M, Pulawski G, Olen RN, et al. Laparoscopy: the preferred method of cholecystectomy in the morbidly obese. Surgery. 1992;112:818–823. [PubMed] [Google Scholar]
- 16.Flancbaum L, Choban PS. Surgical implications of obesity. Annu Rev Med. 1998;49:215–234. doi: 10.1146/annurev.med.49.1.215. [DOI] [PubMed] [Google Scholar]
- 17.Mullen JT, Davenport DL, Hutter MM, Hosokawa PW, Henderson WG, Khuri SF, et al. Impact of body mass index on perioperative outcomes in patients undergoing major intra-abdominal cancer surgery. Ann Surg Oncol. 2008;15:2164–2172. doi: 10.1245/s10434-008-9990-2. [DOI] [PubMed] [Google Scholar]
- 18.Utsunomiya T, Okamoto M, Kameyama T, Matsuyama A, Yamamoto M, Fujiwara M, et al. Impact of obesity on the surgical outcome following repeat hepatic resection in Japanese patients with recurrent hepatocellular carcinoma. World J Gastroenterol. 2008;14:1553–1558. doi: 10.3748/wjg.14.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benoist S, Panis Y, Alves A, Valleur P. Impact of obesity on surgical outcomes after colorectal resection. Am J Surg. 2000;179:275–281. doi: 10.1016/s0002-9610(00)00337-8. [DOI] [PubMed] [Google Scholar]
- 20.Rullier E, Laurent C, Garrelon JL, Michel P, Saric J, Parneix M. Risk factors for anastomotic leakage after resection of rectal cancer. Br J Surg. 1998;85:355–358. doi: 10.1046/j.1365-2168.1998.00615.x. [DOI] [PubMed] [Google Scholar]
- 21.Dhar DK, Kubota H, Tachibana M, Kotoh T, Tabara H, Masunaga R, et al. Body mass index determines the success of lymph node dissection and predicts the outcome of gastric carcinoma patients. Oncology. 2000;59:18–23. doi: 10.1159/000012131. [DOI] [PubMed] [Google Scholar]
- 22.Williams TK, Rosato EL, Kennedy EP, Chojnacki KA, Andrel J, Hyslop T, et al. Impact of obesity on perioperative morbidity and mortality after pancreaticoduodenectomy. J Am Coll Surg. 2009;208:210–217. doi: 10.1016/j.jamcollsurg.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 23.Nair S, Verma S, Thuluvath PJ. Obesity and its effect on survival in patients undergoing orthotopic liver transplantation in the United States. Hepatology. 2002;35:105–109. doi: 10.1053/jhep.2002.30318. [DOI] [PubMed] [Google Scholar]
- 24.Dindo D, Muller MK, Weber M, Clavien PA. Obesity in general elective surgery. Lancet. 2003;361:2032–2035. doi: 10.1016/S0140-6736(03)13640-9. [DOI] [PubMed] [Google Scholar]
- 25.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 26.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 27.Cherqui D, Malassagne B, Colau PI, Brunetti F, Rotman N, Fagniez PL. Hepatic vascular exclusion with preservation of the caval flow for liver resections. Ann Surg. 1999;230:24–30. doi: 10.1097/00000658-199907000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chouillard E, Cherqui D, Tayar C, Brunetti F, Fagniez PL. Anatomical bi- and trisegmentectomies as alternatives to extensive liver resections. Ann Surg. 2003;238:29–34. doi: 10.1097/01.sla.0000075058.37052.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bryant R, Laurent A, Tayar C, Cherqui D. Laparoscopic liver resection – understanding its role in current practice: the Henri Mondor Hospital experience. Ann Surg. 2009;250:103–111. doi: 10.1097/SLA.0b013e3181ad6660. [DOI] [PubMed] [Google Scholar]
- 30.Chang S, Laurent A, Tayar C, Karoui M, Cherqui D. Laparoscopy as a routine approach for left lateral sectionectomy. Br J Surg. 2007;94:58–63. doi: 10.1002/bjs.5562. [DOI] [PubMed] [Google Scholar]
- 31.Vigano L, Laurent A, Tayar C, Tomatis M, Ponti A, Cherqui D. The learning curve in laparoscopic liver resection. Improved feasibility and reproducibility. Ann Surg. 2009;250:772–782. doi: 10.1097/SLA.0b013e3181bd93b2. [DOI] [PubMed] [Google Scholar]
- 32.Terminology Committee of the IHPBA. The Brisbane 2000 terminology of liver anatomy and resection. Terminology Committee of the International Hepato-Pancreato-Biliary Association. HPB. 2000;2:333–339. [Google Scholar]
- 33.Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 34.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The ‘50–50 criteria’ on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–828. doi: 10.1097/01.sla.0000189131.90876.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paradis V, Zalinski S, Chelbi E, Guedj N, Degos F, Vilgrain V, et al. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49:851–859. doi: 10.1002/hep.22734. [DOI] [PubMed] [Google Scholar]
- 37.Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–1206. doi: 10.1001/archsurg.138.11.1198. [DOI] [PubMed] [Google Scholar]
- 38.Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698–710. doi: 10.1097/01.sla.0000141195.66155.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen NT, Goldman C, Rosenquist CJ, Arango A, Cole CJ, Lee SJ, et al. Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life, and costs. Ann Surg. 2001;234:279–289. doi: 10.1097/00000658-200109000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luján JA, Frutos MD, Hernández Q, Liron R, Cuenca JR, Valero G, et al. Laparoscopic versus open gastric bypass in the treatment of morbid obesity: a randomized prospective study. Ann Surg. 2004;239:433–437. doi: 10.1097/01.sla.0000120071.75691.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]


