Abstract
Background
A high prevalence of gallbladder diseases (GBD) in Northern India warranted a population survey into environmental risk factors.
Methods
In 60 villages of Uttar Pradesh and Bihar from 13 334 households, 22 861 persons aged >30 years were interviewed for symptoms of GBD, diet and environmental factors. Subsequently ultrasonography (US) was performed in 5100 and 1448 people with and without symptoms, respectively. Heavy metal and pesticide content in soil and water were estimated.
Results
US revealed a prevalence of GBD of 6.20%. GBD was more common in 5100 persons with symptoms (7.12%) compared with 1448 without (2.99%) (P < 0.05). Adjusted odds ratio (ORs) [95% confidence interval (CI)] revealed a significantly increased risk of GBD in females >50, 1.703 (CI 1.292–2.245); multiparity 1.862 (CI 1.306–2.655) and a genetic history 1.564 (CI 1.049–2.334). An increased risk noted in males with diabetes was 4.271 (CI 2.130–8.566), chickpea consumption 2.546 (CI 1.563–4.146) and drinking unsafe water 3.835 (CI 2.368–6.209). Prevalence of gallstones was 4.15%; more in females 5.59% than males 1.99% (P < 0.05). Cluster analysis identified a positive correlation of nickel, cadmium and chromium in water with a high prevalence of GBD in adjacent villages in Vaishali district, Bihar.
Conclusion
A high risk of GBD was observed in older, multiparous women and men with diabetes, intake of chickpeas, unsafe water and villages with heavy metal water pollution.
Keywords: gallstone, gallbladder cancer, epidemiology, India
Introduction
Northern India has one of the highest reported incidence of gallbladder cancer (GBC) in the World.1 The incidence of GBC has a specific geographic and ethnic variation.1 The highest incidence rates of GBC in the world are 21.5/100 000 in females in Delhi, 13.8/100 000 in Karachi and 12.9 /100 000 in Quito.1 In a review of worldwide incidence, the female-to-male ratio was reported between 2 and 3.1 Gallstones (GST) were said to play a major role.2 Other risk factors are obesity, multiparity and chronic infections.1
In India GBC is most prevalent in northern and northeastern states of Uttar Pradesh, Bihar, Orissa, West Bengal and Assam.3 GBC is two times higher in women than men and is the leading digestive cancer in women in northern Indian cities.4 Six Cancer registries of the Indian Council of Medical Research (1990–96) show a 10 times lower incidence of GBC per 100 000 in South India compared with the North, the age-adjusted incidence rate for females being 0.8 in Chennai in the south and 8.9 in Delhi in the north.5
Although hospital statistics and cancer registries may not reflect actual prevalence, a detailed geographic tracking of 773 GBC patients attending Tata Memorial Hospital in Mumbai between 1990 and 1995 showed that the majority of patients hailed from Uttar Pradesh (41.9%) and Bihar (35.8%).6
Thus the aim of the present study was to undertake a multi-institutional field survey [by the GANGA (Gallbladder Abnormalities in Northern Gangetic Area) study group] to estimate the prevalence of GBD and other risk factors that may be associated with the high prevalence of GBC in this community. The field survey was conducted by International Institute of Population Studies supported by gastroenterologists, surgical oncologists and radiologists.
Methods
As a high prevalence of GBC was reported in the population of the Gangetic basin which shares water and common food habits, the study was therefore planned in three districts in the adjoining states of Uttar Pradesh and Bihar. The districts were Varanasi, Patna and Vaishali (Fig. 1). All subjects were individually interviewed. In this large ‘population-based’ study, ultrasonography (US), a non-invasive, specific and quick screening procedure was chosen to diagnose GBD.
Figure 1.
Map of India showing the States of Uttar Pradesh and Bihar indicating the districts of Varanasi, Patna and Vaishali surveyed
Study design
Considering the prevalence rate of gallstone disease as 6%7 and assuming a 5% coefficient of variation, the required sample size was calculated to be around 7000. A two-stage sampling design was adopted for the selection of the subjects. Villages in a district were subdivided into three strata based on their size (population/household) as high, medium and low. Within each stratum the villages were then ranked according to the level of literacy. In the first stage a total of 60 villages (20 in each district) were selected. The villages were selected systematically with the help of a single random number. The selection of a village was done by probability proportional to its size sampling.
At the second stage, all the households in a selected village were enumerated and every person aged 30 years and above were interviewed for possible selection for a sonography test. Only persons aged 30 and above were selected as the prevalence of GST was low at younger ages.8
Information was elicited concerning socioeconomic status, educational level, marital status, reproductive factors, occupation, tobacco and alcohol habits. Previous medical history including abdominal surgery, other than biliary surgery, was noted. Only medically confirmed diseases were considered. Details of the dietary habits were also collected.
Symptoms ascertained for GBD were: jaundice, pain upper abdomen after taking food or fatty food, acidity, gases, vomiting, loss of appetite and loss of weight. Those with the symptoms were considered ‘symptomatic’. The population was categorized as ‘symptomatic’ and ‘asymptomatic’ and stratified by gender and age. All those with symptoms except pregnant women were directed to undergo US. Asymptomatic persons, selected in a 3:1 ratio, were also directed for US. A conscious effort was made to include adequate asymptomatic persons to get the ratio of 3:1 of symptomatic and asymptomatic persons. The unequal selection (i.e. higher chance of selecting a person with the symptoms) was done to facilitate the risk factor analysis. As the sample was not self-weighted and the response rate was not the same for symptomatic and asymptomatic persons, weights were used separately for symptomatic and asymptomatic males and females to estimate the prevalence rate separately as well as for the total.
Ultrasonography
The prevalence of GBD was estimated by US, a non-invasive technique with high sensitivity and specificity for the detection of GST.9,10 Gallbladder diseases surveyed include acute and chronic cholecystitis, solitary and multiple GST, gallbladder polyps and gallbladder cancer.
As GST were reported to have a major role in the causation of GBC, their prevalence was given separately. All sonography images were assessed independently by a senior radiologist. Only those with an unequivocal mass lesion, liver parenchymal involvement or metastases were diagnosed with GBC.
Statistical analysis
The data were entered using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA).
Double data entry was made to check the consistency. Standard of living index (SLI) was calculated by the weighted sum of consumer durables and household infrastructure.11
Prevalence was determined for GBD and gallstone by considering symptoms, gender and district. The association between male and female prevalence was tested using logistic analysis and normal test for proportion (large sample test). Multivariate analysis was performed by logistic regression using all variables which were significant in bi-variate analysis. Adjusted odds ratios (ORs), as an estimate of relative risk, of GBD and GST, together with their 95% confidence intervals (CI) were obtained by unconditional multiple logistic regression analysis. Models were fitted separately for GBD by gender. All models included specific variables for identifying risk factors for GBD. In logistic analysis, only those variables that were not highly correlated with other independent variables were considered taking into account the importance of the variable.
Data were analyzed separately for males and females, and for asymptomatic and symptomatic individuals. Prevalence was determined for GBD, which includes GST, cholecystitis, polyps and GBC. In this mass screening study, ultrasonography findings were treated as standard as histological confirmation was not possible.
Cluster analysis
Apart from logistic analysis, two-step cluster analysis was used as an exploratory tool designed to reveal natural groupings, or clusters, within a dataset that would otherwise not be apparent. The algorithm employed by this procedure has several desirable features that differentiate it from traditional clustering techniques: it can handle categorical and continuous variables. By assuming variables independently, a joint multinomial-normal distribution can be placed on categorical and continuous variables, with GBD as a categorical variable and soil and water testing results as continuous variables. The difference between the proportions of the different characteristics in the two clusters was tested using the large sample two-tailed tests.
Analysis of water and soil
Samples of water and soil were collected from Patna, and Vaishali in Bihar. Water samples were collected from a tap if present or from tube or bore wells commonly used by the villagers. Soil was collected from three randomly selected fields. All water and soil samples were analysed for presence of nickel, cadmium, chromium and DDT at the Industrial Research Centre, Lucknow, Uttar Pradesh.
Results
The survey data are shown in Table 1. There were 8421 persons with symptoms who were directed for US. Out of 3821 men, 2078 (54.4%) and of 4600 women, 3022 (65.7%) underwent US. In the asymptomatic group of 2971 only 547 out of 1181 (46.3%) men and 901 out of 1790 (50.3%) women underwent US. Further analysis showed that the matching criteria were not influenced by non-response and both symptomatic and asymptomatic persons had a similar age and other characteristics.
Table 1.
Number of households, men and women interviewed in 20 villages each in the three districts and who underwent ultrasonography (US)
| Patna | Varanasi | Vaishali | Total | |
|---|---|---|---|---|
| Number of households | 3885 | 4851 | 4598 | 13 334 |
| Males interviewed | 3355 | 3580 | 3417 | 10 352 |
| Females interviewed | 3323 | 5411 | 3775 | 12 509 |
| Total interviewed | 6678 | 8991 | 7192 | 22 861 |
| Symptomatic males identified | 1454 | 1038 | 1329 | 3 821 |
| Symptomatic females identified | 1433 | 1845 | 1322 | 4 600 |
| Total symptomatic persons | 2887 | 2883 | 3973 | 8 421 |
| Symptomatic males underwent US | 840 | 427 | 811 | 2 078 (54.4%) |
| Symptomatic Females underwent US | 1013 | 1012 | 997 | 3 022 (65.7%) |
| Total symptomatic persons who underwent US | 1853 | 1439 | 1808 | 5 100 (60.6%) |
| Asymptomatic males identified | 454 | 314 | 413 | 1 181 |
| Asymptomatic males underwent US | 246 | 110 | 191 | 547 (46.3%) |
| Asymptomatic females identified | 573 | 709 | 508 | 1 790 |
| Asymptomatic females who underwent US | 296 | 333 | 272 | 901 (50.3%) |
| Total asymptomatic persons identified | 2 971 | |||
| Total asymptomatic persons who underwent US | 542 | 443 | 463 | 1 448 (48.7%) |
| Total number who underwent US | 2395 | 1882 | 2271 | 6 548 |
Characteristics of villages and respondents
Proximity to a river
Households in a 5-km range near the river Ganga were 40% of 4851 in Varanasi; in the Patna District 30% of 3885 were near the Ganga and 25% near the Punpun; in Vaishali 66% of 4598 were near the Gandak river.
Background of Households
Among 13 334 households, 53% had a ‘low’, 36% ‘medium’ and only 11% ‘high SLI’. Among males 68% and females only 18% were literate. Hindus constituted 97%.
Prevalence of GBD
The number of persons screened and the percentage prevalence of GBD with significance and CIs are given in Table 2. In 6548 persons (2625 males and 3923 females) who underwent USG, the prevalence of GBD was 6.20%; 4.45% in males and 7.37% in females. Prevalence of GST was 1.99% in males and 5.59% in females. In all, total GBD was 6.20% and GST accounted for 4.15%. The prevalence of GBD was highest in the Vaishali District at 7.99% compared with Varanasi 6.19% and Patna 4.5%
Table 2.
Prevalence of gallbladder diseases and gallstones among symptomatic and asymptomatic persons by districts and gender
| District | Number USG tested | Symptom/gender | Total gallbladder diseases (%) | 95% CI | Gallstones (%) | 95% CI |
|---|---|---|---|---|---|---|
| 2078 | Symptomatic male | 5.08a | 2.58–7.58 | 2.25a | 1.37–3.14 | |
| 547 | Asymptomatic male | 2.06 | 1.00–3.11 | 0.99 | 0.60–1.38 | |
| 3022 | Symptomatic female | 8.51a | 4.79–12.23 | 6.41a | 3.79–9.04 | |
| 901 | Asymptomatic female | 3.56 | 1.95–5.17 | 2.87 | 1.65–4.11 | |
| 5100 | Symptomatic persons (all) | 7.12a | 2.46–11.76 | 4.72a | 0.19–9.24 | |
| 1448 | Asymptomatic persons (all) | 2.99 | 0.97–5.01 | 2.31 | 0.13–4.49 | |
| Patna | 1086 | Male | 3.25 | 1.43–5.07 | 1.78 | 0.85–2.72 |
| 1309 | Female | 5.53a | 2.45–8.63 | 5.33a | 2.58–8.09 | |
| 2395 | Total | 4.50 | 1.08–7.93 | 3.73 | 0.00–7.80 | |
| Varanasi | 537 | Male | 4.22 | 1.97–6.47 | 1.46 | 0.72–2.18 |
| 1345 | Female | 6.98a | 2.99–10.96 | 4.29a | 1.98–6.59 | |
| 1882 | Total | 6.19 | 1.86–10.52 | 3.48 | 0.28–6.68 | |
| Vaishali | 1002 | Male | 5.85 | 2.89–8.81 | 2.49 | 1.30–3.68 |
| 1269 | Female | 9.69a | 4.66–14.72 | 7.27a | 3.73–1.81 | |
| 2271 | Total | 7.99 | 2.34–13.64 | 5.15 | 0.00–10.57 | |
| All Districts | 2625 | Males | 4.45 | 1.13–7.76 | 1.99 | 0.69–3.28 |
| 3923 | Females | 7.37a | 2.08–12.66 | 5.59a | 1.82–9.37 | |
| 6548 | Total | 6.20 | 0.81–11.59 | 4.15 | 0.00–8.76 | |
Note: Estimates were based on logistic analysis carried out separately for gallbladder diseases and gallstones. Explanatory variables in these analyses were gender, symptoms (presence or absence) and district.
Differences between male and female prevalence were significant at a 5% level of significance (P < 0.05) in three districts and also among symptomatic and asymptomatic persons.
Prevalence of GBD among females was 1.7 times more than males in all three districts: females vs. males in Patna 5.53% vs. 3.25%, Vaishali 9.69% vs. 5.85% and in Varanasi 6.98% vs. 4.22%.
The prevalence of GBD among symptomatic persons in all districts (7.12%) was greater than asymptomatic (2.99%). Symptomatic males (5.08%) had a 2.5 times greater prevalence of GBD than asymptomatic (2.06%). Similarly the prevalence of GBD in symptomatic females (8.51%) was 2.4 times greater than asymptomatic (3.56%).
Females had 2.9 times greater prevalence of GST than males in all three districts: female vs. males, in Patna 5.33% vs.1.78%, in Varanasi 4.29% vs.1.46% and in Vaishali 7.27% vs. 2.49%. The prevalence of GST was more in symptomatic individuals (4.72%) than asymptomatic (2.31%). Prevalence of GST was 2.3 times more in both males and females with symptoms than without symptoms: symptomatic vs. asymptomatic in males 2.25% vs.0.99% and in females 6.41% vs. 2.87%. The differences are significant as shown in Table 2.
Variation in prevalence by individual and socioeconomic factors
Table 3 represents logistic analysis separately for Total (Model I), Males (Model II) and Females (Model III). It gives the number responded under each factor with significance at (P < 0.05). Positive findings in each gender were as follows. Females showed a higher risk (OR 1.853) of having GBD than males. Females older >50 years old had a significantly higher risk (OR 1.703). Risk was significantly higher for women with 4 or more children (OR 1.862) and also with history of previous abdominal surgery (OR 3.335). A history of blood relative suffering from GBD showed a positive association in both genders.
Table 3.
Variation in prevalence of gallbladder disease: logistic analysis
| Demographic characteristics | Total (Model I) | Male (Model II) | Female (Model III) | |||
|---|---|---|---|---|---|---|
| Number | EXP(B) and 95.0% CI | Number | EXP(B) and 95.0% CI | Number | EXP(B) and 95.0% CI | |
| Sex | ||||||
| Male (ref.) | 2526 | 1 | – | – | – | – |
| Female | 3801 | 1.853a | – | – | – | – |
| 1.304–2.635 | – | – | – | – | ||
| Age group | ||||||
| 30–49 (ref.) | 4177 | 1 | 1457 | 1 | 2676 | 1 |
| 50+ | 2150 | 1.626a | 1069 | 1.403 | 1072 | 1.703a |
| 1.293–2.044 | 0.887–2.221 | 1.292–2.245 | ||||
| Children | ||||||
| 0–3 (ref.) | – | – | – | – | 869 | 1 |
| 4+ | – | – | – | – | 2879 | 1.862a |
| – | – | – | – | 1.306–2.655 | ||
| Abd. surgery | ||||||
| No (ref.) | 6096 | 1 | 2424 | 1 | 3622 | 1 |
| Yes | 231 | 2.733a | 102 | 1.957 | 126 | 3.335a |
| 1.822–4.099 | 0.971–3.944 | 1.949–5.705 | ||||
| Diabetes | ||||||
| No (ref.) | 6210 | 1 | 2454 | 1 | 3703 | 1 |
| Yes | 117 | 3.285a | 102 | 4.271a | 45 | 1.724 |
| 2.045–5.276 | 2.130–8.566 | 0.830–3.585 | ||||
| Blood relatives with GBD/GBC | ||||||
| No (ref.) | 5813 | 1 | 2299 | 1 | 3468 | 1 |
| Yes | 514 | 1.795a | 227 | 2.115a | 280 | 1.564a |
| 1.310–2.458 | 1.213–3.688 | 1.049–2.334 | ||||
| Diet – chickpeas | ||||||
| No (ref.) | 5330 | 1 | 2119 | 1 | 3167 | 1 |
| Yes | 997 | 1.636a | 337 | 2.546a | 581 | 1.354 |
| 1.261–2.122 | 1.563–4.146 | 0.981–1.860 | ||||
| Drinking water | ||||||
| Tap/Hand pump (ref.) | 5407 | 1 | 2189 | 1 | 3173 | 1 |
| Well/River/Pond | 920 | 1.758a | 337 | 3.835a | 575 | 1.202 |
| 1.338–2.310 | 2.368–6.209 | 0.814–1.431 | ||||
| SLI | ||||||
| Low (ref.) | 3357 | 1 | 1347 | 1 | 1996 | 1 |
| Medium | 2266 | 1.217 | 893 | 1.427 | 1356 | 1.079 |
| 0.962–1.540 | 0.897– 2.272 | 0.814–1,431 | ||||
| High | 704 | 0.873 | 286 | 0.516 | 406 | 1.098 |
| 0.599–1.273 | 0.227–1.172 | 0.708–1.703 | ||||
| Education | ||||||
| Illiterate (ref.) | 3861 | 1 | 337 | 1 | 3002 | 1 |
| Literate | 2466 | 1.676a | 806 | 1.179 | 728 | 1.849a |
| 1.270–2.212 | 0.717–1.939 | 1.302–2.625 | ||||
| River proximity | ||||||
| No (ref.) | 3719 | 844 | 1119 | |||
| Yes | 2608 | 1.024 | 1682 | 0.608a | 2629 | 1.204 |
| 0.809 –1.296 | 0.393–0.938 | 0.902–1.606 | ||||
| District | ||||||
| Patna | 2311 | 1 | 1026 | 1 | 1268 | 1 |
| Varanasi | 1784 | 1.221 | 509 | 1.694 | 1255 | 0.979 |
| 0.903–1.650 | 0.944–3.040 | 0.679–1.410 | ||||
| Vaishali | 2232 | 1.874a | 991 | 1.222 | 1225 | 2.202a |
| 1.429–2.458 | 0.728–2.050 | 1.592–3.046 | ||||
Significant (P < 0.05); number of patients based on analysis with weights.
‘ref’: reference category.
‘Exp B’– Odds ratio (OR) of having the outcome in a specific category.
SLI, standard of living index.
In males, diabetes showed a positive association with GBD (OR – 4.271). As far as dietary items were concerned, only chickpeas (chana) had higher odds for GBD in all (OR 1.636). It was more significant in males (OR 2.546). Those drinking water from unsafe sources had higher odds for GBD (1.758), with higher odds in males (3.835). Prevalence was significantly higher in literate than illiterate (1.676), more so in females (1.849). This was not significant in males (OR 1.179).
Habits, SLI, occupation, caste and proximity to the river did not show a systematic relationship.
Cluster analysis
Cluster analysis of people surveyed in the districts of Patna and Vaishali in Bihar along with results of water, soil and US (Table 4) revealed that 89.1% of those suffering from GBD were in Cluster I villages. It was also found that the greater number of persons from the Vaishali district came under cluster I. In Vaishali 1066 (73.01%) out of 1460 persons fall into cluster I and in Patna 441 (18.95%) out of 2327 came into cluster I.
Table 4.
Result of cluster analysis with number and mean of selected variables
| Characteristics | Number/mean/standard deviation (SD) | Cluster I | Cluster II |
|---|---|---|---|
| Ultrasound done | Number (%) | 1287 (32.7%) | 2646 (67.3%) |
| Gallbladder disease patients | Number (%) | 212 (89.1%) | 26 (10.9%) |
| Nickel in water | Mean | 7.7764 | 5.8857 |
| SD | 2.4604 | 4.7957 | |
| Cadmium in water | Mean | 2.8727 | 1.9228 |
| SD | 0.5589 | 0.9971 | |
| Chromium in water | Mean | 2.9600 | 0.5400 |
| SD | 2.5870 | 1.0208 | |
| DDT in water | Mean | 0.0358 | 0.0000 |
| SD | 0.0914 | 0.0000 | |
| Nickel in soil | Mean | 10.1841 | 10.9132 |
| SD | 2.4064 | 3.8223 | |
| Cadmium in soil | Mean | 0.8830 | 0.3479 |
| SD | 0.4999 | 0.1581 | |
| Chromium in soil | Mean | 5.3506 | 5.6970 |
| SD | 1.6430 | 4.7140 | |
| DDT in soil | Mean | 12.3089 | 0.1789 |
| SD | 28.5216 | 0.7532 | |
Metal and DDT content in water samples (µg/L) and in soil samples (µg/g).
Note: Levels of pollutants in water and soil between cluster I and cluster II were significantly different (P < 0.001).
Mean values of all pollutants (i.e. nickel, cadmium and chromium) in water and soil samples were higher in the Cluster I than Cluster II. Using the large sample two-tailed tests for differences in proportion for every characteristic, the difference was found to be highly significant (P < 0.001) (shown in Table 4). Figure 2 shows the villages in the Vaishali District falling in cluster I, adjacent to each other indicating a common environmental influence.
Figure 2.
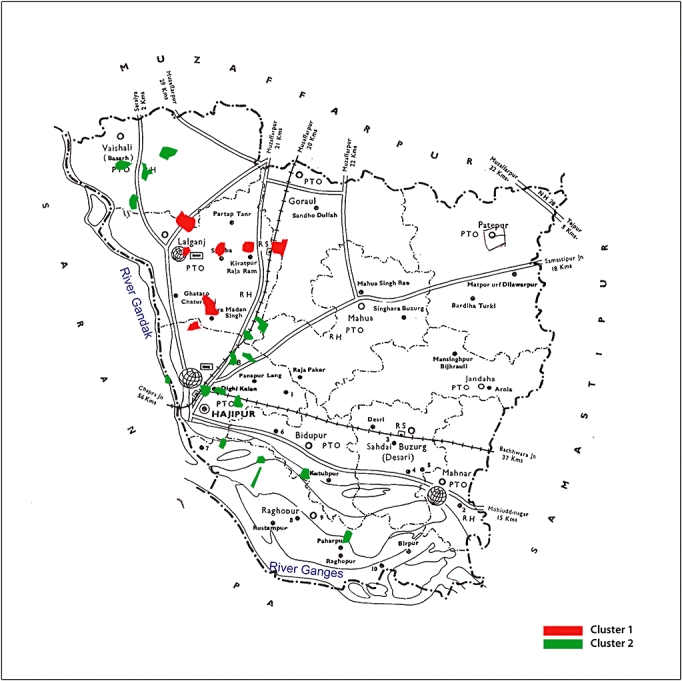
Map of Vaishali District showing villages with high prevalence of GBD in Cluster I (red) and low prevalence in Cluster II (green)
Discussion
The risk factors traditionally linked with GBC include cholelithiasis, obesity, reproductive factors, cholecystitis and specific chemicals. The limitations of epidemiological studies on GBC are small sample sizes and problems in quantifying exposure to putative risk factors.12,13 Other studies relate to prevalence of GST.14 Gallstones are not the only factor. A ‘syndromic approach’ was adopted to investigate risk factors in a high-prevalence population. All gallbladder diseases such as cholecystitis, GST, gallbladder polyp and GBC detectable with US were included together with diet, habits and environmental pollutants.
This is the largest door-to-door survey of a rural population of North India, a region where a high prevalence of GBC was reported. US, a non-invasive technique with high sensitivity and specificity,9,10 was adopted for screening GBD as histological diagnosis was not feasible. The survey was planned to estimate all GBD, which included GBC and the predisposing factors such gallstone disease along with environmental factors. Such a comprehensive study was not undertaken earlier in this area of high prevalence. It was also important to identify the population at risk and plan for prevention by providing safe drinking water and consider other preventive measures.
This rural population belongs mostly to the lower and middle income group which may explain the low prevalence rates of GST. SLI has a bearing on the incidence of GBC. In a case–control study from Chile exploring risk factors for GBC by comparing 114 patients with GBC and 114 patients with GST it was reported that low socioeconomic status and red chilli pepper consumption were significant independent risk factors for GBC.15 Differences in incidence ratios point to variations in GBC aetiology in different populations.1
Logistic analyses were carried out separately for males, females and total for GBD and GST. Several risk factors were examined at the community level with a large number of respondents; hence this helped us to examine factors influencing GBD. GBD prevalence was higher in females in all districts. Others also reported female preponderance with GBC.16,17 GBD was significantly greater in individuals above the age of 50 years and those with previous abdominal surgery and multiparity (females), confirming previous reports.2,16–19
Prevalence was higher in a symptomatic compared with asymptomatic population. Symptomatic males and females had a 2.4 times greater prevalence of GBD than asymptomatic. The ‘syndromic’ approach is important in identifying GBD in a targeted population screening programme. GBD prevalence is higher in literate persons compared with illiterates. A genetic history of blood relatives suffering from GBD showed a significant association in both sexes as reported.20
The factors specifically significant to this area surveyed appear to be items of food and water. The consumption of food item such as chickpeas and drinking unprotected water from wells, ponds and rivers are local factors and these are significant for males.
The cluster analysis revealed a significant association of GBD with environmental pollutants. The majority of the GBD (89%) was identified in cluster I. In the Vaishali District 73% of the people screened by USG were in cluster I, mostly in just eight villages (Fig. 2). The analysis showed a significant association with the levels of heavy metals i.e. nickel, chromium and cadmium in the water. A similar association was also found with soil samples showing high levels of DDT, an organochloride pesticide.
The prevalence of GBD in the present study is lower than in a report from the United States (5.3% to 8.9% for men and 13.9% and 26.7% for women).21 Hospital-based reports in north India showed a higher incidence: Varanasi 13.44% asymptomatic GBD and 11.14% cholelithiasis;22 Chandigarh gallstone 3.3% asymptomatic and 64.9% symptomatic;14 and New Delhi GST 29.8%.23 A referral bias or higher prevalence associated with urban life style could be the reason in urban hospital-based registries.
The prevalence rates for GST in this survey were similar to another smaller survey in Kashmir;9 women 9.6% and men 3.07%. In a rural Bangladesh population where 1058 individuals were surveyed, a prevalence of GST in women and men and asymptomatic individuals was reported as 7.7%, 3.3% and 7.2%, respectively,8 The present survey with 22 861 persons showed a similar prevalence of GST.
The prevalence of GBC was high in the current survey but with small absolute numbers and wide CI. It was estimated at 51 per 100 000 population. Three patients were later confirmed histologically. It is possible that US may underestimate the numbers. Although the identification by US of GBC is lower in this survey, its percentage indicates a higher prevalence. The lower prevalence of GST with high prevalence of GBC suggests other factors. The significant local factors for GBD were consumption of chickpeas and drinking unprotected water. In Vaishali District a cluster analysis also points to role of polluted water.
Conclusion
This population-based study identified that the prevalence of GBD was not as high in rural areas compared with hospital-based registries, probably because the SLI was lower in the areas under study. The total prevalence of GBD was greater in women. Genetic influence, multiparity and previous surgeries were significant risk factors in women. Preventable risk factors identified in men, who normally work in fields, were drinking unprotected water and consumption of excess chickpeas (chana). Cluster analysis has identified an endemic population at high risk in specific villages of Vaishali district showing a strong correlation with heavy metal pollutants in drinking water. Public health measures can reduce the disease burden in this geographically clearly defined population.
GANGA study group members
P. Jagannath, V. Dhir, S. Unisa, C. Shekhar, T.K. Roy, C. Khandelwal and L. Sarangi.
Acknowledgments
The project was partly funded by the International Institute of Population Studies, Mumbai under the Ministry of Health, Government of India. Sir Ratan Tata Trust Mumbai extended financial assistance for sonography units.
References
- 1.Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118:1591–1602. doi: 10.1002/ijc.21683. [DOI] [PubMed] [Google Scholar]
- 2.Zatonski WA, Lowenfels AB, Boyle P, Maisonneuve P, Bueno de Mesquita HB, Ghadirian P, et al. Epidemiologic aspects of gallbladder cancer: a case-control study of the SEARCH Program of the International Agency for Research on Cancer. J Natl Cancer Inst. 1997;89:1132–1138. doi: 10.1093/jnci/89.15.1132. [DOI] [PubMed] [Google Scholar]
- 3.Nandakumar A, Gupta PC, Gangadharan P, Visweswara RN, Parkin DM. Geographic pathology revisited: development of an atlas of cancer in India. Int J Cancer. 2005;116:740–754. doi: 10.1002/ijc.21109. [DOI] [PubMed] [Google Scholar]
- 4.Dhir V, Mohandas KM. Epidemiology of digestive tract cancers in India IV. Gall bladder and pancreas. Indian J Gastroenterol. 1999;18:24–28. [PubMed] [Google Scholar]
- 5.National Cancer Registry Programme. Consolidated Report of the Population Based Cancer Registries 1990–96. New Delhi: Indian Council of Medical Research; 2001. [Google Scholar]
- 6.Jagannath P, Dhir V, Mohandas KM. Geographic patterns in incidence of Gall Bladder cancer in India and the possible etiopathological factors. HPB. 2000;2:168–169. [Google Scholar]
- 7.Khuroo MS, Mahajan R, Zargar SA, Javid G, Sapru S. Prevalence of biliary tract disease in India: a sonographic study in adult population in Kashmir. Gut. 1989;30:201–205. doi: 10.1136/gut.30.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhar SC, Ansari S, Saha M, Ahmed MM, Rahman MT, Hasan M, et al. Gallstone disease in a rural Bangladeshi community. Indian J Gastroenterol. 2001;20:223–226. [PubMed] [Google Scholar]
- 9.Cooperberg PL, Burhenne HJ. Real-time ultrasonography. Diagnostic technique of choice in calculous gallbladder disease. N Engl J Med. 1980;302:1277–1279. doi: 10.1056/NEJM198006053022303. [DOI] [PubMed] [Google Scholar]
- 10.Kratzer W, Mason RA, Kächele V. Prevalence of gallstones in sonographic surveys worldwide. J Clin Ultrasound. 1999;27:1–7. doi: 10.1002/(sici)1097-0096(199901)27:1<1::aid-jcu1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 11.International Institute for Population Sciences (IIPS) and ORC Macro. National Family Health Survey, India 1998–99. Mumbai: IIPS; 2000. [Google Scholar]
- 12.Lazcano-Ponce EC, Miquel JF, Munoz N, Herrero R, Ferrecio C, Wistuba II, et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin. 2001;51:349–364. doi: 10.3322/canjclin.51.6.349. [DOI] [PubMed] [Google Scholar]
- 13.Takiar R, Nadayil D, Nandakumar A. Problem of small numbers in reporting of cancer incidence and mortality rates in Indian cancer registries. Asian Pac J Cancer Prev. 2009;10:657–660. [PubMed] [Google Scholar]
- 14.Singh V, Trikha B, Nain C, Singh K, Bose S. Epidemiology of gallstone disease in Chandigarh: a community-based study. J Gastroenterol Hepatol. 2001;16:560–563. doi: 10.1046/j.1440-1746.2001.02484.x. [DOI] [PubMed] [Google Scholar]
- 15.Serra I, Yamamoto M, Calvo A, Cavada G, Baez S, Endoh K, et al. Association of chili pepper consumption, low socioeconomic status and longstanding gallstones with gallbladder cancer in a Chilean population. Int J Cancer. 2002;102:407–411. doi: 10.1002/ijc.10716. [DOI] [PubMed] [Google Scholar]
- 16.Pandey M, Shukla VK. Lifestyle, parity, menstrual and reproductive factors and risk of gallbladder cancer. Eur J Cancer Prev. 2003;12:269–272. doi: 10.1097/00008469-200308000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Kumar JR, Tewari M, Rai A, Sinha R, Mohapatra SC, Shukla HS. An objective assessment of demography of gallbladder cancer. J Surg Oncol. 2006;93:610–614. doi: 10.1002/jso.20526. [DOI] [PubMed] [Google Scholar]
- 18.Caygill C, Hill M, Kirkham J, Northfield TC. Increased risk of biliary tract cancer following gastric surgery. Br J Cancer. 1988;57:434–436. doi: 10.1038/bjc.1988.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambe M, Trichopoulos D, Hsieh CC, Ekbom A, Adami HO, Pavia M. Parity and cancers of the gall bladder and the extrahepatic bile ducts. Int J Cancer. 1993;54:941–944. doi: 10.1002/ijc.2910540613. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez E, La Vecchia C, D'Avanzo B, Negri E, Franceschi S. Family history and the risk of liver, gallbladder, and pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 1994;3:209–212. [PubMed] [Google Scholar]
- 21.Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology. 1999;117:632–639. doi: 10.1016/s0016-5085(99)70456-7. [DOI] [PubMed] [Google Scholar]
- 22.Pandey M, Khatri AK, Sood BP, Shukla RC, Shukla VK. Cholecystosonographic evaluation of the prevalence of gallbladder diseases. A university hospital experience. Clin Imaging. 1996;20:269–272. doi: 10.1016/0899-7071(95)00034-8. [DOI] [PubMed] [Google Scholar]
- 23.Sharma MP, Duphare HV, Nijhawan S, Dasarathy S. Gallstone disease in north India: clinical and ultrasound profile in a referral hospital. J Clin Gastroenterol. 1990;12:547–549. doi: 10.1097/00004836-199010000-00012. [DOI] [PubMed] [Google Scholar]


