Abstract
Objectives
Postoperative bleeding represents a life-threatening complication after pancreatic surgery. Recent developments in interventional radiology have challenged the role of surgery in bleeding control. This study aimed to assess the management of major haemorrhagic complications after pancreatic surgery at a tertiary referral centre.
Methods
Between August 1998 and June 2009, 18 patients with major bleeding after pancreatic surgery were admitted to the University Hospital of Zurich, Zurich, Switzerland. We retrospectively analysed their medical charts, focusing on diagnosis, therapy and outcome.
Results
Major arterial bleeding occurred after a median postoperative interval of 21.5 days (range: 9–259 days). Seventeen patients demonstrated various symptoms, such as repeated upper gastrointestinal bleeding or haemorrhagic shock. Diagnosis was usually made by contrast-enhanced computed tomography (CT). Leakage of the pancreaticojejunostomy caused the formation of a pseudoaneurysm in 78% of patients. Haemostasis was achieved in 10 patients by interventional radiology. Two patients died of massive re-bleeding. Six patients underwent primary emergency surgery, which five did not survive.
Conclusions
Delayed bleeding after pancreatic surgery is suspicious for a pseudoaneurysm. Contrast-enhanced CT followed by early angiography provides accurate diagnosis and treatment. Interventional radiological treatment should be preferred over primary surgery because it is currently the most life-saving approach.
Keywords: resection, radiological imaging, intervention, outcomes
Introduction
Despite significant reductions in perioperative mortality rates in pancreatic surgery in high-volume centres, reported morbidity rates have remained high during recent years.1 Surgical morbidity mainly concerns three distinct complications: delayed gastric emptying (DGE); pancreatic fistula, and bleeding complications.1,2 Whereas DGE resolves completely with conservative treatment, pancreatic fistula and postoperative bleeding often require interventions to prevent a detrimental outcome. Massive visceral arterial bleeding represents a rare but life-threatening complication that typically occurs with some delay after surgery.3–5 Different mechanisms have been reported to cause major visceral arterial bleeding, including extensive arterial dissection during lymphadenectomy that damages the vessel wall,6,7 and digestive leakage and local abscess formation that may destroy vascular structures and sutures.8–10 Necrosis of the vessel wall has been reported to occur as a result of mechanical irritation caused by drains or ascending infections along the drain.11 Irrespective of the aetiology, the stepwise destruction of the vessel wall promotes the formation of pseudoaneurysms of the superior mesenteric artery (SMA) or branches of the coeliac trunk.
Early identification of bleeding remains difficult because of the lack of specific symptoms. Repeated episodes of gastrointestinal bleeding or a decrease in serum haemoglobin without adequate correlate, recognized as ‘sentinel bleeding’, may precede major bleeding from pseudoaneurysms and should initiate rapid assessment to prevent haemorrhagic shock and associated poor outcome.3 The standard treatment for major visceral arterial bleeding used to be emergency surgery, in which surgical interventions ranged from simple ligature of the bleeding vessel to sophisticated vascular reconstructions. The advent of modern imaging techniques and recent technological developments in interventional radiology apparatus offer novel alternatives to the surgical treatment of this serious complication, which have consequently challenged the role of surgery in these high-risk patients.3,12
The aim of the current analysis was to assess the diagnostic workup and management of major visceral arterial bleeding after pancreatic surgery in our institution during the last decade.
Materials and methods
All patients treated for bleeding complications after pancreatic surgery between August 1998 and June 2009 at the Departments of Surgery and Radiology, University Hospital of Zurich, were included in this current analysis. Patients with bleeding complications related to pancreatic trauma, chronic or necrotizing pancreatitis, or bleeding from primary vascular inflammatory diseases were excluded. Patients were identified from prospective databases in the Departments of Surgery and Radiology. Their medical charts, as well as data pertaining to radiological examinations and interventions, were reviewed.
Definition of bleeding
Postoperative major visceral arterial bleeding was defined as radiologically (angiography, contrast-enhanced computed tomography [ceCT]) or surgically proven bleeding from main branches of the coeliac axis or the SMA beyond the first postoperative week after pancreas resection. This definition allows the exclusion of early postoperative bleeding caused by technical failure to secure haemostasis intraoperatively and pre-existing coagulation disorders. Other sources of bleeding, such as areas of resection, anastomotic suture lines or gastric ulcers, were also excluded.
Interventional radiology
If necessary, resuscitation to restore haemodynamic stability was performed prior to emergency abdominal angiography by an emergency anaesthesiology team.
Unless patients were already intubated, all procedures were performed under local anaesthesia. Angiograms and interventions were carried out on a dedicated system (Intergris V5000®; Philips Medical Systems International BV, Best, the Netherlands) in a standard fashion. Following percutaneous transfemoral visceral angiography with 5-Fr catheters to confirm the bleeding source, embolization was performed with either 5-Fr or coaxial 3-Fr catheters and 0.035″ or, respectively, 0.018″ fibred metal coils.
If balloon-expandable stent grafts (Jostent®; Abbott Vascular Instruments Deutschland GmbH, Rangendingen, Germany) were used to exclude the pseudoaneurysms, they were mounted on low-profile balloons (Savvy®; Cordis BV, Roden, the Netherlands) and introduced with long 6-Fr sheaths positioned in the coeliac trunk or the SMA.
Statistics
Results are given as median and range, respectively.
Results
Eighteen patients with major visceral arterial bleeding related to pancreatic surgery were treated at our institution during the study period. Six patients were referred from outside hospitals and 12 had undergone primary surgery on the pancreas at our institution. Indications for surgery and patient characteristics are summarized in Table 1. Surgical procedures included classical pancreaticoduodenectomy (n= 10), central pancreatic resection (n= 2), pancreatic tail resection (n= 1), combined gastrectomy-splenectomy-pancreatic tail resection (n= 1), double bypass for non-resectable pancreatic cancer (n= 1), choledochal cyst resection followed by hepaticojejunostomy (n= 1) and pancreaticojejunostomy (n= 2).
Table 1.
Patient characteristics
| Patients, n | 18 |
| Gender, male/female, n | 11/7 |
| Median age (range), years | 58 (39–82) |
| Indications for surgery, n | |
| Choledochal cyst | 1 |
| Insulinoma | 1 |
| Chronic pancreatitis | 2 |
| Mucinous cystadenoma | 3 |
| Bile duct cancer | 3 |
| Pancreatic cancer | 6a |
| Giant chronic duodenal ulcer | 1 |
| Intraductal papillary mucinous neoplasm | 1 |
| Type of surgery, n | |
| Duodenopancreatectomy | 10 |
| Central pancreatectomy | 2 |
| Distal pancreatectomyb | 2 |
| Double bypass | 1 |
| Hepaticojejunostomy | 1 |
| Pancreaticojejunostomy | 2 |
n= 1 ampullary cancer
Including splenectomy and gastrectomy
Clinical presentations
Five patients were admitted directly to the Department of Radiology from three different hospitals. These patients were referred for interventional bleeding control after major visceral arterial bleeding had been diagnosed by endoscopy, CT scan or according to the clinical deterioration of the patient. One additional patient was admitted in haemorrhagic shock to the Department of Surgery after a complicated postoperative course with several surgical re-interventions at the referring hospital. Two patients were referred by their general practitioners with new onset of gastrointestinal bleeding to the Department of Surgery, 44 days and 259 days after pancreatic surgery, respectively. The remaining 10 patients developed major visceral arterial bleeding during hospitalization for the primary surgery at our institution.
All but one patient presented clinical symptoms. Seven patients (39%) had repeated gastrointestinal bleeding, which was haemodynamically relevant in one. Another eight patients (44%) developed haemorrhagic shock as a first symptom. Four of them revealed increasing bleeding through an intra-abdominal drain. New onset of abdominal pain was the first symptom in two patients. The final patient demonstrated a steady decrease in serum haemoglobin without clinical symptoms until admission. All nine patients with haemorrhagic shock and haemodynamically relevant gastrointestinal bleeding were transfused. The median interval from the initial operation to major bleeding was 21.5 days (range: 9–259 days).
Diagnosis
The primary diagnosis of major visceral arterial bleeding was made by abdominal ceCT in 16 patients (89%). As spiral CT scanners were developed during the study period, CT technology ranged from single- to multi-slice technology. One patient required immediate surgery in the intensive care unit, where the bleeding suddenly occurred. Another patient primarily underwent diagnostic angiography, which identified a bleeding pseudoaneurysm of the SMA. All patients who were admitted directly to the Department of Radiology had been diagnosed by CT with major visceral arterial bleeding at the referring hospital.
Causes and sources of bleeding
Sources of bleeding in the 18 patients are summarized in Table 2. The most common cause of major visceral arterial bleeding was leakage of the pancreaticojejunostomy, which occurred in 14 patients (78%) and was diagnosed either by high amylase content in peritoneal drains or by local fluid collections at the pancreatic remnant on the CT scan. Of note, in 12 of these 14 patients, a soft pancreas was found intraoperatively during the index operation. One patient revealed a leakage of the hepaticojejunostomy and one had a liver abscess. The cause of major visceral arterial bleeding could not be determined in two patients.
Table 2.
Sources of bleeding and treatment
| Site of bleeding, n | |
| Splenic artery | 4 |
| Superior mesenteric artery | 1 |
| Common hepatic artery | 6 |
| Right hepatic artery | 3 |
| Proper hepatic artery | 1 |
| Gastroduodenal artery | 2 |
| Jejunal artery | 1 |
| Treatment, n | |
| Surgery | 6a |
| Arterial graft | 1 |
| Direct suture | 5 |
| Interventional | 12b |
| Embolization | 11 |
| Stent grafting | 3c |
One patient had an embolization for re-bleeding
Three patients underwent more than one intervention
All three patients underwent additional embolization
Treatments
Twelve patients (67%) were transferred to the interventional radiology suite after resuscitation to restore haemodynamic stability. Immediate haemostasis was achieved in 10 patients by interventional means (primary success rate 83%). Three of the 12 patients required at least two interventions to achieve definitive control of bleeding. Interestingly, one re-bleeding occurred 80 days after the first embolization. Coiling was performed with different types of coil in all 12 patients and three patients underwent additional stent grafting. Two patients died as a result of massive re-bleeding and ischaemia-related multi-organ failure although initial haemostasis had been successfully achieved. After diagnostic angiography had revealed a pseudoaneurysm of the SMA as bleeding source, one patient underwent primary vascular reconstruction with a polytetrafluorethylene (PTFE) graft instead of an interventional treatment.
Six patients (33%) underwent emergency surgery. The hepatic artery and the stump of the gastroduodenal artery represented the source of bleeding in four and two of these patients, respectively. Although two patients did not undergo preoperative CT as a result of sudden haemorrhagic shock, four patients were haemodynamically stabilized and underwent emergency ceCT. One of these patients revealed major bleeding from both the common hepatic artery (CHA) and the main portal vein. These vessels were repaired by resection and direct re-anastomosis or graft interposition, respectively. This patient died of multi-organ failure after three re-operations and one embolization for bleeding control. A ruptured pseudoaneurysm of the CHA was identified as the source of bleeding in another patient during emergency surgery. Massive adhesions in the upper abdomen made the adequate exposition of the bleeding vessel impossible and the patient died within a few hours of surgery because haemostasis could not be achieved. In five patients, adequate exposure of the bleeding vessels was possible when the hepaticojejunostomy was taken down. Completion pancreatectomy was not performed in any patient. Four patients died after multiple surgical revisions failed to control bleeding.
Outcomes
Six patients (one after interventional radiology, four after primary surgery, one after surgery and secondary embolization) died during the postoperative course at a median of 41.5 days (range: 16–81 days) after the index operation. Thus, the overall mortality rate was 33%. All patients who died demonstrated major bleeding for which haemorrhagic shock represented the first clinical manifestation. Five of them had a centrally localized pseudoaneurysm of the CHA and four had a pancreatic fistula.
Discussion
Major visceral arterial bleeding is a rare but typical complication after pancreatic surgery; nevertheless, a generally accepted definition and classification have been lacking for a long time.1 Reported incidence rates range from 1% to 12%, largely because the various series do not differentiate between early and delayed bleeding of various origins.8,11,13–17 The International Study Group of Pancreatic Surgery (ISGPS) recently proposed that postoperative haemorrhage can be defined by the time of onset of bleeding, the cause and localization of bleeding and, finally, by the severity of bleeding.18 Whereas early postoperative bleeding is primarily related to surgical failure to achieve secure haemostasis or is secondary to pre-existing coagulopathy,19 late postoperative haemorrhage is highly suspicious for bleeding from an erosion of a pseudoaneurysm of a major visceral artery. The estimated incidence of delayed major visceral arterial bleeding appears to be 1.5–4.0%.4,9,14,15
In the present series, we analysed outcomes in 18 patients with delayed major bleeding after pancreatic surgery. The vast majority of episodes of bleeding (89%) were caused by pseudoaneurysms; the remaining two (11%) cases involved bleeding from the stump. Furthermore, most episodes (78%) occurred as a consequence of a leak at the pancreaticojejunostomy.
The consensus statement of the ISGPS on the definition of postoperative bleeding after pancreatic surgery distinguishes early from late bleeding.18 The ideal cut-off for the definition of early vs. late bleeding, however, remains controversial and somewhat arbitrary. Although the ISGPS proposes a cut-off of 24 h, others have used cut-offs of 5–7 days post-surgery to define ‘late’ bleeding.19–22 As the likelihood that technical failure and anastomotic bleeding represent the cause of bleeding decreases and that of major visceral arterial bleeding increases with time after surgery, we excluded patients who experienced bleeding episodes during the first week after surgery. This exclusion makes the patient population more homogeneous and increases the power of the analysis.
In accordance with the literature, major visceral arterial bleeding was caused by the formation of pseudoaneurysms in nearly 90% of the patients in our series.8,11,14,23 Pancreatic fistula at the pancreaticojejunostomy caused the formation of a pseudoaneurysm in more than 75% of our patients. A soft pancreas, which represents a well-known risk factor for pancreatic fistula,1 was found in the index operation. Consequently, the presence of postoperative pancreatic fistulae is the most important single factor predisposing to pseudoaneurysm formation and delayed major visceral arterial bleeding. The prevention of pancreatic leakage is therefore of the utmost importance. Patients with known pancreatic fistulae must be carefully followed in order to diagnose any pseudoaneurysm early.
Because the formation of a pseudoaneurysm is a subtle process, minor bleeding episodes may occur repeatedly. Although minor extraluminal bleeding into the abdominal cavity often remains clinically undetected, bleeding into the gastrointestinal tract generally becomes clinically overt faster. In this current series, half of the patients experienced episodes of ‘sentinel bleeding’, a finding that compares well with rates reported in the literature.15,16,18,20 Given the potentially fatal outcome of major visceral arterial bleeding, any episode of sentinel bleeding must prompt immediate diagnostics and treatment.3,15 The absence of a sentinel bleed, however, does not reliably exclude major visceral arterial bleeding.
Surgical access to the bleeding source is usually impaired by the overlying pancreatico-enteric and bilio-enteric reconstruction, postoperative adhesions and inflammatory reactions. As a consequence, a targeted approach is often not possible and completion pancreatectomy becomes unavoidable in the effort to achieve good exposure and obtain haemostasis. Modern radiological equipment offers opportunities for less invasive treatment to control major visceral arterial bleeding,3 with reported success rates ranging from 80% to 100%.6,9,10,12,15,17,23
As a basic principle of interventional treatment, the bleeding vessel is embolized by (micro)-coils proximally and distally to the source of bleeding, as direct embolization of the leak within the pseudoaneurysm is not successful. Embolization should be as selective as possible to minimize organ ischaemia. Pseudoaneurysms of the splenic artery are best treated by the complete embolization of the artery because the blood supply to the spleen is preserved by short gastric arteries. Complete embolization of the main hepatic artery should be avoided to prevent hepatic insufficiency and necrosis formation7,16,19 (Fig. 1). If stent grafting of the CHA is not possible as a result of local anatomy, bleeding control requires complete embolization despite its risks for hepatic ischaemia.7,16 The treatment of pseudoaneurysms of the SMA or the coeliac trunk requires stent grafts of the affected arterial segment to preserve vascular patency and avoid extensive mesenteric infarction.20,24,25 Although two patients required more than one intervention, the overall success rate of interventional radiology was high in our series, as only one patient required surgery after interventional treatment.
Figure 1.
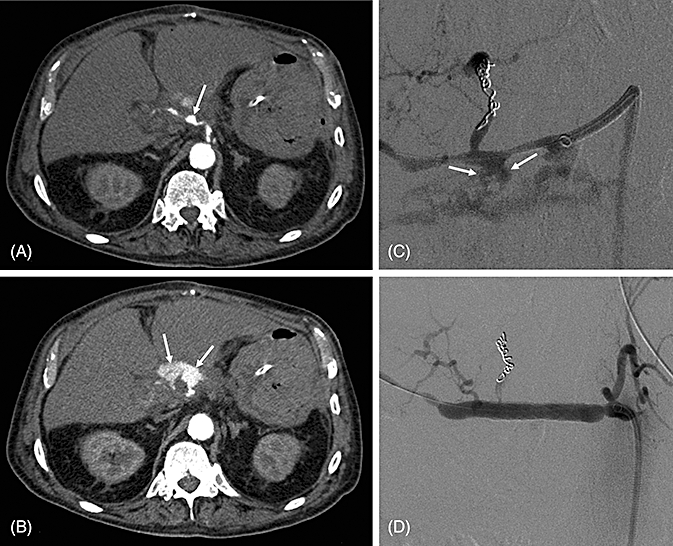
Radiodiagnosis and treatment of a bleeding pseudoaneurysm in a 71-year-old patient with major bleeding after a complicated course with pancreatic fistula 80 days after pancreaticoduodenectomy. (A) An abdominal computed tomography scan demonstrates a pseudoaneurysm of the common hepatic artery (CHA) (arrow). (B) Contrast extravasation indicates an active arterial bleeding (arrow). (C) Angiography shows this extravasation of the CHA after embolization of the left hepatic artery for an additional bleeding (arrow). (D) Post-interventional result after stenting of the CHA
Our results confirm that major visceral arterial bleeding remains a life-threatening condition with an increased mortality rate (33%). Furthermore, our data clearly suggest that interventional treatment is superior to surgery in terms of survival. Interventional treatment does not carry the additional risk of injuring other organs and allows completion pancreatectomy to be avoided. Interventional treatment should therefore be considered a first treatment option. To this end, even in unstable patients, the restoration of haemodynamic stability should always be attempted because angiography will provide diagnostics and treatment within one intervention. Surgery remains a final option in which a deleterious outcome is probable because only severely unstable patients in whom resuscitation has failed eventually undergo emergency surgery.
The role of surgery may be limited to treating the underlying cause of major bleeding (i.e. intra-abdominal abscess and persisting pancreatic leakage).
In conclusion, delayed major haemorrhage after pancreatic surgery must be attributed to a bleeding pseudoaneurysm of a major visceral artery until proven otherwise. Repeated episodes of minor bleeding may precede fatal bleeding and should therefore prompt immediate diagnostic workup to avoid the occurrence of deleterious haemorrhagic shock (Fig. 2). Endoscopy may be useful to exclude intraluminal bleeding caused by ulcer and suture lines. Massive intraluminal bleeding or failure to detect the source of bleeding are highly suspicious for a bleeding pseudoaneurysm of a major visceral artery. Given the pathogenesis of pseudoaneurysm formation, scheduled follow-up CT scans should be considered in patients in whom the perioperative course is protracted, particularly in those with proven pancreatic fistulae. Interventional radiology performed by an experienced radiologist provides the best treatment for major visceral arterial bleeding and should be preferred over emergency surgery whenever feasible.
Figure 2.

Algorithm for the management of delayed visceral arterial bleeding according to the severity of bleeding. CT, computed tomography
Conflicts of interest
None declared.
References
- 1.Schäfer M, Mullhaupt B, Clavien PA. Evidence-based pancreatic head resection for chronic pancreatitis and pancreatic cancer. Ann Surg. 2002;236:137–148. doi: 10.1097/00000658-200208000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Oliveira ML, Winter JM, Schäfer M, Cunningham SC, Cameron JL, Yeo CJ, et al. Assessment of complications after pancreatic surgery: a novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 2006;244:931–939. doi: 10.1097/01.sla.0000246856.03918.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Castro SMM, Busch ORC, Gouma DJ. Management of bleeding and leakage after pancreatic surgery. Best Pract Res Clin Gastroenterol. 2004;18:847–864. doi: 10.1016/j.bpg.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Rumstadt B, Schwab M, Korth P, Samman M, Trede M. Haemorrhage after pancreatoduodenectomy. Ann Surg. 1998;227:236–241. doi: 10.1097/00000658-199802000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Castro SMM, Kuhlmann KF, Busch OR, van Delden OM, Laméris JS, van Gulik TM, et al. Delayed massive haemorrhage after pancreatic and biliary surgery. Ann Surg. 2005;241:85–91. doi: 10.1097/01.sla.0000150169.22834.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reber PU, Baer HU, Patel AG, Wildi S, Triller J, Büchler MW. Superselective microcoil embolization: treatment of choice in high-risk patients with extrahepatic pseudoaneurysm of the hepatic arteries. J Am Coll Surg. 1998;186:325–330. doi: 10.1016/s1072-7515(98)00032-5. [DOI] [PubMed] [Google Scholar]
- 7.Sugimoto H, Kaneko T, Ishiguchi T, Takai K, Ohta T, Yagi Y, et al. Delayed rupture of a pseudoaneurysm following pancreatoduodenectomy: report of a case. Surg Today. 2001;31:932–935. doi: 10.1007/s005950170039. [DOI] [PubMed] [Google Scholar]
- 8.Turrini O, Moutardier V, Guiramand J, Lelong B, Bories E, Sannini A, et al. Haemorrhage after duodenopancreatectomy: impact of neoadjuvant radiochemotherapy and experience with sentinel bleeding. World J Surg. 2005;29:212–216. doi: 10.1007/s00268-004-7557-3. [DOI] [PubMed] [Google Scholar]
- 9.Makowiec F, Riediger H, Euringe W, Uhl M, Hopt UT, Adam U. Management of delayed visceral arterial bleeding after pancreatic head resection. J Gastrointest Surg. 2005;9:1293–1299. doi: 10.1016/j.gassur.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Fujii Y, Shimada H, Endo I, Yoshida K, Matsuo K, Takeda K, et al. Management of massive arterial haemorrhage after pancreatobiliary surgery: does embolotherapy contribute to successful outcome? J Gastrointest Surg. 2007;11:432–438. doi: 10.1007/s11605-006-0076-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otah E, Cushin BJ, Rozenblit GN, Neff R, Otah KE, Cooperman AM. Visceral arterial pseudoaneurysms following pancreatoduodenectomy. Arch Surg. 2002;137:55–59. doi: 10.1001/archsurg.137.1.55. [DOI] [PubMed] [Google Scholar]
- 12.Sohn TA, Yeo CJ, Cameron JL, Geschwind JF, Mitchell SE, Venbrux AC, et al. Pancreaticoduodenectomy: role of interventional radiologists in managing patients and complications. J Gastrointest Surg. 2003;7:209–219. doi: 10.1016/s1091-255x(02)00193-2. [DOI] [PubMed] [Google Scholar]
- 13.Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg. 1990;211:457–458. doi: 10.1097/00000658-199004000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Berge Henegouwen MI, Allema JH, van Gulik TM, Verbeek PC, Obertop H, Gouma DJ. Delayed massive haemorrhage after pancreatic and biliary surgery. Br J Surg. 1995;82:1527–1531. doi: 10.1002/bjs.1800821124. [DOI] [PubMed] [Google Scholar]
- 15.Brodsky JT, Turnbull AD. Arterial haemorrhage after pancreatoduodenectomy. The ‘sentinel bleed’. Arch Surg. 1991;121:1037–1040. doi: 10.1001/archsurg.1991.01410320127019. [DOI] [PubMed] [Google Scholar]
- 16.Sato N, Yamaguchi K, Shimizu S, Morisaki T, Yokohata K, Chijiiwa K, et al. Coil embolization of bleeding visceral pseudoaneurysms following pancreatectomy: the importance of early angiography. Arch Surg. 1998;133:1099–1102. doi: 10.1001/archsurg.133.10.1099. [DOI] [PubMed] [Google Scholar]
- 17.Okuno A, Miyazaki M, Ito H, Ambiru S, Yoshidome H, Shimizu H, et al. Non-surgical management of ruptured pseudoaneurysm in patients with hepatobiliary pancreatic disease. Am J Gastroenterol. 2001;96:1067–1071. doi: 10.1111/j.1572-0241.2001.03691.x. [DOI] [PubMed] [Google Scholar]
- 18.Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Postpancreatectomy haemorrhage (PPH) – an international Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20–25. doi: 10.1016/j.surg.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Yekebas EF, Wolfram L, Cataldegirem G, Habermann CR, Bogoevski D, Koenig AM, et al. Postpancreatectomy haemorrhage: diagnosis and treatment. Ann Surg. 2007;246:269–280. doi: 10.1097/01.sla.0000262953.77735.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tien YW, Lee PH, Ang CN, Ho MC, Chiu YF. Risk factors of massive bleeding related to pancreatic leak after pancreaticoduodenectomy. J Am Coll Surg. 2005;201:554–559. doi: 10.1016/j.jamcollsurg.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Choi SH, Moon HJ, Heo JS, Joh JW, Kim YI. Delayed haemorrhage after pancreaticoduodenectomy. J Am Coll Surg. 2004;199:186–191. doi: 10.1016/j.jamcollsurg.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Blanc T, Cortes A, Goere D, Sibert A, Pessaux P, Belghiti J, et al. Haemorrhage after pancreaticoduodenectomy: when is surgery still indicated? Am J Surg. 2007;194:3–9. doi: 10.1016/j.amjsurg.2006.08.088. [DOI] [PubMed] [Google Scholar]
- 23.Gebauer T, Schulz HU, Tautenhahn J, Halloul Z, Effenberger O, Lippert H, et al. Interventional and surgical management for inflammatory erosion haemorrhage from visceral arteries after pancreatic surgery. Chirurg. 2004;75:1021–1028. doi: 10.1007/s00104-004-0834-8. [DOI] [PubMed] [Google Scholar]
- 24.Heiss P, Bachthaler M, Hamer OW, Piso P, Herold T, Schlitt HJ, et al. Delayed visceral arterial haemorrhage following Whipple's procedure: minimally invasive treatment with covered stents. Ann Surg Oncol. 2008;15:824–832. doi: 10.1245/s10434-007-9715-y. [DOI] [PubMed] [Google Scholar]
- 25.Stoupis C, Ludwig K, Inderbitzin D, Do DD, Triller J. Stent grafting of acute hepatic artery bleeding following pancreatic head resection. Eur Radiol. 2007;17:401–408. doi: 10.1007/s00330-006-0359-2. [DOI] [PubMed] [Google Scholar]


