Abstract
Background
The purpose of the present study was to demonstrate that post-operative morbidity (PM) associated with resections of hilar cholangiocarcinoma (HCCA) is associated with short- and long-term patient survival.
Methods
Between 1998 and 2008, 51 patients with a median age of 64 years underwent resection for HCCA at a single institution. Associations between survival and clinicopathologic factors, including peri- and post-operative variables, were studied using univariate and multivariate models.
Results
Seventy-six per cent of patients underwent major hepatectomy with resection of the extrahepatic bile ducts. The 30- and 90-day operative mortality was 10% and 12%. The overall incidence of PM was 69%, with 68% of all PM as major (Clavien grades III–V). No difference in operative blood loss or peri-operative transfusion rates was observed for patients with major vs. minor or no PM. Patients with major PM received adjuvant chemotherapy less frequently than patients with minor or no complications 29% vs. 52%, P= 0.15. The 1-, 3- and 5-year overall (OS) and disease-specific survival (DSS) rates for all patients were 65%, 36%, 29% and 77%, 46%, 35%, respectively. Using univariate and multivariate analysis, margin status (27% R1), nodal metastasis (35% N1) and major PM were associated with OS and DSS, P < 0.01. Major PM was an independent factor associated with decreased OS and DSS [hazard ratio (HR) = 3.6 and 2.8, respectively, P < 0.05]. The median DSS for patients with major PM was 14 months compared with 40 months for patients who experienced minor or no PM, P < 0.01.
Conclusion
Extensive operations for HCCA can produce substantial post-operative morbidity. In addition to causing early mortality, major post-operative complications are associated with decreased long-term cancer-specific survival after resection of HCCA.
Keywords: cholangiocarcinoma, resection, morbidity, mortality, outcomes
Introduction
Adenocarcinomas arising from the extrahepatic biliary tree are uncommon malignancies. Hilar cholangiocarcinomas (HCCA) represent less than 2% of all gastrointestinal cancers, and few institutions have expertise in their management.1,2 Furthermore, tumours located at the hepatic hilum challenge the capability of even high-resolution pre-operative imaging techniques to determine the local extent of disease accurately. Potential curative therapy for hilar cholangiocarcinoma requires complete operative resection, but more than half of patients with HCCA will not be candidates for resection as a result of the presence of advanced disease, either metastatic or locoregional spread, at the time of clinical presentation.3–7
The median survival of patients with unresectable HCCA is typically less than 12 months, but, for patients with resected tumours, it is in the order of 3 years.5,6,8 As a result of the influence of the surgical resection margin on long-term outcome, more aggressive operative approaches towards HCCA have been adopted and have advocated en bloc hepatectomy with resection of the extrahepatic biliary tree.9–14 An extensive operation to accomplish a margin-negative resection for HCCA poses a major risk for substantial post-operative morbidity and mortality, especially in patients with pre-existing medical conditions.6,10,14–17 Several studies have reported the negative consequences of medical co-morbidities for overall survival of patients with cancer even when these are considered before the introduction of treatment-related adverse events.3,18–20
Operative complications have a negative impact on both short- and long-term outcomes, including cancer-specific survival, for patients with various cancers, e.g. colorectal, hepatocellular and oesophageal.21–27 The effects of post-operative morbidity on long-term outcome after resection of HCCA have not been published widely. The purpose of this single-institution study was to evaluate the specific complications that are encountered during and after the operative management of HCCA and to determine their influence on long-term survival.
Methods
Patients
Between January 1998 and December 2008, 51 consecutive patients underwent operative resection for HCCA at Indiana University Hospital and Methodist Hospital, Indianapolis, IN. Permission to study these patients was obtained from the Indiana University School of Medicine Institutional Review and Privacy Board according to the institutional policy for protected health information. Patients were identified from the institutions' clinical database. Patients' demographic, clinical, operative, pathological and follow-up data were studied retrospectively. The diagnosis of HCCA was verified by a concordant diagnosis contained within the pre-operative radiology report, operative note and pathology description. Patients who underwent liver transplantation as part of treatment for HCCA were excluded.
Clinical data
All clinical data were obtained from the patient electronic medical record system which contains all pre-, peri- and post-operative information. Pre-operative body mass index (BMI) and age-adjusted Charlson co-morbidity index (CCI) were calculated for all patients.28 Pre-operative serum biochemical values, including total bilirubin, creatinine and albumin, were recorded closest to the date of operation. Pre-operative biliary drainage was labelled as percutaneous for drainage procedures which involved percutaneous transhepatic cholangiography (PTC) or endoscopic for patients who underwent endoscopic retrograde cholangiography (ERC) for biliary stenting. Pre-operative portal vein embolization was not performed for any patient.
In the present study, all operations for HCCA involved resection of the extrahepatic biliary tree. Hepatopancreatoduodenectomy was not performed for any patient. Hepatectomy was typically performed en bloc with resection of the biliary tree. Continuity and extent of portal lymphadenectomy could not be ascertained for all cases. Major hepatectomy was defined as a hepatectomy involving resection of at least three liver segments. All biliary reconstructions involved a Roux-en-Y cholangio- or hepaticojejunostomy.
Tumour specimens from both hospitals were analysed histopathologically at a centralized pathology laboratory. Tumour histology was graded as well, moderately or poorly differentiated. Major vascular invasion involving the portal vein and/or hepatic artery was recorded from the operative note or pathology report. Lymph node status was determined from the number of positive and negative lymph nodes recorded in the final pathology report. Operative resection margins were determined from the operative notes and pathology reports and were categorized as R0 (negative microscopic margin), R1 (positive microscopic margin) or R2 (grossly involved margin). Appropriate TNM staging was determined for all HCCA patients according to the AJCC guidelines (6th Edition) for bile duct carcinomas.29
Early post-operative outcomes were analysed for each patient and included type and timing of post-operative complications, length of hospital stay and 30- and 90-day operative mortality. Post-operative complications were graded according to the Clavien grading system.30 Patients were categorized into three groups according to the highest level of post-operative complications: none, minor (Clavien grade I or II) or major (Clavien III, IV, or V) complication.
Adjuvant systemic chemotherapy or combinational chemoradiotherapy was recorded; however, details of adjuvant therapy, including type and duration, were not available for all patients, especially for those treated outside of Indiana University and Methodist Hospitals. Follow-up time was calculated from the date of operation to the date of last clinical encounter captured by the electronic medical record system or the date of death when it occurred. Dates of death were confirmed with the Social Security Database. Data regarding disease recurrence were extracted from either medical records or radiology reports. Overall survival (OS), disease-specific survival (DSS) and recurrence-free survival (RFS) were calculated based on the survivorship status at last follow-up. Location of tumour recurrence was specified as local (liver margin, bile duct, or anastomotic), regional (lymph node), and/or distant (extra-marginal liver or extrahepatic). Time to recurrence was calculated between the date of operation and the first documentation of tumour recurrence.
Statistics
Statistical analyses were performed using SPSS Software Version 17.0 for Windows (SPSS Inc., Chicago, IL, USA) and Microsoft Excel 2007. Categorical variables were compared using a χ2-test. Continuous variables were expressed as median with range or mean ± standard error of mean (SEM). Continuous variables were compared using an independent samples Mann–Whitney U-test or Student's t-test as appropriate. Survival probabilities were estimated using the Kaplan–Meier method and compared with a log-rank test. Mortality, resulting from operative complications, was not excluded from survival analyses. Multivariable analyses of survival were performed with a Cox regression analysis.
Results
Patient, disease and treatment characteristics
Fifty-one patients with a median age of 64 years (range, 40–87) underwent resection of HCCA. Sixteen patients (31%) did not experience a post-operative complication, and 11 patients (22%) experienced only minor (grade I or II) complications. These patients were grouped together and compared with 24 patients (47%) who experienced major (grades III–V) post-operative complications. Table 1 summarizes the clinical, operative and pathological differences between the two groups of patients. The only significant objective differences between the two groups of patients pre-operatively were albumin level and type of biliary drainage procedure. Twenty-two (92%) of the patients in the major complication group underwent a major hepatectomy as part of operative resection; whereas, 17 (63%) patients with no or minor postoperative complications underwent a hepatectomy (P= 0.02).
Table 1.
Complications after resection for hilar cholangiocarcinoma (HCCA) differences in patient, operative, and pathological characteristics
| No or minor complicationsa | Major complicationsa | P-value | |
|---|---|---|---|
| Patients | n= 27 | n= 24 | |
| Ageb | 63 y (40–78) | 65 y (44–87) | 0.14 |
| Gender | 0.97 | ||
| Male | 19 (70%) | 17 (71%) | |
| Female | 8 (30%) | 7 (29%) | |
| Body mass indexb | 26 kg/m2 (21–35) | 24.5 kg/m2 (18–32) | 0.19 |
| Charlson comorbidity indexb | 4 (2–7) | 4 (2–9) | 0.39 |
| Pre-operative serum valuesc | |||
| Albumin | 3.4 ± 0.1 g/dL | 2.8 ± 0.1 g/dL | 0.01 |
| Total bilirubin | 4.1 ± 0.9 mg/dL | 4.4 ± 0.8 mg/dL | 0.81 |
| Creatinine | 1.0 ± 0.04 mg/dl | 0.9 ± 0.04 mg/dL | 0.29 |
| Pre-operative biliary drainage | 0.02 | ||
| Percutaneous | 8 (30%) | 13 (54%) | |
| Endoscopic | 15 (55%) | 9 (38%) | |
| None | 4 (15%) | 2 (8%) | |
| Operative details | |||
| Major hepatectomy | 17 (63%) | 22 (92%) | 0.02 |
| Right hepatectomy | 7 (26%) | 9 (38%) | 0.05 |
| Left hepatectomy | 10 (37%) | 13 (54%) | 0.05 |
| Trisectionectomy | 1 (4%) | 3 (12%) | 0.33 |
| Caudate resection | 1 (4%) | 3 (12%) | 0.33 |
| Portal vein resection | 1 (4%) | 2 (8%) | 0.59 |
| Operative blood lossb | 900 mL (100–6000) | 1250 mL (400–4500) | 0.42 |
| Margin status | 0.38 | ||
| R0 | 21 (78%) | 16 (67%) | |
| R1 | 6 (22%) | 8 (33%) | |
| Adjuvant therapy | 0.15 | ||
| Chemotherapy | 14 (52%) | 7 (29%) | |
| Chemoradiotherapy | 3 (11%) | 1 (4%) | |
| Tumour histological grade | 0.63 | ||
| Well differentiated | 14 (52%) | 12 (50%) | |
| Moderate/poorly differentiated | 13 (48%) | 12 (50%) | |
| Histological invasion | 0.12 | ||
| Lymphovascular | 5 (19%) | 1 (4%) | |
| Perineural | 12 (44%) | 14 (58%) | |
| Both | 2 (7%) | 4 (17%) | |
| None | 8 (30%) | 5 (21%) | |
| Lymph node status | 0.68 | ||
| Positive | 11 (41%) | 7 (29%) | |
| Negative | 13 (48%) | 14 (58%) | |
| Unknown | 3 (11%) | 3 (13%) | |
| Major vascular invasion | 1 (4%) | 3 (12%) | 0.62 |
| AJCC stage (6th ed.) | 0.48 | ||
| IA | 2 (7%) | 4 (17%) | |
| IB | 3 (11%) | 2 (8%) | |
| IIA | 8 (30%) | 11 (46%) | |
| IIB | 13 (48%) | 7 (29%) | |
| III | 1 (4%) | 0 | |
Minor complications: Clavien grade I, II; major complications: grade III–V.
Expressed as median (range).
Expressed as mean (±SEM).
Post-operative morbidity and mortality
The 30- and 90-day mortality rates were 10% and 12%, respectively. The median number of complications for patients who died in the early (<30 days) post-operative period was 4 (range, 2–6). The median number of complications was 1 (range, 1–4) for patients who experienced only minor complications vs. 2 (range, 1–6) for the major complication group. Major post-operative complications were associated with a significantly longer length of hospital stay. Median length of stay for the 24 patients in the major complication group was 17 days (range, 6–62 days) compared with 10 days for the 27 patients with no or minor complications (range, 6–18 days), P < 0.01.
The types and frequencies of post-operative morbidity after resection of HCCA are listed in Table 2. The most common minor complications included cholangitis, bacteraemia and superficial wound infection. The most common major complications were an intra-abdominal abscess requiring percutaneous drainage with radiographic guidance, post-operative bleeding and bile leak requiring intervention (either percutaneous biloma drainage or transhepatic stenting). Three patients required early reoperation to manage major post-operative complications [bleeding (2), portal vein thrombosis]. Post-operative liver failure, defined at our institution as a total serum bilirubin greater than 10 mg/dl accompanied by coagulopathy on or after post-operative day five, was apparent in four patients. Multiorgan failure was present in two of these patients and resulted in death in both cases.
Table 2.
Post-operative complications types and frequency
| Complication type | No. patients (%) |
|---|---|
| Minor complications | 11 (22%) |
| Cholangitis | 5 (10%) |
| Bacteremia | 4 (8%) |
| Superficial wound infection | 4 (8%) |
| Pleural effusion | 3 (6%) |
| DVT/PE | 2 (4%) |
| Bile leak not requiring intervention | 1 (2%) |
| Pancreatitis | 1 (2%) |
| Pneumonia | 1 (2%) |
| Urinary tract infection | 1 (2%) |
| Major complications | 24 (47%) |
| Intra-abdominal abscess treated with drainage | 11 (22%) |
| Death (≤90 day) | 6 (12%) |
| Death (≤30 day) | 5 (10%) |
| Bile leak treated with intervention | 5 (10%) |
| Post-operative bleeding | 4 (8%) |
| Organ failure | 3 (6%) |
| Multiple (including hepatic) | 2 (4%) |
| Hepatic only | 2 (4%) |
| Cardiovascular ischaemia or dysrhythmia | 1 (2%) |
| Fascial dehiscence | 1 (2%) |
| Portal vein thrombosis | 1 (2%) |
Survival analysis
The median follow-up for all patients was 19 months (0–106 months). For survivors, the median follow-up was 28 months (19–106) with no significant difference between patients grouped according to complication grade. There were no differences in OS or DSS for patients with no post-operative morbidity or minor complications only. The estimated 1-, 3- and 5-year OS for patients who experienced no or minor post-operative morbidity were 81%, 56%, and 38%, respectively. The actuarial 3- and 5-year DSS for this same group of patients were 51% and 40%, respectively. Comparatively, these survival rates were statistically better than those for patients who experienced major post-operative morbidity (Fig. 1). Unlike the OS plots, post-operative mortality did not serve as an event for DSS estimations. After excluding patients who died within 90 days of operation (n= 6), there was still a significant difference in survival between patients who experienced no or minor post-operative morbidity (median DSS = 40 months) and those with major, non-fatal complications (median DSS = 14 months), P= 0.05.
Figure 1.
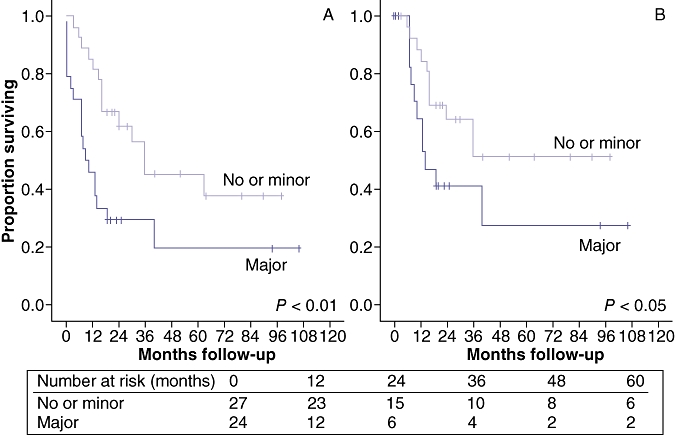
(a) Actuarial overall (OS) and (b) disease-specific survival (DSS) for patients undergoing resection for hilar cholangiocarcinoma (HCCA). Patients are categorized into two groups according to post-operative complications: none or minor (grade I or II, n= 27) and major (grade III–V, n= 24). Proportion surviving is shown over time (months)
Tumour recurrence was detected in 14 patients (27%) during the follow-up period. The median time to recurrence for all patients was 18 months; 35 months for patients with no or minor post-operative complications compared with 10 months for the major complications group, P= 0.15. The most common location for tumour recurrence was the distant liver (i.e. outside the liver margin) and occurred in 10 patients. One local recurrence involved the hepaticojejunostomy, four involved regional lymph nodes and four distant extrahepatic recurrences were observed.
Multivariable analysis of survival
Post-operative morbidity, resection margin status, pre-operative Charlson comorbidity index, pre-operative biliary drainage procedure and lymph node status were associated with OS and DSS using univariate analysis. These factors were included in a proportional hazards model to identify independent predictors of outcome, namely OS and DSS. Using multivariable analysis, major post-operative morbidity was associated with a significant decline in OS (Table 3) as well as DSS [not shown, hazard ratio (HR) = 2.8, 95% confidence interval (CI) = 1.3–6.5; P= 0.04]. The other factors associated with a significantly worse OS (Table 3) and DSS were R1 resection and pre-operative Charlson comorbidity index. The DSS HR for an R1 resection was 4.3, P= 0.003 and that for a pre-operative Charlson index ≥4 was 4.8, P= 0.007. The median number of lymph nodes examined for all patients was four. Positive lymph node status was an independent factor associated with OS but not with DSS.
Table 3.
Predictors of survival
| No. patients (%) | OS | DSS | Univariate analysis | Multivariable analysis (OS) | |||
|---|---|---|---|---|---|---|---|
| Median (1, 3, 5 year) | Median (1, 3, 5 year) | P-value* (OS, DSS, RFS) | HR | 95% CI | P-value | ||
| Complication | 0.008, 0.04, 0.15 | ||||||
| None or minor | 27 (53%) | 36 m (81,56,38%) | 40 m (85,51,40%) | ||||
| Major | 24 (47%) | 9 m (46,29,19%) | 14 m (64,41,27%) | 3.58 | 1.47–8.27 | 0.005 | |
| Resection margin status | 0.01, 0.02, 0.009 | ||||||
| R0 | 37 (72%) | 30 m (70,53,43%) | NR (81,55,55%) | ||||
| R1 | 14 (28%) | 12 m (50,19,0%) | 15 m (64,24,0%) | 4.23 | 1.89–9.52 | <0.01 | |
| Charlson comorbidity index | 0.006, 0.05, 0.02 | ||||||
| Low (<4) | 18 (35%) | 40 m (94,69,43%) | 40 m (94,58,47%) | ||||
| High (≥4) | 33 (65%) | 10 m (49,30,20%) | 16 m (64,39,39%) | 7.36 | 2.68–12.12 | <0.01 | |
| Pre-operative biliary drainage | 0.03, 0.01, 0.01 | ||||||
| Endoscopic | 24 (47%) | 36 m (75,69,40%) | NR (86,62,62%) | ||||
| Percutaneous | 21 (41%) | 13 m (52,23,16%) | 14 m (65,29,20%) | 0.57 | |||
| None | 6 (12%) | 16 m (67,33,33%) | 19 m (80,40,40%) | 0.71 | |||
| LN status | 0.03, 0.006, 0.03 | ||||||
| Negative | 27 (53%) | 40 m (70,51,46%) | NR (79,58,51%) | ||||
| Positive | 18 (35%) | 30 m (67,44,22%) | 36 m (86,36,36%) | 1.27 | 1.10–5.45 | 0.05 | |
| Not assessed | 6 (12%) | 7 m (33,20,20%) | 12 m (50,35,35%) | 5.95 | 2.10–16.67 | 0.001 | |
| Tumour histological grade | 0.11, 0.21, 0.24 | ||||||
| Well differentiated | 26 (51%) | 40 m | NR | ||||
| Mod/poorly diff | 25 (49%) | 15 m | 16 m | ||||
| Pre-operative bilirubin | 0.45, 0.49, 0.75 | ||||||
| <10 mg/dl | 44 (86%) | 16 m | 36 m | ||||
| ≥10 mg/dl | 7 (14%) | 15 m | NR | ||||
| Pre-operative albumin | 0.19, 0.37, 0.26 | ||||||
| ≤3.0 g/dl | 23 (45%) | 13 m | 36 m | ||||
| >3.0 g/dl | 28 (55%) | 30 m | NR | ||||
| Operation | 0.42, 0.10, 0.01 | ||||||
| Hepatectomy | 39 (76%) | 30 m | 40 m | ||||
| No hepatectomy | 12 (24%) | 15 m | 15 m | ||||
| EBL | 0.27, 0.25, 0.52 | ||||||
| <1000 ml | 30 (59%) | 16 m | 16 m | ||||
| ≥1000 ml | 21 (41%) | 16 m | 36 m | ||||
| Transfusion (RBC peri-operative) | |||||||
| Yes | 26 (51%) | 19 m | 40 m | 0.37, 0.30, 0.06 | |||
| No | 25 (49%) | 13 m | 16 m | ||||
| Adjuvant therapy | 0.24, 0.65, 0.14 | ||||||
| Yes | 21 (41%) | 36 m | 36 m | ||||
| No | 30 (59%) | 13 m | NR | ||||
OS, overall survival; DSS, disease-specific survival; RFS, recurrence-free survival.
Log-rank test.
NR = not reached.
Discussion
In order to accomplish complete tumour clearance, the operative management of HCCA typically requires resection of the extrahepatic biliary tree in continuity with a large portion of the liver including the caudate. These procedures have substantial potential for major post-operative complications including liver failure. Extensive hepatobiliary operations also require technically challenging reconstructions of the bile ducts which pose risks for bile leakage and intra-abdominal sepsis. Our single institution experience highlights the objective complications which are encountered after extensive operations for HCCA. Seventy-six per cent of the patients in the present study underwent a major hepatectomy; however, unlike other recently published series, the caudate resection and trisectionectomy rates were under 10%.31–33 Pre-operative portal vein embolization was not utilized, but nearly 90% of patients received pre-operative biliary drainage. The R0 resection rate of 73% is consistent with other published reports from highly experienced centers.12,34–36 A comparison of outcomes for patients who underwent resection of HCCA before or after the year 2003, when a formal hepatopancreatobiliary unit was organized at our institution, did not reveal major differences between the two eras with the exception that patients were more likely to undergo hepatectomy and hepatectomy with en bloc caudate resection in the recent era (i.e. years 2003 to 2008). Despite better pre-operative imaging to improve patient selection combined with advancements in operative techniques and post-operative care, there has been no significant decrease in post-operative morbidity or mortality over time at our institution. This finding suggests that more aggressive operative approaches to patients with advanced, higher-risk tumours offset the benefits of improved peri- and post-operative care for HCCA.
Comparing patients who did or did not experience major post-operative complications or mortality, we did not unveil many objective differences between these two groups of patients except for the extent of hepatectomy and type of pre-operative biliary drainage. Pre-operative percutaneous drainage and major hepatectomy were both associated with more post-operative morbidity. On the other hand, operative blood loss and peri-operative transfusion rate, which are often interpreted as surrogates for extent of tumour burden and extent of resection, did not correlate with early or late post-operative outcomes. The negative impact of peri-operative blood transfusion on survival has been published for patients undergoing hepatectomy for hepatocellular carcinoma and colorectal metastases; however, controversy remains with regards to a true cause and effect relationship.22,24,37
Although hypoalbuminaemia did not correlate with poorer long-term outcomes, pre-operative albumin levels were significantly lower in the patients who developed major complications. Malnutrition is a major concern for patients scheduled to undergo complex abdominal operations, and nutritional assessment should be a routine component of pre-operative evaluation. A nutrition risk index has been developed that stratifies patients into groups according to the severity of malnutrition.38 This index is calculated from the albumin level and deficit of lean body mass. Severe degrees of malnutrition are associated with post-operative complication rates over 50% and mortality rates approaching 20%.35 Our centre currently employs a full-time nutritionist who is dedicated solely to the pre-operative clinical setting.
Since its inception, the Charlson comorbidity index (CCI) has provided validated prognostic information for predicting mid- and long-term survival for cancer patients.19,28,39,40 One potential deficiency of the CCI for predicting survival after resection of HCCA is the formulary dependence on chronic medical diseases (e.g. diabetes, renal failure, etc.) which could become somewhat irrelevant in the setting of aggressive cancers. In the present study, CCI correlated with post-operative complications and with long-term survival on multivariable analysis, which considered multiple factors reflective of cancer treatment. Fernandez-Ruiz et al. also reported that CCI has a negative impact on survival for patients with extrahepatic cholangiocarcinoma treated either operatively or medically.41 Given its easy application, CCI can be implemented in the risk stratification process for patients being considered for resection of HCCA.
The majority of patients with HCCA present with various degrees of liver dysfunction related to obstructive cholestasis. Many experienced centres have adopted treatment algorithms that include routine preoperative biliary drainage procedures, either percutaneous or endoscopic, while some centres use biliary drainage selectively with or without portal vein embolization after consideration of the status and volume of the future liver remnant.6,17,42,43 In this retrospective analysis, we were not able to determine how patients were selected for pre-operative biliary drainage. Pre-operative cholangitis has been reported as an independent predictor of post-operative morbidity.3,6 In our study, however, we were not able to determine accurately how many patients had clinical cholangitis before operative resection. The use of pre-operative endoscopic biliary drainage was associated with an improvement in complication rates and even long-term survival, but this finding could represent a confounding bias with less extensive hilar tumours. It is possible that reliable biliary decompression with ERC poses less risk for pre-operative cholangitis.
In this series, the overall complication rate was 69% with over two-thirds categorized as major morbidity according to the Clavien grading system.30 Whereas, many experienced centres have reported operative mortality rates less than 10% and post-operative morbidity rates of approximately 50%, our 90-day mortality rate was 12% and post-operative complications occurred in 69% of patients.6,12,15,33,42 The most common major post-operative complication was intra-abdominal sepsis which in many cases resulted from difficult bile leaks. In addition to the short-term impact of septic complications on operative mortality, several studies have demonstrated a correlation between long-term outcomes after curative resections of solid tumours and post-operative sepsis.21–23,44 Infection and sepsis potentiate proinflammatory cytokine cascades including tumour necrosis factor-α and interleukins 1, 6, and 8. These immune modulators can affect the function and regulation of natural killer cells, cytotoxic T-lymphocytes and antigen-presenting cells.45–47 Hypothetically, occult micrometastases may progress rapidly during brief and prolonged periods of relative immunosuppression resulting from systemic infection. Thus, the future development and clinical use of immunomodulators may play a role in offsetting the potentially detrimental oncologic effects of the systemic inflammatory responses during periods of post-operative sepsis and organ dysfunction.
A major goal for the operative management of HCCA must focus on reducing peri- and post-operative complications. Sano and colleagues at the National Cancer Center Hospital in Tokyo, Japan performed 102 consecutive hepatobiliary resections for perihilar cholangiocarcinoma without a single case of operative mortality and with a major complication rate under 7%.6 These impressive results and others have served as a benchmark for many centres who treat patients with cholangiocarcinoma.10,48
Based on the significant impact of existing pre-operative co-morbidities on long-term outcomes, patient selection must consider both cancer- and patient-related factors. Despite the limitations of a retrospective design and small sample size, the results of the present study show a clear association between post-operative morbidity and short-term mortality and long-term cancer-specific survival for patients undergoing resection for HCCA. Operations for hilar bile duct tumours must be conducted safely with minimal post-operative mortality and acceptable morbidity. Multiple parameters must be considered to improve short- and long-term outcomes in patients with HCCA. Proper patient selection with judicious use of pre-operative biliary drainage and portal vein embolization is crucial. Performance of a safe operation with minimal blood loss and careful anatomic reconstruction is important for reducing post-operative complications. Avoidance of morbidity will increase the pool of patients who are eligible to receive more effective adjuvant therapies which should therefore improve long-term survival.
Conflict of interest
None declared.
References
- 1.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 2.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 3.Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463–475. doi: 10.1097/00000658-199610000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakeeb A, Tran KQ, Black MJ, Erickson BA, Ritch PS, Quebbeman EJ, et al. Improved survival in resected biliary malignancies. Surgery. 2002;132:555–564. doi: 10.1067/msy.2002.127555. [DOI] [PubMed] [Google Scholar]
- 5.Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BJ, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–519. doi: 10.1097/00000658-200110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sano T, Shimada K, Sakamoto Y, Yamamoto J, Yamasaki S, Kosuge T. One hundred two consecutive hepatobiliary resections for perihilar cholangiocarcinoma with zero mortality. Ann Surg. 2006;244:240–247. doi: 10.1097/01.sla.0000217605.66519.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemming AW, Reed AI, Fujita S, Foley DP, Howard RJ. Surgical management of hilar cholangiocarcinoma. Ann Surg. 2005;241:693–699. doi: 10.1097/01.sla.0000160701.38945.82. discussion 699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 9.Ikeyama T, Nagino M, Oda K, Ebata T, Nishio H, Nimura Y. Surgical approach to bismuth Type I and II hilar cholangiocarcinomas: audit of 54 consecutive cases. Ann Surg. 2007;246:1052–1057. doi: 10.1097/SLA.0b013e318142d97e. [DOI] [PubMed] [Google Scholar]
- 10.Nishio H, Nagino M, Nimura Y. Surgical management of hilar cholangiocarcinoma: the Nagoya experience. HPB (Oxford) 2005;7:259–262. doi: 10.1080/13651820500373010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baton O, Azoulay D, Adam DV, Castaing D. Major hepatectomy for hilar cholangiocarcinoma type 3 and 4: prognostic factors and longterm outcomes. J Am Coll Surg. 2007;204:250–260. doi: 10.1016/j.jamcollsurg.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 12.Endo I, House MG, Klimstra DS, Gonen M, D'Angelica M, DeMatteo RP, et al. Clinical significance of intraoperative bile duct margin assessment for hilar cholangiocarcinoma. Ann Surg Oncol. 2008;15:2104–2112. doi: 10.1245/s10434-008-0003-2. [DOI] [PubMed] [Google Scholar]
- 13.Otto G, Romaneehsen B, Hoppe-Lotichius M, Bittinger F. Hilar cholangiocarcinoma: resectability and radicality after routine diagnostic imaging. J Hepatobiliary Pancreat Surg. 2004;11:310–318. doi: 10.1007/s00534-004-0912-9. [DOI] [PubMed] [Google Scholar]
- 14.Todoroki T, Kawamoto T, Koike N, Takahashi H, Yoshida S, Kashiwagi H, et al. Radical resection of hilar bile duct carcinoma and predictors of survival. Br J Surg. 2000;87:306–313. doi: 10.1046/j.1365-2168.2000.01343.x. [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa S, Ikai I, Fujii H, Hatano E, Shimahara Y. Surgical resection of hilar cholangiocarcinoma: analysis of survival and postoperative complications. World J Surg. 2007;31:1256–1263. doi: 10.1007/s00268-007-9001-y. [DOI] [PubMed] [Google Scholar]
- 16.Otani K, Chijiiwa K, Kai M, Ohuchida J, Nagano M, Tsuchiya K, et al. Outcome of surgical treatment of hilar cholangiocarcinoma. J Gastrointest Surg. 2008;12:1033–1040. doi: 10.1007/s11605-007-0453-z. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy TJ, Yopp A, Qin Y, Zhao B, Guo P, Liu F, et al. Role of preoperative biliary drainage of liver remnant prior to extended liver resection for hilar cholangiocarcinoma. HPB (Oxford) 2009;11:445–451. doi: 10.1111/j.1477-2574.2009.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garman KS, Pieper CF, Seo P, Cohen HJ. Function in elderly cancer survivors depends on comorbidities. J Gerontol A Biol Sci Med Sci. 2003;58:M1119–M1124. doi: 10.1093/gerona/58.12.m1119. [DOI] [PubMed] [Google Scholar]
- 19.Ouellette JR, Small DG, Termuhlen PM. Evaluation of Charlson-Age Comorbidity Index as predictor of morbidity and mortality in patients with colorectal carcinoma. J Gastrointest Surg. 2004;8:1061–1067. doi: 10.1016/j.gassur.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 20.Seo PH, Pieper CF, Cohen HJ. Effects of cancer history and comorbid conditions on mortality and healthcare use among older cancer survivors. Cancer. 2004;101:2276–2284. doi: 10.1002/cncr.20606. [DOI] [PubMed] [Google Scholar]
- 21.Chok KS, Ng KK, Poon RT, Lo CM, Fan ST. Impact of postoperative complications on long-term outcome of curative resection for hepatocellular carcinoma. Br J Surg. 2009;96:81–87. doi: 10.1002/bjs.6358. [DOI] [PubMed] [Google Scholar]
- 22.Farid SG, Aldouri A, Morris-Stiff G, Khan AZ, Toogood GJ, Lodge JP, et al. Correlation between postoperative infective complications and long-term outcomes after hepatic resection for colorectal liver metastasis. Ann Surg. 2010;251:91–100. doi: 10.1097/SLA.0b013e3181bfda3c. [DOI] [PubMed] [Google Scholar]
- 23.Ito H, Are C, Gonen M, D'Angelica M, DeMatteo RP, Kemeny NE, et al. Effect of postoperative morbidity on long-term survival after hepatic resection for metastatic colorectal cancer. Ann Surg. 2008;247:994–1002. doi: 10.1097/SLA.0b013e31816c405f. [DOI] [PubMed] [Google Scholar]
- 24.Katz SC, Shia J, Liau KH, Gonen M, Ruo L, Jarnagin WR, et al. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg. 2009;249:617–623. doi: 10.1097/SLA.0b013e31819ed22f. [DOI] [PubMed] [Google Scholar]
- 25.Rizk NP, Bach PB, Schrag D, Bains MS, Turnbull AD, Karpeh M, et al. The impact of complications on outcomes after resection for esophageal and gastroesophageal junction carcinoma. J Am Coll Surg. 2004;198:42–50. doi: 10.1016/j.jamcollsurg.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Law WL, Choi HK, Lee YM, Ho JW. The impact of postoperative complications on long-term outcomes following curative resection for colorectal cancer. Ann Surg Oncol. 2007;14:2559–2566. doi: 10.1245/s10434-007-9434-4. [DOI] [PubMed] [Google Scholar]
- 27.Schiesser M, Chen JW, Maddern GJ, Padbury RT. Perioperative morbidity affects long-term survival in patients following liver resection for colorectal metastases. J Gastrointest Surg. 2008;12:1054–1060. doi: 10.1007/s11605-007-0438-y. [DOI] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 29.Greene F, Page D, Fleming I, Fritz A, Balch C, Haller D, et al. AJCC Cancer Staging Manual. 6th edn. New York, NY: Springer; 2002. [Google Scholar]
- 30.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dinant S, Gerhards MF, Busch OR, Obertop H, Guoma DJ, Van Gulik TM. The importance of complete excision of the caudate lobe in resection of hilar cholangiocarcinoma. HPB (Oxford) 2005;7:263–267. doi: 10.1080/13651820500372376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paik KY, Choi DW, Chung JC, Kang KT, Kim SB. Improved survival following right trisectionectomy with caudate lobectomy without operative mortality: surgical treatment for hilar cholangiocarcinoma. J Gastrointest Surg. 2008;12:1268–1274. doi: 10.1007/s11605-008-0503-1. [DOI] [PubMed] [Google Scholar]
- 33.Hidalgo E, Asthana S, Nishio H, Wyatt J, Toogood GJ, Prasad KR, et al. Surgery for hilar cholangiocarcinoma: the Leeds experience. Eur J Surg Oncol. 2008;34:787–794. doi: 10.1016/j.ejso.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Jarnagin WR, Ruo L, Little SA, Klimstra D, D'Angelica M, DeMatteo RP, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98:1689–1700. doi: 10.1002/cncr.11699. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki R, Takeda Y, Funato O, Nitta H, Kawamura H, Uesugi N, et al. Significance of ductal margin status in patients undergoing surgical resection for extrahepatic cholangiocarcinoma. World J Surg. 2007;31:1788–1796. doi: 10.1007/s00268-007-9102-7. [DOI] [PubMed] [Google Scholar]
- 36.Wakai T, Shirai Y, Moroda T, Yokoyama N, Hatakeyama K. Impact of ductal resection margin status on long-term survival in patients undergoing resection for extrahepatic cholangiocarcinoma. Cancer. 2005;103:1210–1216. doi: 10.1002/cncr.20906. [DOI] [PubMed] [Google Scholar]
- 37.Tralhao JG, Kayal S, Dagher I, Sanhueza M, Vons C, Franco D. Resection of hepatocellular carcinoma: the effect of surgical margin and blood transfusion on long-term survival. Analysis of 209 consecutive patients. Hepatogastroenterology. 2007;54:1200–1206. [PubMed] [Google Scholar]
- 38.Sungurtekin H, Sungurtekin U, Balci C, Zencir M, Erdem E. The influence of nutritional status on complications after major intraabdominal surgery. J Am Coll Nutr. 2004;23:227–232. doi: 10.1080/07315724.2004.10719365. [DOI] [PubMed] [Google Scholar]
- 39.Birim O, Kappetein AP, Bogers AJ. Charlson comorbidity index as a predictor of long-term outcome after surgery for nonsmall cell lung cancer. Eur J Cardiothorac Surg. 2005;28:759–762. doi: 10.1016/j.ejcts.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 40.Singh B, Bhaya M, Stern J, Roland JT, Zimbler M, Rosenfield RM, et al. Validation of the Charlson comorbidity index in patients with head and neck cancer: a multi-institutional study. Laryngoscope. 1997;107(Part 1):1469–1475. doi: 10.1097/00005537-199711000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez-Ruiz M, Guerra-Vales JM, Colina-Ruizdelgado F. Comorbidity negatively influences prognosis in patients with extrahepatic cholangiocarcinoma. World J Gastroenterol. 2009;15:5279–5286. doi: 10.3748/wjg.15.5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawasaki S, Imamura H, Kobayashi A, Noike T, Miwa S, Miyagawa S. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg. 2003;238:84–92. doi: 10.1097/01.SLA.0000074984.83031.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parikh AA, Abdalla EK, Vauthey JN. Operative considerations in resection of hilar cholangiocarcinoma. HPB (Oxford) 2005;7:254–258. doi: 10.1080/13651820500373093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kressner U, Graf W, Mahteme H, Pahlman L, Glimelius B. Septic complications and prognosis after surgery for rectal cancer. Dis Colon Rectum. 2002;45:316–321. doi: 10.1007/s10350-004-6174-4. [DOI] [PubMed] [Google Scholar]
- 45.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 46.Menetrier-Caux C, Montmain G, Dieu MC, Bain C, Favrot MC, Caux C, et al. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood. 1998;92:4778–4791. [PubMed] [Google Scholar]
- 47.Horn F, Henze C, Heidrich K. Interleukin-6 signal transduction and lymphocyte function. Immunobiology. 2000;202:151–167. doi: 10.1016/S0171-2985(00)80061-3. [DOI] [PubMed] [Google Scholar]
- 48.Hirano S, Kondo SKS, Tanaka E, Shichinohe T, Tsuchikawa T, Kato K, et al. Outcome of surgical treatment of hilar cholangiocarcinoma: a special reference to postoperative morbidity and mortality. J Hepatobiliary Pancreat Surg. 2009;17:455–462. doi: 10.1007/s00534-009-0208-1. [DOI] [PubMed] [Google Scholar]


